Abstract
Glecaprevir/pibrentasvir (G/P) are direct-acting antivirals (DAAs) that achieve a high sustained virological response (SVR) rate for hepatitis C virus (HCV) infection. We investigated G/P effectiveness for HCV patients based on real-world experience and the clinical features of retreatment cases. HCV patients (n = 182) were compared for clinical features and outcomes between first treatment (n = 159) and retreatment (n = 23) G/P groups. Overall, 77 patients (42.3%) were male, the median age was 68 years, and 86/66/1/4 cases had genotype 1/2/1 + 2/3, respectively. An SVR was achieved in 97.8% (178/182) of cases by intention-to-treat analysis and 99.4% (178/179) of cases by per-protocol analysis. There were no remarkable differences between the first treatment and retreatment groups for male (42.8% vs. 39.1%, p = 0.70), median age (68 vs. 68 years, p = 0.36), prior hepatocellular carcinoma (5.8% vs. 8.7%, p = 0.59), or the fibrosis markers AST-to-platelet ratio index (APRI) (0.5 vs. 0.5, p = 0.80) and fibrosis-4 (FIB-4) index (2.2 vs. 2.6, p = 0.59). The retreatment group had a significantly more frequent history of interferon treatment (12.3% vs. 52.2%, p < 0.01) and the Y93H mutation (25.0% vs. 64.7%, p = 0.02). The number of retreatment patients who had experienced 3, 2, and 1 DAA treatment failures was 1, 3, and 19, respectively, all of whom ultimately achieved an SVR by G/P treatment. In conclusion, G/P was effective and safe for both HCV first treatment and retreatment cases despite the retreatment group having specific resistance mutations for other prior DAAs. As G/P treatment failure has been reported for P32 deletions, clinicians should consider resistance mutations during DAA selection.
1. Introduction
With an estimated 130–170 million people chronically infected worldwide, including 1.5 million cases in Japan, hepatitis C virus (HCV) infection has become a global health concern, especially considering eventual disease progression to cirrhosis, hepatic failure, and hepatocellular carcinoma (HCC) [1,2,3,4]. Viral eradication is the most effective treatment to halt HCV progression. The introduction of direct-acting antiviral (DAA)-based therapies for chronic HCV infection has revolutionized HCV treatment, resulting in highly efficacious and well-tolerated therapies for nearly all patients. Recently, the effectiveness and safety of glecaprevir/pibrentasvir (G/P) was reported in a multicenter Northern Italy population, in which the treatment provided an excellent sustained virological response (SVR) rate (99.2%) with only mild adverse events [5] comparable to those in a Japanese population [6,7]. Resistance-associated substitutions (RAS) should be considered when treating patients with DAA because it was reported that 989 of 5107 patients (19.4%) had RASs in the HCV-NS5A region in a Real-World, Nationwide, Multicenter Study in Japan [8]. However, the real-world clinical impact of G/P for HCV retreatment remains unknown. We therefore examined the effectiveness and safety of G/P in a Japanese cohort with a specific focus on first treatment and retreatment cases.
2. Methods
2.1. Patients and Methods
A total of 1637 patients who had received DAA treatment and achieved 12 weeks of follow-up periods after completion of G/P treatment at Shinshu University Hospital in Matsumoto, Japan, or its affiliated hospitals, between 2015 and 2018 were initially included. Of them, 304/328 patients under daclatasvir + asunaprevir (DCV + ASV) for 24 weeks (SVR rate: 92.6%), 342/348 patients under ledipasvir/sofosbuvir (LDV/SOF) for 12 weeks (SVR rate: 98.2%), 39/44 patients under ombitasvir/paritaprevir/ritonavir (OMV/PTV/r) for 12 weeks (SVR rate: 88.6%), 80/82 patients under elbasvir + grazoprevir (EBR + GZR) for 12 weeks (SVR rate: 97.6%), and 256/264 patients under sofosbuvir + ribavirin (SOF + RBV) for 12 weeks (SVR rate: 97.0%) achieved an SVR, as shown in Figure 1. Ultimately, a total of 182 patients (median age: 68 years, 77 (42.8%) male), including 23 prior DAA failure patients, who received G/P treatment between December 2017 and November 2018 according to the Japan Society of Hepatology (JSH) clinical guidelines [9] were analyzed in this study (Table 1 and Table 2). The racial background of all patients was Japanese. The diagnosis of chronic HCV was based on JSH criteria [9] as the presence of serum HCV antibodies and detectable HCV-RNA by the real-time polymerase chain reaction (RT-PCR) at therapy initiation. Liver cirrhosis was diagnosed based on the characteristic clinical signs of advanced liver disease and/or imaging studies by attending physicians. An SVR12 was defined as undetectable HCV RNA at 12 weeks after completion of DAA therapy. Treatment failure was defined as detectable HCV RNA during treatment or within 12 weeks after completion or discontinuation of DAAs. We suspected that the reason why repeat treatment worked but the first treatment did not, was that no patient received the same DAA therapy twice. As all retreatment cases were given a different DAA (Figure 1), the initiation of differing drugs might be a point of consideration in retreatment cases.
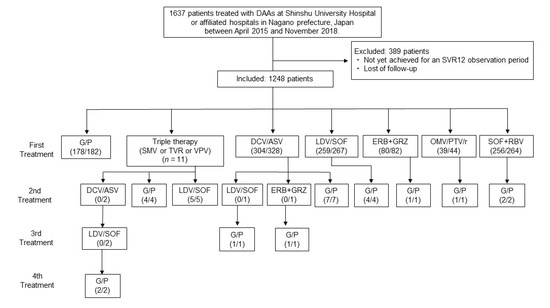
Figure 1.
Study flowchart showing the efficacy of direct-acting antiviral (DAA) treatment at SVR12. The respective number of patients who achieved a sustained virological response (SVR) and received DAA treatment is shown. Abbreviations: DAA, direct-acting antiviral; SVR, sustained virological response; G/P, glecaprevir/pibrentasvir; SMV, simeprevir; TVR, telaprevir; VPV, vaniprevir; DCV + ASV, daclatasvir + asunaprevir; LDV/SOF, ledipasvir/sofosbuvir; OMV/PTV/r, ombitasvir/paritaprevir/ritonavir; EBR + GRZ, elbasvir + grazoprevir; SOF + RBV, sofosbuvir + ribavirin.

Table 1.
Baseline patient characteristics.

Table 2.
Comparison of clinical characteristics between first treatment and retreatment cases.
This study was reviewed and approved by the Institutional Review Board of Shinshu University School of Medicine (approval number: 3244) and its affiliated hospitals. Written informed consent was obtained from all participating subjects. The study was conducted according to the principals of the Declaration of Helsinki.
2.2. Laboratory Testing
All laboratory data, including white blood cell count, hemoglobin, platelet count, albumin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alpha fetoprotein, were determined using standard methods at respective institutions. The fibrosis-4 (FIB-4) index and AST-to-platelet ratio index (APRI) were respectively calculated as: age (years) × AST (U/L)/platelet count (x109/L) × ALT (U/L)1/2 [10] and (AST/upper limit of normal; 40 (U/L)) × (100/platelet count (109/L)) [11].
2.3. Testing for RAS of the HCV-RNA Genome
Among 159 patients who were treated with G/P as the first DAA treatment, 19 were analyzed for the NS5A-Y93H RAS mutation by RT-PCR prior to G/P treatment as described previously [12], with a value of 20% or more defined as NS5A-Y93H positivity [13] in a commercially based laboratory [14]. All retreatment cases with HCV genotype 1, except for 3 with a failed combination of interferon (IFN) and DAA treatment, were analyzed for RAS in the NS5A and NS3 regions (Table 3). All cases with HCV genotype 2 were analyzed for RAS in NS5B (Table 4) by a direct sequencing method.

Table 3.
Resistance-associated substitutions of the HCV-RNA genome in genotype 1 retreatment cases.

Table 4.
Resistance-associated substitutions of the HCV-RNA genome in genotype 2 retreatment cases and a G/P failure case.
2.4. Statistical Analysis
Statistical analysis and data visualization were carried out using StatFlex version 7.0.7 (Artech Co., Ltd., Osaka, Japan). Continuous baseline data are expressed as the median ± interquartile range and statistically evaluated by means of the Mann–Whitney U test. Categorical variables are presented as the frequency (percentage) and analyzed using the chi-square test. All statistical tests were two-sided and evaluated at the 0.05 level of significance.
3. Results
3.1. Baseline Clinical Characteristics
The baseline clinical characteristics in this study are summarized in Table 1. Of the 182 enrolled patients, 77 (42.3%) were male, 105 (57.7%) were female, and the median age was 68 years. The number of patients at the chronic hepatitis stage or liver cirrhosis stage was 134 and 25 cases, respectively. Patients infected with HCV genotype 1, 2, 1 + 2, or 3 numbered 86, 66, 1, and 4, respectively, as shown in Table 1. Roughly half of the patients had the Y93 mutation in the NS5A region. Hypertension was the most frequent complication (51 patients; 37%), followed next by diabetes mellitus in 20 patients (16%), dyslipidemia in 12 patients (8.7%), and renal failure under hemodialysis in eight patients (5.0%). Twenty-nine patients (18%) had interferon (IFN) treatment experience, and 10 patients (6.2%) had a history of HCC. Overall, 23 patients (13.6%) were prior DAA treatment failure cases (retreatment group), which included three cases of triple therapy (simeprevir or vaniprevir/pegylated IFN/ribavirin), seven cases of DCV/ASV, three cases of LDV/SOF, one case of EBR + GZR, one case of OMV/PTV/r, four cases of SOF + RBV, two cases of DCV/ASV followed by LDV/SOF, one case of DCV/ASV followed by EBR + GRZ, and one case of SMV followed by DCV/ASV followed by LDV/SOF.
3.2. G/P Outcomes
In total, an SVR was achieved in 97.8% (178/182) of cases by intention-to-treat analysis and 99.4% (178/179) of cases by per-protocol analysis. One (0.5%) treatment-naïve, non-cirrhotic stage, genotype 2a-infected male patient experienced a virological relapse at eight weeks of follow-up. In the retreatment group, the number of patients who had experienced 3, 2, and 1 DAA treatment failures was 1, 3, and 19, respectively, all of whom ultimately achieved an SVR by G/P treatment.
Of 182 patients treated with G/P, only three discontinued treatment, which supported previous studies on the safety of G/P therapy [5,6]. Specifically, one female patient (0.5%) discontinued treatment due to the adverse event of diarrhea 2 weeks after commencing G/P. Her HCV-RNA was negative 4 weeks after starting treatment but had become positive at 12 weeks. Another female patient (0.5%) elected to stop therapy for personal reasons despite negativity for HCV-RNA at 4 weeks. A male patient (0.5%) died of cerebral infarction unrelated to G/P treatment. His HCV-RNA had been negative after 4 weeks of drug administration. Although patients with HCV genotype 3 have been reported to achieve comparatively lower SVR rates [15], all such patients (n = 4) in this study attained an SVR by G/P, thus confirming a previous study demonstrating high SVR rates regardless of genotype [16].
3.3. Comparisons between First Treatment and Retreatment Groups
Comparisons of the clinical features and outcomes between the first treatment (n = 159) and retreatment (n = 23) G/P therapy groups are presented in Table 2. There were no remarkable differences between the groups for male gender frequency (43.3% vs. 39.1%, p = 0.705), median age (68 vs. 68 years, p = 0.362), frequency of cirrhosis stage (17.4% vs. 9.1%, p = 0.357), frequency of prior HCC history (5.8% vs. 8.7%, p = 0.593), or the liver fibrosis markers of APRI (0.5 vs. 0.5, p = 0.805) or FIB-4 index (2.2 vs. 2.6, p = 0.592). However, the retreatment group had a significantly higher frequency of IFN treatment history (12.3% vs. 52.2%, p < 0.001) and the Y93H mutation (25.0% vs. 75.0%, p < 0.001). Y93H was the most frequent mutation in the retreatment group (Table 3). A variety of RASs were detected in the NS5A, NS3, and NS5B regions, but G/P could successfully achieve an SVR in all cases.
3.4. RASs in HCV-RNA Genome in Retreatment Cases
As shown in Table 3, retreatment cases in genotype 1 had a variety of types of RASs in NS5A while those in genotype 2 had no RASs in NS5B.
4. Discussion
This study presents important evidence on G/P treatment efficacy and safety for HCV eradication in first and retreatment cases based on our real-world experience.
In the cohort, one case with HCV genotype 2a had unsuccessful G/P treatment as the first DAA therapy. RAS had not been analyzed before G/P, but later testing revealed none in the NS5B region. Another case was previously reported as a failed 8-week course of G/P therapy [6], which suggested that 8 weeks of treatment was insufficient to achieve an SVR for genotype 2a HCV, as evidenced in the present series.
It was noteworthy that eight patients receiving hemodialysis achieved an SVR under G/P, which supported previous reports that even individuals with accompanying conditions could achieve an SVR safely and effectively by a G/P regimen [17]. We very recently described that a history of HCC was an independent risk factor of DAA treatment failure [18]. However, all 10 patients (6.2%) with prior HCC in our cohort achieved an SVR to further highlight the broad utility of G/P. Unfortunately, six of 49 DAA treatment failure patients were unable to receive further treatment, including G/P, due to complicating HCC after DAA failure, suggesting the need for earlier intervention.
G/P is the first pan-genotype DAA treatment for HCV in the world. Although genotype 3 patients are a minority in Japan, genotype 3 HCV has been resistant to SOF + RBV regimens, with an SVR rate of approximately 35% [19]. In Japan, a DAA-based regimen of SOF + RBV for 24 weeks was approved for genotype 3 in 2017, which could achieve an SVR rate of 85% [20]. Our study included four patients with this genotype, all of whom achieved an SVR. Genotype 3 HCV was reported to show relatively rapid liver fibrosis progression [21]. Therefore, G/P may be a good option for treatment before advancement to liver fibrosis.
The retreatment group had a significantly higher frequency of IFN treatment history and the Y93H mutation, suggesting a resistance to prior IFN therapy and the acquisition of a resistance mutation following prior DAA treatment. Although the Y93H mutation on NS5A has been associated with DAA treatment failure [22], all 12 patients with this mutation achieved an SVR, indicating that it was unrelated to G/P outcome. The P32 deletion has also been strongly linked to G/P therapy ineffectiveness by previous clinical reports [23,24], but we were unable to conclude a relationship with G/P failure as there was only one P32 deletion patient in our cohort who had acquired the mutation after DCV/ASV failure and did not receive G/P due to prior identification of the deletion. Regarding the administration of DCV + ASV, if patients harbored the NS5A-Y93H RAS and could no longer postpone treatment due to age, disease progression, or other clinical reasons, they commenced the regimen instead of waiting for next-generation DAAs, which could achieve a high SVR rate of 91.7% as reported previously [18]. The relatively low frequency of the P32 deletion in our cohort was presumably due to the above strategy. It has also been confirmed by basic research evidence that a P32 deletion conferred severe drug resistance even to second-generation NS5A inhibitors [25], thus supporting a strategy of resistance mutation testing before treatment to halt the creation of new unidentified mutations in the HCV genome. Taken together, our data confirmed that G/P was effective not only for HCV first treatment, but also for retreatment, despite the retreatment group having specific resistance mutations to other DAAs.
There are several limitations to this study. It was retrospectively conducted, and the assessed data on safety and tolerability were based on medical records by attending physicians. Additionally, RASs measurement was limited in some cases because it is not covered by the health insurance system in our country. G/P efficacy and safety should also be analyzed in other ethnicities to confirm the results of the present study.
5. Conclusions
G/P treatment appears effective and safe for patients with HCV, regardless of DAA treatment history, which highlights the value of a micro-elimination approach to global HCV eradication. As G/P treatment failure has been reported for P32 deletions, clinicians should consider resistance mutations during DAA selection.
Author Contributions
Conceptualization: A.S., S.J.; Data curation: A.S., S.J.; Formal analysis: A.S., Methodology: A.S., S.J.; Project administration: A.S., S.J.; Resources: A.S., S.J., Y.Y., T.Y., N.F., T.K., A.M. (Akihiro Matsumoto), S.W., H.M., S.S., K.Y., S.M., K.F., A.K., A.I., S.K., A.M. (Atsushi Maruyama), M.K. (Masakazu Kobayashi), M.K. (Michiharu Komatsu), M.M., C.M., T.I., A.T., Y.K., Y.G., T.T., H.I., Y.N., S.U., K.K., E.T., T.U.; Software: A.S.; Supervision: T.U.; Validation: A.S., S.J., T.U.; Visualization: A.S., S.J.; Writing-original draft: A.S., S.J.; Writing-review & editing: S.J., T.U. All authors have read and agreed to the published version of the manuscript.
Funding
We sincerely appreciate the research support provided in part by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant-in-Aid for Scientific Research (C) (18K07907) and the Taiju Life Social Welfare Foundation.
Acknowledgments
The authors thank Yuki Akahane and Asami Yamazaki for their technical assistance and Trevor Ralph for his English editorial assistance.
Conflicts of Interest
S.J. has received research grants from AbbVie and MSD. E.T. has received research grants from AbbVie, Takeda Pharmaceutical, Chugai Pharmaceutical, Astellas Pharma Inc, Sumitomo Dainippon Pharma, Eisai, and Nippon Kayaku. T.U. has received research grants from AbbVie, MSD, Daiichi Sankyo, and Otsuka Pharmaceutical. The other authors declare nothing to disclose regarding funding from industries or conflicts of interest with respect to this manuscript.
References
- Kiyosawa, K.; Sodeyama, T.; Tanaka, E.; Gibo, Y.; Yoshizawa, K.; Nakano, Y.; Furuta, S.; Akahane, Y.; Nishioka, K.; Purcell, R.H.; et al. Interrelationship of blood transfusion, non-A, non-B hepatitis and hepatocellular carcinoma: Analysis by detection of antibody to hepatitis C virus. Hepatology 1990, 12, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Kiyosawa, K.; Tanaka, E.; Sodeyama, T.; Furuta, S. Natural History of Hepatitis C. Intervirology 1994, 37, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, E.; Kiyosawa, K. Natural history of acute hepatitis C. J. Gastroenterol. Hepatol. 2000, 15, E97–E104. [Google Scholar] [CrossRef] [PubMed]
- Kiyosawa, K.; Umemura, T.; Ichijo, T.; Matsumoto, A.; Yoshizawa, K.; Gad, A.; Tanaka, E. Hepatocellular carcinoma: Recent trends in Japan. Gastroenterology 2004, 127, S17–S26. [Google Scholar] [CrossRef]
- D’Ambrosio, R.; Pasulo, L.; Puoti, M.; Vinci, M.; Schiavini, M.; Lazzaroni, S.; Soria, A.; Gatti, F.; Menzaghi, B.; Aghemo, A.; et al. Real-world effectiveness and safety of glecaprevir/pibrentasvir in 723 patients with chronic hepatitis C. J. Hepatol. 2019, 70, 379–387. [Google Scholar] [CrossRef]
- Ogawa, E.; Furusyo, N.; Nakamuta, M.; Nomura, H.; Satoh, T.; Takahashi, K.; Koyanagi, T.; Kajiwara, E.; Dohmen, K.; Kawano, A.; et al. Glecaprevir and pibrentasvir for Japanese patients with chronic hepatitis C genotype 1 or 2 infection: Results from a multicenter, real-world cohort study. Hepatol. Res. 2019, 49, 617–626. [Google Scholar] [CrossRef]
- Sezaki, H.; Suzuki, F.; Hosaka, T.; Fujiyama, S.; Kawamura, Y.; Akuta, N.; Kobayashi, M.; Suzuki, Y.; Saitoh, S.; Arase, Y.; et al. Initial- and re-treatment effectiveness of glecaprevir and pibrentasvir for Japanese patients with chronic hepatitis C virus-genotype 1/2/3 infections. J. Gastroenterol. 2019, 54, 916–927. [Google Scholar] [CrossRef]
- Toyoda, H.; Atsukawa, M.; Uojima, H.; Nozaki, A.; Tamai, H.; Takaguchi, K.; Fujioka, S.; Nakamuta, M.; Tada, T.; Yasuda, S.; et al. Trends and Efficacy of Interferon-Free Anti-hepatitis C Virus Therapy in the Region of High Prevalence of Elderly Patients, Cirrhosis, and Hepatocellular Carcinoma: A Real-World, Nationwide, Multicenter Study of 10 688 Patients in Japan. Open Forum Infect. Dis. 2019, 6, ofz185. [Google Scholar] [CrossRef]
- Asahina, Y.; Izumi, N.; Hiromitsu, K.; Kurosaki, M.; Koike, K.; Suzuki, F.; Takikawa, H.; Tanaka, A.; Tanaka, E.; Tanaka, Y.; et al. JSH Guidelines for the Management of Hepatitis C Virus Infection: A 2016 update for genotype 1 and 2. Hepatol. Res. 2016, 46, 129–165. [Google Scholar] [CrossRef]
- Vallet-Pichard, A.; Mallet, V.; Nalpas, B.; Verkarre, V.; Nalpas, A.; Dhalluin-Venier, V.; Fontaine, H.; Pol, S. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology 2007, 46, 32–36. [Google Scholar] [CrossRef]
- Castera, L.; Vergniol, J.; Foucher, J.; Le Bail, B.; Chanteloup, E.; Haaser, M.; Darriet, M.; Couzigou, P.; De Ledinghen, V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 2005, 128, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, F.; Sezaki, H.; Akuta, N.; Suzuki, Y.; Seko, Y.; Kawamura, Y.; Hosaka, T.; Kobayashi, M.; Saito, S.; Arase, Y.; et al. Prevalence of hepatitis C virus variants resistant to NS3 protease inhibitors or the NS5A inhibitor (BMS-790052) in hepatitis patients with genotype 1b. J. Clin. Virol. 2012, 54, 352–354. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Yasui, S.; Nakamura, M.; Suzuki, E.; Arai, M.; Haga, Y.; Sasaki, R.; Wu, S.; Nakamoto, S.; Imazeki, F.; et al. Daclatasvir plus Asunaprevir Treatment for Real-World HCV Genotype 1-Infected Patients in Japan. Int. J. Med. Sci. 2016, 13, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Miura, M.; Maekawa, S.; Sato, M.; Komatsu, N.; Tatsumi, A.; Takano, S.; Amemiya, F.; Nakayama, Y.; Inoue, T.; Sakamoto, M.; et al. Deep sequencing analysis of variants resistant to the non-structural 5A inhibitor daclatasvir in patients with genotype 1b hepatitis C virus infection. Hepatol. Res. 2014, 44, E360–E367. [Google Scholar] [CrossRef]
- Goossens, N.; Negro, F. Is genotype 3 of the hepatitis C virus the new villain? Hepatology 2014, 59, 2403–2412. [Google Scholar] [CrossRef]
- Zeuzem, S.; Foster, G.R.; Wang, S.; Asatryan, A.; Gane, E.; Feld, J.J.; Asselah, T.; Bourliere, M.; Ruane, P.J.; Wedemeyer, H.; et al. Glecaprevir-Pibrentasvir for 8 or 12 Weeks in HCV Genotype 1 or 3 Infection. N. Engl. J. Med. 2018, 378, 354–369. [Google Scholar] [CrossRef] [PubMed]
- Kumada, H.; Watanabe, T.; Suzuki, F.; Ikeda, K.; Sato, K.; Toyoda, H.; Atsukawa, M.; Ido, A.; Takaki, A.; Enomoto, N.; et al. Efficacy and safety of glecaprevir/pibrentasvir in HCV-infected Japanese patients with prior DAA experience, severe renal impairment, or genotype 3 infection. J. Gastroenterol. 2017, 53, 566–575. [Google Scholar] [CrossRef]
- Sugiura, A.; Joshita, S.; Umemura, T.; Yamazaki, T.; Fujimori, N.; Kimura, T.; Matsumoto, A.; Igarashi, K.; Usami, Y.; Wada, S.; et al. Past history of hepatocellular carcinoma is an independent risk factor of treatment failure in patients with chronic hepatitis C virus infection receiving direct-acting antivirals. J. Viral Hepat. 2018, 25, 1462–1471. [Google Scholar] [CrossRef]
- Reddy, K.R.; Lim, J.K.; Kuo, A.; Di Bisceglie, A.M.; Galati, J.S.; Morelli, G.; Everson, G.T.; Kwo, P.Y.; Brown, R.S.J.; Sulkowski, M.S.; et al. All-oral direct-acting antiviral therapy in HCV-advanced liver disease is effective in real-world practice: Observations through HCV-TARGET database. Aliment. Pharmacol. Ther. 2017, 45, 115–126. [Google Scholar] [CrossRef]
- Zeuzem, S.; Dusheiko, G.M.; Salupere, R.; Mangia, A.; Flisiak, R.; Hyland, R.H.; Illeperuma, A.; Svarovskaia, E.; Brainard, D.M.; Symonds, W.T.; et al. Sofosbuvir and Ribavirin in HCV Genotypes 2 and 3. N. Engl. J. Med. 2014, 370, 1993–2001. [Google Scholar] [CrossRef]
- Probst, A.; Dang, T.; Bochud, M.; Egger, M.; Negro, F.; Bochud, P.-Y. Role of Hepatitis C virus genotype 3 in liver fibrosis progression—A systematic review and meta-analysis. J. Viral Hepat. 2011, 18, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Lemm, J.A.; O’Boyle, D.; Liu, M.; Nower, P.T.; Colonno, R.; Deshpande, M.S.; Snyder, L.B.; Martin, S.W.; St Laurent, D.R.; Serrano-Wu, M.H.; et al. Identification of hepatitis C virus NS5A inhibitors. J. Virol. 2010, 84, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Uemura, H.; Uchida, Y.; Kouyama, J.; Naiki, K.; Tsuji, S.; Sugawara, K.; Nakao, M.; Motoya, D.; Nakayama, N.; Imai, Y.; et al. NS5A-P32 deletion as a factor involved in virologic failure in patients receiving glecaprevir and pibrentasvir. J. Gastroenterol. 2019, 54, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Osawa, M.; Imamura, M.; Teraoka, Y.; Uchida, T.; Morio, K.; Fujino, H.; Nakahara, T.; Ono, A.; Murakami, E.; Kawaoka, T.; et al. Real-world efficacy of glecaprevir plus pibrentasvir for chronic hepatitis C patient with previous direct-acting antiviral therapy failures. J. Gastroenterol. 2019, 54, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Nitta, S.; Asahina, Y.; Kato, T.; Tsuchiya, J.; Inoue-Shinomiya, E.; Sato, A.; Tsunoda, T.; Miyoshi, M.; Kawai-Kitahata, F.; Murakawa, M.; et al. Impact of novel NS5A resistance-associated substitutions of hepatitis C virus detected in treatment-experienced patients. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).