Increased Encapsulation Efficiency of Methotrexate in Liposomes for Rheumatoid Arthritis Therapy
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Liposome Production by Ethanol Injection Method
2.2.1. Conventional Method
2.2.2. Pre-Concentration Method
2.3. Determination of MTX Concentration
2.4. Physicochemical Characterization of Liposomes
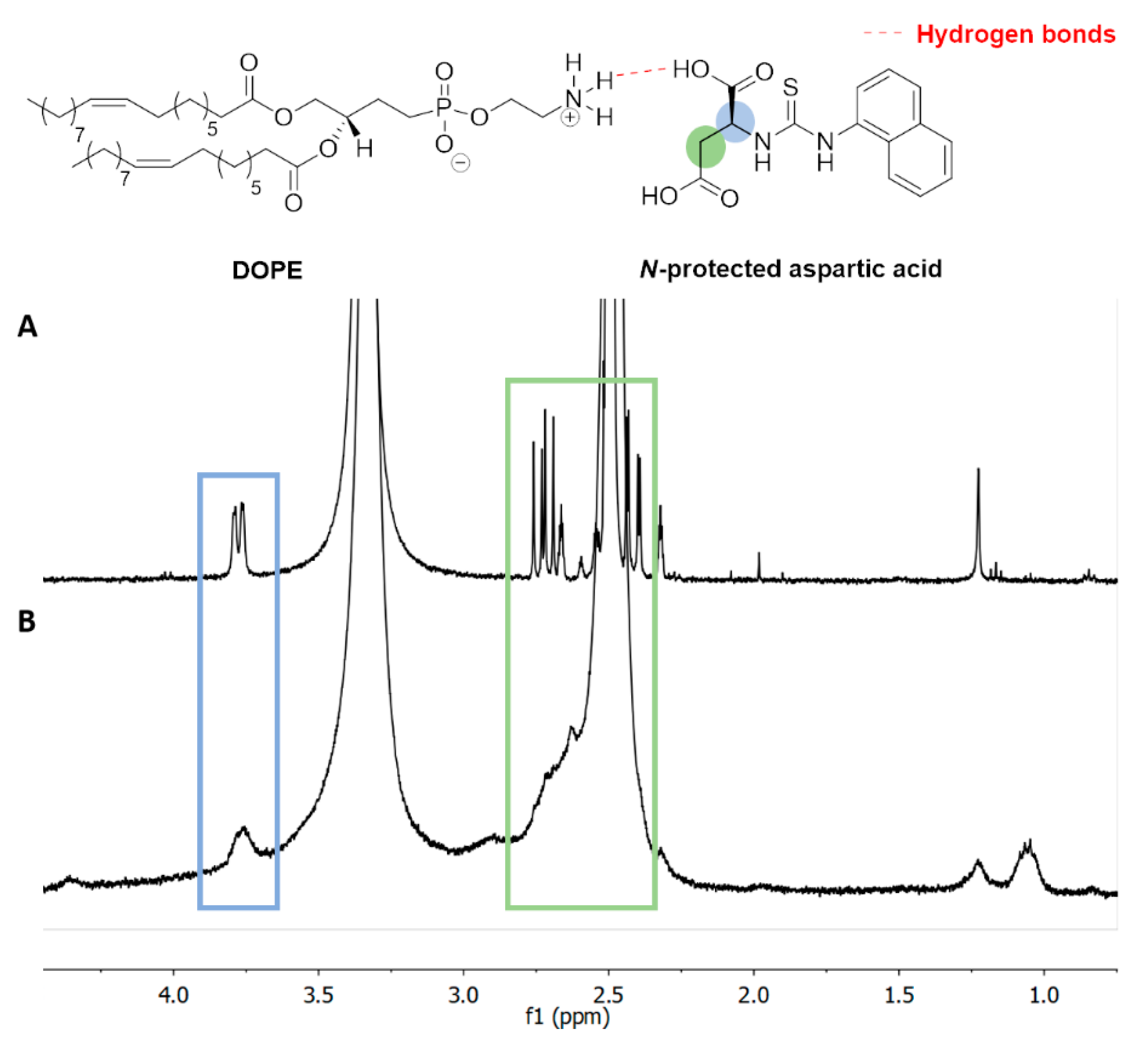
2.5. Evaluation of Compound Interactions by 1H NMR
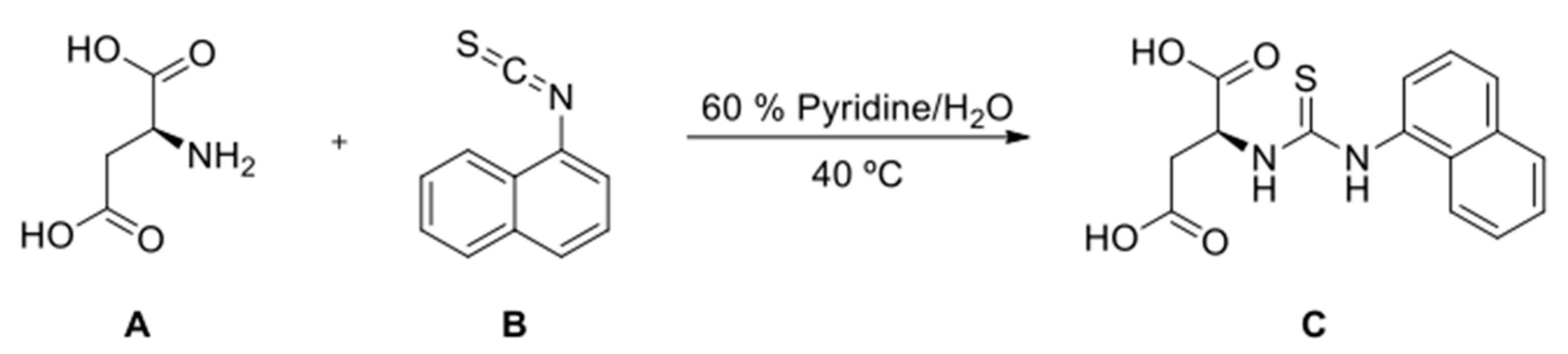
2.6. Synthesis of (S)-2-(3-(Naphthalen-1-yl)Thioureido)Succinic Acid
2.7. Collagen-Induced Arthritis (CIA)
2.8. Statistical Analysis
3. Results
3.1. Liposome-Encapsulated MTX Prepared by the Conventional Ethanol Injection Method
3.2. Liposome-Encapsulated MTX Prepared by the Pre-Concentration Ethanol Injection Method
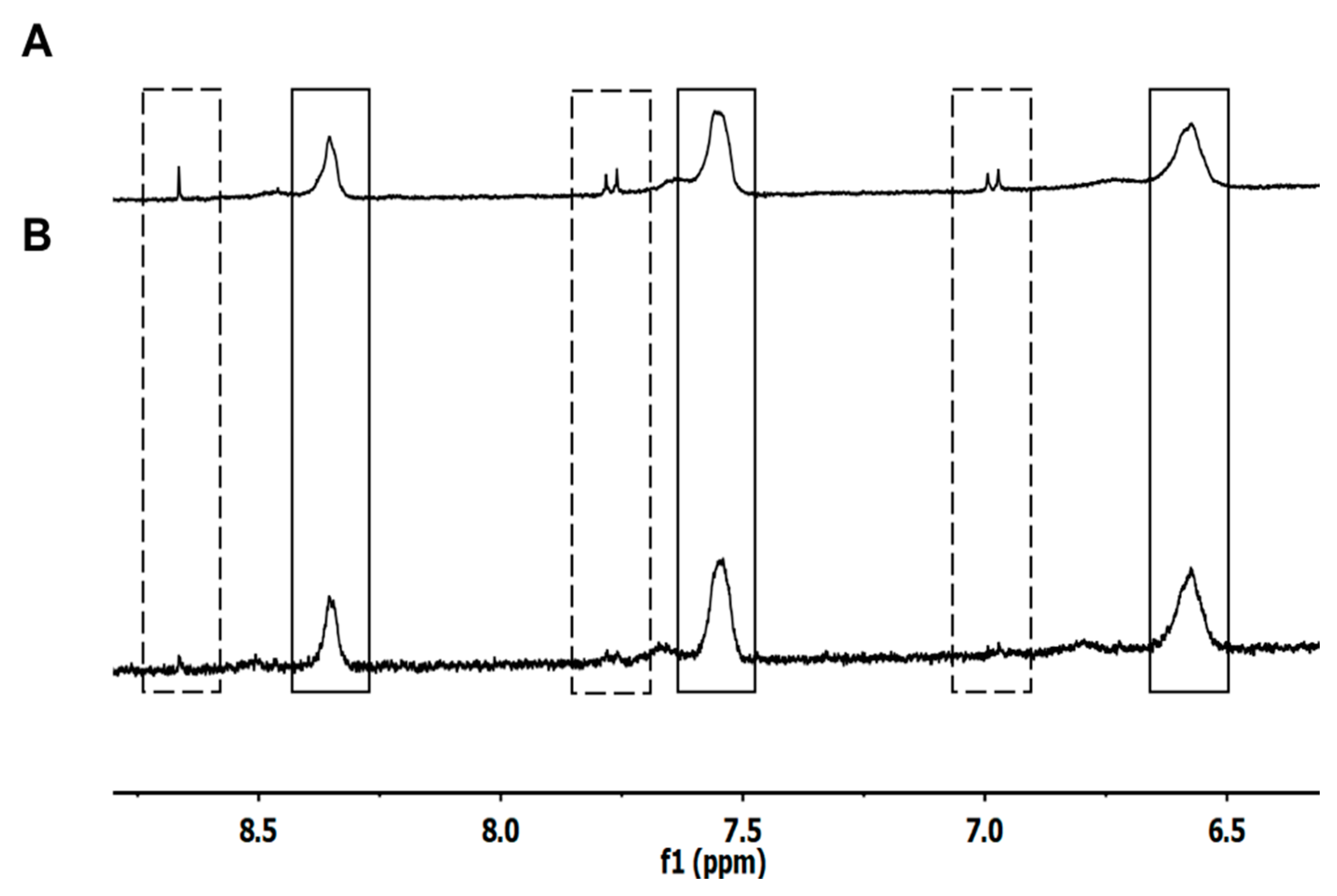
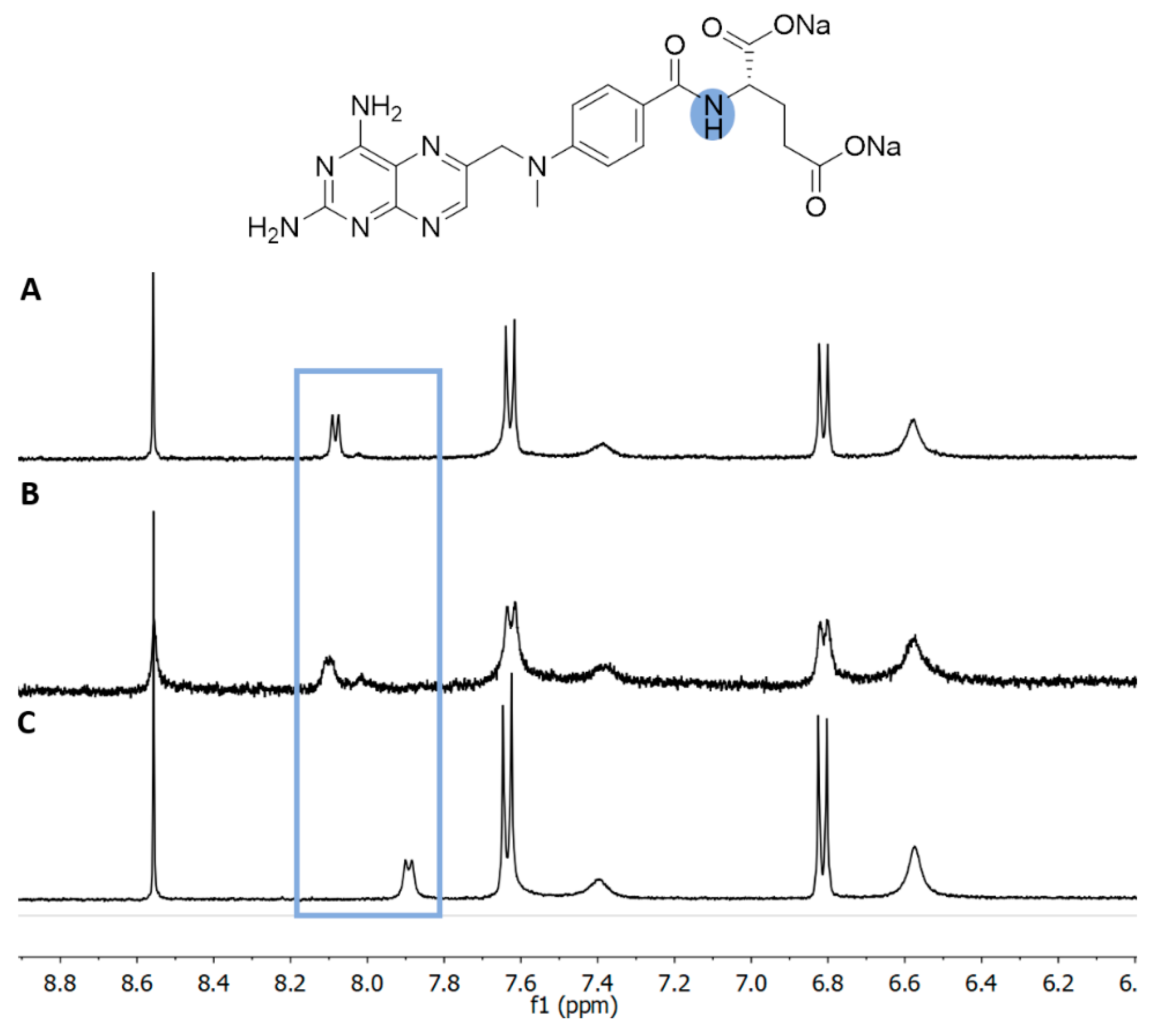
3.3. H NMR of Liposome-Encapsulated MTX
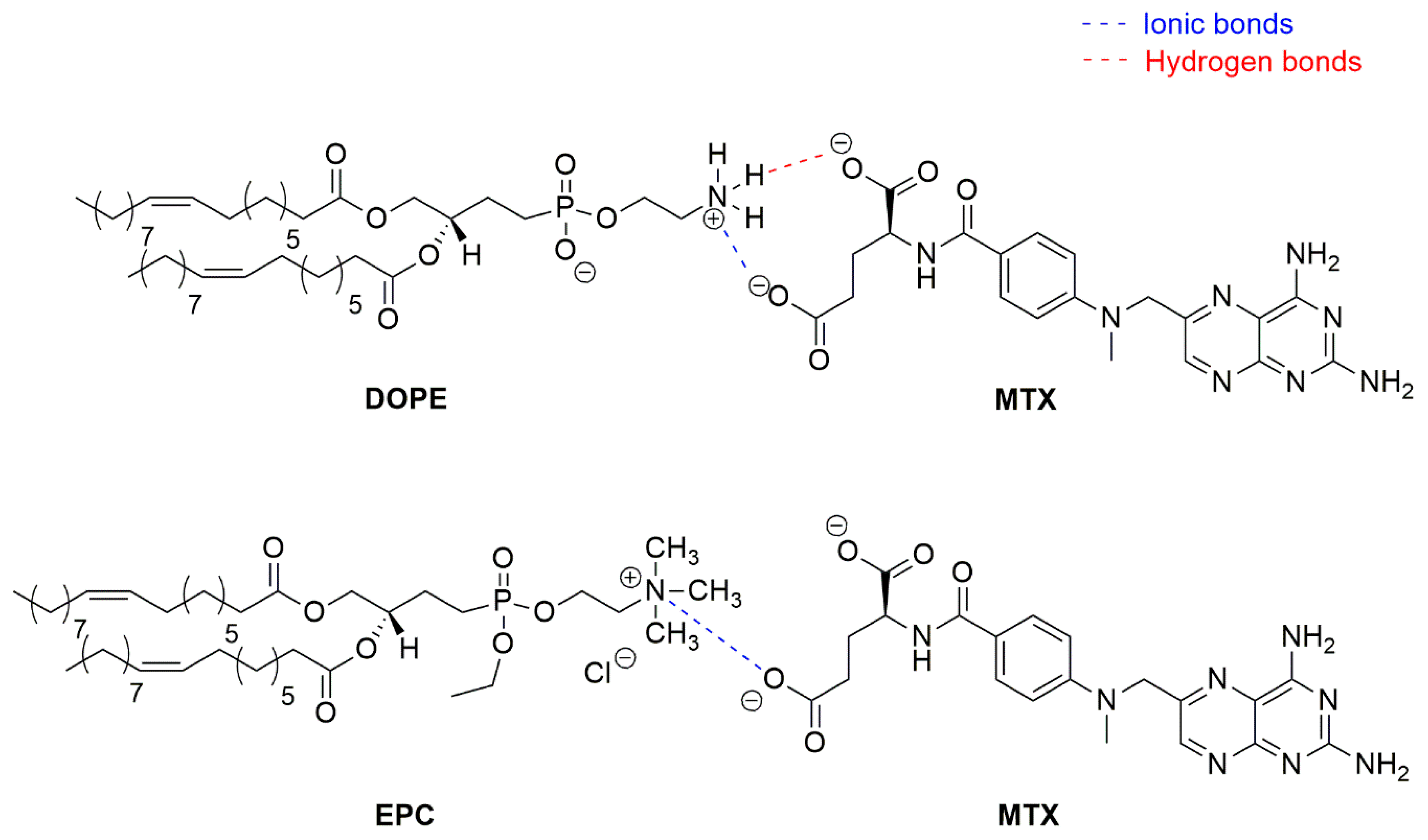
3.4. Role of the Lipid in the Encapsulation of MTX
3.5. Interaction of DOPE with N-Protected L-Aspartic Acid
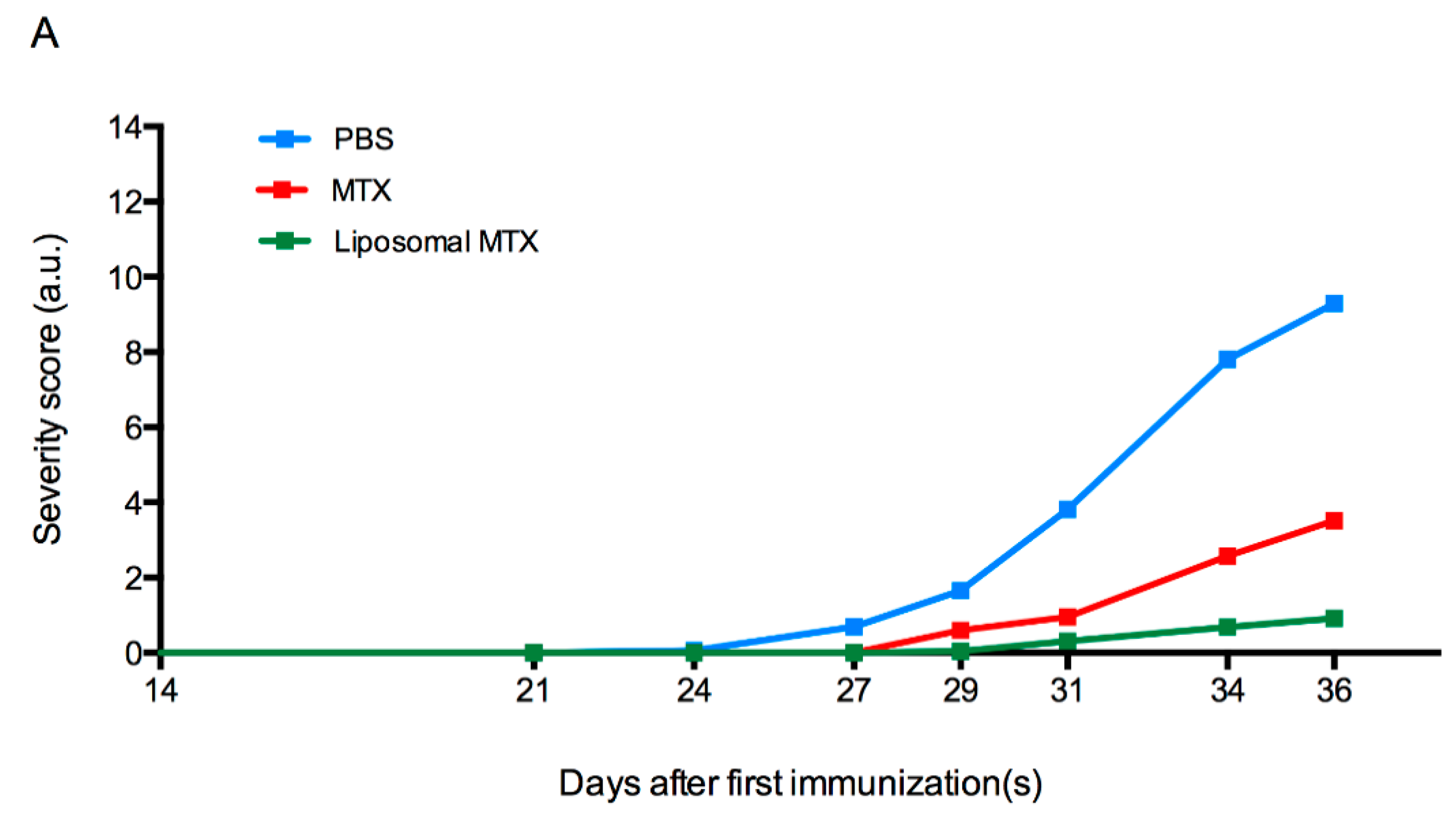
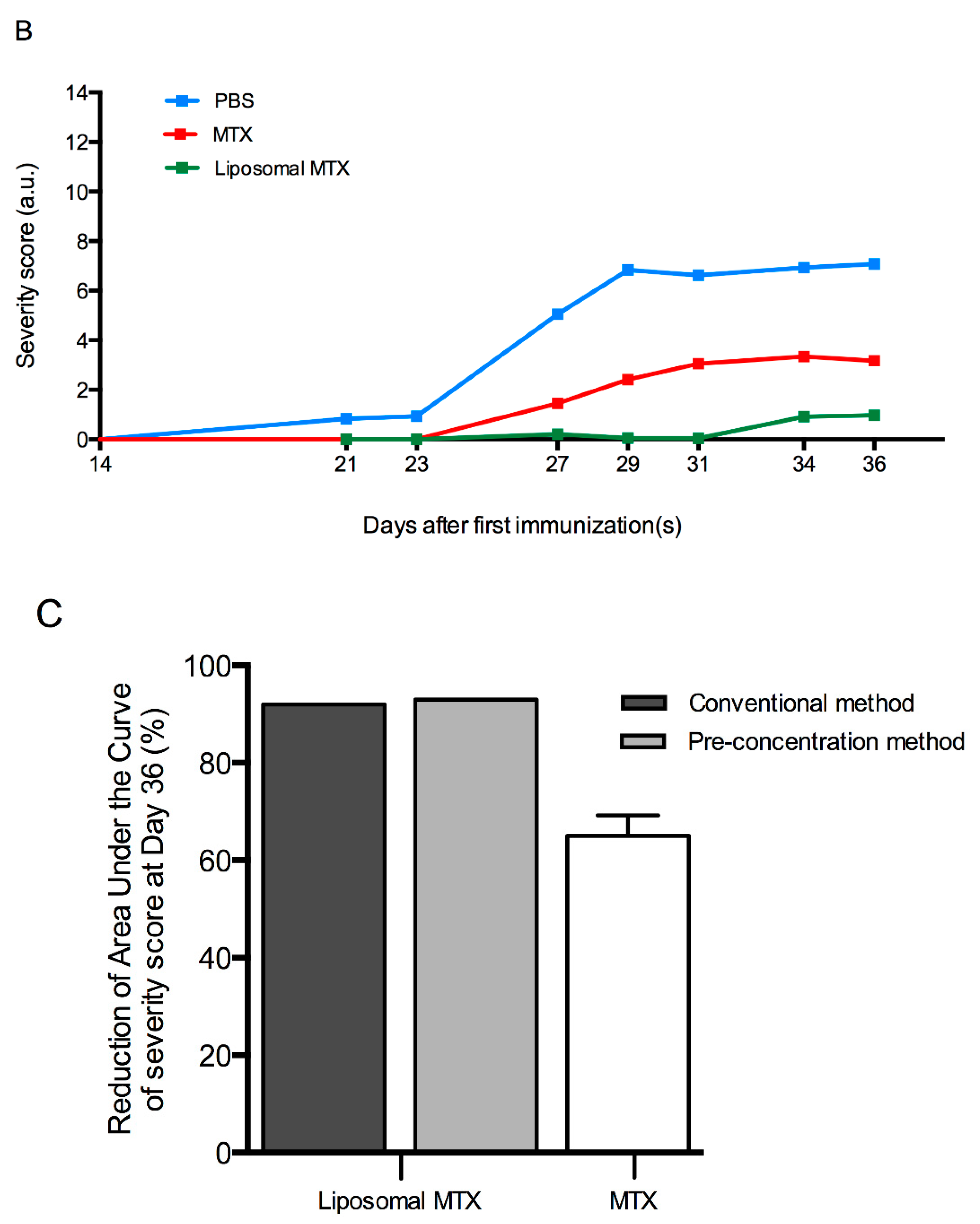
3.6. Biological Effect of Liposome-Encapsulated MTX
4. Discussion
5. Patents
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AUC | Area under the curve |
| CIA | Collagen-induced arthritis |
| CII | Type II bovine collagen |
| D2O | Deuterium oxide |
| DMSO-d6 | Deuterated dimethyl sulfoxide |
| DOPE | 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine |
| DSPE–mPEG | 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] |
| EE | Encapsulation efficiency |
| EPC | Egg phosphatidylcholine |
| IP | Intraperitoneally |
| MTX | Methotrexate |
| NMR | Nuclear magnetic resonance |
| PBS | Phosphate-buffered saline |
| PDI | Polydispersity index |
| SD | Standard deviation |
References
- Setoguchi, S.; Solomon, D.H.; Weinblatt, M.E.; Katz, J.N.; Avorn, J.; Glynn, R.J.; Cook, E.F.; Carney, G.; Schneeweiss, S. Tumor necrosis factor α antagonist use and cancer in patients with rheumatoid arthritis. Arthritis Rheum. 2006, 54, 2757–2764. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.; Rau, R. An update on methotrexate. Curr. Opin. Rheumatol. 2009, 21, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.-H.; Jeong, S.-H.; Lee, Y. Preparation and In Vitro/In Vivo Characterization of Polymeric Nanoparticles Containing Methotrexate to Improve Lymphatic Delivery. Int. J. Mol. Sci. 2019, 20, 3312. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Pentak, D.; Kozik, V.; Bak, A.; Dybal, P.; Sochanik, A.; Jampilek, J. Methotrexate and Cytarabine—Loaded Nanocarriers for Multidrug Cancer Therapy. Spectroscopic Study. Molecules 2016, 21, 1689. [Google Scholar] [CrossRef]
- Hong, M.-S.; Lim, S.J.; Lee, M.-K.; Kim, Y.B.; Kim, C.-K. Prolonged Blood Circulation of Methotrexate by Modulation of Liposomal Composition. Drug Deliv. 2001, 8, 231–237. [Google Scholar] [CrossRef]
- Sartori, T.; Murakami, F.S.; Cruz, A.P.; De Campos, A.M. Development and validation of a fast RP-HPLC method for determination of methotrexate entrapment efficiency in polymeric nanocapsules. J. Chromatogr. Sci. 2008, 46, 505–509. [Google Scholar] [CrossRef]
- Byeon, J.C.; Lee, S.-E.; Kim, T.-H.; Bin Ahn, J.; Kim, D.-H.; Choi, J.-S.; Park, J.-S. Design of novel proliposome formulation for antioxidant peptide, glutathione with enhanced oral bioavailability and stability. Drug Deliv. 2019, 26, 216–225. [Google Scholar] [CrossRef]
- Nogueira, E.S.D.C.; Mangialavori, I.C.; Loureiro, A.; Azoia, N.; Sárria, M.P.; Nogueira, P.; Freitas, J.; Härmark, J.; Shimanovich, U.; Rollett, A.; et al. Peptide Anchor for Folate-Targeted Liposomal Delivery. Biomacromolecules 2015, 16, 2904–2910. [Google Scholar] [CrossRef]
- Nogueira, E.S.D.C.; Lager, F.; Le Roux, D.; Nogueira, P.; Freitas, J.; Charvet, C.; Renault, G.; Loureiro, A.; Almeida, C.R.; Ohradanova-Repic, A.; et al. Enhancing Methotrexate Tolerance with Folate Tagged Liposomes in Arthritic Mice. J. Biomed. Nanotechnol. 2015, 11, 2243–2252. [Google Scholar] [CrossRef]
- Worsham, R.D.; Thomas, V.; Farid, S.S. Potential of Continuous Manufacturing for Liposomal Drug Products. Biotechnol. J. 2019, 14, e1700740. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.M.; Nguyen, D.X.; Patel, P.; Cote, B.; Al-Fatease, A.; Pham, Y.; Huynh, M.G.; Woo, Y.; Alani, A.W. Liposomes produced by microfluidics and extrusion: A comparison for scale-up purposes. Nanomed. Nanotechnol. Biol. Med. 2019, 18, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A.G.; Syed, S.; Marshall, K.; Hosseinidoust, Z. Liposomal Nanovesicles for Efficient Encapsulation of Staphylococcal Antibiotics. ACS Omega 2019, 4, 10866–10876. [Google Scholar] [CrossRef] [PubMed]
- Shaker, S.; Gardouh, A.R.; Ghorab, M.M. Factors affecting liposomes particle size prepared by ethanol injection method. Res. Pharm. Sci. 2017, 12, 346–352. [Google Scholar] [CrossRef]
- Meure, L.A.; Foster, N.R.; Dehghani, F. Conventional and Dense Gas Techniques for the Production of Liposomes: A Review. AAPS PharmSciTech 2008, 9, 798–809. [Google Scholar] [CrossRef]
- Gentine, P.; Bourel-Bonnet, L.; Frisch, B. Modified and derived ethanol injection toward liposomes: Development of the process. J. Liposome Res. 2012, 23, 11–19. [Google Scholar] [CrossRef]
- Ong, S.G.M.; Chitneni, M.; Lee, K.S.; Ming, L.C.; Yuen, K.H. Evaluation of Extrusion Technique for Nanosizing Liposomes. Pharmaceutics 2016, 8, 36. [Google Scholar] [CrossRef]
- Nii, T.; Ishii, F. Encapsulation efficiency of water-soluble and insoluble drugs in liposomes prepared by the microencapsulation vesicle method. Int. J. Pharm. 2005, 298, 198–205. [Google Scholar] [CrossRef]
- Kulkarni, S.B.; Betageri, G.V.; Singh, M. Factors affecting microencapsulation of drugs in liposomes. J. Microencapsul. 1995, 12, 229–246. [Google Scholar] [CrossRef]
- Barenholz, Y. Relevancy of Drug Loading to Liposomal Formulation Therapeutic Efficacy. J. Liposome Res. 2003, 13, 1–8. [Google Scholar] [CrossRef]
- Alekseeva, A.A.; Moiseeva, E.V.; Onishchenko, N.; Boldyrev, I.A.; Singin, A.S.; Budko, A.P.; Shprakh, Z.S.; Molotkovsky, J.G.; Vodovozova, E.L. Liposomal formulation of a methotrexate lipophilic prodrug: Assessment in tumor cells and mouse T-cell leukemic lymphoma. Int. J. Nanomed. 2017, 12, 3735–3749. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, A.M.; Bârcă, M.; Manda, G.; Dragomiroiu, G.T.A.B.; Baconi, D.L. Methotrexate Liposomes—A Reliable Therapeutic Option. In Liposomes; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Guimarães, D.; Noro, J.; Loureiro, A.; Cavaco-Paulo, A.; Nogueira, E. Quantification of drugs encapsulated in liposomes by 1H NMR. Colloids Surf. B Biointerfaces 2019, 179, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Rosloniec, E.F.; Cremer, M.; Kang, A.; Myers, L.K. Collagen-Induced Arthritis. Curr. Protoc. Immunol. 1996, 20, 15.5.1–15.5.24. [Google Scholar] [CrossRef] [PubMed]
- Jelicks, L.A.; Broido, M.S.; Becker, J.M.; Naider, F.R. Interaction of the Saccharomyces cerevisiae. alpha.-factor with phospholipid vesicles as revealed by proton and phosphorus NMR. Biochemistry 1989, 28, 4233–4240. [Google Scholar] [CrossRef]
- Pamunuwa, G.; Karunaratne, V.; Karunaratne, D.N. Effect of Lipid Composition onIn VitroRelease and Skin Deposition of Curcumin Encapsulated Liposomes. J. Nanomater. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Friedman, B.; Cronstein, B.N. Methotrexate mechanism in treatment of rheumatoid arthritis. Jt. Bone Spine 2019, 86, 301–307. [Google Scholar] [CrossRef]
- Justo, O.R.; Moraes, Â.M. Analysis of process parameters on the characteristics of liposomes prepared by ethanol injection with a view to process scale-up: Effect of temperature and batch volume. Chem. Eng. Res. Des. 2011, 89, 785–792. [Google Scholar] [CrossRef]
- Maitani, Y.; Soeda, H.; Junping, W.; Takayama, K. Modified ethanol injection method for liposomes containing β-sitosterol β-d-glucoside. J. Liposome Res. 2001, 11, 115–125. [Google Scholar] [CrossRef]
- Jaafar-Maalej, C.; Diab, R.; Andrieu, V.; Elaissari, A.; Fessi, H. Ethanol injection method for hydrophilic and lipophilic drug-loaded liposome preparation. J. Liposome Res. 2010, 20, 228–243. [Google Scholar] [CrossRef]
- Leo, E.; Brina, B.; Forni, F.; Vandelli, M.A. In vitro evaluation of PLA nanoparticles containing a lipophilic drug in water-soluble or insoluble form. Int. J. Pharm. 2004, 278, 133–141. [Google Scholar] [CrossRef]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New Developments in Liposomal Drug Delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef] [PubMed]
- Pentak, D. Evaluation of the physicochemical properties of liposomes as potential carriers of anticancer drugs: Spectroscopic study. J. Nanoparticle Res. 2016, 18. [Google Scholar] [CrossRef]
- Seddon, A.M.; Casey, D.; Law, R.V.; Gee, A.; Templer, R.H.; Ces, O. Drug interactions with lipid membranes. Chem. Soc. Rev. 2009, 38, 2509–2519. [Google Scholar] [CrossRef] [PubMed]

| Ethanol Injection Method | Liposomes * | Initial Ratio Organic: Aqueous Phase (v/v) | EtOH (%) # | Extruder | Z-Average (d.nm) | Polydispersity Index | [Methotrexate] (mg/mL) | Encapsulation Efficiency (%) |
|---|---|---|---|---|---|---|---|---|
| Conventional | A | 1:5 | 20 | − | 160.60 ± 1.84 | 0.300 ± 0.01 | 0.26 ± 0.04 | 1.32 ± 0.19 |
| + | 110.20 ± 1.84 | 0.083 ± 0.04 | 0.17 ± 0.03 | 0.88 ± 0.16 | ||||
| Pre-concentration | B | 1:2 | 10 | − | 187.9 ± 14.14 | 0.272 ± 0.01 | 2.92 ± 0.60 | 14.60 ± 3.01 |
| + | 122.55 ± 11.24 | 0.079 ± 0.02 | 0.75 ± 0.04 | 3.75 ± 0.22 | ||||
| C | 1:1 | 20 | − | 128.76 ± 7.78 | 0.107 ± 0.02 | 4.58 ± 0.03 | 22.90 ± 0.17 | |
| D | 2:1 | 40 | − | 257.7 ± 15.98 | 0.201 ± 0.02 | 6.47 ± 0.94 | 32.33 ± 4.70 | |
| + | 129.35 ± 2.33 | 0.060 ± 0.02 | 1.10 ± 0.43 | 5.52 ± 2.17 | ||||
| E | 1:1 | 20 | − | 201.1 ± 7.47 | 0.123 ± 0.03 | 2.37 ± 0.04 | 11.85 ± 0.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guimarães, D.; Noro, J.; Loureiro, A.; Lager, F.; Renault, G.; Cavaco-Paulo, A.; Nogueira, E. Increased Encapsulation Efficiency of Methotrexate in Liposomes for Rheumatoid Arthritis Therapy. Biomedicines 2020, 8, 630. https://doi.org/10.3390/biomedicines8120630
Guimarães D, Noro J, Loureiro A, Lager F, Renault G, Cavaco-Paulo A, Nogueira E. Increased Encapsulation Efficiency of Methotrexate in Liposomes for Rheumatoid Arthritis Therapy. Biomedicines. 2020; 8(12):630. https://doi.org/10.3390/biomedicines8120630
Chicago/Turabian StyleGuimarães, Diana, Jennifer Noro, Ana Loureiro, Franck Lager, Gilles Renault, Artur Cavaco-Paulo, and Eugénia Nogueira. 2020. "Increased Encapsulation Efficiency of Methotrexate in Liposomes for Rheumatoid Arthritis Therapy" Biomedicines 8, no. 12: 630. https://doi.org/10.3390/biomedicines8120630
APA StyleGuimarães, D., Noro, J., Loureiro, A., Lager, F., Renault, G., Cavaco-Paulo, A., & Nogueira, E. (2020). Increased Encapsulation Efficiency of Methotrexate in Liposomes for Rheumatoid Arthritis Therapy. Biomedicines, 8(12), 630. https://doi.org/10.3390/biomedicines8120630







