Exploring the Multifaceted Therapeutic Potential of Withaferin A and Its Derivatives
Abstract
1. Introduction
2. Pharmacokinetics and Bioavailability Studies of Withaferin A
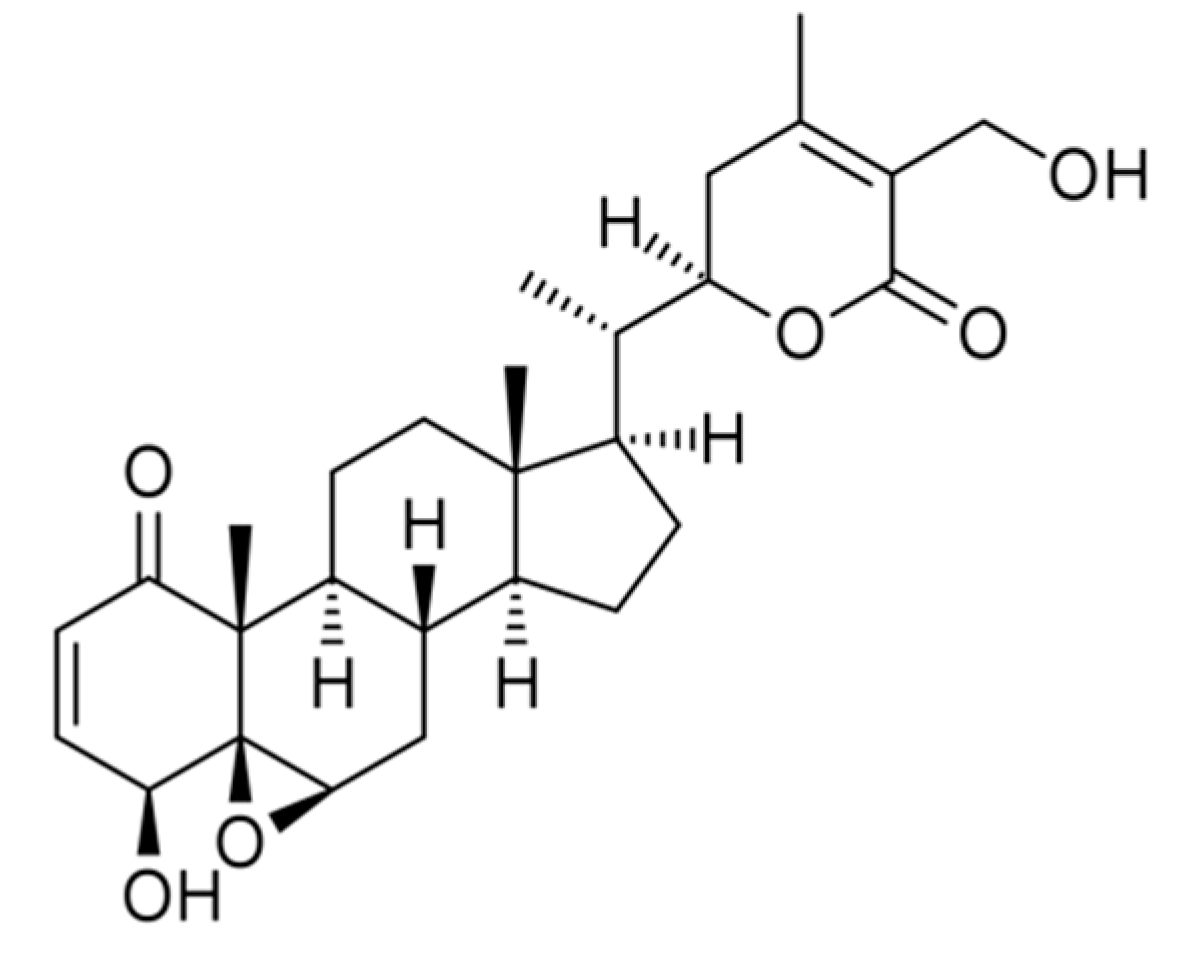
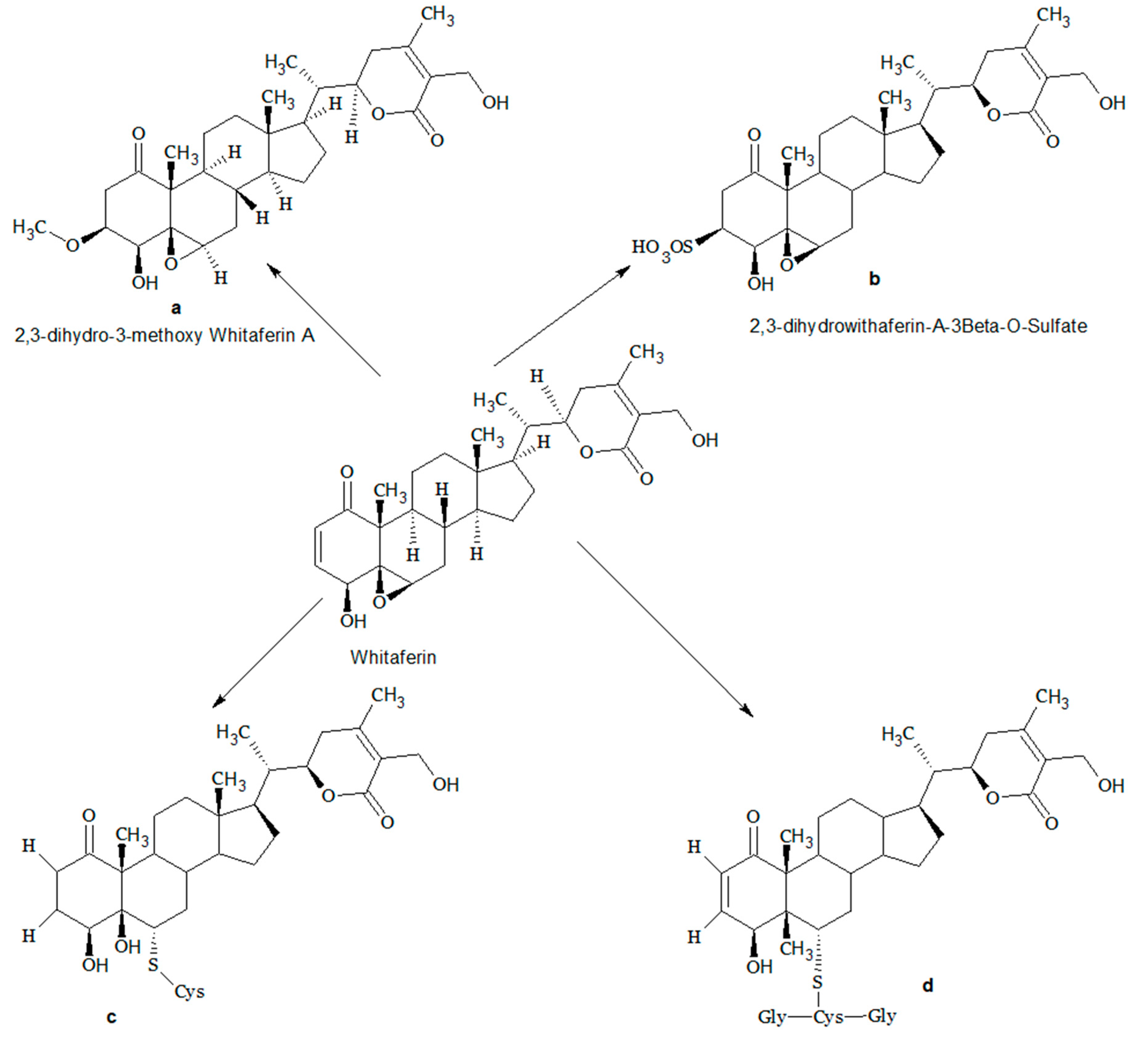
3. Structural Modifications of Withaferin A
4. Pharmacological Activities of Withaferin A
4.1. Anti-Cancer Activity
4.1.1. Breast Cancer
4.1.2. Ovarian Cancer
4.1.3. Prostate Cancer
4.1.4. Colorectal Cancer
4.1.5. Lung Cancer
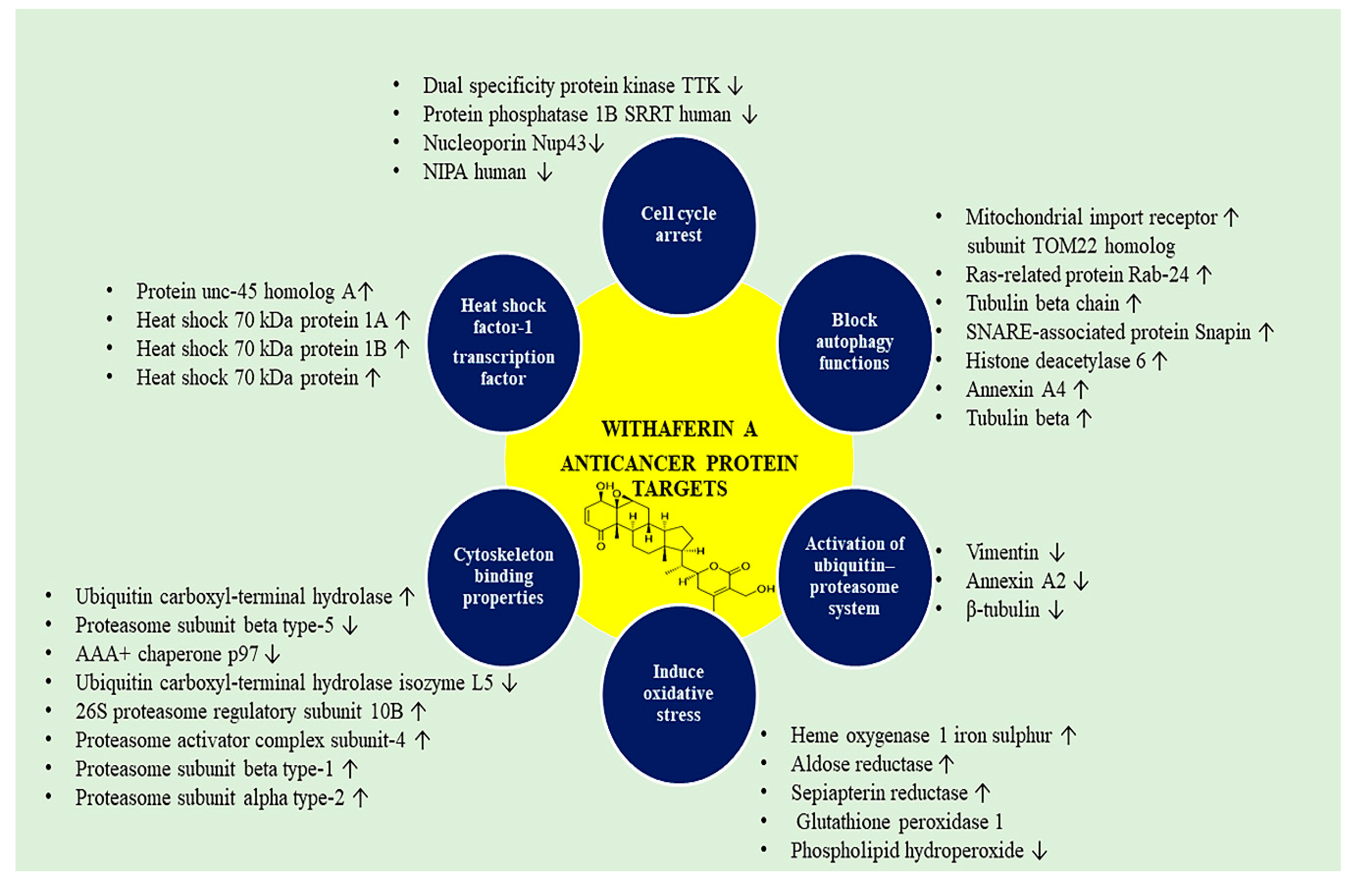
4.2. WA-Responsive Proteins in Cancer
Oxidative Stress Response and Red Cell Proteins
4.3. Anti Diabetic Activity
4.4. Neuroprotective Activity
4.5. Cardioprotective Activity
4.6. COVID 19
4.7. Anti Hepatitis Activity
4.8. Osteoporosis
5. Formulations Prepared from Withaferin A
6. Synergistic Combination of Withaferin A
7. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maurya, R. Chemistry and pharmacology of Withania coagulans: An Ayurvedic remedy. J. Pharm. Pharmacol. 2010, 62, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.S.; Zhang, S. Polypharmacology: Drug discovery for the future. Expert Rev. Clin. Pharmacol. 2013, 6, 41–47. [Google Scholar] [CrossRef] [PubMed]
- John, J. Therapeutic potential of Withania somnifera: A report on phytopharmacological properties. Int. J. Pharm. Sci. Res. 2014, 5, 2131–2148. [Google Scholar]
- Ríos, J.L.; Rec9io, M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef]
- Lemma, M.T.; Ahmed, A.M.; Elhady, M.T.; Ngo, H.T.; Vu, T.L.H.; Sang, T.K.; Campos-Alberto, E.; Sayed, A.; Mizukami, S.; Na-Bangchang, K. Medicinal plants for in vitro antiplasmodial activities: A systematic review of literature. Parasitol. Int. 2017, 66713–66720. [Google Scholar]
- Naghibi, F.; Esmaeili, S.; Abdullah, N.R.; Nateghpour, M.; Taghvai, M.; Kamkar, S.; Mosaddegh, M. In vitro and in vivo antimalarial evaluations of myrtle extract, a plant traditionally used for treatment of parasitic disorders. Biomed. Res. Int. 2013, 2013, 316185. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta 2013, 1830, 3670–3695. [Google Scholar] [CrossRef]
- Orlikova, B.; Diederich, M. Power from the garden: Plant compounds as inhibitors of the hallmarks of cancer. Curr. Med. Chem. 2012, 19, 2061–2087. [Google Scholar] [CrossRef]
- Mirjalili, M.H.E.; Moyano, M.; Bonfill, R.M.; Palazon, C.J. Steroidal lactones from Withania somnifera, an ancient plant for novel medicine. Molecules 2009, 14, 2373–2393. [Google Scholar] [CrossRef]
- Dutta, R.; Khalil, R.; Green, R.; Mohapatra, S.S.; Mohapatra, S. Withania Somnifera (Ashwagandha) and Withaferin A: Potential in Integrative Oncology. Int. J. Mol. Sci. 2019, 20, 5310. [Google Scholar] [CrossRef]
- Huang, M.; He, J.X.; Hu, H.X.; Zhang, K.; Wang, X.N.; Zhao, B.B.; Lou, H.X.; Ren, D.M.; Shen, T. Withanolides from the genus Physalis: A review on their phytochemical and pharmacological aspects. J. Pharm. Pharmacol. 2020, 72, 649–669. [Google Scholar] [CrossRef]
- Hassannia, B.; Wiernicki, B.; Ingold, I.; Qu, F.; Van Herck, S.; Tyurina, Y.Y.; Bayir, H.; Abhari, B.A.; Angeli, J.P.F.; Choi, S.M.; et al. Nano-targeted induction of dual ferroptotic mechanisms eradicates high-risk neuroblastoma. J. Clin. Invest. 2018, 128, 3341–3355. [Google Scholar] [CrossRef] [PubMed]
- Berghe, W.V.; Sabbe, L.; Kaileh, M.; Haegeman, G.; Heyninck, K. Molecular insight in the multifunctional activities of Withaferin, A. Biochem. Pharmacol. 2012, 84, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Patil, D.; Gautam, M.; Mishra, S.; Karupothula, S.; Gairola, S.; Jadhav, S.; Pawar, S.; Patwardhan, B. Determination of withaferin A and withanolide A in mice plasma using high-performance liquid chromatography-tandem mass spectrometry: Application to pharmacokinetics after oral administration of Withania somnifera aqueous extract. J. Pharmaceut. Biomed. 2013, 80, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Devkar, S.T.; Kandhare, A.D.; Sloley, B.D.; Jagtap, S.D.; Lin, J.; Tam, Y.K.; Katyare, S.S.; Bodhankar, S.L.; Hegde, M.V. Evaluation of the bioavailability of major withanolides of Withania somnifera using an in vitro absorption model system. J. Adv. Pharm. Technol. Res. 2015, 6, 159–164. [Google Scholar] [PubMed]
- Dai, T.; Jiang, W.; Guo, Z.; Wang, Z.; Huang, M.; Zhong, G.; Liang, C.; Pei, X.; Dai, R. Studies on oral bioavailability and first-pass metabolism of withaferin A in rats using LC-MS/MS and Q-TRAP. Biomed. Chromatogr. 2019, 33, e4573. [Google Scholar] [CrossRef]
- Pires, N.; Gota, V.; Gulia, A.; Hingorani, L.; Agarwal, M.; Puri, A. Safety and pharmacokinetics of Withaferin-A in advanced stage high grade osteosarcoma: A phase I trial. J. Ayurveda Integr. Med. 2020, 11, 68–72. [Google Scholar] [CrossRef]
- Wijeratne, E.M.; Xu, Y.M.; Scherz-Shouval, R.; Marron, M.T.; Rocha, D.D.; Liu, M.X.; Costa-Lotufo, L.V.; Santagata, S.; Lindquist, S.; Whitesell, L.; et al. Structure-activity relationships for withanolides as inducers of the cellular heatshock response. J. Med. Chem. 2014, 57, 2851–2863. [Google Scholar] [CrossRef]
- Sy-Cordero, A.A.; Graf, T.N.; Runyon, S.P.; Wani, M.C.; Kroll, D.J.; Agarwal, R.; Brantley, S.J.; Paine, M.F.; Polyak, S.J.; Oberlies, N.H. Enhanced bioactivity of Silybin B methylation products. Bioorg. Med. Chem. 2013, 21, 742–747. [Google Scholar] [CrossRef]
- Walle, T. Methylation of dietary flavones increases their metabolic stability and chemopreventive effects. Int. J. Mol. Sci. 2009, 10, 5002–5019. [Google Scholar] [CrossRef]
- Deocaris, C.C.; Lu, W.J.; Kaul, S.C.; Wadhwa, R. Druggability of mortalin for cancer and neuro-degenerative disorders. Curr. Pharm. Des. 2013, 19, 418–429. [Google Scholar] [CrossRef]
- Sane, S.; Abdullah, A.; Boudreau, D.A.; Autenried, R.K.; Gupta, B.K.; Wang, X.; Wang, H.; Schlenker, E.H.; Zhang, D.; Telleria, C.; et al. Ubiquitin-like (UBX)-domain-containing protein, UBXN2A, promotes cell death by interfering with the p53-Mortalin interactions in colon cancer cells. Cell Death Dis. 2014, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Vaishnavi, K.; Kalra, R.S.; Zhang, Z.; Sekar, K.; Kaul, S.C.; Wadhwa, R. 3β-Methoxy Derivation of Withaferin-a attenuates its anticancer Potency. Bioinfo Mol. Evid. Med. Aromat. Plants 2015, 4, 1–8. [Google Scholar]
- Rabhi, C.; Arcile, G.; Goff, G.L.; Noble, C.D.C.; Ouazzan, J. Neuroprotective effect of CR-777, a glutathione derivative of Withaferin A, obtained through the bioconversion of Withania somnifera (L.) Dunal extract by the fungus Beauveria bassiana. Molecules 2019, 24, 4599. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.; Kalra, R.S.; Malik, V.; Katiyar, S.P.; Sundar, D.; Kaul, S.C.; Wadhwa, R. 2, 3-Dihydro-3β-methoxy Withaferin-A Lacks Anti-Metastasis Potency: Bioinformatics and experimental evidences. Sci. Rep. 2019, 9, 17344. [Google Scholar] [CrossRef]
- Yousuf, S.K.; Majeed, R.; Ahmad, M.; Sangwana, P.L.; Purnimaa, B.; Saxsena, A.K.; Suri, K.A.; Mukherjeea, D.; Tanejaa, S.C. Ring A structural modified derivatives of Withaferin A and the evaluation of their cytotoxic potential. Steroids 2011, 76, 1213–1222. [Google Scholar] [CrossRef]
- Shohat, B.; Gitter, S.; Abraham, A.; Lavie, D. Antitumor activity of withaferin-A (NSC-101088). Cancer Chemother. Rep. 1967, 51, 271–276. [Google Scholar]
- Sanchez-Martin, M.; Ambesi Impiombato, A.; Qin, Y.; Herranz, D.; Bansal, M.; Girardi, T.; Paietta, M.S.; Tallman, E.; Rowe, J.M.; De Keersmaecker, K.; et al. Synergistic antileukemic therapies in NOTCH1-induced T-ALL. Proc. Natl. Acad. Sci. USA 2017, 114, 2006–2011. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar]
- O’Regan, R.M.; Jordan, V.C. The evolution of tamoxifen therapy in breast cancer: Selective oestrogen-receptor modulators and down regulators. Lancet Oncol. 2002, 3, 207–214. [Google Scholar] [CrossRef]
- Simpson, E.R.; Dowsett, M. Aromatase and its inhibitors: Significance for breast cancer therapy. Recent Prog. Horm. Res. 2002, 57, 317–338. [Google Scholar] [CrossRef]
- Stan, S.D.; Hahm, E.R.; Warin, R.; Singh, S.V. Withaferin A causes FOXO3a- and Bim-dependent apoptosis and inhibits growth of human breast cancer cells in vivo. Cancer Res. 2008, 68, 7661–7669. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Hahm, E.R.; Singh, S.V. Withaferin A inhibits activation of signal transducer and activator of transcription 3 in human breast cancer cells. Carcinogenesis 2010, 31, 1991–1998. [Google Scholar] [CrossRef] [PubMed]
- Hahm, E.R.; Moura, M.B.; Kelley, E.E.; Van Houten, B.; Shiva, S.; Singh, S.V. Withaferin A induced apoptosis in human breast cancer cells is mediated by reactive oxygen species. PLoS ONE 2011, 6, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Stan, S.D.; Zeng, Y.; Singh, S.V. Ayurvedic medicine constituent withaferin a causes G2 and M phase cell cycle arrest in human breast cancer cells. Nutr. Cancer 2008, 60, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Thaiparambil, J.T.; Bender, L.; Ganesh, T.; Kline, E.; Patel, P.; Liu, Y.; Tighiouart, M.; Vertino, P.M.; Harvey, R.D.; Garcia, A.L.; et al. Withaferin-A inhibits breast cancer invasion and metastasis at sub-cytotoxic doses by inducing vimentin disassembly and serine 56 phosphorylation. Int. J. Cancer 2011, 129, 2744–2755. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, G.; Palovcak, A.; Li, Y.; Hao, S.; Liu, Z.J.; Landgraf, R.; Yuan, F.; Zhang, Y. Impeding the single-strand annealing pathway of DNA double-strand break repair by withaferin A-mediated FANCA degradation. DNA Repair. 2019, 1, 10–17. [Google Scholar] [CrossRef]
- Muniraj, N.; Siddharth, S.; Nagalingam, A.; Walker, A.; Woo, J.; Győrffy, B.; Gabrielson, E.; Saxena, N.K.; Sharma, D. Withaferin A inhibits lysosomal activity to block autophagic flux and induces apoptosis via energetic impairment in breast cancer cells. Carcinogenesis 2019, 40, 1110–1120. [Google Scholar] [CrossRef]
- Sivasankarapillai, V.S.; Nair, R.; Rahdar, A.; Bungau, S.; Zaha, D.C.; Aleya, L.; Tit, D.M. Overview of the anticancer activity of withaferin A, an active constituent of the Indian ginseng Withania somnifera. Environ. Sci. Pollut. Res. 2020, 27, 26025–26035. [Google Scholar] [CrossRef]
- Fong, Y.; Jin, S.; Rane, M.; Singh, R.K.; Gupta, R.; Kakar, S.S. Withaferin A Synergizes the Therapeutic Effect of Doxorubicin through ROS-Mediated Autophagy in Ovarian Cancer. PLoS ONE 2012, 7, e42265. [Google Scholar] [CrossRef]
- Kakar, S.S.; Jala, V.R.; Fong, M.Y. Synergistic cytotoxic action of cisplatin and Withaferin-A on ovarian cancer cell lines. Biochem. Biophys. Res. Commun. 2012, 423, 819–825. [Google Scholar] [CrossRef]
- Devi, P.U.; Kamath, R.; Rao, B.S. Radiosensitization of a mouse melanoma by withaferin A: In vivo studies. Indian J. Exp. Biol. 2000, 38, 432–437. [Google Scholar] [PubMed]
- Zhang, X.; Samadi, A.K.; Roby, K.F.; Timmermann, B.; Cohen, M.S. Inhibition of cell growth and induction of apoptosis in ovarian carcinoma cell lines CaOV3 and SKOV3 by natural withanolide Withaferin-A. Gynecol. Oncol. 2012, 124, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Straughn, A.R.; Kakar, S.S. Withaferin A ameliorates ovarian cancer-induced cachexia and proinflammatory signaling. J. Ovarian Res. 2019, 12, 1–14. [Google Scholar] [CrossRef]
- Kelm, N.Q.; Straughn, A.R.; Kakar, S.S. Withaferin A attenuates ovarian cancer-induced cardiac cachexia. PLoS ONE 2020, 15, e0236680. [Google Scholar] [CrossRef] [PubMed]
- Kakar, S.S.; Parte, S.; Carter, K.; Joshua, I.G.; Worth, C.; Rameshwar, P.; Ratajczak, M.Z. Withaferin A (WFA) inhibits tumor growth and metastasis by targeting ovarian cancer stem cells. Oncotarget 2017, 26, 74494–74505. [Google Scholar] [CrossRef]
- Cereda, V.; Formica, V.; Massimiani, G.; Tosetto, L.; Roselli, M. Targeting metastatic castration-resistant prostate cancer: Mechanisms of progression and novel early therapeutic approaches. Expert Opin. Investig. Drugs 2014, 223, 469–487. [Google Scholar] [CrossRef]
- Fan, X.; Liu, S.; Su, F.; Pan, Q.; Lin, T. Effective enrichment of prostate cancer stem cells from spheres in a suspension culture system. Urol. Oncol. 2012, 30, 314–318. [Google Scholar] [CrossRef]
- Sheng, X.; Li, Z.; Wang, D.L.; Li, W.B.; Luo, Z.; Chen, K.H.; Cao, J.J.; Yu, C.; Liu, W.J. Isolation and enrichment of PC-3 prostate cancer stem-like cells using MACS and serum-free medium. Oncol. Lett. 2013, 5, 787–792. [Google Scholar] [CrossRef]
- Wang, L.; Huang, X.; Zheng, X.; Wang, X.; Li, S.; Zhang, L.; Yang, Z.; Xia, Z. Enrichment of prostate cancer stem like cells from human prostate cancer cell lines by culture in serum-free medium and chemoradiotherapy. Int. J. Biol. Sci. 2013, 9, 472–479. [Google Scholar] [CrossRef]
- Luo, Y.; Cui, X.; Zhao, J.; Han, Y.; Li, M.; Lin, Y.; Jiang, Y.; Lan, L. Cells susceptible to epithelial-mesenchymal transition are enriched in stem-like side population cells from prostate cancer. Oncol. Rep. 2014, 31, 874–884. [Google Scholar] [CrossRef][Green Version]
- Kreso, A.; Dick, J.E. Evolution of the Cancer Stem Cell Model. Cell Stem Cell 2014, 14, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Bargagna-Mohan, P.; Deokule, S.P.; Thompson, K.; Wizeman, J.; Srinivasan, C.; Vooturi, S.; Wendschlag, N.; Liu, J.; Evans, R.M.; Markovitz, D.M.; et al. Withaferin A effectively targets soluble vimentin in the glaucoma filtration surgical model of fibrosis. PLoS ONE 2013, 8, e63881. [Google Scholar] [CrossRef] [PubMed]
- Bargagna-Mohan, P.; Hamza, A.; Kim, Y.E.; Khuan Abby Ho, Y.; Mor-Vaknin, N.; Wendschlag, N.; Liu, J.; Evans, R.M.; Markovitz, D.M.; Zhan, C.G.; et al. The tumor inhibitor and antiangiogenic agent withaferin A targets the intermediate filament protein vimentin. Chem. Biol. 2007, 14, 623–634. [Google Scholar] [CrossRef]
- Grin, B.; Mahammad, S.; Wedig, T.; Cleland, M.M.; Tsai, L.; Herrmann, H.; Goldman, R.D. Withaferin alters intermediate filament organization, cell shape and behavior. PLoS ONE 2012, 7, e39065. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Hamza, A.; Zhang, T.; Gu, M.; Zou, P.; Newman, B.; Li, Y.; Leslie Gunatilaka, A.A.; Whitesell, L.; Zhan, C.G.; et al. Withaferin A targets heat shock protein 90 in pancreatic cancer cells. Biochem. Pharmacol. 2010, 79, 542–551. [Google Scholar] [CrossRef]
- Moselhy, J.; Suman, S.; Alghamdi, M.; Chandarasekharan, B.; Das, T.P.; Houda, A.; Murali Ankem, M.; Damodaran, C. Withaferin A inhibits prostate carcinogenesis in a PTEN-deficient Mouse Model of Prostate Cancer. Neoplasia 2017, 19, 451–459. [Google Scholar] [CrossRef]
- Deng, Y.; Chan, S.S.; Chang, S. Telomere dysfunction and tumour suppression: The senescence connection. Nat. Rev. Cancer 2008, 8, 450–458. [Google Scholar] [CrossRef]
- Bartkova, J.; Horejsi, Z.; Koed, K.; Kramer, A.; Tort, F.; Zieger, K.; Guldberg, P.; Sehested, M.; Nesland, J.M.; Lukas, C.; et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005, 434, 864–870. [Google Scholar] [CrossRef]
- Bryan, T.M.; Englezou, A.; Gupta, J.; Bacchetti, S.; Reddel, R.R. Telomere elongation in immortal human cells without detectable telomerase activity. EMBO J. 1995, 14, 4240–4248. [Google Scholar] [CrossRef]
- Greider, C.W.; Blackburn, E.H. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell 1985, 43, 405–413. [Google Scholar] [CrossRef]
- Yu, Y.; Katiyar, S.P.; Sundar, D.; Kaul, Z.; Miyako, E.; Zhang, Z.; Kaul, S.C.; Reddel, R.R.; Wadhwa, R. Withaferin-A kills cancer cells with and without telomerase: Chemical, computational and experimental evidences. Cell Death Disease 2017, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Suman, S.; Das, T.P.; Moseley, J.; Pal, D.; Kolluru, V.; Alatassi, H.; Ankem, M.K.; Damodaran, C. Oral administration of withaferin A inhibits carcinogenesis of prostate in TRAMP model. Oncotarget 2016, 7, 53751–53761. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Guan, H.; Fang, L.; Yang, Y.; Zhu, X.; Yuan, J.; Wu, J. MicroRNA-374a activates Wnt/beta-catenin signaling to promote breast cancer metastasis. J. Clin. Invest. 2013, 123, 566–579. [Google Scholar]
- Du, Y.; Wang, Y.; Zhang, F.; Wu, W.; Wang, W.; Li, H.; Xia, S.; Liu, H. Regulation of metastasis of bladder cancer cells through the WNT signaling pathway. Tumour. Biol. 2015, 36, 8839–8844. [Google Scholar] [CrossRef]
- Ormanns, S.; Neumann, J.; Horst, D.; Kirchner, T.; Jung, A. WNT signaling and distant metastasis in colon cancer through transcriptional activity of nuclear beta-Catenin depend on active PI3K signaling. Oncotarget 2014, 5, 2999–3011. [Google Scholar] [CrossRef]
- Weeraratna, A.T.; Jiang, Y.; Hostetter, G.; Rosenblatt, K.; Duray, P.; Bittner, M.; Trent, J.M. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell 2002, 1, 279–288. [Google Scholar] [CrossRef]
- Fu, C.; Liang, X.; Cui, W.; Ober-Blobaum, J.L.; Vazzana, J.; Shrikant, P.A.; Lee, K.P.; Clausen, B.E.; Mellman, I.; Jiang, A. Beta-Catenin in dendritic cells exerts opposite functions in cross-priming and maintenance of CD8+ T cells through regulation of IL-10. Proc. Natl. Acad. Sci. USA 2015, 112, 2823–2828. [Google Scholar] [CrossRef]
- Spranger, S.; Gajewski, T.F. A new paradigm for tumor immune escape: Beta-catenin-driven immune exclusion. J. Immunother. Cancer 2015, 3, 43. [Google Scholar] [CrossRef]
- Amin, H.; Nayak, D.; Ur Rasool, R.; Chakraborty, S.; Kumar, A.; Yousuf, K.; Sharma, P.R.; Ahmed, Z.; Sharma, N.; Magotra, A.; et al. Par-4 dependent modulation of cellular beta-catenin by medicinal plant natural product derivative 3-azido Withaferin A. Mol. Carcinog. 2016, 55, 864–881. [Google Scholar] [CrossRef]
- Srinivasan, S.; Ranga, R.S.; Burikhanov, R.; Han, S.S.; Chendil, D. Par-4-dependent apoptosis by the dietary compound Withaferin A in prostate cancer cells. Cancer Res. 2007, 67, 246–253. [Google Scholar] [CrossRef]
- Kim, S.H.; Hahm, E.R.; Singh, K.B.; Shiva, S.; Ornstein, J.S. Singh SV RNA-seq reveals novel mechanistic targets of withaferin A in prostate cancer cells. Carcinogenesis 2020, 41, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef] [PubMed]
- Gusella, M.; Frigo, A.C.; Bolzonella, C.; Marinelli, R.; Barile, C.; Bononi, A.; Crepaldi, G.; Menon, D.; Stievano, L.; Toso, S.; et al. Predictors of survival and toxicity in patients on adjuvant therapy with 5-fluorouracil for colorectal cancer. Br. J. Cancer 2009, 100, 1549–1557. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pallag, A.; Rosca, E.; Tit, D.M.; Mutiu, G.; Bungau, S.G.; Pop, O.L. Monitoring the effects of treatment in colon cancer cells using immunohistochemical and histoenzymatic techniques. Rom. J. Morph. Embriol. 2015, 56, 1103–1109. [Google Scholar]
- Das, T.; Roy, K.S.; Chakrabarti, T.; Mukhopadhyay, S.; Roychoudhury, S. Withaferin-A modulates the Spindle assembly checkpoint by degradation of Mad2-Cdc20 complex in colorectal cancer cell lines. Biochem. Pharmacol. 2014, 91, 31–39. [Google Scholar] [CrossRef]
- Koduru, S.; Kumar, R.; Srinivasan, S.; Evers, M.B.; Damodaran, C. Notch-1 inhibition by Withaferin-A: A therapeutic target against colon carcinogenesis. Mol. Cancer Ther. 2010, 9, 202–210. [Google Scholar] [CrossRef]
- Pal, D.; Tyagi, A.; Chandrasekaran, B.; Alattasi, H.; Murali, K.; Sharma, A.K.; Damodaran, C. Suppression of Notch1 and AKT mediated epithelial to mesenchymal transition by Verrucarin J in metastatic colon cancer. Cell Death Disease 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Chandrasekaran, B.; Pal, D.; Kolluru, V.; Tyagi, A.; Baby, B.; Dahiya, N.R.; Youssef, K.; Alatassi, H.; Ankem, M.K.; Sharma, A.K.; et al. The chemopreventive effect of withaferin a on spontaneous and inflammation-associated colon carcinogenesis models. Carcinogenesis 2018, 39, 1537–1547. [Google Scholar] [CrossRef]
- Kyakulaga, A.H.; Aqil, F.; Munagala, R.C.; Gupta, R. Withaferin A inhibits Epithelial to Mesenchymal Transition in Non-Small Cell Lung Cancer Cells. Sci. Rep. 2018, 15737, 1–13. [Google Scholar] [CrossRef]
- Li, X.; Zhu, F.; Jiang, J.; Sun, C.; Wang, X.; Shen, M.; Tian, R.; Shi, C.; Xu, M.; Peng, F.; et al. Synergistic antitumor activity of withaferin A combined with oxaliplatin triggers reactive oxygen species-mediated inactivation of the PI3K/AKT pathway in human pancreatic cancer cells. Cancer. Lett. 2014, 357, 219–230. [Google Scholar] [CrossRef]
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. Semin. Cell. Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, W.; Su, Z. AN Kong T The complexity of the Nrf2 pathway: Beyond the antioxidant response. J. Nutr. Biochem. 2015, 26, 1401–1413. [Google Scholar] [CrossRef] [PubMed]
- Dom, M.; Offner, F.; Vanden Berghe, W.; Van Ostade, X. Proteomic characterization of Withaferin A-targeted protein networks for the treatment of monoclonal myeloma gammopathies. J. Proteom. 2018, 179, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Narayan, M.; Seeley, K.W.; Jinwal, U.K. Identification and quantitative analysis of cellular proteins affected by treatment with withaferin a using a SILAC-based proteomics approach. J. Ethnopharmacol. 2015, 175, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Narayan, M.; Zhang, J.; Braswell, K.; Gibson, C.; Zitnyar, A.; Lee, D.C.; Gupta, S.V.; Jinwal, U.K. Withaferin A Regulates LRRK2 Levels by Interfering with the Hsp90- Cdc37 chaperone complex. Curr. Aging Sci. 2015, 8, 259–265. [Google Scholar] [CrossRef]
- Zhang, L.; Nemzow, L.; Chen, H.; Lubin, A.; Rong, X.; Sun, Z.; Harris, T.K.; Gong, F. The Deubiquitinating Enzyme. USP24 Is a Regulator of the UV Damage Response. Cell. Rep. 2015, 10, 140–147. [Google Scholar] [CrossRef]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef]
- Monastyrska, I.; Rieter, E.; Klionsky, D.J.; Reggiori, F. Multiple roles of the cytoskeleton in autophagy. Biol. Rev. Camb. Philos Soc. 2009, 84, 431–448. [Google Scholar] [CrossRef]
- Dokladny, K.; Myers, O.B.; Moseley, P.L. Heat shock response and autophagy cooperation and control. Autophagy 2015, 11, 200–213. [Google Scholar] [CrossRef]
- Santagata, S.; Xu, Y.; Wijeratne, E.M.K.; Kontnik, R.; Rooney, C.; Perley, C.C.; Kwon, H.; Clardy, J.; Kesari, S.; Whitesell, L.; et al. Using the Heat-Shock response to discover anticancer compounds that target protein homeostasis. ACS Chem. Biol. 2012, 7, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.S.; An, S.; Kim, E.; Yu, J.; Hong, K.Y.; Lee, J.S.; Jang, S.K. An mRNA-specific tRNAi carrier eIF2A plays a pivotal role in cell proliferation under stress conditions: Stress-resistant translation of c-Src mRNA is mediated by eIF2A. Nucleic Acids Res. 2017, 45, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Ivaska, J.; Vuoriluoto, K.; Huovinen, T.; Izawa, I.; Inagaki, M.; Parker, P.J. PKC epsilon mediated phosphorylation of vimentin controls integrin recycling and motility. EMBO J. 2005, 24, 3834–3845. [Google Scholar] [CrossRef] [PubMed]
- Janosch, P.; Kieser, A.; Eulitz, M.; Lovric, J.; Sauer, G.; Reichert, M.; Gounari, F.; Büscher, D.; Baccarini, M.; Mischak, H.; et al. The Raf-1 kinase associates with vimentin kinases and regulates the structure of vimentin filaments. FASEB J. 2000, 14, 2008–2021. [Google Scholar] [CrossRef] [PubMed]
- Perlson, E.; Michaelevski, I.; Kowalsman, N.; Ben-Yaakov, K.; Shaked, M.; Seger, R.; Eisenstein, M.; Fainzilber, M. Vimentin binding to phosphorylated Erk sterically hinders enzymatic dephosphorylation of the kinase. J. Mol. Biol. 2006, 364, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Kidd, M.E.; Shumaker, D.K.; Ridge, K.M. The Role of Vimentin Intermediate Filaments in the Progression of Lung Cancer. Am. J. Respir. Cell Mol. Biol. 2014, 50, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, L.; Dong, X.; Liu, L.; Huo, L.; Chen, H. High Expression of Vimentin is Associated with Progression and a Poor Outcome in Glioblastoma. Appl. Immunohistochem. Mol. Morphol. 2018, 26, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Falsey, R.R.; Marron, M.T.; Gunaherath, G.K.B.; Shirahatti, N.; Mahadevan, D.; Gunatilaka, A.A.L.; Whitesell, L. Actin microfilament aggregation induced by withaferin A is mediated by annexin II. Nat. Chem. Biol. 2006, 2, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Ozorowski, G.; Ryan, C.M.; Whitelegge, J.P.; Luecke, H. Withaferin A binds covalently to the N-terminal domain of annexin A2. Biol. Chem. 2012, 393, 1151–1163. [Google Scholar] [CrossRef]
- Baud, V.; Karin, M. Is NF-[kappa] B a good target for cancer therapy? Hopes and pitfalls. Nat. Rev. Drug Discov. 2009, 8, 33–40. [Google Scholar] [CrossRef]
- Labbozzetta, M.; Notarbartolo, M.; Poma, P. Can NF-κB be considered a valid drug target in Neoplastic Diseases? Our Point of View. Int. J. Mol. Sci. 2020, 21, 3070. [Google Scholar] [CrossRef] [PubMed]
- Su, H.T.; Weng, C.C.; Hsiao, P.J.; Chen, L.H.; Kuo, T.L.; Chen, Y.W.; Kuo, K.K.; Cheng, K.H. Stem cell marker nestin is critical for TGF-β1-mediated tumor progression in pancreatic cancer. Mol. Cancer Res. 2013, 11, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Singh, S.V. Mammary cancer chemoprevention by withaferin-A is accompanied by in vivo suppression of self-renewal of cancer stem cells. Cancer Prev. Res. 2014, 7, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.W.; Li, R.N.; Wang, H.R.; Liu, J.R.; Tang, J.Y.; Huang, H.W.; Chan, Y.H.; Yen, C.Y. Withaferin A induces oxidative stress-mediated apoptosis and DNA damage in oral cancer cells. Front. Physiol. 2017, 8, 634. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Miao, Y.; Liu, S. Withaferin A induces apoptosis by ROS-dependent mitochondrial dysfunction in human colorectal cancer cells. Biochem. Biophys. Res. Commun. 2018, 503, 2363–2369. [Google Scholar] [CrossRef]
- Kim, G.; Kim, T.H.; Hwang, E.H.; Chang, K.T.; Hong, J.J.; Park, J.H. Withaferin A inhibits the proliferation of gastric cancer cells by inducing G2/M cell cycle arrest and apoptosis. Oncol. Lett. 2017, 14, 416–422. [Google Scholar] [CrossRef]
- Yu, T.J.; Tang, J.Y.; Yang, F.O.; Wang, Y.Y.; Yuan, S.S.F.; Tseng, K.; Lin, L.C.; Chang, H.W. Low Concentration of Withaferin a Inhibits Oxidative Stress-Mediated Migration and Invasion in Oral Cancer Cells. Biomolecules 2020, 10, 777. [Google Scholar] [CrossRef]
- Dabelsteen, E. Cell surface carbohydrates as prognostic markers in human carcinomas. J. Pathol. 1996, 179, 358–369. [Google Scholar] [CrossRef]
- Aranganathan, S.; Senthil, K.; Nalini, N. A case control study of glycoprotein status in ovarian carcinoma. Clin. Biochem. 2005, 38, 535–539. [Google Scholar] [CrossRef]
- Senthil, N.; Manoharan, S.; Balakrishnan, S.; Ramachandran, C.R.; Muralinaidu, R.; Rajalingam, K. Modifying effects of Piper longum on cell surface abnormalities in 7,12-dimethylbenz[a]anthracene induced hamster buccal pouch carcinogenesis. Int. J. Pharmacol. 2007, 3, 290–294. [Google Scholar]
- Suresh, K.; Manoharan, S.; Panjamurthy, K.; Senthil, N. Modifying effects of Annona squamosa on glycoconjugates levels in 7,12-dimethylbenz(a)anthracene induced hamster buccal pouch carcinogenesis. J. Med. Sci. 2007, 7, 100–105. [Google Scholar]
- Thirunavukarasu, C.; Sakthisekaran, D. Influence of sodium selenite on glycoprotein contents in normal and N-nitrosodiethylamine initiated and phenobarbital promoted rat liver tumors. Pharmacol Res. 2003, 48, 167–171. [Google Scholar] [CrossRef]
- Selvendiran, K.; Sakthisekaran, D. Chemopreventive effect of piperine on modulating lipid peroxidation and membrane bound enzymes in benzo(a)pyrene induced lung carcinogenesis. Biomed. Pharmacother. 2004, 58, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, S.; Panjamurthy, K.; Pugalendi, P.; Balakrishnan, S.; Rajalingam, K.; Vellaichamy, L.; Alias, L.M. Protective Role of Withaferin-A on Red Blood Cell Integrity during 7,12-Dimethylbenz[a]anthracene Induced Oral Carcinogenesis. Afr. J. Tradit Complement. Altern Med. 2009, 6, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Heyninck, K.; Sabbe, L.; Chirumamilla, C.S.; Szic, K.S.V.; Veken, P.V.; Lemmens, K.J.A.; Kakkonen, M.L.; Naulaerts, S.; Beeck, K.O.; Laukens, K.; et al. Withaferin A induces heme oxygenase (HO-1) expression in endothelial cells via activation of the Keap1/Nrf2 pathway. Biochem. Pharmacol. 2016, 1, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Rathmann, W.; Giani, G. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 2568–2569. [Google Scholar] [CrossRef]
- Vesa, C.M.; Popa, L.; Popa, A.R.; Rus, M.; Zaha, A.A.; Bungau, S.; Tit, D.M.; Aron, R.A.C.; Zaha, D.C. Current Data Regarding the Relationship between Type 2 Diabetes Mellitus and Cardiovascular Risk Factors. Diagnostics 2020, 10, 314. [Google Scholar] [CrossRef]
- Khalilpourfarshbafi, M.; Devi, M.D.; Abdul Sattar, M.Z.; Sucedaram, Y.; Abdullah, N.A. Withaferin A inhibits adipogenesis in 3T3- F442A cell line, improves insulin sensitivity and promotes weight loss in high fat diet-induced obese mice. PLoS ONE 2019, 14, e0218792. [Google Scholar] [CrossRef]
- Riboulet-Chavey, A.; Diraison, F.D.R.; Siew, L.K.; Wong, F.S.; Rutter, G.A. Inhibition of AMP-activated protein kinase protects pancreatic Î2-cells from cytokine-mediated apoptosis and CD8+ T-Cells induced cytotoxicity. Diabetes 2008, 57, 415–423. [Google Scholar] [CrossRef][Green Version]
- Vesa, C.M.; Popa, A.R.; Bungau, S.; Daina, L.G.; Buhas, C.; Judea-Pusta, C.T.; Pasca, B.; Dimulescu (Nica), I.; Zaha, D.C. Exploration of insulin sensitivity, insulin resistance, early insulin secretion and β-cell function, and their relationship with glycated hemoglobin level in normal weight patients with newly diagnosed type 2 diabetes mellitus. Rev. Chim. 2019, 70, 4217–4223. [Google Scholar]
- Tekula, S.; Khurana, A.; Anchi, P.; Godugu, C. Withaferin-A attenuates multiple low doses of Streptozotocin (MLD-STZ) induced type 1 diabetes. Biomed. Pharmacother. 2018, 106, 1428–1440. [Google Scholar] [CrossRef] [PubMed]
- Palliyaguru, D.L.; Chartoumpekis, D.V.; Wakabayashi, N.; Skoko, J.J.; Yagishita, Y.; Singh, S.V.; Kensler, T.W. Withaferin A induces Nrf2-dependent protection against liver injury: Role of Keap1-independent mechanisms. Free Radic. Biol. Med. 2016, 101, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Batumalaie, K.; Arif Amin, M.; Murugan, D.D.; Zubaid Abdul Sattar, M.; Abdullah, N.A. Withaferin A protects against palmitic acid-induced endothelial insulin resistance and dysfunction through suppression of oxidative stress and inflammation. Sci. Rep. 2016, 6, 27236. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Atluri, V.S.R.; Yndart Arias, A.; Jayant, R.D.; Kaushik, A.; Geiger, J.; Nair, M.N. Withaferin A Suppresses Beta Amyloid in APP Expressing Cells: Studies for Tat and Cocaine Associated Neurological Dysfunctions. Front. Aging Neurosci. 2018, 10, 1–12. [Google Scholar] [CrossRef]
- Choudhary, M.I.; Yousuf, S.; Nawaz, S.A.; Ahmed, S.; Rahman, A.U. Cholinesterase inhibiting withanolides from Withania somnifera. Chem. Pharm. Bull. (Tokyo) 2004, 52, 1358–1361. [Google Scholar] [CrossRef]
- Banu, M.R.; Ibrahim, M.; Prabhu, K.; Rajasankar, S. Withaferin-A Protects the Nigral Dopamine Neuron and Recovers Motor Activity in Aged Rats. Cells Tissues Organs 2019, 208, 59–65. [Google Scholar]
- Zhou, Z.; Xiang, W.; Jiang, Y.; Tian, N.; Wei, Z.; Wen, X.; Wang, W.; Liao, W.; Xia, X.; Li, Q.; et al. Withaferin A alleviates traumatic brain injury induced secondary brain injury via suppressing apoptosis in endothelia cells and modulating activation in the microglia. Eur. J. Pharmaco 2020, 5, 1–13. [Google Scholar] [CrossRef]
- Mandrekar-Colucci, S.; Landreth, G.E. Microglia and inflammation in Alzheimer’s disease. CNS Neurol. Disord. Drug Targets 2010, 9, 156–167. [Google Scholar] [CrossRef]
- Heneka, M.T.; Kummer, M.P.; Stutz, A.; Delekate, A.; Schwartz, S.; Saecker, A.; Griep, A.; Axt, D.; Remus, A.; Tzeng, T.C.; et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 2013, 493, 674–678. [Google Scholar] [CrossRef]
- Rao Atluri, V.S.; Tiwari, S.; Rodriguez, M.; Kaushik, A.; Yndart, A.; Nagesh Kolishetti, N.; Yatham, M.; Nair, M. Inhibition of Amyloid-Beta production, associated neuroinflammation, and Histone Deacetylase 2-mediated epigenetic modifications prevent neuropathology in Alzheimer’s disease in vitro Model. Front. Aging Neurosci. 2019, 11, 1–11. [Google Scholar] [CrossRef]
- Khan, Z.H.; Ghosh, A.R. Withaferin-A displays enhanced anxiolytic efficacy without tolerance in rats following sub chronic administration. Afr. J. Biotechnol. 2011, 10, 12973–12978. [Google Scholar]
- Guo, R.; Gan, L.; Lau, W.B.; Yan, Z.; Xie, D.; Gao, E.; Christopher, T.A.; Lopez, B.L.; Ma, X.; Wang, Y. Withaferin A prevents myocardial ischemia/reperfusion injury by upregulating AMP-activated protein kinase dependent B-Cell Lymphoma2 signaling. Circ. J. 2019, 83, 1726–1736. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.A.; Waterboer, T.; Choi, S.K.; Lindquist, J.N.; Stefanovic, B.; Burchardt, E.; Yamauchi, M.; Gillan, A.; Rinne, R.A. New aspects of hepatic fibrosis. J. Hepatol. 2000, 32, 32–38. [Google Scholar] [CrossRef]
- Rona, G. Catecholamine cardiotoxicity. J. Mol. Cell. Cardiol. 1985, 17, 291–306. [Google Scholar] [CrossRef]
- Diez, J. Mechanisms of cardiac fibrosis in hypertension. J. Clin. Hypertens. (Greenwich) 2007, 9, 546–550. [Google Scholar] [CrossRef]
- Cuspidi, C.; Ciulla, M.; Zanchetti, A. Hypertensive myocardial fibrosis. Nephrol. Dial. Transplant. 2006, 21, 20–23. [Google Scholar] [CrossRef]
- Heling, A.; Zimmermann, R.; Kostin, S.; Maeno, Y.; Hein, S.; Devaux, B.; Bauer, E.; Klövekorn, W.P.; Schlepper, M.; Schaper, W.; et al. Increased expression of cytoskeletal, linkage, and extracellular proteins in failing human myocardium. Circ. Res. 2000, 86, 846–853. [Google Scholar] [CrossRef]
- Lombardi, R.; Betocchi, S.; Losi, M.A.; Tocchetti, C.G.; Aversa, M.; Miranda, M.; Alessandro, G.D.; Cacace, A.; Ciampi, Q.; Chiariello, M. Myocardial collagen turnover in hypertrophic cardiomyopathy. Circulation 2003, 108, 1455–1460. [Google Scholar] [CrossRef]
- Ku, S.K.; Bae, J.S. Antiplatelet, anticoagulant, and profibrinolytic activities of withaferin A. Vasc. Pharmacol. 2014, 60, 120–126. [Google Scholar] [CrossRef]
- Brunetti, O.; Derakhshani, A.; Baradaran, B.; Galvano, A.; Russo, A.; Silvestris, N. COVID-19 infection in Cancer patients: How can oncologists Deal with these patients? Front. Oncol. 2020, 734, 1–3. [Google Scholar] [CrossRef]
- Fratino, L.; Procopio, G.; Di Maio, M.; Cinieri, S.; Leo, S.; Beretta, G. Coronavirus: Older persons with Cancer in Italy in the COVID-19 pandemic. Front. Oncol. 2020, 10, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Liu, D.; Liu, M.; Zhou, F.; Li, G.; Chen, Z.; Zhang, Z.; You, H.; Wu, M.; Zheng, Q.; et al. Patients with Cancer appear more vulnerable to SARS-COV-2: A multicenter study during the COVID-19 outbreak. Cancer Discov. 2020, 10, 783–791. [Google Scholar]
- Liang, W.; Guan, W.; Chen, R.; Wang, W.; Li, J.; Xu, K.; Li, C.; Ai, Q.; Lu, W.; Liang, H.; et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020, 21, 335–337. [Google Scholar] [CrossRef]
- Mehta, V.; Goel, S.; Kabarriti, R.; Cole, D.; Goldfinger, M.; Acuna-Villaorduna, A.; Pradhan, K.; Thota, R.; Reissman, S.; Sparano, J.A.; et al. A Case fatality rate of Cancer patients with COVID-19 in a New York hospital system. Cancer Discov. 2020, 10, 935–941. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, B.; Mao, J. The pathogenesis and treatment of the ‘cytokine Storm’ in COVID-19. J. Inf. Secur. 2020, 80, 607–613. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, I.; Bungau, S.; Kumar, A.; Uddin, M.S.; Kumar, C.; Pal, G.; Shrivastava, K.; Zengin, G.; Arora, S. The dual impact of ACE2 in COVID-19 and ironical actions in geriatrics and pediatrics with possible therapeutic solutions. Life Sci. 2020, 257, 118075. [Google Scholar]
- Maurya, D.K.; Sharma, D. Evaluation of Traditional Ayurvedic Preparation for Prevention and Management of the Novel Coronavirus (Sars-Cov-2) Using Molecular Docking Approach; Anushaktinagar: Mumbai, India, 2020. [Google Scholar]
- Kumar, V.; Dhanjal, J.K.; Kaul, S.C.; Wadhwa, R.; Sundar, D. Withanone and caffeic acid phenethyl ester are predicted to interact with main protease (Mpro) of SARS-CoV-2 and inhibit its activity. J. Biomol. Struct. Dyn. 2020, 1–13. [Google Scholar] [CrossRef]
- Straughn, A.R.; Kakar, S.S. Withaferin A: A potential therapeutic agent against COVID-19 infection. J. Ovarian Res. 2020, 13, 1–5. [Google Scholar] [CrossRef]
- Farrell, G.C.; Larter, C.Z. Nonalcoholic fatty liver disease: From steatosis to cirrhosis. Hepatology 2006, 43, S99–S112. [Google Scholar] [CrossRef]
- Michelotti, G.A.; Machado, M.V.; Diehl, A.M. NAFLD, NASH and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 656–665. [Google Scholar] [CrossRef]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.P.; Yan, T.; Kim, D.; Dias, H.B.; Krausz, K.W.; Kimura, S.; Gonzalez, F.J. Withaferin A improves non-alcoholic steatohepatitis in mice. J. Pharmacol. Exp. Ther. 2020, 2020, 256792. [Google Scholar]
- Tit, D.M.; Bungau, S.; Iovan, C.; Nistor Cseppento, D.C.; Endres, L.; Sava, C.; Sabau, A.M.; Furau, G.; Furau, C. Effects of the hormone replacement therapy and of soy isoflavones on bone resorption in postmenopause. J. Clin. Med. 2018, 7, 297. [Google Scholar] [CrossRef]
- Khedgikar, V.; Kushwaha, P.; Gautam, J.; Verma, A.; Changkija, B.; Kumar, A.; Sharma, S.; Nagar, G.K.; Singh, D.; Trivedi, P.K.; et al. Withaferin A: A proteasomal inhibitor promotes healing after injury and exerts anabolic effect on osteoporotic bone. Cell Death Dis. 2013, 4, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Agarwalla, P.; Mukherjee, S.; Sreedhar, B.; Banerjee, R. Glucocorticoid receptor-mediated delivery of nano gold–withaferin conjugates for reversal of epithelial-to-mesenchymal transition and tumor regression. Nanomedicine 2016, 11, 2529–2546. [Google Scholar] [CrossRef]
- Sultana, F.; Neog, M.K.; Rasool, M.K. Withaferin-A, a steroidal lactone encapsulated mannose decorated liposomes ameliorates rheumatoid arthritis by intriguing the macrophage repolarization in adjuvant-induced arthritic rats. Colloids Surfaces B Biointerfaces 2017, 155, 349–365. [Google Scholar] [CrossRef] [PubMed]
- Jaggarapua, M.M.C.S.; Rachamall, H.K.; Nimmuc, N.V.; Banerjeea, R. NGRKC16-lipopeptide assisted liposomal-withaferin delivery for efficient killing of CD13 receptor-expressing pancreatic cancer and angiogenic endothelial cells. J. Drug Deliv. Sci. Technol. 2020, 58, 1–12. [Google Scholar]
- Shaha, H.S.; Usman, F.; Ashfaq–Khanc, M.; Khalild, R.; Ul-Haqd, Z.; Mushtaqe, A.; Qaiserf, R.; Iqbalg, J. Preparation and characterization of anticancer niosomal withaferin–A formulation for improved delivery to cancer cells: An in vitro and in vivo evaluation. J. Drug Deliv. Sci. Technol. 2020, 59, 101863. [Google Scholar] [CrossRef]
- Gupta, R.C.; Bansal, S.S.; Aqil, F.; Jeyabalan, J.; Cao, P.; Kausar, H.; Russell, G.K.; Munagala, R.; Ravoori, S.; Vadhanam, M.V. Controlled-release systemic delivery—A new concept in cancer chemoprevention. Carcinogenesis 2012, 33, 1608–1615. [Google Scholar] [CrossRef]
- Yu, Y. Withaferin A Targets Hsp90 in Pancreatic Cancer Cells. Ph.D. Thesis, University of Michigan, Ann Arbor, MI, USA, 2011. Available online: https://deepblue.lib.umich.edu/bitstream/handle/2027.42/89629/yorkyu_1.pdf?sequence=1&isAllowed=y (accessed on 20 October 2020).
- Cohen, S.M.; Mukerji, R.; Timmermann, B.N.; Samadi, A.K.; Cohen, M.S. A novel combination of withaferin A and sorafenib shows synergistic efficacy against both papillary and anaplastic thyroid cancers. Am. J. Surg. 2012, 204, 895–901. [Google Scholar] [CrossRef]
- Kakar, S.S.; Worth, C.A.; Wang, Z.; Carter, K.; Ratajczak, M.; Gunjal, P. DOXIL when combined with Withaferin A (WFA) targets ALDH1 positive cancer stem cells in ovarian cancer. J. Cancer Stem Cell. Res. 2016, 4, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Kyakulaga, A.H.; Aqil, F.; Munagala, R.; Gupta, R.C. Synergistic combinations of paclitaxel and withaferin A against human non-small cell lung cancer cells. Oncotarget 2020, 11, 1399–1416. [Google Scholar] [CrossRef] [PubMed]
- Alnuqaydan, A.M.; Rah, B.; Almutary, A.G.; Chauhan, S.S. Synergistic antitumor effect of 5-fluorouracil and withaferin-A induces endoplasmic reticulum stress-mediated autophagy and apoptosis in colorectal cancer cells. Am. J. Cancer Res. 2020, 10, 799–815. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behl, T.; Sharma, A.; Sharma, L.; Sehgal, A.; Zengin, G.; Brata, R.; Fratila, O.; Bungau, S. Exploring the Multifaceted Therapeutic Potential of Withaferin A and Its Derivatives. Biomedicines 2020, 8, 571. https://doi.org/10.3390/biomedicines8120571
Behl T, Sharma A, Sharma L, Sehgal A, Zengin G, Brata R, Fratila O, Bungau S. Exploring the Multifaceted Therapeutic Potential of Withaferin A and Its Derivatives. Biomedicines. 2020; 8(12):571. https://doi.org/10.3390/biomedicines8120571
Chicago/Turabian StyleBehl, Tapan, Aditi Sharma, Lalit Sharma, Aayush Sehgal, Gokhan Zengin, Roxana Brata, Ovidiu Fratila, and Simona Bungau. 2020. "Exploring the Multifaceted Therapeutic Potential of Withaferin A and Its Derivatives" Biomedicines 8, no. 12: 571. https://doi.org/10.3390/biomedicines8120571
APA StyleBehl, T., Sharma, A., Sharma, L., Sehgal, A., Zengin, G., Brata, R., Fratila, O., & Bungau, S. (2020). Exploring the Multifaceted Therapeutic Potential of Withaferin A and Its Derivatives. Biomedicines, 8(12), 571. https://doi.org/10.3390/biomedicines8120571







