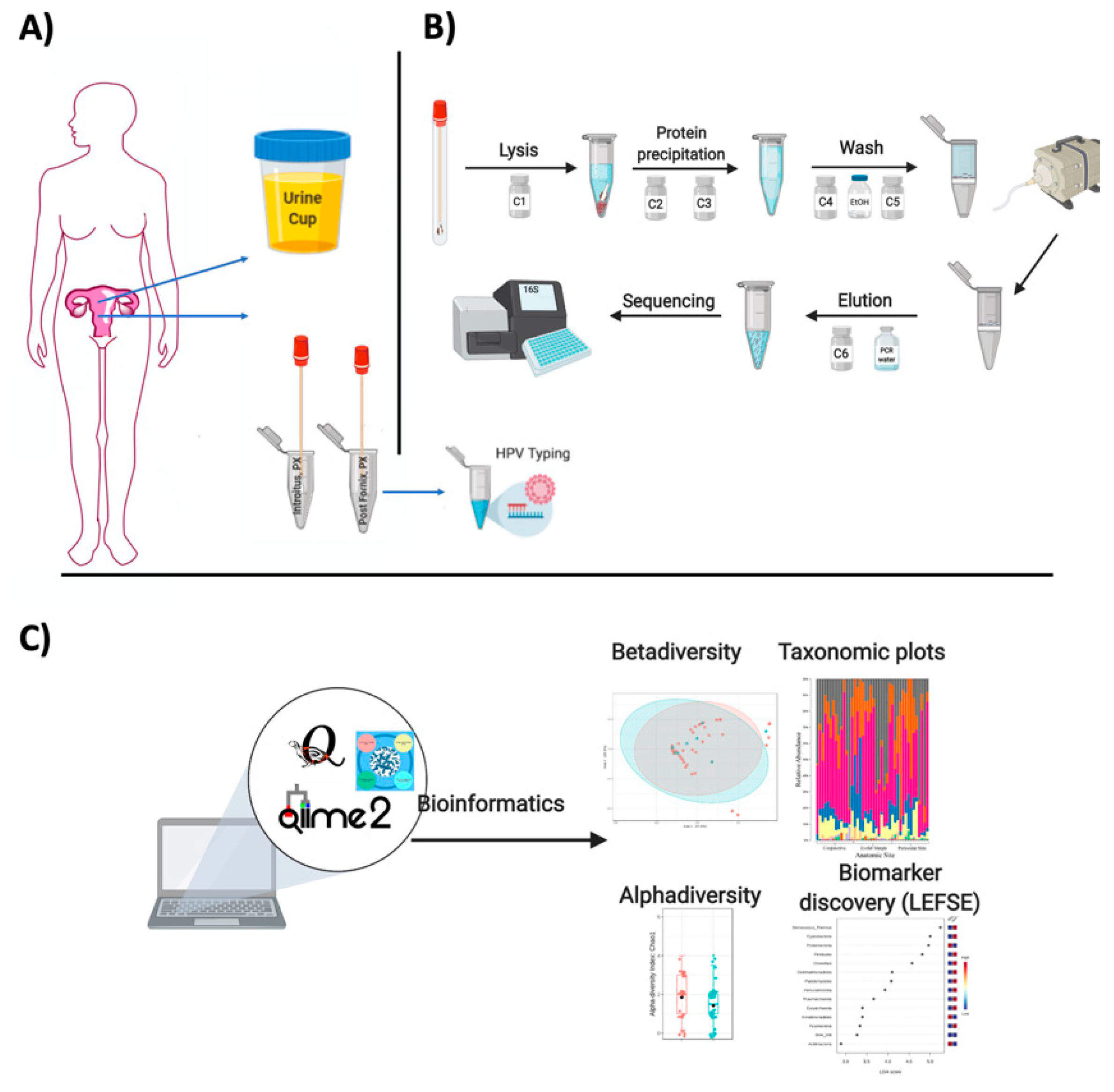
A Protocol for the Multi-Omic Integration of Cervical Microbiota and Urine Metabolomics to Understand Human Papillomavirus (HPV)-Driven Dysbiosis
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials for Microbiota Production
2.1.1. Patient Recruitment and Sampling
- With the approved IRB protocol, explain the informed consent verbally to the patient.
- Deliver the questionnaire.
- Gather the data collection form—recording the patient health record, sample ID, weight and height, age, and check exclusion criteria such as those suggested in the Manual of Procedures of the Human Microbiome [23]: (1) taken antibiotics in the last month (30 days); (2) have a history of regular urinary incontinence; (3) Treatment for or suspicion of, ever having had toxic shock syndrome; (4) have candidiasis; (5) urinary tract infections; (5) active STD: (6) vaginal irritation at the time of screening and (7) a total hysterectomy (or other criteria specific for each given project).
- Deliver the educational material (flyer) and the copy of the signed consent.
- Give the Sterile 4oz Specimen Cups, and ask the patient to collect the urine.
- During the oB/Gyn visit, during the pelvic examination, labia are spread for introitus visualization, and the specimen is collected by placing one swab at the vagina, posterior to the hymenal ring/tissue. The swab should be rotated along the lumen with a circular motion. Swabs will then be placed in the 1.5 mL Eppendorf.
- For the cervical samples (posterior fornix), a Pederson speculum is inserted for access and visualization of the cervix. The sterile swab will be placed in the posterior fornix and rotated along the lumen with a circular motion and swabs immediately placed in the sterile tubes.
- All vaginal and cervical swab samples will be stored at ultra-low temperatures (−80 °C) until DNA extractions.
2.1.2. Collection of Cervical Swabs for Microbiota and HPV Typing
- Cotton tipped swab applicator with semi-flexible polystyrene handle
- 1.5mL Eppendorf tubes
- Zip-Lock bags
2.2. Genomic DNA Extraction for Microbiota and HPV Typing
- The first step is an alkaline lysis. Solution C1 contains Sodium Dodecyl Sulfate (SDS)—an anionic detergent that breaks down fatty acids and lipids of cell membranes to aid in cell lysis. Should it form a precipitate (cold temperature), just warm the solution in a water bath (up to 60 °C). The solution can be used while it is still warm.
- Vortex Power Bead tubes with the swabs briefly.
- Add 60 μL of C1 to the power bead tube with the swab and vortex briefly.
- Vortex horizontally at maximum speed for 10 min (physical cell disruption).
- Centrifuge for 30 s at 13,000 rpm.
- Transfer all supernatant to a clean 2ml collection tube (~750 μL volume).
- In the Second step (solution C2), what occurs is the precipitation of non-DNA organic and inorganic material, including cell debris and proteins. Given that cervical swabs are low biomass samples, this step will be joined with inhibitor removal (solution C3).
- Add 100 μL of C2 and 100 μL of C3. Vortex for 5 s.
- Incubate at −20 °C for 5 min.
- Centrifuge at room temperature for 1 min at 13,000 rpm.
- Avoiding the pellet, transfer up to, but no more than 750 μL of supernatant to a clean 2 mL tube.
- The next step with solution 4 (rich in salt), is aimed at binding the DNA to spin filter.
- Add 1200 μL of C4 (previously shaken) to the former supernatant and vortex for 5 s.
- Place the spin filters in the vacuum manifold and load approximately 675 μL onto a spin filter. Turn on the vacuum until all the supernatant flows through. Add an additional 675 μL of the supernatant to the spin filter and vacuum.
- Load the remaining of the supernatant and repeat it. A total of three loads for each sample processed are required.
- The next step includes washing the DNA bound to the membrane and remove contaminants.
- Add 650 μL of cold 100% ethanol (−20 °C) to all the tubes and turn on the vacuum.
- Add 500 μL of solution C5 to all tubes and turn on the vacuum. Carefully place the spin filter in a clean 2ml collection tube. Avoid splashing any Solution C5 onto the spin filter. It is critical to remove all traces of Solution C5 as it can interfere with many downstream DNA applications.
- The final step is DNA elution from the membrane using sterile solution C6 (10 mM Tris) or sterile PCR water.
- Add 60–100 μL of C6 (55 °C) to the center of the white filter membrane and let sit for 5 min. Volume range depends on how diluted you wish the sample to be, If low biomass, lower volumes of C6 are suggested.
- Centrifuge for 1 min at room temperature at 13,000 rpm.
- Discard the spin filter and store DNA at −20 °C (Figure 1).
2.2.1. Human Papilloma Virus Genotyping
- Thaw the DNA previously extracted from clinical samples, mix by vortexing and spin down the tubes to collect all liquid at the bottom of the tube.
- The assay uses SPF10 primers to amplify a 65-bp fragment of the L1 open reading frame of HPV genotypes, followed by a reverse-hybridization step. The 65-bp PCR fragment assay amplifies the following common mucosal HPV genotypes: 6, 11, 16, 18, 31, 33, 34, 35, 39, 40, 42, 43, 44, 45, 51, 52, 53, 54, 56, 58, 59, 66, 68/73, 70, and 74. This step uses Boxes A and B.
- Prepare the master mix for the PCR. At first use of the PCR master mix (vial A1), it is recommended to mix the contents of the vial on a Vortex mixer and aliquot the contents in ready-to-use portions of 40 μL. This further reduces the risk of contamination. After aliquoting, store at −20 °C
- Prepare the tubes/96-well plates (depending on the number of samples) considering the number of the samples to process and include negative and positive controls provided in the kit.
- Mark each PCR reaction vial/PCR plate.
- Spin down the liquid in the tubes with the processed specimen for 15 s at 14,000 rpm in a minicentrifuge.
- Add (one by one) 10 μL of the supernatant of the processed sample to the appropriate vials with PCR master mix. The vials intended for the HPV negative and positive PCR controls remain closed. Cap each vial after the addition of DNA before proceeding with the next.
- Mix HPV positive PCR control (vial B1) on a Vortex mixer, add 10 μL to the appropriate vial, and close the vial.
- Use clean filter tips for each sample and mix each sample with the PCR mix by pipetting up and down a few times.
- Insert the vials into the automatic thermal cycler and run the appropriate cycling program:9 min at 94 °C
- 40 cycles of:
- 30 s denaturation at 94 °C
- 45 s annealing at 52 °C
- 45 s elongation at 72 °C
3 min at 72 °C (final elongation)Keep the amplified products at 2–8 °C for short-term storage or freeze at −20 °C for long term storage. - In the second step, the amplified fragments undergo a line probe assay by reverse-hybridization assay performed by using the portion of the kit named RHA kit HPV SPF10-LiPA25. In this second step, the biotinylated amplicons are denatured and then hybridized with specific oligonucleotide probes immobilized as parallel lines in membrane strips. After immobilization of the oligonucleotides on the strips, these were washed, and streptavidin alkaline phosphatase (SAP) is added to bind to the biotinylated hybrid formed, yielding a purple precipitate in the strip that visually determines the specific HPV type compared to the kit-provided controls. The LiPA strips are visually inspected and interpreted following the standardized reference guide provided by the kit’s manual.
- Prewarm the vials with the hybridization Solution (HS) and the Stringent Wash Solution (SW) to at least 37 °C but must not exceed the hybridization temperature of 49 °C (all crystals should be dissolved before the opening of the vial).
- Use 2 mL of hybridization solution for each strip + 15 mL in excess for each run.
- Use 6 mL of stringent wash solution for each strip + 30 mL in excess for each run.
- Rinse solution (RS) should be prepared by diluting the concentrated rinse solution 1/5 (1 part concentrated solution + 4 parts of water). Prepare 10 mL rinse solution for each strip + 55 mL in excess.
- Conjugate (C) solution and substrate solution should be prepared by diluting the concentrated conjugate or substrate 1/100 in conjugate diluent or substrate buffer, respectively. Mix gently.
- Prepare 2 mL conjugate solution and substrate solution for each strip + 5 mL in excess for 22 or less strips.
- In order to reduce the excess of buffer for each run with 22 or less strips, use a 50 mL conical tube and place it in the bottle provided with the ProfiBlotTM 48 T. When testing more than 22 strips an excess of 20 mL for each run is needed.
- Use 2 mL of substrate buffer for each strip + 5 mL in excess for each run with 22 or less strips. In order to reduce the excess of buffer for each run with 22 or less strips, use a 50 mL conical tube and place it in the bottle provided with the ProfiBlotTM 48 T.
- When testing more than 22 strips an excess of 20 mL for each run is needed.
- All vessels used to prepare conjugate and substrate solutions should be cleaned thoroughly and rinsed with water.
- The next step is a stringent wash using rinse solution and conjugate.
- Remove the tray from the water bath
- Aspirate the liquid with a pipette. Add 2ml prewarmed SW to each strip.
- Shake the strips for 10 to 20 s at room temperature (RT). Aspirate the solution.
- Repeat the washing step.
- Aspirate the solution.
- Add 2ml of SW to each strip.
- Incubate in the shaking water at 50 °C for 30 min. Close the lid of the bath.
- Aspirate the Stringent Wash Solution.
- The final step is the strip color development. These steps will be at RT on a shaker (not on a water bath).
- Wash each strip twice for 1 min using 2 mL of Rinse Solution (RS). Aspirate.
- Add 2 mL of C (conjugate solution) to each sample and incubate for 30 min while shaking. At this point, prepare the substrate solution (S).
- Aspirate the C solution.
- Wash each strip twice for 1 min using 2ml of RS. Aspirate.
- Wash once more using 2 mL of substrate buffer (SB). Aspirate.
- Add 2 mL of the S solution an incubate for 30 min in the dark (cover with aluminium foil) while shaking. Stop the colour development by washing the strip twice in 2 mL of water while shaking for 3 min.
- Remove and place the strip with forceps in absorbent paper.
- Wait for the strip to dry completely.
- Compare strip bands with the interpretation sheet provided with the kit.
2.2.2. 16S rDNA (Bacterial) Read Quality Control and Data Analyses
- Currently, it is cheaper to extract good quality DNA and send directly to any company for outsourced sequencing of 16S rDNA genes.
- To characterize the microbiota, one sequences the V4 hypervariable region of the 16S ribosomal RNA using the universal bacterial primers: 515F (5′ GTGCCAGCMGCCGCGGTAA 3′) and 806R (5′ GGACTACHVGGGTWTCTAAT 3′) as described in the Earth Microbiome Project (EMP; http://www.earthmicrobiome.org/emp-standard-protocols/16s/;) [27].
- Once the facility sends the data, raw FASTQ reads can be uploaded to QIITA https://qiita.ucsd.edu/, an entirely open-source microbial study management platform [28]. It allows users to keep track of multiple studies with multiple omics data, and use stringent quality criteria PHRED scores (quality score of the nucleotides generated by automated DNA sequencing) with an ASCII offset of 33, and the user can choose the length of the reads for trimming, demultiplexing and binning using a given database (Greengenes, SILVA, or the Ribosomal Database Project-RDP). The usefulness of this platform is that it allows data to be directly made public via EBI (the European Bioinformatics Institute), a great service offered by the QIITA team at the University of California San Diego (UCSD).
- After the OTU table is prepared in QIITA, an alternative is to use QIIME2 for analyses or R packages such as Bioconductor [29] or the quick tool microbiomeanalyst (https://www.microbiomeanalyst.ca) for preliminary analyses [30].
- Reads matching chloroplast, mitochondria, and unassigned sequences should be removed as well as rare singletons.
- Downstream taxonomy plots, alpha and beta analyses should follow a given rarefaction level considering a minimum number of reads shared by most samples.
- Heatmaps can be built using the plot_heatmap function from the heatmap library [33].
- For beta diversity, samples can be visualized using pairwise Bray–Curtis distance between samples using the R package [35] with vegdist function in vegan [36]. The global differences in bacteria can be visualized with Principal Coordinates Analysis (PCoA) or non-metric multidimensional scaling (NMDS). Alternatively, beta diversity can be visualized using UniFRAC [37].
2.3. Collection of Urine Samples for Metabolomics
- Wet ice
- Gloves
- Sterile 4oz Specimen Cups
- Eppendorf vials of 1.5 mL
- Zip-Lock bags
2.4. Extraction of Metabolites from Urine
- An amount of 200 μL of liquefied urine samples
- Extraction solution: Methanol/Water mixture 8:1 (by volume)
- Borosilicate glass disposable culture tubes 16 × 100 mm
- Glass pipettes, 10 mL
- plastic beaker, 50 mL
- Pasteur pipettes and pipette rubber bulbs
- Vortex
- Reaction tube centrifuge (e.g., Eppendorf 5810 R)
- Vials, screw top, clear glass, 1.5 mL, thread 8-425
- Assembled screw cap with the hole with PTFE/silicone septum, thread 8-425 (Cat# 27093-U Sigma-Aldrich)
- Plastic caps solid-top, thread 8-425
- Rotary vacuum evaporator
2.5. Metabolites Derivatization
- Derivatization solution 1: 20 mg/mL Methoxyamine hydrochloride in pyridine (see Note 1)
- Derivatization solution 2: N-tert-Butyldimethylsilyl-N-methyltrifluoroacetamide with 1% tert-Butyldimethylchlorosilane (MTBSTFA+1% TBDMSCl, mixture 99:1, Cat # 375934-10X1ML, Sigma-Aldrich)
- Glass pipettes, 10 mL
- Vials, screw top, clear glass, 1.5 mL, thread 8-425
- Black plastic caps with a solid top, white rubber liner, thread 8-425
- Vials, screw top, clear glass, 4 mL, thread 13-425
- Black plastic caps, solid top, white rubber liner, thread 13-425
- Dry Block Incubator for glass vials (diameter of the well ~14 mm)
- Eppendorf vial of 1.5 mL
- Reaction tube centrifuge (e.g., Eppendorf 5415D)
- Glass inserts (Cat # 29445-U, Sigma-Aldrich) (see Note 2).
- Assembled screw cap with a hole with PTFE/silicone septum, thread 8-425 (Cat# 27093-U Sigma-Aldrich
- Hexane ≥ 95% for HPLC
2.5.1. Gas Chromatography/Mass Spectrometry (GC/MS) Analysis and Data Acquisition
- GC/MS
- Fused-silica capillary column RXI-5MS (0.25 mm inner diameter, 0.25 μm D.F., 30 m)
- Hexane ≥ 95% for HPLC
- NIST/EPA/NIH Mass spectral Library (NIST14)
2.5.2. Extraction of Metabolites
- Separate 200 μL of liquefied urine samples and place them on ice.
- Mix with 800 μL of the cold methanol-water mixture (8:1 v/v)
- Vortex mixture for 1 min.
- Keep the sample at 4 °C or ice for 20 min.
- Centrifuge at 6000 rpm for 8 min at 4 °C.
- De-assemble the screw cap and remove silicone septum. Perforate it using scissors and mount vials onto a rotary vacuum evaporator. (see Note 3).
- Remove 200 μL of the supernatant from the top phase and transfer to a glass vials, thread 8-425.
- Prepare reference pool quality control samples (see Note 4).
- Attach vials to a rotary vacuum evaporator (see Note 5).
- Evaporate supernatants to dryness.
- Replace caps with solid tops.
- Put vials in desiccator and store at −80 °C for at least four weeks.
2.5.3. Metabolites Derivatization
- Prepare the derivatization solution 1: mix 20 mg Methoxyamine hydrochloride in 1 mL pyridine using 4 mL glass vials with plastic caps solid-top, thread 13-425 (see Note 6). Briefly, vortex the obtained solution at RT until Methoxyamine hydrochloride is fully dissolved.
- Take out dried samples from storage and allow them to warm up to room temperature for at least 15 min before derivatization (see Note 7) and add 50 µL Derivatization Solution 1, close tightly the vials with closed caps and incubate at 37 °C for two hours.
- Remove samples, add 50 µL of the Derivatization Solution 2 (see Note 8) directly to the reaction mixture in the vial, close tightly the vials with closed caps and incubate for an additional 1 h at 60 °C.
- Transfer the reaction mixture to labeled Eppendorf vial and centrifuge at 13,000 rpm for 10 min at RT.
- Transfer the supernatant in the new glass vial and immediately close each sample with a solid plastic cap, thread 8-425. Store for a long time at −20 °C and for a short time at 4 °C (see Note 9).
- For the analysis, place a glass insert in the new glass vial and dilute each sample 1:50 with hexane prior to GC/MS analysis (see Note 10).
2.6. GC/MS Analysis
- The mass spectrometer must be tuned according to the manufacturer’s manuals for optimal parameters for ion lenses, detector voltage, and other settings. Usually, this can be performed in autotune operation.
- Inject 1 μL of each sample including quality control samples in the GC-MS.
- Separate metabolites using a GC temperature ramping program. The GC oven can be programmed from 100 °C to 280 °C at a rate of 4 °C/min. The injection port temperature will be 280 °C, and helium is used as the carrier gas at a constant linear velocity of 39 cm/s. Injection mode—split (15:1).
- Detect metabolites by setting the ion source filament energy to 70 eV, the ion source temperature −200 °C, and the scan range (mass-to-charge, m/z) 35–600 Da.
Metabolomic Data Acquisition and Analyses
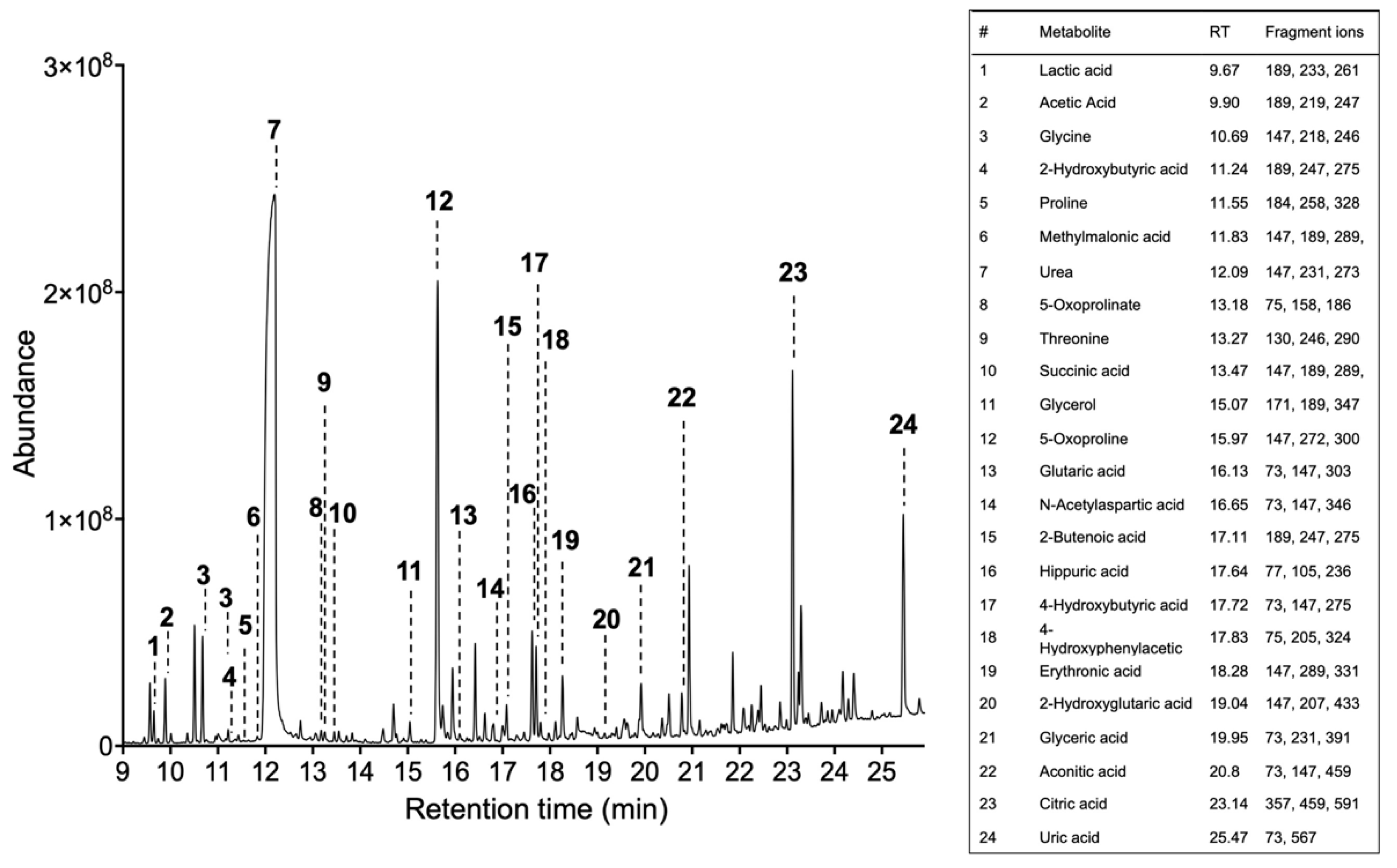
- Obtain the total ion chromatogram and mass chromatograms for each detected metabolite GC/MS instruments generate a single file per sample, a list of mass spectra together with their corresponding retention times (RT). These spectra are commonly shown on a chromatogram represented by RT on the horizontal axis and signal intensity on the vertical axis (Figure 2).
- Identify metabolites based on the fragmentation pattern of molecular ions (characteristic fragment ions) using the GC/MS manufacturer’s software equipped with NIST/EPA/NIH Mass spectral Library (NIST14) (see Note 11).
2.7. Statistical Analysis
2.7.1. Microbiota
- Statistical tests on the beta diversity can be completed via community-level differences between sample groups, assessed using the PERMANOVA test, which allows sample-sample distance to be applied to an Analyses of Variance (ANOVA)-like framework [38]. This PERMANOVA test is determined through permutations and provides strength and statistical significance on sample groupings using a Bray–Curtis distance matrix as the primary input.
- Analyses of Variance tests using the aov function in R (Team, 2008) must be used on the rarefied richness (alpha) values as well as for the Shannon diversity values, to find significant differences related to a given category. p-Values should be considered using an FDR of 0.05 to be considered significant.
- The differential abundance test for OTUs, to identify possible signatures significantly associated with the metadata categories (biomarkers), can be completed with non-parametric Wilcoxon rank sum tests that have lower false positive rates and are more robust to outliers, without needing a normal distribution assumption.
- Biomarker analyses can be identified also with LEFSE [34].
2.7.2. Metabolomics
- Quantify each peak using the maximum peak intensity value and organize data as a “peak intensity table” using MS Excel or similar software (see Note 12). The table must include metabolite identities and peak intensity values and saved in Comma Separated Values (.csv) or Tab Delimited Text (.txt). Perform statistical analyses using free online tool MetaboAnalyst.ca [39,40] (see Note 13).
- Open MetaboAnalyst.ca, choose “Statistical Analysis”, upload the “peak intensity table” and submit. If the table was organized correctly and saved in the required format, the “Data Integrity Check” will pass the information for further analysis. Click the “Skip” button to accept the default practice (see Note 14).
- In “Data Filtering” click “None”.
- Perform data normalization to remove unwanted variations between the samples. First, perform “sample-specific normalization. Click “Proceed” (see Note 15).
- For two groups (control and experimental) perform univariate analysis (fold change analysis, T-tests, volcano plot, correlation analysis).
- For more groups, perform chemometrics analysis (Principal Component Analysis (PCA), Partial Least Squares-Discriminant Analysis (PLS-DA), feature identification and cluster analysis. Detect outliers to improve downstream results (see Note 16).
3. Multi-Omic Integration of Microbiota and Metabolomics
- Prepare the output data files and save in txt format for each sample in either control or experimental groups separately.
- For “microbiome”: From QIITA or QIIME, export the species table (BIOM) with Greengenes ID as OTU identifiers [43]. This will allow for PICRUST [44] to use the OTU table and result in a functional-gene-count matrix, telling the count of each functional-gene in each of the samples surveyed, based on the reference genome.
- For “metabolome”—use KEGG [45] IDs for each identified metabolite and it’s identified concentration.
- Log in to borensteinlab.com/software_MIMOSA2.html. The following options will be used for the analysis:
- B
- Microbiome data—create a table using Greengenes 13_5 or 13_8 OTUs;
- C
- Metabolic model settings—PICRUSt and KEGG metabolic model;
- D
- Metabolome data—create a table using KEGG compound IDs, that can be logarithmically transformed if required;
- E
- Algorithm settings—the least-squares (OLS) regression estimation could be used for comparing metabolite levels with community metabolic potential (CMP) scores identified with positive model slope and a model p-value < 0.1. (see Note 17).
- F
- Log in to borensteinlab.com/software_MIMOSA2.html.
- MIMOSA2 will calculate CMP scores for each metabolite and generate a tab-delimited file for each bacterial species in each condition. Additionally, a contribution table will be generated as a tab-delimited file which will relate taxa, to KEGG metabolic pathways with its associated p-value, model slope and VarShare (represent the fraction of the variation) for each metabolite explained by the taxon in question. VarShare values could be used to build heatmaps in R, in the same format as those for microbial taxa. Finally, MIMOSA2 will identify microbial features that may underlie differences in microbial metabolite concentrations between similar communities. It answers questions such as: (1) Do the metabolites in a given dataset appear to vary depending on microbiome composition? Which ones? (2) Can differences in microbiome metabolic capabilities explain metabolite variation? (3) Which taxa, genes, and reactions appear to be playing a role in metabolite differences? [42].
Notes
- Methoxyamine hydrochloride is very sensitive to moisture. Storage is recommended under the vacuum at RT.
- Glass inserts decrease the surface area inside the vial allowing to achieve maximum sample recovery and easier sample removal.
- De-assemble the screw cap and remove silicone septum. Perforate it using scissors and mount the vials onto a rotary vacuum evaporator. This step is required to minimize sample cross-contamination during evaporation.
- Preparation of the reference pool quality control samples. Prepare a large pool sample during the preparation of sample extracts by aliquoting 100 µl from each 1 mL authentic subject sample. After a collection of a large pool sample, aliquot all for 1 mL in separate vials (thread 8-425), label all as reference quality controls and process similar to authentic subject samples. Continue as instructed in Section 3.3.7. The derivatization solution without the sample only, and empty vials, must also be included as quality controls in the analysis to ensure that identified metabolites are derived from the sample [46].
- Attach the glass vials to the rotary evaporator [47].
- Derivatization Solution 1 must be freshly prepared.
- During derivatization, avoid contact with moisture, which will result in metabolite degradation. If samples are not dried, it is necessary to proceed to additional evaporation as said in Sections 3.4.9–3.4.11.
- Derivatization Solution 2 must be used from freshly opened vials.
- If an analysis is performed after samples preparation, wait two hours before injecting the first sample into the GC-MS.
- The sample concentration must be adequately adjusted to give sufficient chromatogram, then it needs to be concentrated before injecting the sample into the gas chromatograph. The dilution of a sample (1:50) was found to be sufficient for the analysis. However, if the signal is too low, the concentration could be increased. Derivatization solution without the sample only, and empty vials, must also be included as quality controls in the analysis to ensure that identified metabolites
- MS detects the mass of the molecular ions and the masses of the fragment ions. The database contains extensive information on many compounds including the masses of fragment ions that help to perform successful metabolite identification. In addition, to ensure a valid peak identification, data can be also processed using a deconvolution method, which is a signal processing technique that estimates the relative area corresponding to each peak when multiple peaks overlap within the same spectral region. This is especially important for low abundant metabolites that might co-elute with abundant major peaks. For general quadrupole mass spectrometers, data deconvolution by the freely available software AMDIS is recommended (http://chemdata.nist.gov/mass-spc/amdis/).
- Some of the metabolite’s so-called “missing values” will not be identified in chromatograms. Therefore, when composing the table for the analysis, missing values should be presented as empty values or NA without quotes.
- MetaboAnalyst is a web server designed to permit comprehensive metabolomics data analysis, visualization, and interpretation [39]. The web interfaces of MetaboAnalyst are designed to be self-explanatory. Therefore, most steps are documented on top of the corresponding pages. Available tutorials and sample data sets complement the information by providing step-by-step instructions for several most common tasks.
- This step is strongly recommended for metabolomics datasets with a large number of variables (more than 2000 metabolites) since many of them are from baseline noises. Usually, the GC/MS analysis of brain tissues could identify less than 60 variables.
- The normalization procedures are grouped into three categories. The sample normalization allows general-purpose adjustment for differences among your sample, which can include the protein content in the sample or intensity value of internal standard spiked into the derivatization mixture before GC/MS analysis. Data can be further transformed by “log” or “cube root” and/or scaled via “mean centering, autoscaling, Pareto scaling, and range scaling” (please see the description of each in the MetaboAnalyst program). According to [48] autoscaling and range scaling performed better than the other pretreatment methods and showed biologically sensible results in chemometrics analysis. Data transformation and scaling are two different approaches to make individual features more comparable that can be used individually or in combination. Scaling is a useful procedure when variables are of very different orders of magnitude [49].
- Detect outliers. This can be carried out by visual inspection of the PCA plot to identify samples-outliers outside the Hotelling T2 95% confidence ellipse [40]. Many outliers could be corrected by normalization or excluded from the analysis. In many cases, outliers are the result of operational errors during the analytical process. If those values cannot be corrected, they should be removed from the analysis, but always justified [39]. Please refer to MetaboAnalyst.ca tutorials, or FAQ to select the statistical analysis method.
- Well-predicted metabolites were identified by the Community Metabolic Potential (CMP) scores model in groups by examining of the total pool of metabolites with positive model slope and a model p-value < 0.1. Given that MIMOSA2 is prone to false negatives because its model is approximate, it requires a weak statistical threshold (p-value < 0.1) to capture more relationships of possible interest [18,41,42]. Additional information could be found at borensteinlab.com/software_MIMOSA2.html helper.
4. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dethlefsen, L.; McFall-Ngai, M.; Relman, D.A. An ecological and evolutionary perspective on human-microbe mutualism and disease. Nature 2007, 449, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Vitorino, F. Human microbial ecology and the rising new medicine. Ann. Transl. Med. 2019, 7, 342. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Bello, M.G.; Godoy-Vitorino, F.; Knight, R.; Blaser, M.J. Role of the microbiome in human development. Gut 2019, 68, 1108–1114. [Google Scholar] [CrossRef]
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godoy-Vitorino, F.; Romaguera, J.; Zhao, C.; Vargas-Robles, D.; Ortiz-Morales, G.; Vazquez-Sanchez, F.; Sanchez-Vazquez, M.; de la Garza-Casillas, M.; Martinez-Ferrer, M.; White, J.R.; et al. Cervicovaginal Fungi and Bacteria Associated With Cervical Intraepithelial Neoplasia and High-Risk Human Papillomavirus Infections in a Hispanic Population. Front. Microbiol. 2018, 9, 2533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, B.C.; McAndrew, T.; Chen, Z.; Harari, A.; Barris, D.M.; Viswanathan, S.; Rodriguez, A.C.; Castle, P.; Herrero, R.; Schiffman, M. The cervical microbiome over 7 years and a comparison of methodologies for its characterization. PLoS ONE 2012, 7, e40425. [Google Scholar] [CrossRef] [PubMed]
- Audirac-Chalifour, A.; Torres-Poveda, K.; Bahena-Roman, M.; Tellez-Sosa, J.; Martinez-Barnetche, J.; Cortina-Ceballos, B.; Lopez-Estrada, G.; Delgado-Romero, K.; Burguete-Garcia, A.I.; Cantu, D.; et al. Cervical Microbiome and Cytokine Profile at Various Stages of Cervical Cancer: A Pilot Study. PLoS ONE 2016, 11, e0153274. [Google Scholar] [CrossRef]
- Brotman, R.M.; Ghanem, K.G.; Klebanoff, M.A.; Taha, T.E.; Scharfstein, D.O.; Zenilman, J.M. The effect of vaginal douching cessation on bacterial vaginosis: A pilot study. Am. J. Obstet. Gynecol. 2008, 198, 628.e1–628.e7. [Google Scholar] [CrossRef] [Green Version]
- Oakley, B.B.; Fiedler, T.L.; Marrazzo, J.M.; Fredricks, D.N. Diversity of human vaginal bacterial communities and associations with clinically defined bacterial vaginosis. Appl. Environ. Microbiol. 2008, 74, 4898–4909. [Google Scholar] [CrossRef] [Green Version]
- Castle, P.E.; Hillier, S.L.; Rabe, L.K.; Hildesheim, A.; Herrero, R.; Bratti, M.C.; Sherman, M.E.; Burk, R.D.; Rodriguez, A.C.; Alfaro, M.; et al. An association of cervical inflammation with high-grade cervical neoplasia in women infected with oncogenic human papillomavirus (HPV). Cancer Epidemiol. Prev. Biomark. 2001, 10, 1021–1027. [Google Scholar]
- Ilhan, Z.E.; Laniewski, P.; Thomas, N.; Roe, D.J.; Chase, D.M.; Herbst-Kralovetz, M.M. Deciphering the complex interplay between microbiota, HPV, inflammation and cancer through cervicovaginal metabolic profiling. EBioMedicine 2019, 44, 675–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gjerdingen, D.; Fontaine, P.; Bixby, M.; Santilli, J.; Welsh, J. The impact of regular vaginal pH screening on the diagnosis of bacterial vaginosis in pregnancy. J. Fam. Pract. 2000, 49, 39–43. [Google Scholar] [PubMed]
- Sendag, F.; Kazandi, M.; Akercan, F.; Kazandi, A.C.; Karadadas, N.; Sagol, S. Vaginal fluid pH, cervicovaginitis and cervical length in pregnancy. Clin. Exp. Obstet. Gynecol. 2010, 37, 127–130. [Google Scholar] [PubMed]
- Bosch, F.X.; Lorincz, A.; Muñoz, N.; Meijer, C.J.L.M.; Shah, K.V. The causal relation between human papillomavirus and cervical cancer. J. Clin. Pathol. 2002, 55, 244–265. [Google Scholar] [CrossRef] [Green Version]
- Herrero, R.; Castellsague, X.; Pawlita, M.; Lissowska, J.; Kee, F.; Balaram, P.; Rajkumar, T.; Sridhar, H.; Rose, B.; Pintos, J.; et al. Human papillomavirus and oral cancer: The International Agency for Research on Cancer multicenter study. J. Natl. Cancer Inst. 2003, 95, 1772–1783. [Google Scholar] [CrossRef]
- Romaguera, J.; Ortiz-Morales, G.; Vázquez-Sánchez, F.; Dominicci-Maura, A.; Ortiz, A.; Godoy-Vitorino, F. The microbiota associated to cervical and anal HPV infections in a Hispanic population. In Proceedings of the AACR Annual Meeting 2019, Atlanta, GA, USA, 29 March–3 April 2019. [Google Scholar]
- Yu, M.; Jia, H.; Zhou, C.; Yang, Y.; Zhao, Y.; Yang, M.; Zou, Z. Variations in gut microbiota and fecal metabolic phenotype associated with depression by 16S rRNA gene sequencing and LC/MS-based metabolomics. J. Pharm. Biomed. Anal. 2017, 138, 231–239. [Google Scholar] [CrossRef]
- Chorna, N.; Romaguera, J.; Godoy-Vitorino, F. Cervicovaginal Microbiome and Urine Metabolome Paired Analysis Reveals Niche Partitioning of the Microbiota in Patients with Human Papilloma Virus Infections. Metabolites 2020, 10, 36. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Yang, S.; Wang, Y.; Shen, Q.; Yang, Y.; Deng, X.; Zhang, W.; Delwart, E. Identification of a novel human papillomavirus by metagenomic analysis of vaginal swab samples from pregnant women. Virol. J. 2016, 13, 122. [Google Scholar] [CrossRef] [Green Version]
- Cottier, F.; Srinivasan, K.G.; Yurieva, M.; Liao, W.; Poidinger, M.; Zolezzi, F.; Pavelka, N. Advantages of meta-total RNA sequencing (MeTRS) over shotgun metagenomics and amplicon-based sequencing in the profiling of complex microbial communities. NPJ Biofilms Microbiomes 2018, 4, 2. [Google Scholar] [CrossRef]
- Ibarlucea, B.; Rim, T.; Baek, C.K.; de Visser, J.; Baraban, L.; Cuniberti, G. Nanowire sensors monitor bacterial growth kinetics and response to antibiotics. Lab Chip 2017, 17, 4283–4293. [Google Scholar] [CrossRef]
- Fehr, M.; Okumoto, S.; Deuschle, K.; Lager, I.; Looger, L.L.; Persson, J.; Kozhukh, L.; Lalonde, S.; Frommer, W.B. Development and use of fluorescent nanosensors for metabolite imaging in living cells. Biochem. Soc. Trans. 2005, 33, 287–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McInnes, P.; Cutting, M. Manual of Procedures—Human Microbiome Project: Core Microbiome Sampling, Protocol A, HMP Protocol # 07-001. 2010; p. 114. Available online: https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/GetPdf.cgi?id=phd003190.2 (accessed on 2 December 2019).
- Kleter, B.; van Doorn, L.J.; Schrauwen, L.; Molijn, A.; Sastrowijoto, S.; ter Schegget, J.; Lindeman, J.; ter Harmsel, B.; Burger, M.; Quint, W. Development and clinical evaluation of a highly sensitive PCR-reverse hybridization line probe assay for detection and identification of anogenital human papillomavirus. J. Clin. Microbiol. 1999, 37, 2508–2517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinten, F.; Hilbrands, L.B.; Meeuwis, K.A.P.; IntHout, J.; Quint, W.G.V.; Hoitsma, A.J.; Massuger, L.; Melchers, W.J.G.; de Hullu, J.A. Reactivation of Latent HPV Infections After Renal Transplantation. Am. J. Transplant. 2017, 17, 1563–1573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vargas-Robles, D.; Magris, M.; Morales, N.; de Koning, M.N.C.; Rodríguez, I.; Nieves, T.; Godoy-Vitorino, F.; Sánchez, G.I.; Alcaraz, L.D.; Forney, L.J.; et al. High Rate of Infection by Only Oncogenic Human Papillomavirus in Amerindians. mSphere 2018, 3, e00176-18. [Google Scholar] [CrossRef] [Green Version]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, A.; Navas-Molina, J.A.; Kosciolek, T.; McDonald, D.; Vazquez-Baeza, Y.; Ackermann, G.; DeReus, J.; Janssen, S.; Swafford, A.D.; Orchanian, S.B.; et al. Qiita: Rapid, web-enabled microbiome meta-analysis. Nat. Methods 2018, 15, 796–798. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [Green Version]
- Dhariwal, A.; Chong, J.; Habib, S.; King, I.L.; Agellon, L.B.; Xia, J. MicrobiomeAnalyst: A web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res. 2017, 45, W180–W188. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Champaign, IL, USA, 1949; ISBN 0-252-72548-4. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2009. [Google Scholar]
- Kolde, R. R pheatmap-package: Pretty Heatmaps. 2015. Available online: http://www.r-project.org/ (accessed on 2 December 2019).
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [Green Version]
- Team, R.D.C. R: A Language and Environment for Statistical Computing; Foundation for statistical computing: Vienna, Austria, 2011. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; O’hara, R.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package. R Package Version 2.4-4. R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2010. [Google Scholar]
- Lozupone, C.; Hamady, M.; Knight, R. UniFrac—An online tool for comparing microbial community diversity in a phylogenetic context. BMC Bioinform. 2006, 7, 371. [Google Scholar] [CrossRef] [Green Version]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar] [CrossRef]
- Xia, J.; Sinelnikov, I.V.; Han, B.; Wishart, D.S. MetaboAnalyst 3.0—Making metabolomics more meaningful. Nucleic Acids Res. 2015, 43, W251–W257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, J.; Wishart, D.S. Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nat. Protoc. 2011, 6, 743–760. [Google Scholar] [CrossRef] [PubMed]
- Noecker, C.; Chiu, H.-C.; McNally, C.P.; Borenstein, E. Defining and Evaluating Microbial Contributions to Metabolite Variation in Microbiome-Metabolome Association Studies. bioRxiv 2019, 402040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noecker, C.; Eng, A.; Srinivasan, S.; Theriot, C.M.; Young, V.B.; Jansson, J.K.; Fredricks, D.N.; Borenstein, E. Metabolic Model-Based Integration of Microbiome Taxonomic and Metabolomic Profiles Elucidates Mechanistic Links between Ecological and Metabolic Variation. mSystems 2016, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langille, M.G.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Kanehisa, M. The KEGG database. Discussion 101–103, 119–128, 244–152. Novartis Found. Symp. 2002, 247, 91–101. [Google Scholar]
- Fiehn, O. Metabolomics by Gas Chromatography-Mass Spectrometry: Combined Targeted and Untargeted Profiling. Curr. Protoc. Mol. Biol. 2016, 114, 30–34. [Google Scholar] [CrossRef]
- Chorna, N.E.; Chornyy, A.P. Metabolomics Analysis of Glutamate Receptor Function. Methods Mol. Biol. 2019, 1941, 155–165. [Google Scholar] [CrossRef]
- van den Berg, R.A.; Hoefsloot, H.C.; Westerhuis, J.A.; Smilde, A.K.; van der Werf, M.J. Centering, scaling, and transformations: Improving the biological information content of metabolomics data. BMC Genom. 2006, 7, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanani, H.; Chrysanthopoulos, P.K.; Klapa, M.I. Standardizing GC–MS metabolomics. J. Chromatogr. B 2008, 871, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Flezar, M.S. Urine and bladder washing cytology for detection of urothelial carcinoma: Standard test with new possibilities. Radiol. Oncol. 2010, 44, 207–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, T.W.; Hostetter, T.H. Uremic solutes from colon microbes. Kidney Int. 2012, 81, 949–954. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Li, X.H.; Chen, W.N. An untargeted fecal and urine metabolomics analysis of the interplay between the gut microbiome, diet and human metabolism in Indian and Chinese adults. Sci. Rep. 2019, 9, 9191. [Google Scholar] [CrossRef] [Green Version]
- Holmes, E.; MacIntyre, D.; Modi, N.; Marchesi, J.R. Chapter 8—Handing on Health to the Next Generation: Early Life Exposures. In Metabolic Phenotyping in Personalized and Public Healthcare; Holmes, E., Nicholson, J.K., Darzi, A.W., Lindon, J.C., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 213–264. [Google Scholar]
- Godoy-Vitorino, F.; Ortiz-Morales, G.; Romaguera, J.; Sanchez, M.M.; Martinez-Ferrer, M.; Chorna, N. Discriminating high-risk cervical Human Papilloma Virus infections with urinary biomarkers via non-targeted GC-MS-based metabolomics. PLoS ONE 2018, 13, e0209936. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chorna, N.; Godoy-Vitorino, F. A Protocol for the Multi-Omic Integration of Cervical Microbiota and Urine Metabolomics to Understand Human Papillomavirus (HPV)-Driven Dysbiosis. Biomedicines 2020, 8, 81. https://doi.org/10.3390/biomedicines8040081
Chorna N, Godoy-Vitorino F. A Protocol for the Multi-Omic Integration of Cervical Microbiota and Urine Metabolomics to Understand Human Papillomavirus (HPV)-Driven Dysbiosis. Biomedicines. 2020; 8(4):81. https://doi.org/10.3390/biomedicines8040081
Chicago/Turabian StyleChorna, Nataliya, and Filipa Godoy-Vitorino. 2020. "A Protocol for the Multi-Omic Integration of Cervical Microbiota and Urine Metabolomics to Understand Human Papillomavirus (HPV)-Driven Dysbiosis" Biomedicines 8, no. 4: 81. https://doi.org/10.3390/biomedicines8040081
APA StyleChorna, N., & Godoy-Vitorino, F. (2020). A Protocol for the Multi-Omic Integration of Cervical Microbiota and Urine Metabolomics to Understand Human Papillomavirus (HPV)-Driven Dysbiosis. Biomedicines, 8(4), 81. https://doi.org/10.3390/biomedicines8040081




