Impact of Statin Use on Dementia Incidence in Elderly Men and Women with Ischemic Heart Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
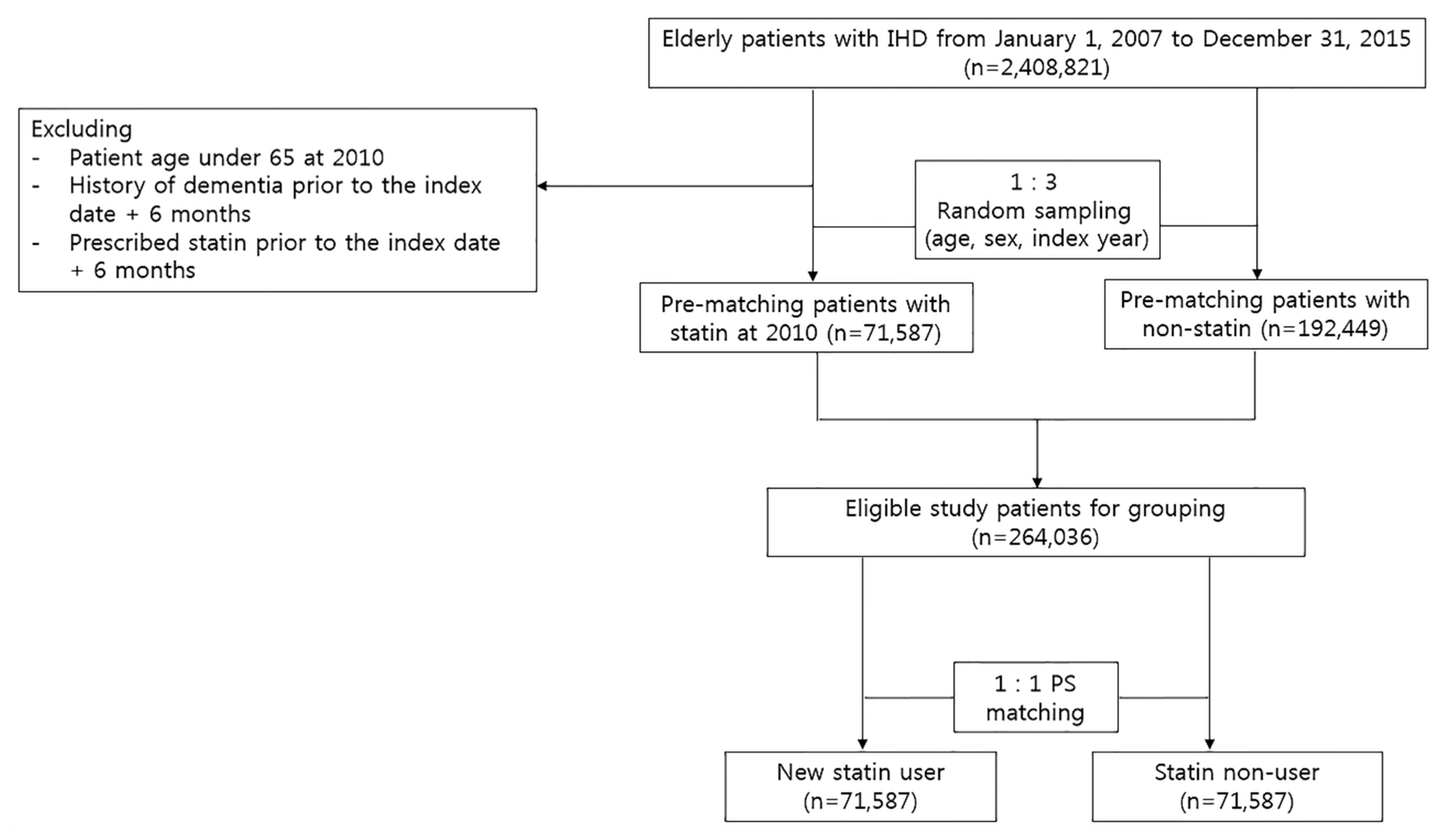
2.2. Patient Population and Study Design
2.3. Variables
2.4. Study Outcomes
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. The Impact of Statin Use on Dementia Risk in Men and Women with IHD
3.3. Association between Statin Use and Dementia Risk According to the Exposure Duration of Statin
3.4. Association between Statin Use and Dementia Risk According to Age
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| IHD | Ischemic heart disease |
| CCI | Charlson comorbidity index |
| ACEI | Angiotensin-converting enzyme inhibitor |
| ARB | Angiotensin II receptor blocker |
| CCB | Calcium channel blockers |
| PPI | Proton pump inhibitors |
| PS | Propensity score |
| ICD | International Classification of Diseases |
| HIRA | Health Insurance Review and Assessment Service |
References
- Larson, E.B.; Yaffe, K.; Langa, K.M. New insights into the dementia epidemic. N. Engl. J. Med. 2013, 369, 2275. [Google Scholar] [CrossRef]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Burns, A.; Iliffe, S. Dementia. BMJ. Br. Med. J. 2009, 338, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J. Dementia prevention, intervention, and care. Lancet 2017, 390, 2673–2734. [Google Scholar] [CrossRef]
- Sahathevan, R.; Brodtmann, A.; Donnan, G.A. Dementia, stroke, and vascular risk factors; a review. Int. J. Stroke 2012, 7, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-T.; Fratiglioni, L.; Matthews, F.E.; Lobo, A.; Breteler, M.M.; Skoog, I.; Brayne, C. Dementia in western europe: Epidemiological evidence and implications for policy making. Lancet Neurol. 2016, 15, 116–124. [Google Scholar] [CrossRef]
- Zuccalà, G.; Onder, G.; Pedone, C.; Carosella, L.; Pahor, M.; Bernabei, R.; Cocchi, A. Hypotension and cognitive impairment: Selective association in patients with heart failure. Neurology 2001, 57, 1986–1992. [Google Scholar] [CrossRef]
- Breteler, M.M.; Claus, J.J.; Grobbee, D.E.; Hofman, A. Cardiovascular disease and distribution of cognitive function in elderly people: The rotterdam study. BMJ 1994, 308, 1604–1608. [Google Scholar] [CrossRef]
- Aronson, M.; Ooi, W.; Morgenstern, H.; Hafner, A.; Masur, D.; Crystal, H.; Frishman, W.; Fisher, D.; Katzman, R. Women, myocardial infarction, and dementia in the very old. Neurology 1990, 40, 1102. [Google Scholar] [CrossRef]
- Ikram, M.A.; van Oijen, M.; de Jong, F.J.; Kors, J.A.; Koudstaal, P.J.; Hofman, A.; Witteman, J.C.; Breteler, M.M. Unrecognized myocardial infarction in relation to risk of dementia and cerebral small vessel disease. Stroke 2008, 39, 1421–1426. [Google Scholar] [CrossRef]
- Shepherd, J.; Blauw, G.J.; Murphy, M.B.; Bollen, E.L.; Buckley, B.M.; Cobbe, S.M.; Ford, I.; Gaw, A.; Hyland, M.; Jukema, J.W. Pravastatin in elderly individuals at risk of vascular disease (prosper): A randomised controlled trial. Lancet 2002, 360, 1623–1630. [Google Scholar] [CrossRef]
- Kuwabara, M.; Kondo, F.; Hamada, T.; Takahashi, J.-I.; Takenaka, N.; Furuno, T. Impact of statins therapy for ischemic heart disease patients with low-density lipoprotein cholesterol levels less than 100 mg/dl. Acta Cardiol. Sin. 2016, 32, 565. [Google Scholar] [PubMed]
- Jick, H.; Zornberg, G.L.; Jick, S.S.; Seshadri, S.; Drachman, D.A. Statins and the risk of dementia. Lancet 2000, 356, 1627–1631. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Beason-Held, L.L.; Kitner-Triolo, M.H.; Beydoun, H.A.; Ferrucci, L.; Resnick, S.M.; Zonderman, A.B. Statins and serum cholesterol’s associations with incident dementia and mild cognitive impairment. J. Epidemiol. Community Health 2011, 65, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Bettermann, K.; Arnold, A.M.; Williamson, J.; Rapp, S.; Sink, K.; Toole, J.F.; Carlson, M.C.; Yasar, S.; DeKosky, S.; Burke, G.L. Statins, risk of dementia, and cognitive function: Secondary analysis of the ginkgo evaluation of memory study. J. Stroke Cerebrovasc. Dis. 2012, 21, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Kandiah, N.; Feldman, H.H. Therapeutic potential of statins in alzheimer’s disease. J. Neurol. Sci. 2009, 283, 230–234. [Google Scholar] [CrossRef]
- Shepardson, N.E.; Shankar, G.M.; Selkoe, D.J. Cholesterol level and statin use in alzheimer disease: Ii. Review of human trials and recommendations. Arch. Neurol. 2011, 68, 1385–1392. [Google Scholar] [CrossRef]
- Group, H.P.S.C. Mrc/bhf heart protection study of cholesterol lowering with simvastatin in 20 536 high-risk individuals: A randomised placebocontrolled trial. Lancet 2002, 360, 7–22. [Google Scholar]
- Zucker, I.; Beery, A.K. Males still dominate animal studies. Nature 2010, 465, 690. [Google Scholar] [CrossRef]
- Keitt, S.K.; Fagan, T.F.; Marts, S.A. Understanding sex differences in environmental health: A thought leaders’ roundtable. Environ. Health Perspect. 2004, 112, 604–609. [Google Scholar] [CrossRef]
- Franconi, F.; Brunelleschi, S.; Steardo, L.; Cuomo, V. Gender differences in drug responses. Pharmacol. Res. 2007, 55, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Schmetzer, O.; Flörcken, A. Sex differences in the drug therapy for oncologic diseases. In Sex and Gender Differences in Pharmacology; Springer: Berlin/Heidelberg, Germany, 2013; pp. 411–442. [Google Scholar]
- Seeland, U.; Regitz-Zagrosek, V. Sex and gender differences in cardiovascular drug therapy. In Sex and Gender Differences in Pharmacology; Springer: Berlin/Heidelberg, Germany, 2013; pp. 211–236. [Google Scholar]
- Kim, H.-I.; Lim, H.; Moon, A. Sex differences in cancer: Epidemiology, genetics and therapy. Biomol. Ther. 2018, 26, 335. [Google Scholar] [CrossRef] [PubMed]
- Zissimopoulos, J.M.; Barthold, D.; Brinton, R.D.; Joyce, G. Sex and race differences in the association between statin use and the incidence of alzheimer disease. JAMA Neurol. 2017, 74, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Sundararajan, V.; Henderson, T.; Perry, C.; Muggivan, A.; Quan, H.; Ghali, W.A. New icd-10 version of the charlson comorbidity index predicted in-hospital mortality. J. Clin. Epidemiol. 2004, 57, 1288–1294. [Google Scholar] [CrossRef]
- Chou, C.-Y.; Chou, Y.-C.; Chou, Y.-J.; Yang, Y.-F.; Huang, N. Statin use and incident dementia: A nationwide cohort study of taiwan. Int. J. Cardiol. 2014, 173, 305–310. [Google Scholar] [CrossRef]
- Wolozin, B.; Wang, S.W.; Li, N.-C.; Lee, A.; Lee, T.A.; Kazis, L.E. Simvastatin is associated with a reduced incidence of dementia and parkinson’s disease. BMC Med. 2007, 5, 20. [Google Scholar] [CrossRef]
- Hippisley-Cox, J.; Coupland, C. Unintended effects of statins in men and women in england and wales: Population based cohort study using the qresearch database. BMJ 2010, 340, c2197. [Google Scholar] [CrossRef]
- Swiger, K.J.; Manalac, R.J.; Blumenthal, R.S.; Blaha, M.J.; Martin, S.S. Statins and Cognition: A Systematic Review and Meta-Analysis of Short-and Long-Term Cognitive Effects, Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1213–1221. [Google Scholar]
- Chen, P.-Y.; Liu, S.-K.; Chen, C.-L.; Wu, C.-S. Long-term statin use and dementia risk in Taiwan. J. Geriatr. Psychiatry Neurol. 2014, 27, 165–171. [Google Scholar] [CrossRef]
- Naranjo, C.A.; Busto, U.; Sellers, E.M.; Sandor, P.; Ruiz, I.; Roberts, E.; Janecek, E.; Domecq, C.; Greenblatt, D. A method for estimating the probability of adverse drug reactions. Clin. Pharmacol. Ther. 1981, 30, 239–245. [Google Scholar] [CrossRef]
- Schultz, B.G.; Patten, D.K.; Berlau, D.J. The role of statins in both cognitive impairment and protection against dementia: A tale of two mechanisms. Transl. Neurodegener. 2018, 7, 5. [Google Scholar] [CrossRef]
- Refolo, L.M.; Pappolla, M.A.; LaFrancois, J.; Malester, B.; Schmidt, S.D.; Thomas-Bryant, T.; Tint, G.S.; Wang, R.; Mercken, M.; Petanceska, S.S. A cholesterol-lowering drug reduces β-amyloid pathology in a transgenic mouse model of alzheimer’s disease. Neurobiol. Dis. 2001, 8, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Reed, B.; Villeneuve, S.; Mack, W.; DeCarli, C.; Chui, H.C.; Jagust, W. Associations between serum cholesterol levels and cerebral amyloidosis. JAMA Neurol. 2014, 71, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Liao, J.K. Pleiotropic effects of statins. Circ. J. 2010, 74, 818–826. [Google Scholar] [CrossRef]
- Kim, M.-Y.; Noh, Y.; Son, S.J.; Shin, S.; Paik, H.-Y.; Lee, S.; Jung, Y.-S. Effect of cilostazol on incident dementia in elderly men and women with ischemic heart disease. J. Alzheimer’s Dis. 2018, 63, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-W.; Horng, J.-T.; Hsu, C.-C.; Chen, J.-M. Mean daily dosage of aspirin and the risk of incident alzheimer’s dementia in patients with type 2 diabetes mellitus: A nationwide retrospective cohort study in taiwan. J. Diabetes Res. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nanna, M.G.; Wang, T.Y.; Xiang, Q.; Goldberg, A.C.; Robinson, J.G.; Roger, V.L.; Virani, S.S.; Wilson, P.W.; Louie, M.J.; Koren, A. Sex differences in the use of statins in community practice: Patient and provider assessment of lipid management registry. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005562. [Google Scholar] [CrossRef] [PubMed]
- Raparelli, V.; Pannitteri, G.; Todisco, T.; Toriello, F.; Napoleone, L.; Manfredini, R.; Basili, S. Treatment and response to statins: Gender-related differences. Curr. Med. Chem. 2017, 24, 2628–2638. [Google Scholar] [CrossRef]
- Karp, I.; Chen, S.-F.; Pilote, L. Sex differences in the effectiveness of statins after myocardial infarction. CMAJ 2007, 176, 333–338. [Google Scholar] [CrossRef]
- Uchiyama, N.; Kagami, Y.; Saitoh, Y.; Ohtawa, M. Male-specific metabolism of simvastatin by rat liver microsomes. Chem. Pharm. Bull. 1991, 39, 236–238. [Google Scholar] [CrossRef]
- Ohtawa, M.; Uchiyama, N. Sex difference in metabolism of simvastatin by rat hepatic microsomes. Eur. J. Drug Metab. Pharmacokinet. 1992, 17, 175–181. [Google Scholar] [CrossRef]
- Jacobson, T.A.; Jokubaitis, L.A.; Amorosa, L.F. Fluvastatin and niacin in hypercholesterolemia: A preliminary report on gender differences in efficacy. Am. J. Med. 1994, 96, S64–S68. [Google Scholar] [CrossRef]
- Nakajima, K. Sex-related differences in response of plasma lipids to simvastatin: The saitama postmenopausal lipid intervention study. Clin. Ther. 1999, 21, 2047–2057. [Google Scholar] [CrossRef]
- Wolbold, R.; Klein, K.; Burk, O.; Nüssler, A.K.; Neuhaus, P.; Eichelbaum, M.; Schwab, M.; Zanger, U.M. Sex is a major determinant of cyp3a4 expression in human liver. Hepatology 2003, 38, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Vermes, A.; Vermes, I. Genetic polymorphisms in cytochrome p450 enzymes. Am. J. Cardiovasc. Drugs 2004, 4, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Husain, I.; Akhtar, M.; Vohora, D.; Abdin, M.Z.; Islamuddin, M.; Akhtar, M.J.; Najmi, A.K. Rosuvastatin attenuates high-salt and cholesterol diet induced neuroinflammation and cognitive impairment via preventing nuclear factor kappab pathway. Neurochem. Res. 2017, 42, 2404–2416. [Google Scholar] [CrossRef] [PubMed]
- Vuletic, S.; Riekse, R.G.; Marcovina, S.M.; Peskind, E.R.; Hazzard, W.R.; Albers, J.J. Statins of different brain penetrability differentially affect csf pltp activity. Dement. Geriatr. Cogn. Disord. 2006, 22, 392–398. [Google Scholar] [CrossRef]
- Glasser, S.P.; Wadley, V.; Judd, S.; Kana, B.; Prince, V.; Jenny, N.; Kissela, B.; Safford, M.; Prineas, R.; Howard, G. The association of statin use and statin type and cognitive performance: Analysis of the reasons for geographic and racial differences in stroke (regards) study. Clin. Cardiol. Int. Index. Peer-Rev. J. Adv. Treat. Cardiovasc. Dis. 2010, 33, 280–288. [Google Scholar] [CrossRef]


| Total (n = 143,174) | Male (n = 57,737) | Female (n = 85,437) | ||||
|---|---|---|---|---|---|---|
| Non–Statin (n = 71,587) | Statin (n = 71,587) | Non–Statin (n = 28,725) | Statin (n = 29,012) | Non–Statin (n = 42,862) | Statin (n = 42,575) | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Age | ||||||
| mean ± SD | 72.3 ± 5.3 | 72.1 ± 5.4 | 71.7 ± 5.1 | 71.6 ± 5.2 | 72.6 ± 5.4 | 72.4 ± 5.5 |
| 65–75 years | 54,292 (75.8) | 54,281 (75.8) | 22,685 (31.7) | 22,913 (32.0) | 31,607 (44.2) | 31,368 (43.8) |
| 75–85 years | 16,014 (22.4) | 15,959 (22.3) | 5607 (7.8) | 5660 (7.9) | 10,407 (14.5) | 10,299 (14.4) |
| >85 years | 1281 (1.8) | 1347 (1.9) | 433 (0.6) | 439 (0.6) | 848 (1.2) | 908 (1.3) |
| Comorbidities | ||||||
| Hypertension | 53,606 (74.9) | 53,541 (74.8) | 21,233 (29.7) | 21,464 (30.0) | 32,373 (45.2) | 32,077 (44.8) |
| Diabetes | 25,682 (35.9) | 25,951 (36.3) | 10,895 (15.2) | 11,210 (15.7) | 14,787 (20.7) | 14,741 (20.6) |
| Ischemic stroke | 8639 (12.1) | 8935 (12.5) | 4029 (5.6) | 4334 (6.1) | 4610 (6.4) | 4601 (6.4) |
| Depression | 6013 (8.4) | 5720 (8.0) | 1087 (1.5) | 1783 (2.5) | 4206 (5.9) | 3937 (5.5) |
| Parkinson | 767 (1.1) | 712 (1.0) | 287 (0.4) | 263 (0.4) | 480 (0.7) | 449 (0.6) |
| Schizophrenia | 262 (0.4) | 137 (0.2) | 130 (0.2) | 62 (0.1) | 132 (0.2) | 75 (0.1) |
| CCI score | ||||||
| ≤1 | 30,328 (42.4) | 30,319 (42.4) | 11,600 (16.2) | 11,607 (16.2) | 18,728 (26.2) | 18,712 (26.1) |
| 2 | 15,887 (22.2) | 15,816 (22.1) | 6276 (8.8) | 6296 (8.8) | 9611 (13.4) | 9520 (13.3) |
| ≥3 | 25,372 (35.4) | 25,452 (35.6) | 10,849 (15.2) | 11,109 (15.5) | 14,523 (20.3) | 14,343 (20.0) |
| Concurrent medication | ||||||
| Antiplatelet agents | 47,713 (66.7) | 61,540 (86.0) | 19,609 (27.4) | 26,364 (36.8) | 28,104 (39.3) | 35,176 (49.1) |
| ACEIs or ARBs | 43,101 (60.2) | 52,675 (73.6) | 17,200 (24.0) | 21,614 (30.2) | 25,901 (36.2) | 31,061 (43.4) |
| Beta Blockers | 25,479 (35.6) | 36,279 (50.7) | 9822 (13.7) | 15,377 (21.5) | 15,657 (21.9) | 20.902 (29.2) |
| CCBs | 42,245 (59.0) | 43,666 (61.0) | 16,367 (22.9) | 16,673 (23.3) | 25,878 (36.1) | 26,993 (37.7) |
| Antidiabetic agents | 21,770 (30.4) | 26,268 (36.7) | 9220 (12.9) | 11,093 (15.5) | 12,550 (17.5) | 15,175 (21.2) |
| PPIs | 30,867 (43.1) | 37,237 (52.0) | 12,043 (16.8) | 14,423 (20.1) | 18,824 (26.3) | 22,814 (31.9) |
| Follow-up duration (year) | ||||||
| mean ± SD | 5.2 ± 1.7 | 5.0 ± 1.4 | 5.2 ± 1.8 | 5.0 ± 1.3 | 5.1 ± 1.7 | 4.9 ± 1.5 |
| Dementia Incidence, n (%) | |||||
|---|---|---|---|---|---|
| Total (n = 143,174) | |||||
| n | case, n | (%) | HR (95% CI) | p-value | |
| Non–user | 71,587 | 14,122 | 19.7 | reference | |
| Statin user | 71,587 | 14,249 | 19.9 | 0.95 (0.92–0.97) | <0.001 |
| Atorvastatin | 45,753 | 9312 | 20.4 | 0.96 (0.93–0.99) | 0.003 |
| Simvastatin | 10,888 | 2282 | 21.0 | 1.02 (0.98–1.07) | 0.320 |
| Rosuvastatin | 8231 | 1428 | 17.3 | 0.82 (0.78–0.87) | <0.001 |
| Pitavastatin | 2676 | 498 | 18.6 | 0.89 (0.82–0.98) | 0.013 |
| Pravastatin | 2538 | 468 | 18.4 | 0.86 (0.78–0.94) | 0.001 |
| Fluvastatin | 934 | 162 | 17.3 | 0.84 (0.72–0.98) | 0.032 |
| Lovastatin | 567 | 99 | 17.5 | 0.87 (0.71–1.06) | 0.164 |
| Male (n = 57,737) | |||||
| n | case, n | (%) | HR (95% CI) | p-value | |
| Non–user | 28,725 | 4619 | 16.1 | reference | |
| Statin user | 29,012 | 4644 | 16.0 | 0.92 (0.88–0.96) | < 0.001 |
| Atorvastatin | 17,970 | 2919 | 16.2 | 0.92 (0.88–0.97) | 0.001 |
| Simvastatin | 4075 | 675 | 16.6 | 0.98 (0.90–1.06) | 0.582 |
| Rosuvastatin | 4038 | 603 | 14.9 | 0.87 (0.80–0.95) | 0.002 |
| Pitavastatin | 1153 | 178 | 15.4 | 0.90 (0.78–1.04) | 0.157 |
| Pravastatin | 1130 | 170 | 15.0 | 0.83 (0.71–0.97) | 0.016 |
| Fluvastatin | 448 | 62 | 13.8 | 0.85 (0.66–1.08) | 0.188 |
| Lovastatin | 198 | 37 | 18.7 | 1.15 (0.84–1.60) | 0.385 |
| Female (n = 85,437) | |||||
| n | case, n | (%) | HR (95% CI) | p-value | |
| Non–user | 42,862 | 9503 | 22.2 | reference | |
| Statin user | 42,575 | 9605 | 22.6 | 0.96 (0.93–0.99) | 0.003 |
| Atorvastatin | 27,783 | 6393 | 23.0 | 0.97 (0.94–1.00) | 0.075 |
| Simvastatin | 6813 | 1607 | 23.6 | 1.03 (0.98–1.09) | 0.275 |
| Rosuvastatin | 4193 | 825 | 19.7 | 0.82 (0.76–0.88) | <0.001 |
| Pitavastatin | 1523 | 320 | 21.0 | 0.90 (0.80–1.01) | 0.063 |
| Pravastatin | 1408 | 298 | 21.2 | 0.89 (0.79–0.99) | 0.048 |
| Fluvastatin | 486 | 100 | 20.6 | 0.87 (0.71–1.06) | 0.164 |
| Lovastatin | 369 | 62 | 16.8 | 0.74 (0.58–0.95) | 0.018 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-Y.; Jung, M.; Noh, Y.; Shin, S.; Hong, C.H.; Lee, S.; Jung, Y.-S. Impact of Statin Use on Dementia Incidence in Elderly Men and Women with Ischemic Heart Disease. Biomedicines 2020, 8, 30. https://doi.org/10.3390/biomedicines8020030
Kim M-Y, Jung M, Noh Y, Shin S, Hong CH, Lee S, Jung Y-S. Impact of Statin Use on Dementia Incidence in Elderly Men and Women with Ischemic Heart Disease. Biomedicines. 2020; 8(2):30. https://doi.org/10.3390/biomedicines8020030
Chicago/Turabian StyleKim, Mi-Young, Minji Jung, Yoojin Noh, Sooyoung Shin, Chang Hyung Hong, Sukhyang Lee, and Yi-Sook Jung. 2020. "Impact of Statin Use on Dementia Incidence in Elderly Men and Women with Ischemic Heart Disease" Biomedicines 8, no. 2: 30. https://doi.org/10.3390/biomedicines8020030
APA StyleKim, M.-Y., Jung, M., Noh, Y., Shin, S., Hong, C. H., Lee, S., & Jung, Y.-S. (2020). Impact of Statin Use on Dementia Incidence in Elderly Men and Women with Ischemic Heart Disease. Biomedicines, 8(2), 30. https://doi.org/10.3390/biomedicines8020030






