Aptamers for Proteins Associated with Rheumatic Diseases: Progress, Challenges, and Prospects of Diagnostic and Therapeutic Applications
Abstract
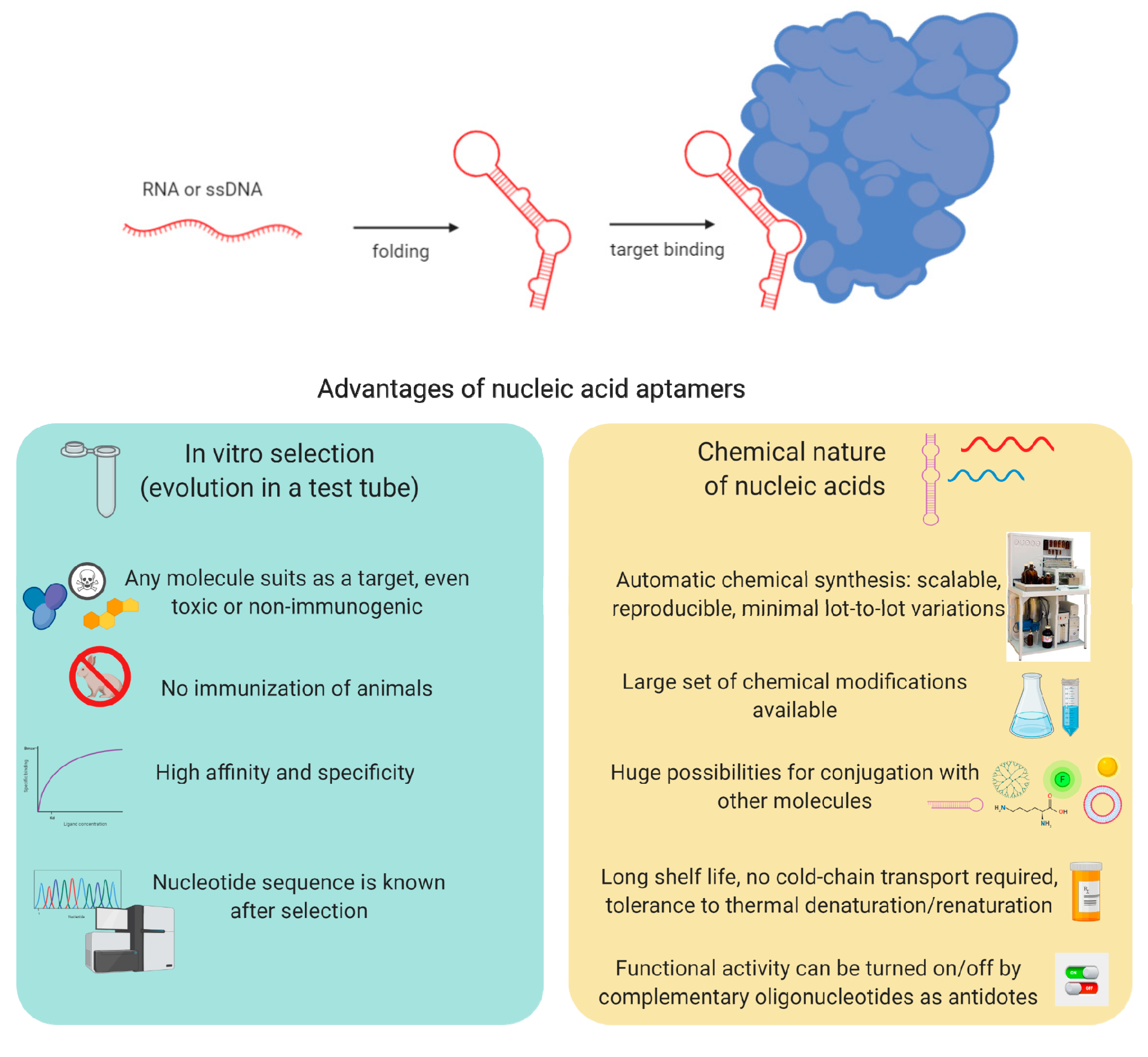
1. Introduction
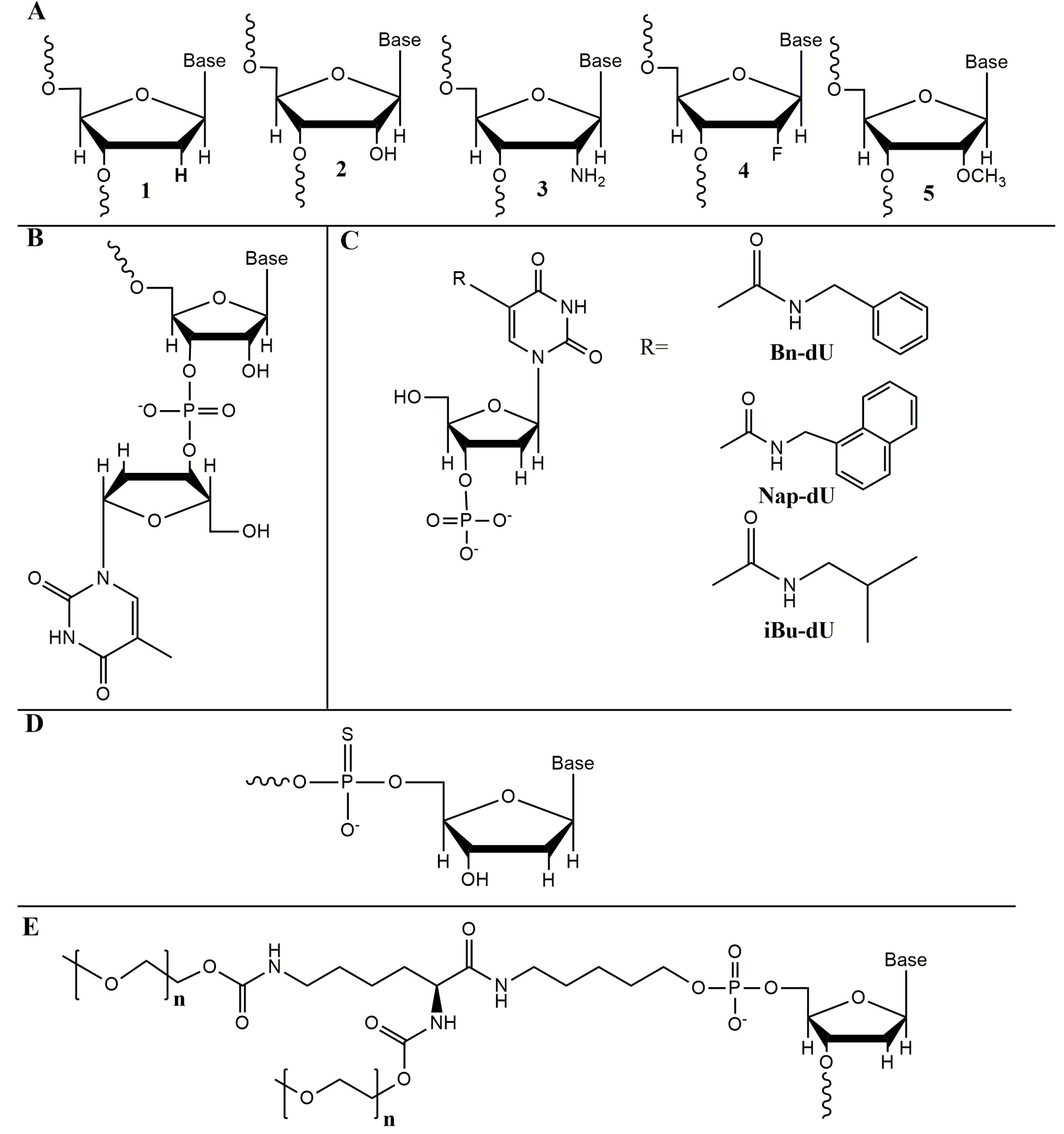
2. Selection and Chemical Modifications of Nucleic Acid Aptamers
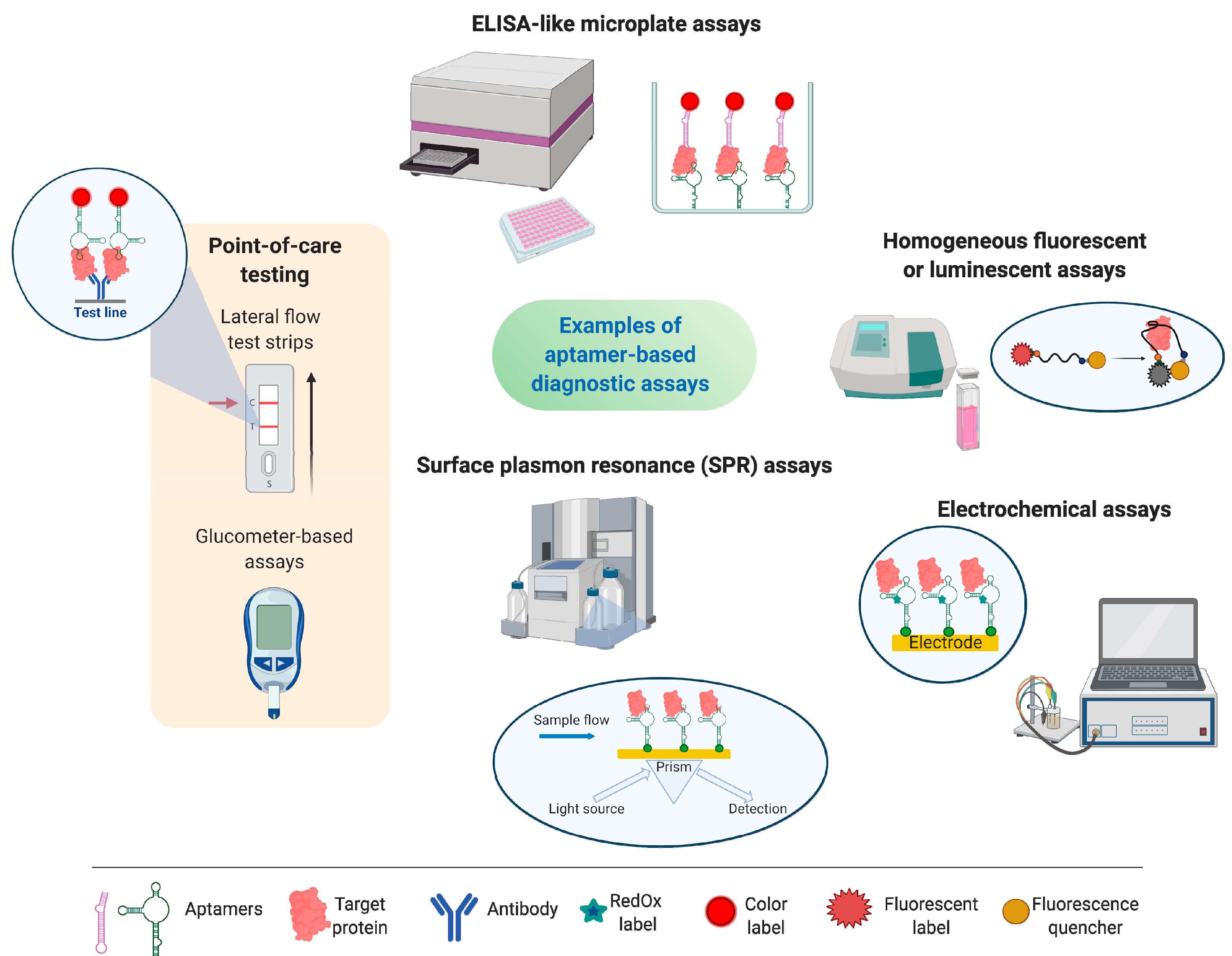
3. Aptasensors—Aptamer-Based Bioanalytical Systems
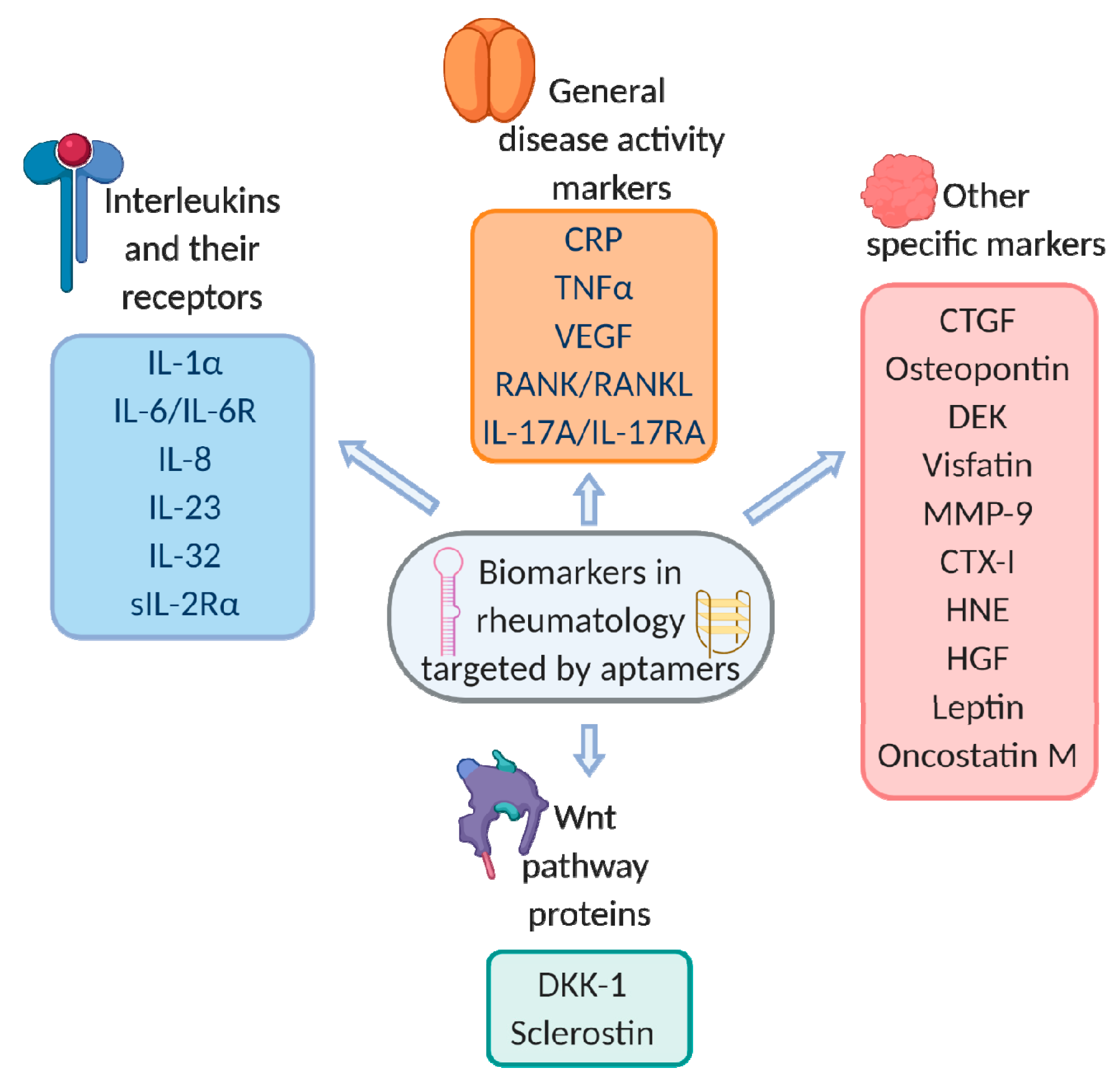
4. Aptamers for Protein Biomarkers of Rheumatic Disorders
4.1. General Disease Activity Markers
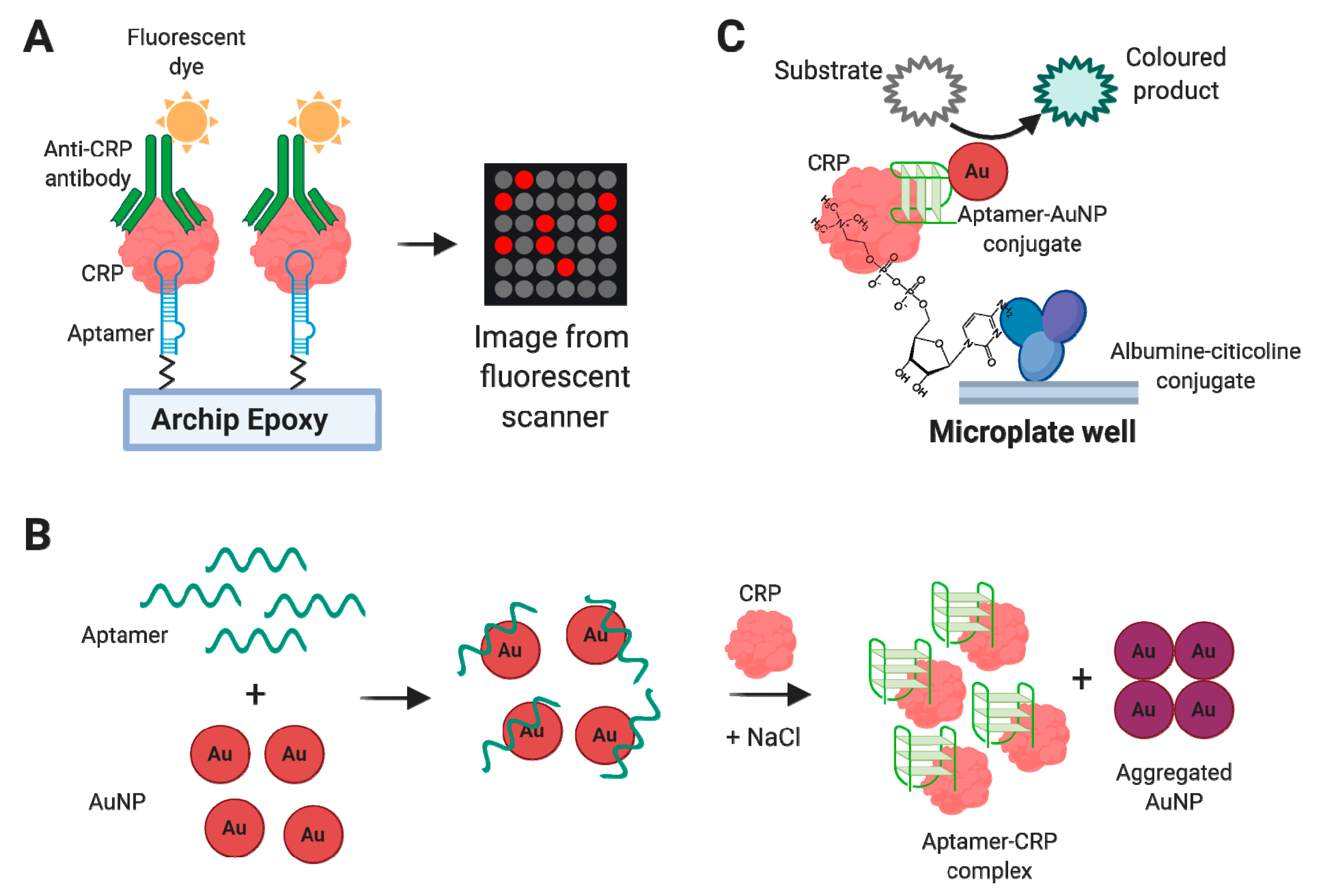
4.1.1. C-Reactive Protein (CRP)
Aptamer-Based CRP Detection Assays
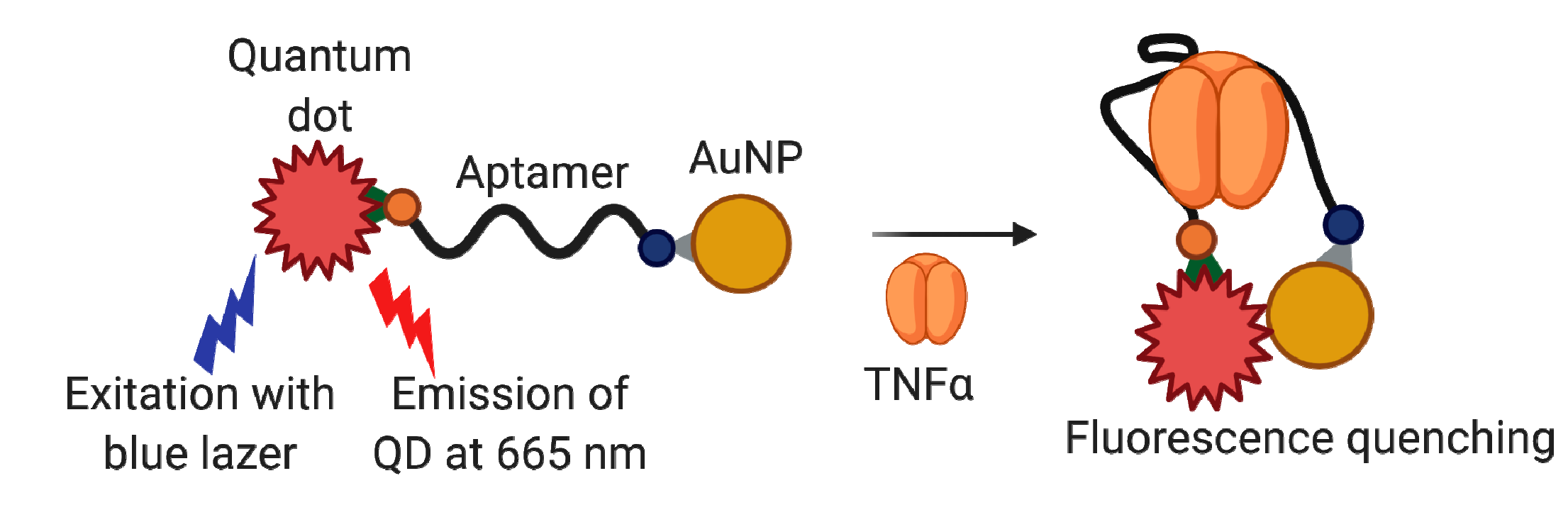
4.1.2. Tumor Necrosis Factor Alpha (TNFα)
Aptamer-Based TNFα Inhibitors
Aptamer-Based TNFα Detection Assays
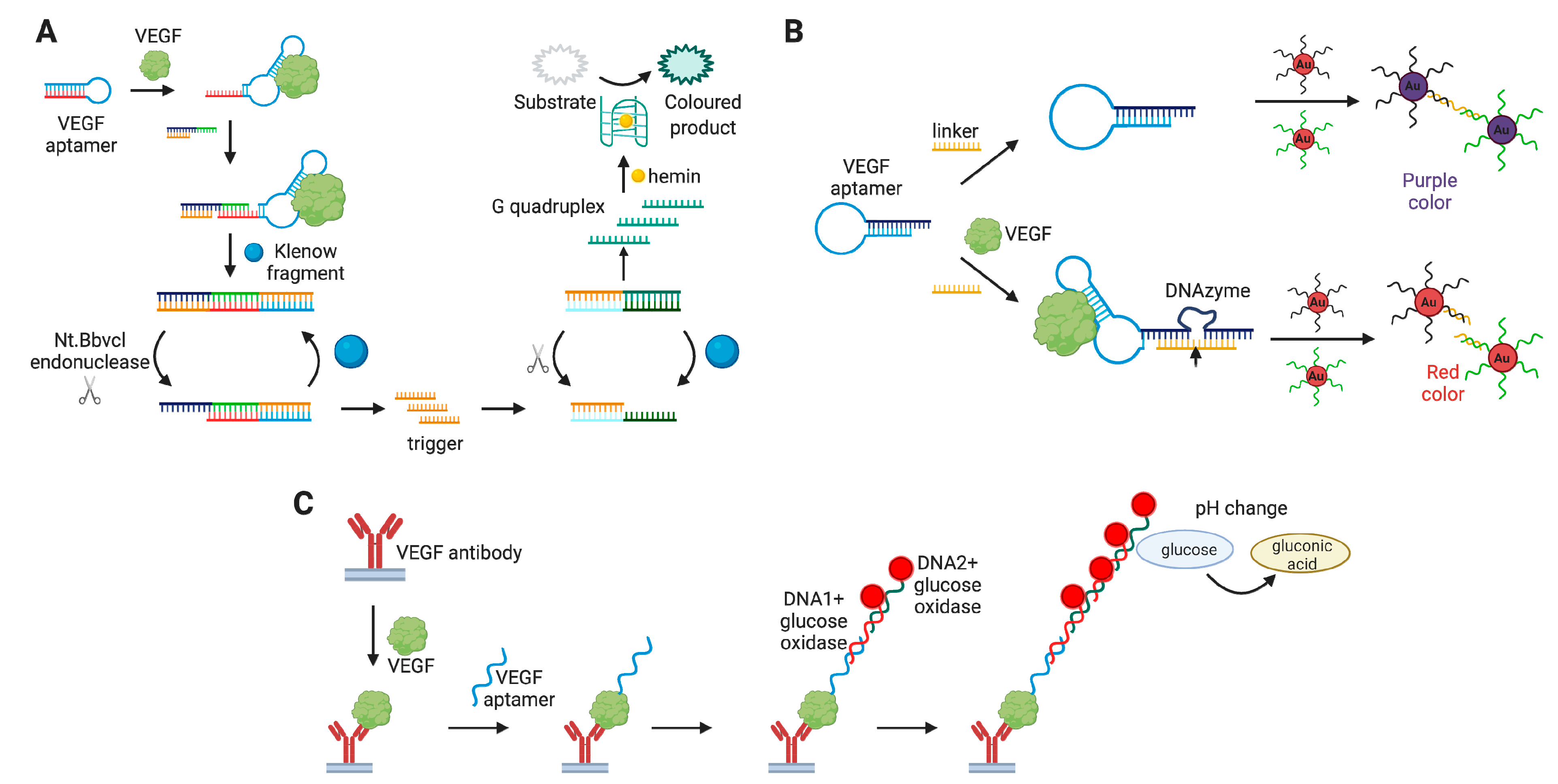
4.1.3. Vascular Endothelial Growth Factor (VEGF)
Aptamer-Based VEGF Detection Assays
4.1.4. Receptor Activator of Nuclear Factor Kappa-Β (RANK)
4.2. Interleukins and Their Receptors
4.2.1. Interleukin 17A (IL-17A) and Its Receptor (IL-17AR)
Aptamer-Based Inhibitors of IL-17A/IL-17RA
Aptamer-Based IL-17A/IL-17RA Detection Assays
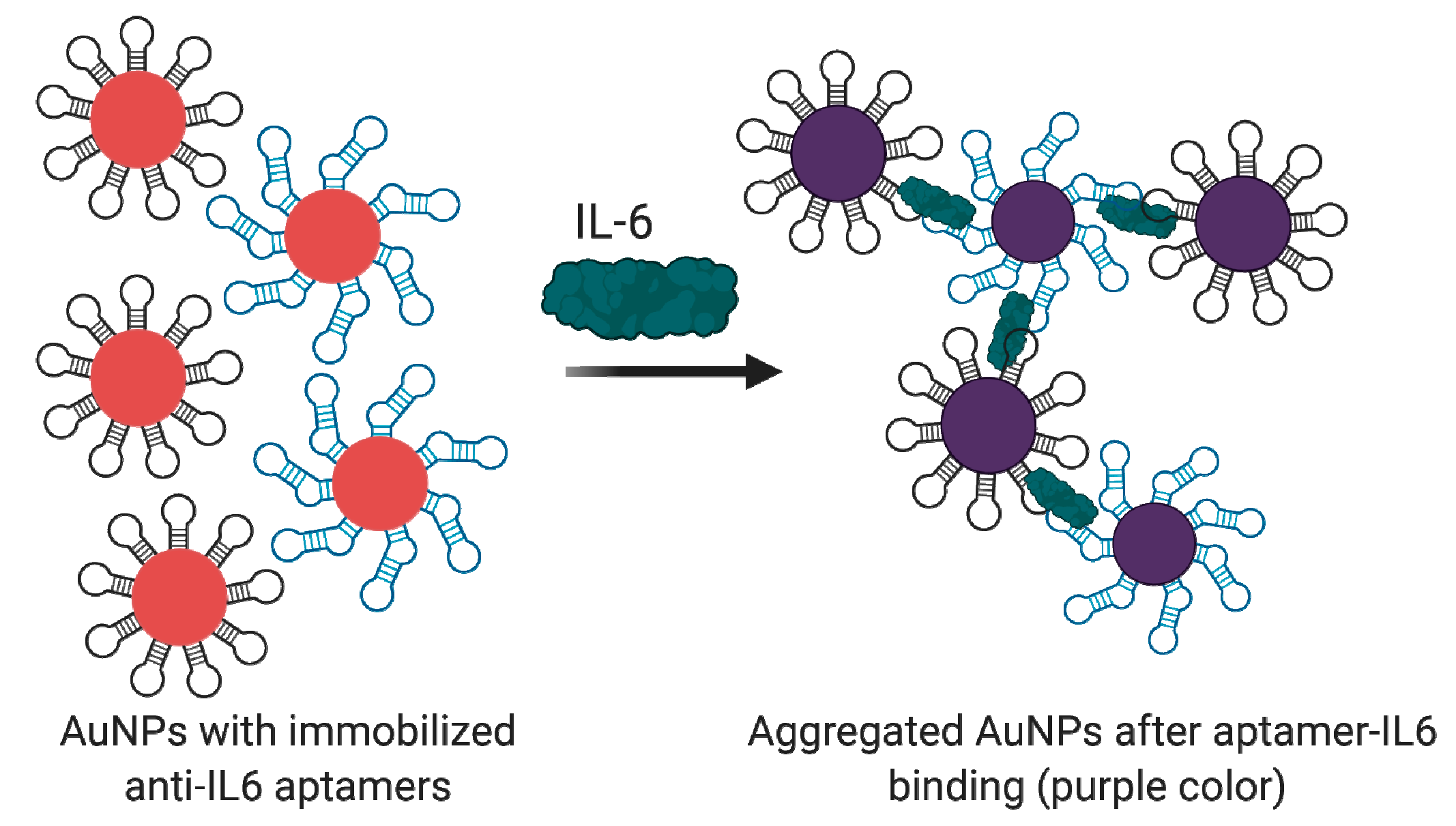
4.2.2. Interleukin 6 (IL-6) and Its Receptor (IL-6R)
Aptamer-Based Inhibitors of IL-6/IL-6R
Aptamer-Based IL-6/IL-6R Detection Assays
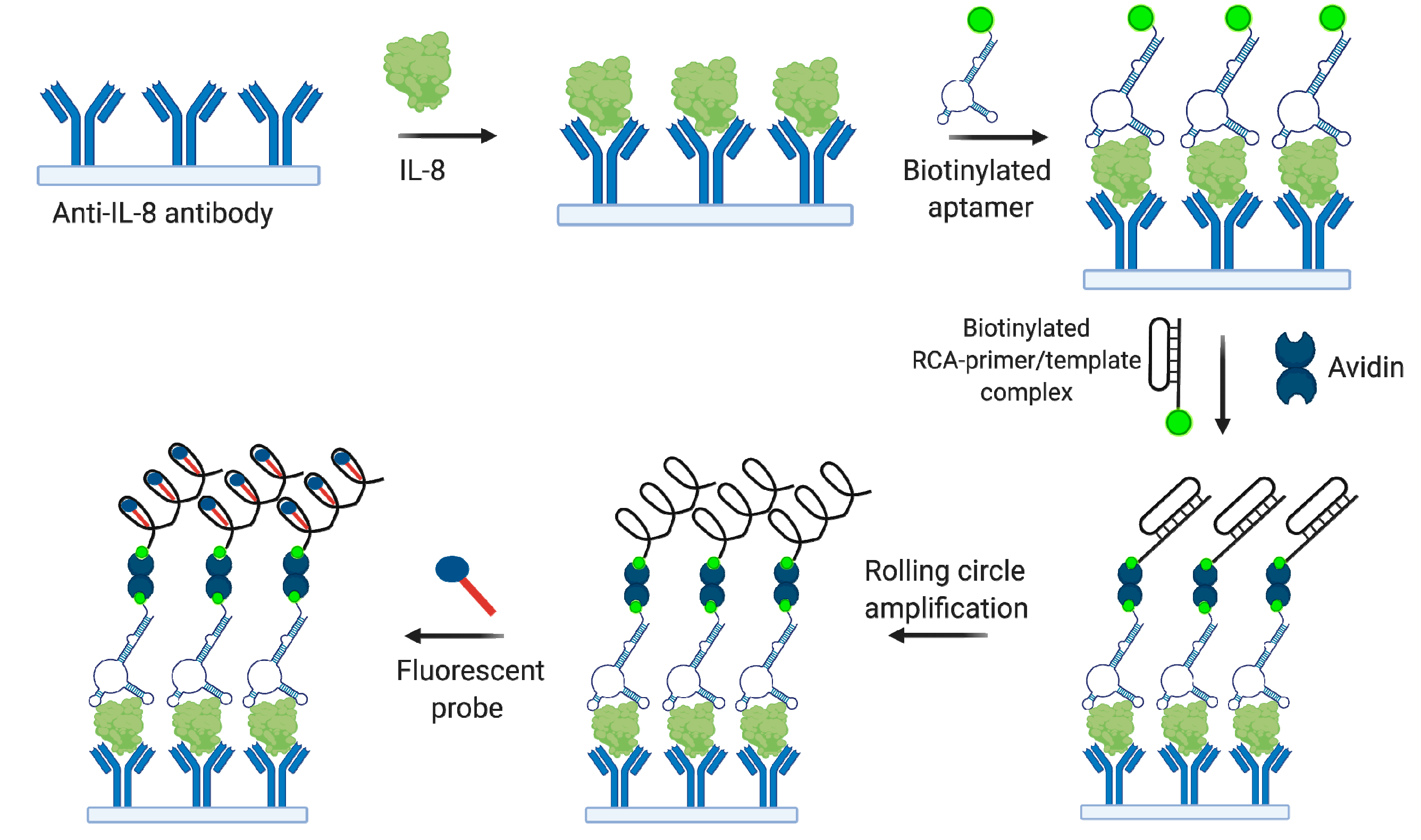
4.2.3. Interleukin 8 (IL-8)
4.2.4. Interleukin 23 (IL-23)
Aptamer-Based Inhibitors of IL-23
4.2.5. Other Interleukins
Aptamer-Based Inhibitors of Interleukins
Aptamer-Based Detection Assays
4.3. Other Specific Markers
4.3.1. WNT Pathway
Aptamer-Based Inhibitors of WNT Proteins
Aptamer-Based Detection Assays for WNT Proteins
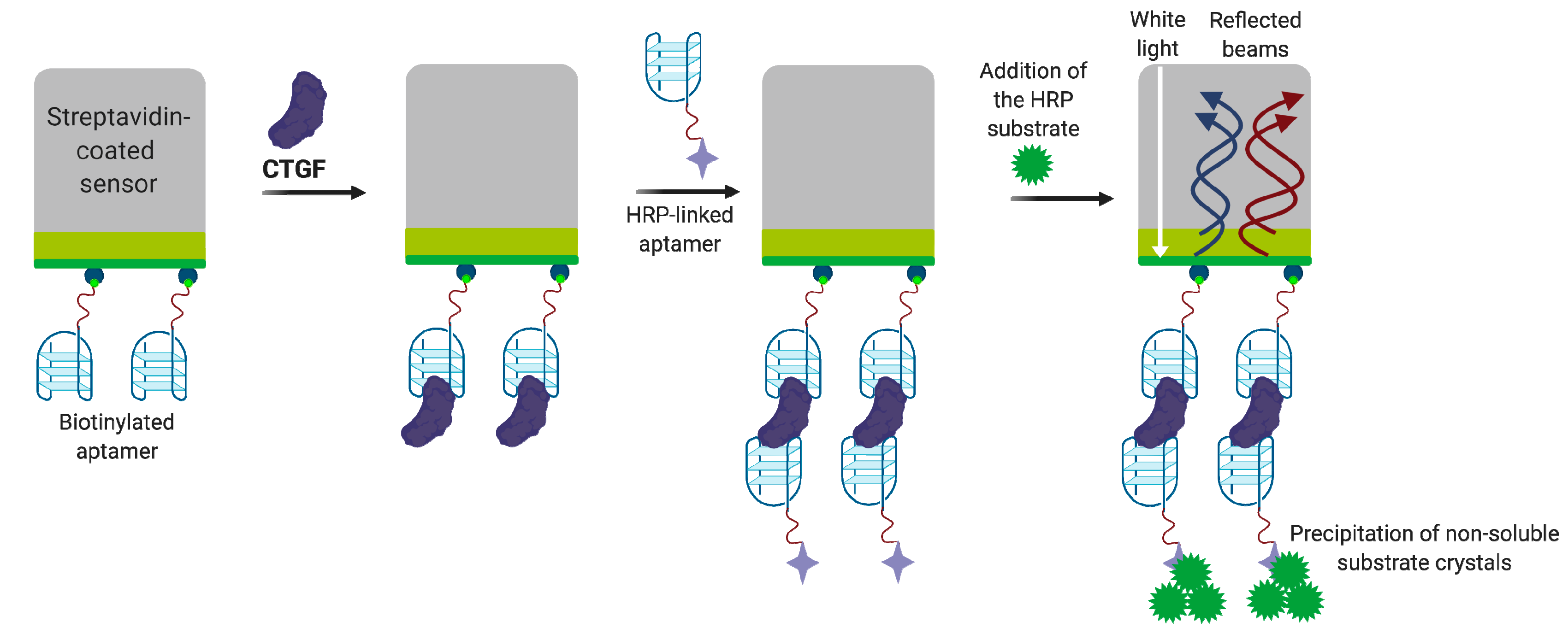
4.3.2. Connective Tissue Growth Factor (CTGF)
Aptamer-Based CTGF Detection Assays
4.3.3. Osteopontin
Aptamer-Based OPN Inhibitors
Aptamer-Based OPN Detection Assays
DEK Protein
Aptamer-Based DEK Inhibitors
4.3.4. Visfatin
Aptamer-Based Visfatin Detection Assays
4.3.5. Matrix Metalloproteinase 9 (MMP-9)
Aptamer-Based MMP-9 Detection Assays
4.3.6. C-Terminal Telopeptide (CTX-I)
Aptamer-Based CTX-I Detection Assays
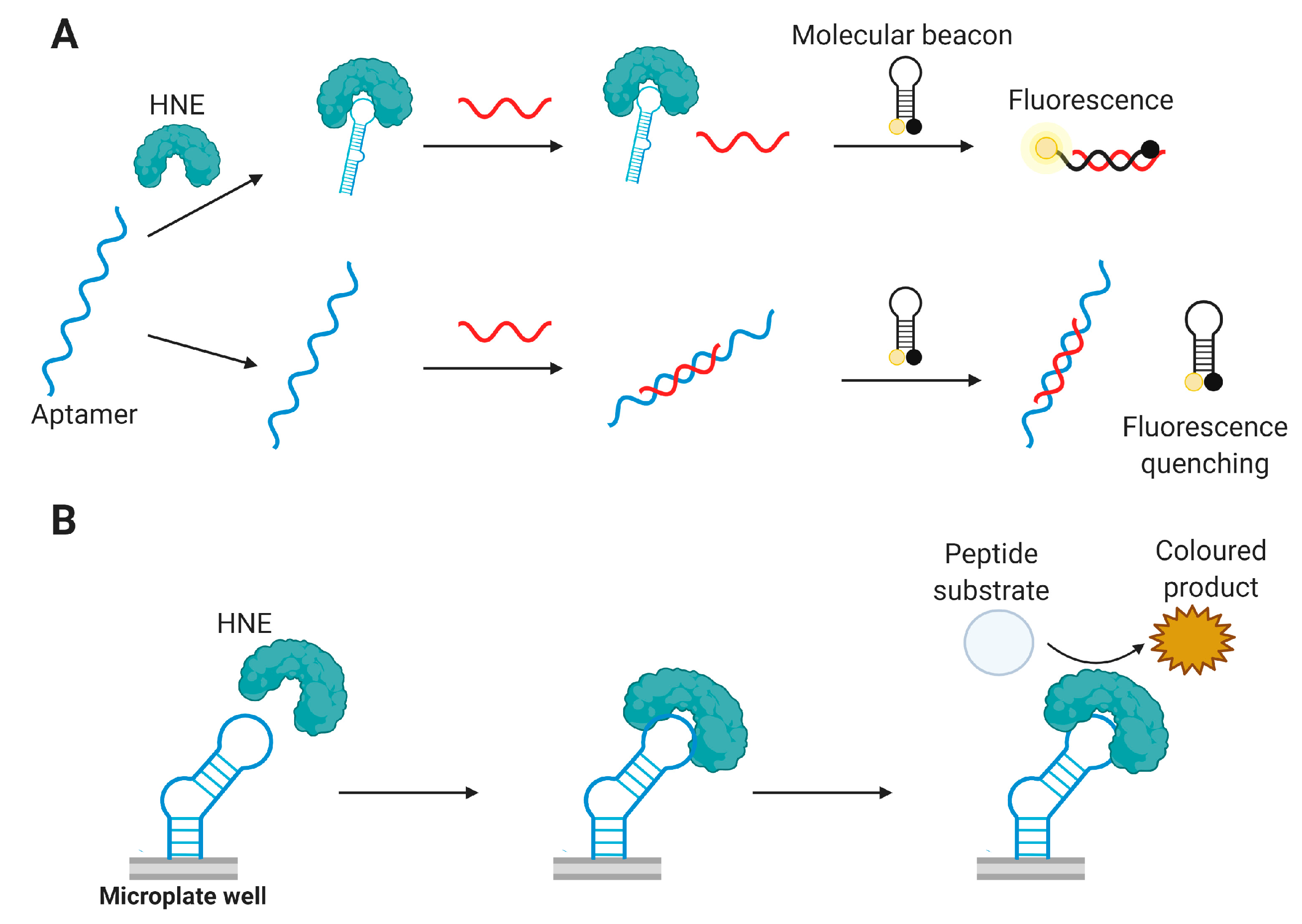
4.3.7. Human Neutrophil Elastase (HNE)
Aptamer-Based HNE Detection Assays
4.3.8. Hepatocyte Growth Factor (HGF)
Aptamer-Based HGF Inhibitors
4.3.9. Leptin (Lp)
4.3.10. Oncostatin M (OSM)
Aptamer-Based OSM Inhibitors
5. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ALF | Acute liver failure |
| ALI | Acute lung injury |
| ANCAs | Anti-neutrophil cytoplasmic antibodies |
| AS | Ankylosing spondylitis |
| AuNP | Gold nanoparticles |
| BLI | Biolayer interferometry |
| BMD | Bone mineral density |
| BSA | Bovine serum albumin |
| CE | Capillary electrophoresis |
| CEA | Carcinoembryonic antigen |
| CRP | C-reactive protein |
| CTGF | Connective tissue growth factor |
| CTX-I | C-terminal telopeptide |
| DKK-1 | Dikkopf 1 |
| DNMT1 | DNA methyltransferase 1 |
| EGFR | Epidermal growth factor receptor |
| ELISA | Enzyme linked immunosorbent assay |
| FDA | U.S. Food and Drug Administration |
| FET | Field effect transistor |
| FRET | Förster resonance energy transfer |
| GFET | Graphene-based field effect transistor |
| GO-SELEX | Graphene oxide-based SELEX |
| HGF | Hepatocyte growth factor |
| HNE | Human neutrophil elastase |
| HSA | Human serum albumin |
| LOD | Limit of detection |
| MMP-9 | Matrix metalloproteinase 9 |
| NAMPT | Nicotinamide phosphoribosyl transferase |
| OP | Osteoporosis |
| OPG | Osteoprotegerin |
| OPN | Osteopontin |
| OSM | Oncostatin M |
| PDGF-BB | Platelet derived growth factor-BB |
| PEG | Polyethylene glycol |
| PoC | Point-of-care |
| QD | Quantum dots |
| RA | Rheumatoid arthritis |
| RANK | Receptor activator of nuclear factor kappa-Β |
| SELEX | Systematic evolution of ligands by exponential enrichment |
| SERS | Surface-enhanced Raman spectroscopy |
| SLE | Systemic lupus erythematosus |
| Sn | Sclerostin |
| SOMAmer | Slow off-rate modified aptamer |
| SPR | Surface plasmon resonance |
| TMB | tetramethylbenzidine |
| TNFα | Tumor necrosis factor α |
| VEGF | Vascular endothelial growth factor |
References
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar]
- Robertson, D.L.; Joyce, G.F. Selection in vitro of an RNA enzyme that specifically cleaves single-stranded DNA. Nature 1990, 344, 467–468. [Google Scholar] [CrossRef]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [PubMed]
- Nimjee, S.M.; White, R.R.; Becker, R.C.; Sullenger, B.A. Aptamers as Therapeutics. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lai, B.; Juhas, M. Recent advances in aptamer discovery and applications. Molecules 2019, 24, 941. [Google Scholar] [CrossRef]
- Adachi, T.; Nakamura, Y. Aptamers: A review of their chemical properties and modifications for therapeutic application. Molecules 2019, 24, 4229. [Google Scholar] [CrossRef]
- Haßel, S.K.; Mayer, G. Aptamers as therapeutic agents: Has the initial euphoria subsided? Mol. Diagnosis Ther. 2019, 23, 301–309. [Google Scholar] [CrossRef]
- Kumar Kulabhusan, P.; Hussain, B.; Yüce, M. Current perspectives on aptamers as diagnostic tools and therapeutic agents. Pharmaceutics 2020, 12, 646. [Google Scholar] [CrossRef]
- Kou, X.; Zhang, X.; Shao, X.; Jiang, C.; Ning, L. Recent advances in optical aptasensor technology for amplification strategies in cancer diagnostics. Anal. Bioanal. Chem. 2020, 412, 6691–6705. [Google Scholar] [CrossRef]
- Pirzada, M.; Altintas, Z. Recent progress in optical sensors for biomedical diagnostics. Micromachines 2020, 11, 356. [Google Scholar] [CrossRef]
- Yan, S.R.; Foroughi, M.M.; Safaei, M.; Jahani, S.; Ebrahimpour, N.; Borhani, F.; Rezaei Zade Baravati, N.; Aramesh-Boroujeni, Z.; Foong, L.K. A review: Recent advances in ultrasensitive and highly specific recognition aptasensors with various detection strategies. Int. J. Biol. Macromol. 2020, 155, 184–207. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.K.; Bruno, J.G.; Dhiman, A. ABCs of DNA aptamer and related assay development. Biotechnol. Adv. 2017, 35, 275–301. [Google Scholar] [CrossRef] [PubMed]
- Kalra, P.; Dhiman, A.; Cho, W.C.; Bruno, J.G.; Sharma, T.K. Simple methods and rational design for enhancing aptamer sensitivity and specificity. Front. Mol. Biosci. 2018, 5, 1–16. [Google Scholar] [CrossRef]
- Baker, M. Blame it on the antibodies. Nature 2015, 521, 274–276. [Google Scholar] [CrossRef]
- Bradbury, A.; Plückthun, A. Reproducibility: Standardize antibodies used in research. Nature 2015, 518, 27–29. [Google Scholar]
- Weller, M.G. Quality issues of research antibodies. Anal. Chem. Insights 2016, 2016, 21–27. [Google Scholar] [CrossRef]
- Maimaitiyiming, Y.; Hong, D.F.; Yang, C.; Naranmandura, H. Novel insights into the role of aptamers in the fight against cancer. J. Cancer Res. Clin. Oncol. 2019, 145, 797–810. [Google Scholar] [CrossRef]
- Ponce, A.T.; Hong, K.L. A Mini-Review: Clinical development and potential of aptamers for thrombotic events treatment and monitoring. Biomedicines 2019, 7, 55. [Google Scholar]
- Davydova, A.; Vorobjeva, M.; Pyshnyi, D.; Altman, S.; Vlassov, V.; Venyaminova, A. Aptamers against pathogenic microorganisms. Crit. Rev. Microbiol. 2016, 42, 847–865. [Google Scholar] [CrossRef]
- Park, K.S. Nucleic acid aptamer-based methods for diagnosis of infections. Biosens. Bioelectron. 2018, 102, 179–188. [Google Scholar] [CrossRef]
- Aletaha, D.; Maa, J.; Chen, S.; Park, S.-H.; Nicholls, D.; Florentinus, S.; Furtner, D.; Smolen, J.S. Effect of disease duration and prior disease-modifying antirheumatic drug use on treatment outcomes in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2019, 78, 1609–1615. [Google Scholar] [CrossRef] [PubMed]
- Deminger, A.; Klingberg, E.; Geijer, M.; Göthlin, J.; Hedberg, M.; Rehnberg, E.; Carlsten, H.; Jacobsson, L.T.; Forsblad-d’Elia, H. A five-year prospective study of spinal radiographic progression and its predictors in men and women with ankylosing spondylitis. Arthritis Res. Ther. 2018, 20, 1–14. [Google Scholar] [CrossRef]
- Landewé, R.; Nurminen, T.; Davies, O.; Baeten, D. A single determination of C-reactive protein does not suffice to declare a patient with a diagnosis of axial spondyloarthritis “CRP-negative”. Arthritis Res. Ther. 2018, 20, 4–9. [Google Scholar] [CrossRef]
- Gravallese, E.M.; Schett, G. Effects of the IL-23–IL-17 pathway on bone in spondyloarthritis. Nat. Rev. Rheumatol. 2018, 14, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Pan, F.; Gao, J.; Ge, R.; Duan, Z.; Zeng, Z.; Liao, F.; Xia, G.; Wang, S.; Xu, S.; et al. Increased serum IL-17 and IL-23 in the patient with ankylosing spondylitis. Clin. Rheumatol. 2011, 30, 269–273. [Google Scholar] [CrossRef]
- Bayat, P.; Nosrati, R.; Alibolandi, M.; Rafatpanah, H.; Abnous, K.; Khedri, M.; Ramezani, M. SELEX methods on the road to protein targeting with nucleic acid aptamers. Biochimie 2018, 154, 132–155. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.H.; Elsherbiny, M.E.; Emara, M. Updates on aptamer research. Int. J. Mol. Sci. 2019, 20, 2511. [Google Scholar] [CrossRef]
- Komarova, N.; Kuznetsov, A. Inside the Black Box: What Makes SELEX Better? Molecules 2019, 24, 3598. [Google Scholar]
- Elskens, J.P.; Elskens, J.M.; Madder, A. Chemical Modification of Aptamers for Increased Binding Affinity in Diagnostic Applications: Current Status and Future Prospects. Int. J. Mol. Sci. 2020, 21, 4522. [Google Scholar] [CrossRef]
- Vorobyeva, M.; Davydova, A.; Vorobjev, P.; Pyshnyi, D.; Venyaminova, A. Key Aspects of Nucleic Acid Library Design for in Vitro Selection. Int. J. Mol. Sci. 2018, 19, 470. [Google Scholar] [CrossRef]
- Röthlisberger, P.; Hollenstein, M. Aptamer chemistry. Adv. Drug Deliv. Rev. 2018, 134, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Odeh, F.; Nsairat, H.; Alshaer, W.; Ismail, M.A.; Esawi, E.; Qaqish, B.; Bawab, A.A.; Ismail, S.I. Aptamers chemistry: Chemical modifications and conjugation strategies. Molecules 2020, 25, 3. [Google Scholar] [CrossRef]
- Moreno, A.; Pitoc, G.A.; Ganson, N.J.; Layzer, J.M.; Hershfield, M.S.; Tarantal, A.F.; Sullenger, B.A. Anti-PEG Antibodies inhibit the anticoagulant activity of PEGylated aptamers. Cell Chem. Biol. 2019, 26, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sun, F.; Liu, S.; Jiang, S. Anti-PEG antibodies in the clinic: Current issues and beyond PEGylation. J. Control. Release 2016, 244, 184–193. [Google Scholar] [CrossRef]
- Vasilescu, A.; Marty, J.L. Electrochemical aptasensors for the assessment of food quality and safety. Trends Anal. Chem. 2016, 79, 60–70. [Google Scholar]
- Schmitz, F.R.W.; Valério, A.; de Oliveira, D.; Hotza, D. An overview and future prospects on aptamers for food safety. Appl. Microbiol. Biotechnol. 2020, 104, 6929–6939. [Google Scholar] [CrossRef]
- Mishra, G.; Sharma, V.; Mishra, R. Electrochemical aptasensors for food and environmental safeguarding: A review. Biosensors 2018, 8, 28. [Google Scholar] [CrossRef]
- Li, Z.; Mohamed, M.A.; Vinu Mohan, A.M.; Zhu, Z.; Sharma, V.; Mishra, G.K.; Mishra, R.K. Application of electrochemical aptasensors toward clinical diagnostics, food, and environmental monitoring: Review. Sensors 2019, 19, 5435. [Google Scholar] [CrossRef]
- McConnell, E.M.; Nguyen, J.; Li, Y. Aptamer-based biosensors for environmental monitoring. Front. Chem. 2020, 8, 434. [Google Scholar] [CrossRef]
- Heydari, M.; Gholoobi, A.; Ranjbar, G.; Rahbar, N.; Sany, S.B.T.; Mobarhan, M.G.; Ferns, G.A.; Rezayi, M. Aptamers as potential recognition elements for detection of vitamins and minerals: A systematic and critical review. Crit. Rev. Clin. Lab. Sci. 2020, 57, 126–144. [Google Scholar]
- Şahin, S.; Caglayan, M.O.; Üstündağ, Z. Recent advances in aptamer-based sensors for breast cancer diagnosis: Special cases for nanomaterial-based VEGF, HER2, and MUC1 aptasensors. Microchim. Acta 2020, 187, 549. [Google Scholar] [CrossRef]
- Han, K.; Liu, T.; Wang, Y.; Miao, P. Electrochemical aptasensors for detection of small molecules, macromolecules, and cells. Rev. Anal. Chem. 2016, 35, 201–211. [Google Scholar]
- Xu, Y.; Cheng, G.; He, P.; Fang, Y. A Review: Electrochemical aptasensors with various detection strategies. Electroanalysis 2009, 21, 1251–1259. [Google Scholar] [CrossRef]
- Reid, R.; Chatterjee, B.; Das, S.J.; Ghosh, S.; Sharma, T.K. Application of aptamers as molecular recognition elements in lateral flow assays. Anal. Biochem. 2020, 593, 113574. [Google Scholar] [CrossRef] [PubMed]
- Citartan, M.; Tang, T.-H. Recent developments of aptasensors expedient for point-of-care (POC) diagnostics. Talanta 2019, 199, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Li, R.; Gan, Y.Z.; Zhang, R.J.; Li, J.; Cai, Y.M.; Zhao, J.X.; Liao, H.; Xu, J.; Shi, L.J.; et al. Clinical deep remission and related factors in a large cohort of patients with rheumatoid arthritis. Chin. Med. J. 2019, 132, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Chui, E.T.F.; Lee, K.H.; Tsang, H.H.L.; Chan, S.C.W.; Lau, C.S. ASDAS is associated with both the extent and intensity of DW-MRI spinal inflammation in active axial spondyloarthritis. RMD Open 2019, 5, e001008. [Google Scholar] [CrossRef]
- Yahagi, A.; Saika, T.; Hirano, H.; Takai-Imamura, M.; Tsuji, F.; Aono, H.; Iseki, M.; Morita, Y.; Igarashi, H.; Saeki, Y.; et al. IL-6-PAD4 axis in the earliest phase of arthritis in knock-in gp130F759 mice, a model for rheumatoid arthritis. RMD Open 2019, 5, e000853. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sugiyama, N.; Toyoizumi, S.; Lukic, T.; Lamba, M.; Zhang, R.; Chen, C.; Stock, T.; Valdez, H.; Mojcik, C.; et al. Modified-versus immediate-release tofacitinib in Japanese rheumatoid arthritis patients: A randomized, phase III, non-inferiority study. Rheumatology 2019, 58, 70–79. [Google Scholar] [CrossRef]
- Su, J.; Cui, L.; Yang, W.; Shi, H.; Jin, C.; Shu, R.; Li, H.; Zeng, X.; Wu, S.; Gao, X. Baseline high-sensitivity C-reactive protein predicts the risk of incident ankylosing spondylitis: Results of a community-based prospective study. PLoS ONE 2019, 14, e0211946. [Google Scholar] [CrossRef]
- Chan, F.L.Y.; Lester, S.; Whittle, S.L.; Hill, C.L. The utility of ESR, CRP and platelets in the diagnosis of GCA. BMC Rheumatol. 2019, 3, 14. [Google Scholar] [CrossRef]
- Ing, E.B.; Lahaie Luna, G.; Toren, A.; Ing, R.; Chen, J.; Arora, N.; Torun, N.; Jakpor, O.A.; Fraser, J.A.; Tyndel, F.J.; et al. Multivariate prediction model for suspected giant cell arteritis: Development and validation. Clin. Ophthalmol. 2017, 11, 2031–2042. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.; Graf, N.; Sauter, R.; Allanore, Y.; Curram, J.; Denton, C.P.; Khanna, D.; Matucci-Cerinic, M.; de Oliveira Pena, J.; Pope, J.E.; et al. Predictors of disease worsening defined by progression of organ damage in diffuse systemic sclerosis: A European Scleroderma Trials and Research (EUSTAR) analysis. Ann. Rheum. Dis. 2019, 78, 1242–1248. [Google Scholar] [CrossRef]
- Lis-Święty, A.; Widuchowska, M.; Brzezińska-Wcisło, L.; Kucharz, E. High acute phase protein levels correlate with pulmonary and skin involvement in patients with diffuse systemic sclerosis. J. Int. Med. Res. 2018, 46, 1634–1639. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Niu, R.; Jiang, L.; Wang, Y.; Shao, X.; Wu, M.; Ma, Y. The diagnostic values of C-reactive protein and procalcitonin in identifying systemic lupus erythematosus infection and disease activity. Medicine 2019, 98, e16798. [Google Scholar] [CrossRef]
- Littlejohn, E.; Marder, W.; Lewis, E.; Francis, S.; Jackish, J.; McCune, W.J.; Somers, E.C. The ratio of erythrocyte sedimentation rate to C-reactive protein is useful in distinguishing infection from flare in systemic lupus erythematosus patients presenting with fever. Lupus 2018, 27, 1123–1129. [Google Scholar] [CrossRef]
- Bay-Jensen, A.C.; Platt, A.; Jenkins, M.A.; Weinblatt, M.E.; Byrjalsen, I.; Musa, K.; Genovese, M.C.; Karsdal, M.A. Tissue metabolite of type I collagen, C1M, and CRP predicts structural progression of rheumatoid arthritis. BMC Rheumatol. 2019, 3, 3. [Google Scholar] [CrossRef]
- Yeh, J.-C.; Wu, C.-C.; Choy, C.-S.; Chang, S.-W.; Liou, J.-C.; Chen, K.-S.; Tung, T.-H.; Lin, W.-N.; Hsieh, C.-Y.; Ho, C.-T.; et al. Non-hepatic alkaline phosphatase, hs-CRP and progression of vertebral fracture in patients with rheumatoid arthritis: A population-based longitudinal study. J. Clin. Med. 2018, 7, 439. [Google Scholar] [CrossRef]
- Yu, Z.; Kim, S.C.; Vanni, K.; Huang, J.; Desai, R.; Murphy, S.N.; Solomon, D.H.; Liao, K.P. Association between inflammation and systolic blood pressure in RA compared to patients without RA. Arthritis Res. Ther. 2018, 20, 107. [Google Scholar] [CrossRef]
- Azevedo, S.; Santos-Faria, D.; Leite Silva, J.; Ramos Rodrigues, J.; Sousa Neves, J.; Peixoto, D.; Tavares-Costa, J.; Alcino, S.; Afonso, C.; Teixeira, F. Obesity, metabolic syndrome and other comorbidities in rheumatoid arthritis and psoriatic arthritis: Influence on disease activity and quality of life. Acta Reumatol. Port. 2019, 44, 322–324. [Google Scholar]
- Ferguson, L.D.; Siebert, S.; McInnes, I.B.; Sattar, N. Cardiometabolic comorbidities in RA and PsA: Lessons learned and future directions. Nat. Rev. Rheumatol. 2019, 15, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Dimitroulas, T.; Hodson, J.; Sandoo, A.; Smith, J.; Kitas, G.D. Endothelial injury in rheumatoid arthritis: A crosstalk between dimethylarginines and systemic inflammation. Arthritis Res. Ther. 2017, 19, 32. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Wang, T. Features of cardiac remodeling in patients with acute coronary syndrome complicated with rheumatoid arthritis. Sci. Rep. 2017, 7, 10268. [Google Scholar] [CrossRef] [PubMed]
- Nash, P.; Ohson, K.; Walsh, J.; Delev, N.; Nguyen, D.; Teng, L.; Gómez-Reino, J.J.; Aelion, J.A. Early and sustained efficacy with apremilast monotherapy in biological-naïve patients with psoriatic arthritis: A phase IIIB, randomised controlled trial (ACTIVE). Ann. Rheum. Dis. 2018, 77, 690–698. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Chakravarty, S.D.; Apaolaza, I.; Kafka, S.; Hsia, E.C.; You, Y.; Kavanaugh, A. Efficacy of ustekinumab in biologic-naïve patients with psoriatic arthritis by prior treatment exposure and disease duration: Data from PSUMMIT 1 and PSUMMIT 2. RMD Open 2019, 5, e000990. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, H.-A.; Shin, K.; Park, Y.-B.; Kim, T.-H.; Song, Y.W.; Lee, E.Y. Effects of tapering tumor necrosis factor inhibitor on the achievement of inactive disease in patients with axial spondyloarthritis: A nationwide cohort study. Arthritis Res. Ther. 2019, 21, 163. [Google Scholar] [CrossRef]
- Cohen, S.; Pablos, J.L.; Pavelka, K.; Müller, G.A.; Matsumoto, A.; Kivitz, A.; Wang, H.; Krishnan, E. An open-label extension study to demonstrate long-term safety and efficacy of ABP 501 in patients with rheumatoid arthritis. Arthritis Res. Ther. 2019, 21, 84. [Google Scholar] [CrossRef]
- Park, W.; Božić-Majstorović, L.; Milakovic, D.; Berrocal Kasay, A.; El-Khouri, E.C.; Irazoque-Palazuelos, F.; Molina, F.F.C.; Shesternya, P.; Miranda, P.; Medina-Rodriguez, F.G.; et al. Comparison of biosimilar CT-P10 and innovator rituximab in patients with rheumatoid arthritis: A randomized controlled Phase 3 trial. MAbs 2018, 10, 934–943. [Google Scholar] [CrossRef]
- Fleischmann, R.M.; Alten, R.; Pileckyte, M.; Lobello, K.; Hua, S.Y.; Cronenberger, C.; Alvarez, D.; Bock, A.E.; Sewell, K.L. A comparative clinical study of PF-06410293, a candidate adalimumab biosimilar, and adalimumab reference product (Humira®) in the treatment of active rheumatoid arthritis. Arthritis Res. Ther. 2018, 20, 178. [Google Scholar] [CrossRef]
- Taylor, P.C.; Saurigny, D.; Vencovsky, J.; Takeuchi, T.; Nakamura, T.; Matsievskaia, G.; Hunt, B.; Wagner, T.; Souberbielle, B. Efficacy and safety of namilumab, a human monoclonal antibody against granulocyte-macrophage colony-stimulating factor (GM-CSF) ligand in patients with rheumatoid arthritis (RA) with either an inadequate response to background methotrexate therapy or an i. Arthritis Res. Ther. 2019, 21, 101. [Google Scholar] [CrossRef]
- Braun, J.; Deodhar, A.; Landewé, R.; Baraliakos, X.; Miceli-Richard, C.; Sieper, J.; Quebe-Fehling, E.; Martin, R.; Porter, B.; Gandhi, K.K.; et al. Impact of baseline C-reactive protein levels on the response to secukinumab in ankylosing spondylitis: 3-year pooled data from two phase III studies. RMD Open 2018, 4, e000749. [Google Scholar] [CrossRef] [PubMed]
- Strand, V.; Alemao, E.; Lehman, T.; Johnsen, A.; Banerjee, S.; Ahmad, H.A.; Mease, P.J. Improved patient-reported outcomes in patients with psoriatic arthritis treated with abatacept: Results from a phase 3 trial. Arthritis Res. Ther. 2018, 20, 269. [Google Scholar] [CrossRef] [PubMed]
- Bini, A.; Centi, S.; Tombelli, S.; Minunni, M.; Mascini, M. Development of an optical RNA-based aptasensor for C-reactive protein. Anal. Bioanal. Chem. 2008, 390, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Orito, N.; Umekage, S.; Sato, K.; Kawauchi, S.; Tanaka, H.; Sakai, E.; Tanaka, T.; Kikuchi, Y. High-affinity RNA aptamers to C-reactive protein (CRP): Newly developed pre-elution methods for aptamer selection. J. Phys. Conf. Ser. 2012, 352. [Google Scholar] [CrossRef]
- Huang, C.; Lin, H.; Shiesh, S.; Lee, G. Integrated microfluidic system for rapid screening of CRP aptamers utilizing systematic evolution of ligands by exponential enrichment (SELEX). Biosens. Bioelectron. 2010, 25, 1761–1766. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Jiang, R.; Wang, Q.; Huang, J.; Yang, X.; Wang, K.; Li, W.; Chen, N.; Li, Q. Detection of C-reactive protein using nanoparticle-enhanced surface plasmon resonance using an aptamer-antibody sandwich assay. Chem. Commun. 2016, 52, 3568–3571. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Wang, K.; Wang, Q.; Wang, P.; Lin, M.; Chen, N.; Tan, Y. DNA aptamer-based surface plasmon resonance sensing of human C-reactive protein. RSC Adv. 2014, 4, 30934–30937. [Google Scholar] [CrossRef]
- Lai, W.Y.; Wang, J.W.; Huang, B.T.; Lin, E.P.Y.; Yang, P.C. A Novel TNF-α-targeting aptamer for TNF-α-mediated acute lung injury and acute liver failure. Theranostics 2019, 9, 1741–1751. [Google Scholar] [CrossRef]
- Orava, E.W.; Jarvik, N.; Shek, Y.L.; Sidhu, S.S.; Gariépy, J. A Short DNA aptamer that recognizes TNFα and blocks its activity in vitro. ACS Chem. Biol. 2013, 8, 170–178. [Google Scholar] [CrossRef]
- Yan, X.; Gao, X.; Zhang, Z. Isolation and characterization of 2′-amino-modified RNA aptamers for human TNFalpha. Genom. Proteom. Bioinform. 2004, 2, 32–42. [Google Scholar] [CrossRef]
- Mashayekhi, K.; Ganji, A.; Sankian, M. Designing a new dimerized anti human TNF-α aptamer with blocking activity. Biotechnol. Prog. 2020, 36, e2969. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; He, Z.; Mao, S.; Jie, M.; Yi, L.; Lin, J.M. Quantitative determination of VEGF165 in cell culture medium by aptamer sandwich based chemiluminescence assay. Talanta 2017, 171, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Oguro, A.; Ohtsu, T.; Nakamura, Y. RNA aptamers selected against the receptor activator of NF-κB acquire general affinity to proteins of the tumor necrosis factor receptor family. Nucleic Acids Res. 2004, 32, 6120–6128. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, D.Q.; Zhong, J.; Wu, X.L.; Chen, Q.; Peng, H.; Liu, S.Q. IL-17RA aptamer-mediated repression of IL-6 inhibits synovium inflammation in a murine model of osteoarthritis. Osteoarthr. Cartil. 2011, 19, 711–718. [Google Scholar] [CrossRef]
- Ishiguro, A.; Akiyama, T.; Adachi, H.; Inoue, J.I.; Nakamura, Y. Therapeutic potential of anti-interleukin-17A aptamer: Suppression of interleukin-17A signaling and attenuation of autoimmunity in two mouse models. Arthritis Rheum. 2011, 63, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Adachi, H.; Ishiguro, A.; Hamada, M.; Sakota, E.; Asai, K.; Nakamura, Y. Antagonistic RNA aptamer specific to a heterodimeric form of human interleukin-17A/F. Biochimie 2011, 93, 1081–1088. [Google Scholar] [CrossRef]
- Gupta, S.; Hirota, M.; Waugh, S.M.; Murakami, I.; Suzuki, T.; Muraguchi, M.; Shibamori, M.; Ishikawa, Y.; Jarvis, T.C.; Carter, J.D.; et al. Chemically modified DNA aptamers bind interleukin-6 with high affinity and inhibit signaling by blocking its interaction with interleukin-6 receptor. J. Biol. Chem. 2014, 289, 8706–8719. [Google Scholar] [CrossRef]
- Meyer, C.; Eydeler-Haeder, K.; Magbanua, E.; Tijana, Z.; Piganeau, N.; Lorenzen, I.; Grötzinger, J.; Mayer, G.; Rose-John, S.; Hahn, U. Interleukin-6 receptor specific RNA aptamers for cargo delivery into target cells. RNA Biol. 2012, 9, 67–80. [Google Scholar] [CrossRef]
- Meyer, C.; Berg, K.; Eydeler-Haeder, K.; Lorenzen, I.; Grötzinger, J.; Rose-John, S.; Hahn, U. Stabilized interleukin-6 receptor binding RNA aptamers. RNA Biol. 2014, 11, 57–65. [Google Scholar] [CrossRef]
- Mittelberger, F.; Meyer, C.; Waetzig, G.H.; Zacharias, M.; Valentini, E.; Svergun, D.I.; Rose-john, S.; Hahn, U. RAID3—An interleukin-6 receptor-binding aptamer with post-selective modification-resistant affinity. RNA Biol. 2015, 130, 1043–1053. [Google Scholar]
- Lenn, J.D.; Neil, J.; Donahue, C.; Demock, K.; Tibbetts, C.V.; Cote-Sierra, J.; Smith, S.H.; Rubenstein, D.; Therrien, J.P.; Pendergrast, P.S.; et al. RNA aptamer delivery through intact human skin. J. Invest. Dermatol. 2018, 138, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, J.H.; Yoon, S.; Kim, K.S.; Yoon, M.Y.; Yoon, D.Y.; Kim, D.E. Generation of antagonistic RNA aptamers specific to proinflammatory cytokine interleukin-32. Bull. Korean Chem. Soc. 2010, 31, 3561–3566. [Google Scholar] [CrossRef]
- Sung, H.J.; Choi, S.; Lee, J.W.; Ok, C.Y.; Bae, Y.S.; Kim, Y.H.; Lee, W.; Heo, K.; Kim, I.H. Inhibition of human neutrophil activity by an RNA aptamer bound to interleukin-8. Biomaterials 2014, 35, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Gelinas, A.D.; von Carlowitz, I.; Janjic, N.; Pyle, A.M. Structural basis for IL-1α recognition by a modified DNA aptamer that specifically inhibits IL-1α signaling. Nat. Commun. 2017, 8, 810. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, W.; Tseng, Y.; Zhang, J.; Liu, J. Developing slow-off dickkopf-1 aptamers for early-diagnosis of hepatocellular carcinoma. Talanta 2019, 194, 422–429. [Google Scholar] [CrossRef]
- Shum, K.T.; Chan, C.; Leung, C.M.; Tanner, J.A. Identification of a DNA aptamer that inhibits sclerostin’s antagonistic effect on Wnt signalling. Biochem. J. 2011, 434, 493–501. [Google Scholar] [CrossRef]
- Gao, S.; Hu, W.; Zheng, X.; Cai, S.; Wu, J. Functionalized aptamer with an antiparallel G-quadruplex: Structural remodeling, recognition mechanism, and diagnostic applications targeting CTGF. Biosens. Bioelectron. 2019, 142, 111475. [Google Scholar] [CrossRef]
- Li, S.; Huo, Y.; Tian, H.; Zhang, Q.; Lv, Y.; Hao, Z. In vitro selection and characterization of deoxyribonucleic acid aptamers against connective tissue growth factor. Biochem. Biophys. Res. Commun. 2015, 457, 640–646. [Google Scholar] [CrossRef]
- Mi, Z.; Guo, H.; Russell, M.B.; Liu, Y.; Sullenger, B.A.; Kuo, P.C. RNA aptamer blockade of osteopontin inhibits growth and metastasis of MDA-MB231 breast cancer cells. Mol. Ther. 2009, 17, 153–161. [Google Scholar] [CrossRef]
- Mor-Vaknin, N.; Saha, A.; Legendre, M.; Carmona-Rivera, C.; Amin, M.A.; Rabquer, B.J.; Gonzales-Hernandez, M.J.; Jorns, J.; Mohan, S.; Yalavarthi, S.; et al. DEK-targeting DNA aptamers as therapeutics for inflammatory arthritis. Nat. Commun. 2017, 8, 14252. [Google Scholar] [CrossRef]
- Park, J.-W.; Saravan Kallempudi, S.; Niazi, J.H.; Gurbuz, Y.; Youn, B.-S.; Gu, M.B. Rapid and sensitive detection of Nampt (PBEF/visfatin) in human serum using an ssDNA aptamer-based capacitive biosensor. Biosens. Bioelectron. 2012, 38, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Da Rocha Gomes, S.; Miguel, J.; Azéma, L.; Eimer, S.; Ries, C.; Dausse, E.; Loiseau, H.; Allard, M.; Toulmé, J.J. 99mTc-MAG3-aptamer for imaging human tumors associated with high level of matrix metalloprotease-9. Bioconjug. Chem. 2012, 23, 2192–2200. [Google Scholar] [CrossRef] [PubMed]
- Scarano, S.; Dausse, E.; Crispo, F.; Toulmé, J.-J.; Minunni, M. Design of a dual aptamer-based recognition strategy for human matrix metalloproteinase 9 protein by piezoelectric biosensors. Anal. Chim. Acta 2015, 897, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bruno, J.G.; Carrillo, M.P.; Phillips, T.; Hanson, D.; Bohmann, J.A. DNA aptamer beacon assay for C-telopeptide and handheld fluorometer to monitor bone resorption. J. Fluoresc. 2011, 21, 2021–2033. [Google Scholar] [CrossRef]
- Lin, Y.; Padmapriya, A.; Morden, K.M.; Jayasena, S.D. Peptide conjugation to an in vitro-selected DNA ligand improves enzyme inhibition. Proc. Natl. Acad. Sci. USA 1995, 92, 11044–11048. [Google Scholar] [CrossRef]
- Saito, T.; Tomida, M. Generation of inhibitory DNA aptamers against human hepatocyte growth factor. DNA Cell Biol. 2005, 24, 624–633. [Google Scholar] [CrossRef]
- Ashley, J.; Li, S.F.Y. Three-dimensional selection of leptin aptamers using capillary electrophoresis and implications for clone validation. Anal. Biochem. 2013, 434, 146–152. [Google Scholar] [CrossRef]
- Rhodes, A.; Deakin, A.; Spaull, J.; Coomber, B.; Aitken, A.; Life, P.; Rees, S. The generation and characterization of antagonist RNA aptamers to human oncostatin M. J. Biol. Chem. 2000, 275, 28555–28561. [Google Scholar] [CrossRef]
- Qureshi, A.; Gurbuz, Y.; Kallempudi, S.; Niazi, J.H. Label-free RNA aptamer-based capacitive biosensor for the detection of C-reactive protein. Phys. Chem. Chem. Phys. 2010, 12, 9176–9182. [Google Scholar] [CrossRef]
- Jarczewska, M.; Rębiś, J.; Górski, Ł.; Malinowska, E. Development of DNA aptamer-based sensor for electrochemical detection of C-reactive protein. Talanta 2018, 189, 45–54. [Google Scholar] [CrossRef]
- Pultar, J.; Sauer, U.; Domnanich, P.; Preininger, C. Aptamer-antibody on-chip sandwich immunoassay for detection of CRP in spiked serum. Biosens. Bioelectron. 2009, 24, 1456–1461. [Google Scholar] [CrossRef] [PubMed]
- António, M.; Ferreira, R.; Vitorino, R.; Daniel-da-Silva, A.L. A simple aptamer-based colorimetric assay for rapid detection of C-reactive protein using gold nanoparticles. Talanta 2020, 214, 120868. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Tang, M.Q.; Chen, J.; Zhu, Y.H.; Lei, C.B.; He, H.W.; Xu, X.H. A sandwich ELISA-like detection of C-reactive protein in blood by citicoline-bovine serum albumin conjugate and aptamer-functionalized gold nanoparticles nanozyme. Talanta 2020, 217, 121070. [Google Scholar] [CrossRef] [PubMed]
- Centi, S.; Sanmartin, L.B.; Tombelli, S.; Palchetti, I.; Mascini, M. Detection of C reactive protein (CRP) in serum by an electrochemical aptamer-based sandwich assay. Electroanalysis 2009, 21, 1309–1315. [Google Scholar] [CrossRef]
- Wang, J.; Guo, J.; Zhang, J.; Zhang, W.; Zhang, Y. RNA aptamer-based electrochemical aptasensor for C-reactive protein detection using functionalized silica microspheres as immunoprobes. Biosens. Bioelectron. 2017, 95, 100–105. [Google Scholar] [CrossRef]
- Bernard, E.D.; Nguyen, K.C.; DeRosa, M.C.; Tayabali, A.F.; Aranda-Rodriguez, R. Development of a bead-based aptamer/antibody detection system for C-reactive protein. Anal. Biochem. 2015, 472, 67–74. [Google Scholar] [CrossRef]
- Eid, C.; Palko, J.W.; Katilius, E.; Santiago, J.G. Rapid Slow Off-Rate Modified Aptamer (SOMAmer)-Based Detection of C-Reactive Protein Using Isotachophoresis and an Ionic Spacer. Anal. Chem. 2015, 87, 6736–6743. [Google Scholar] [CrossRef]
- Kao, W.-C.; Chen, Y.-W.; Chu, C.-H.; Chang, W.-H.; Shiesh, S.-C.; Wang, Y.-L.; Lee, G.-B. Detection of C-reactive protein on an integrated microfluidic system by utilizing field-effect transistors and aptamers. Biomicrofluidics 2017, 11, 044105. [Google Scholar] [CrossRef]
- Wu, B.; Chen, N.; Wang, Q.; Yang, X.; Wang, K.; Li, W.; Li, Q.; Liu, W.; Fang, H. A simple label-free aptamer-based method for C-reactive protein detection. Anal. Methods 2016, 8, 4177–4180. [Google Scholar] [CrossRef]
- Zubiate, P.; Zamarreño, C.R.; Sánchez, P.; Matias, I.R.; Arregui, F.J. High sensitive and selective C-reactive protein detection by means of lossy mode resonance based optical fiber devices. Biosens. Bioelectron. 2017, 93, 176–181. [Google Scholar] [CrossRef]
- Ghalehno, M.H.; Mirzaei, M.; Torkzadeh-Mahani, M. Aptamer-based determination of tumor necrosis factor α using a screen-printed graphite electrode modified with gold hexacyanoferrate. Mikrochim. Acta 2018, 185, 165. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Datta, D.; Chaudhry, S.; Dutta, M.; Stroscio, M.A. Rapid detection of tumor necrosis factor-alpha using quantum dot-based optical aptasensor. IEEE Trans. Nanobiosci. 2018, 17, 417–423. [Google Scholar] [CrossRef]
- Hao, Z.; Wang, Z.; Li, Y.; Zhu, Y.; Wang, X.; De Moraes, C.G.; Pan, Y.; Zhao, X.; Lin, Q. Measurement of cytokine biomarkers using an aptamer-based affinity graphene nanosensor on a flexible substrate toward wearable applications. Nanoscale 2018, 10, 21681–21688. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.D.; Lai, R.Y. Effects of redox label location on the performance of an electrochemical aptamer-based tumor necrosis factor-alpha sensor. Talanta 2018, 189, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, Q.; Revzin, A. An aptasensor for electrochemical detection of tumor necrosis factor in human blood. Analyst 2013, 138, 4321–4326. [Google Scholar] [CrossRef]
- Dong, J.; He, L.; Wang, Y.; Yu, F.; Yu, S.; Liu, L.; Wang, J.; Tian, Y.; Qu, L.; Han, R.; et al. A highly sensitive colorimetric aptasensor for the detection of the vascular endothelial growth factor in human serum. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 226, 117622. [Google Scholar] [CrossRef]
- Zhang, H.; Peng, L.; Li, M.; Ma, J.; Qi, S.; Chen, H.; Zhou, L.; Chen, X. A label-free colorimetric biosensor for sensitive detection of vascular endothelial growth factor-165. Analyst 2017, 142, 2419–2425. [Google Scholar] [CrossRef]
- Chang, C.C.; Chen, C.Y.; Chuang, T.L.; Wu, T.H.; Wei, S.C.; Liao, H.; Lin, C.W. Aptamer-based colorimetric detection of proteins using a branched DNA cascade amplification strategy and unmodified gold nanoparticles. Biosens. Bioelectron. 2016, 78, 200–205. [Google Scholar] [CrossRef]
- Wu, D.; Gao, T.; Lei, L.; Yang, D.; Mao, X.; Li, G. Colorimetric detection of proteins based on target-induced activation of aptazyme. Anal. Chim. Acta 2016, 942, 68–73. [Google Scholar] [CrossRef]
- Freeman, R.; Girsh, J.; Fang-Ju Jou, A.; Ho, J.A.A.; Hug, T.; Dernedde, J.; Willner, I. Optical aptasensors for the analysis of the vascular endothelial growth factor (VEGF). Anal. Chem. 2012, 84, 6192–6198. [Google Scholar] [CrossRef]
- Xu, H.; Kou, F.; Ye, H.; Wang, Z.; Huang, S.; Liu, X.; Zhu, X.; Lin, Z.; Chen, G. Highly sensitive antibody-aptamer sensor for vascular endothelial growth factor based on hybridization chain reaction and pH meter/indicator. Talanta 2017, 175, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Kou, F.; Xu, H.; Lin, L.; Yang, G.; Lin, Z. A highly sensitive aptamer-immunoassay for vascular endothelial growth factor coupled with portable glucose meter and hybridization chain reaction. Sens. Actuators B Chem. 2017, 253, 660–665. [Google Scholar] [CrossRef]
- Jo, H.; Kim, S.H.S.K.; Youn, H.; Lee, H.; Lee, K.; Jeong, J.; Mok, J.; Kim, S.H.S.K.; Park, H.S.; Ban, C. A highly sensitive and selective impedimetric aptasensor for interleukin-17 receptor A. Biosens. Bioelectron. 2016, 81, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Pan, Y.; Huang, C.; Wang, Z.; Zhao, X. Sensitive detection of lung cancer biomarkers using an aptameric graphene-based nanosensor with enhanced stability. Biomed. Microdev. 2019, 21, 65. [Google Scholar] [CrossRef]
- Tertis, M.; Leva, P.I.; Bogdan, D.; Suciu, M.; Graur, F.; Cristea, C. Impedimetric aptasensor for the label-free and selective detection of Interleukin-6 for colorectal cancer screening. Biosens. Bioelectron. 2019, 137, 123–132. [Google Scholar] [CrossRef]
- Giorgi-Coll, S.; Marín, M.J.; Sule, O.; Hutchinson, P.J.; Carpenter, K.L.H. Aptamer-modified gold nanoparticles for rapid aggregation-based detection of inflammation: An optical assay for interleukin-6. Microchim. Acta 2020, 187, 13. [Google Scholar] [CrossRef]
- Jeon, J.; Jo, H.; Her, J.; Youn, H.; Park, J.; Jo, J.; Lee, J.; Chang, C.L.; Ban, C. A Rapid colorimetric sensor for soluble Interleukin-2 receptor α, based on aptamer-adsorbed AuNP. ChemBioChem 2019, 2236–2240. [Google Scholar] [CrossRef]
- Zhang, W.; He, Z.; Yi, L.; Mao, S.; Li, H.; Lin, J.M. A dual-functional microfluidic chip for on-line detection of interleukin-8 based on rolling circle amplification. Biosens. Bioelectron. 2018, 102, 652–660. [Google Scholar] [CrossRef]
- Mukama, O.; Wu, W.; Wu, J.; Lu, X.; Liu, Y.; Liu, Y.; Liu, J.; Zeng, L. A highly sensitive and specific lateral flow aptasensor for the detection of human osteopontin. Talanta 2020, 210, 120624. [Google Scholar] [CrossRef]
- He, J.L.; Wu, Z.S.; Zhang, S.B.; Shen, G.L.; Yu, R.Q. Fluorescence aptasensor based on competitive-binding for human neutrophil elastase detection. Talanta 2010, 80, 1264–1268. [Google Scholar] [CrossRef]
- Cheng, L.; Zhao, Q. Aptamer-capture based assays for human neutrophil elastase. Talanta 2013, 106, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Wang, H.; Zhao, Q. Detection of human neutrophil elastase by aptamer affinity capillary electrophoresis coupled with laser-induced fluorescence using specified site fluorescently labeled aptamer. Anal. Bioanal. Chem. 2017, 409, 6843–6849. [Google Scholar] [CrossRef] [PubMed]
- Camussi, G.; Albano, E.; Tetta, C.; Bussolino, F. The molecular action of tumor necrosis factor-alpha. Eur. J. Biochem. 1991, 202, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Acar, L.; Atalan, N.; Karagedik, E.H.; Ergen, A. Tumour necrosis factor-alpha and nuclear factor-kappa b gene variants in sepsis. Balkan Med. J. 2018, 35, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, T.; Mitoma, H.; Harashima, S. -i.; Tsukamoto, H.; Shimoda, T. Transmembrane TNF-: Structure, function and interaction with anti-TNF agents. Rheumatology 2010, 49, 1215–1228. [Google Scholar] [CrossRef]
- Keffer, J.; Probert, L.; Cazlaris, H.; Georgopoulos, S.; Kaslaris, E.; Kioussis, D.; Kollias, G. Transgenic mice expressing human tumour necrosis factor: A predictive genetic model of arthritis. EMBO J. 1991, 10, 4025–4031. [Google Scholar]
- Zwerina, J.; Redlich, K.; Polzer, K.; Joosten, L.; Kronke, G.; Distler, J.; Hess, A.; Pundt, N.; Pap, T.; Hoffmann, O.; et al. TNF-induced structural joint damage is mediated by IL-1. Proc. Natl. Acad. Sci. USA 2007, 104, 11742–11747. [Google Scholar] [CrossRef]
- Gorth, D.J.; Shapiro, I.M.; Risbud, M.V. Transgenic mice overexpressing human TNF-α experience early onset spontaneous intervertebral disc herniation in the absence of overt degeneration. Cell Death Dis. 2019, 10, 7. [Google Scholar] [CrossRef]
- Merola, J.F.; Espinoza, L.R.; Fleischmann, R. Distinguishing rheumatoid arthritis from psoriatic arthritis. RMD Open 2018, 4, e000656. [Google Scholar] [CrossRef]
- Jung, M.K.; Lee, J.S.; Kwak, J.E.; Shin, E.C. Tumor necrosis factor and regulatory T cells. Yonsei Med. J. 2019, 60, 126–131. [Google Scholar] [CrossRef]
- Liu, Y.; Kwa, T.; Revzin, A. Simultaneous detection of cell-secreted TNF-α and IFN-γ using micropatterned aptamer-modified electrodes. Biomaterials 2012, 33, 7347–7355. [Google Scholar] [CrossRef] [PubMed]
- Russo Krauss, I.; Merlino, A.; Randazzo, A.; Novellino, E.; Mazzarella, L.; Sica, F. High-resolution structures of two complexes between thrombin and thrombin-binding aptamer shed light on the role of cations in the aptamer inhibitory activity. Nucleic Acids Res. 2012, 40, 8119–8128. [Google Scholar] [CrossRef] [PubMed]
- Dolot, R.; Lam, C.H.; Sierant, M.; Zhao, Q.; Liu, F.-W.; Nawrot, B.; Egli, M.; Yang, X. Crystal structures of thrombin in complex with chemically modified thrombin DNA aptamers reveal the origins of enhanced affinity. Nucleic Acids Res. 2018, 46, 4819–4830. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Bae, S.C. Correlation between circulating VEGF levels and disease activity in rheumatoid arthritis: A meta-analysis. Z. Rheumatol. 2018, 77, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Fromm, S.; Cunningham, C.C.; Dunne, M.R.; Veale, D.J.; Fearon, U.; Wade, S.M. Enhanced angiogenic function in response to fibroblasts from psoriatic arthritis synovium compared to rheumatoid arthritis. Arthritis Res. Ther. 2019, 21. [Google Scholar] [CrossRef]
- Supuran, C.T. Agents for the prevention and treatment of age-related macular degeneration and macular edema: A literature and patent review. Expert Opin. Ther. Pat. 2019, 29, 761–767. [Google Scholar] [CrossRef]
- Dehghani, S.; Nosrati, R.; Yousefi, M.; Nezami, A.; Soltani, F.; Taghdisi, S.M.; Abnous, K.; Alibolandi, M.; Ramezani, M. Aptamer-based biosensors and nanosensors for the detection of vascular endothelial growth factor (VEGF): A review. Biosens. Bioelectron. 2018, 110, 23–37. [Google Scholar] [CrossRef]
- Tanaka, Y.; Ohira, T. Mechanisms and therapeutic targets for bone damage in rheumatoid arthritis, in particular the RANK-RANKL system. Curr. Opin. Pharmacol. 2018, 40, 110–119. [Google Scholar] [CrossRef]
- Yang, H.; Liu, W.; Zhou, X.; Rui, H.; Zhang, H.; Liu, R. The association between RANK, RANKL and OPG gene polymorphisms and the risk of rheumatoid arthritis: A case-controlled study and meta-analysis. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef]
- Takayanagi, H.; Ogasawara, K.; Hida, S.; Chiba, T.; Murata, S.; Sato, K.; Takaoka, A.; Yokochi, T.; Oda, H.; Tanaka, K.; et al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-γ. Nature 2000, 408, 600–605. [Google Scholar] [CrossRef]
- Komatsu, N.; Okamoto, K.; Sawa, S.; Nakashima, T.; Oh-hora, M.; Kodama, T.; Tanaka, S.; Bluestone, J.A.; Takayanagi, H. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat. Med. 2014, 20, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Itonaga, I.; Fujikawa, Y.; Sabokbar, A.; Murray, D.W.; Athanasou, N.A. Rheumatoid arthritis synovial macrophage-osteoclast differentiation is osteoprotegerin ligand-dependent. J. Pathol. 2000, 192, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Danks, L. Synovial macrophage-osteoclast differentiation in inflammatory arthritis. Ann. Rheum. Dis. 2002, 61, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Cummings, S.R.; Martin, J.S.; McClung, M.R.; Siris, E.S.; Eastell, R.; Reid, I.R.; Delmas, P.; Zoog, H.B.; Austin, M.; Wang, A.; et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N. Engl. J. Med. 2009, 361, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Henry, D.H.; Costa, L.; Goldwasser, F.; Hirsh, V.; Hungria, V.; Prausova, J.; Scagliotti, G.V.; Sleeboom, H.; Spencer, A.; Vadhan-Raj, S.; et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J. Clin. Oncol. 2011, 29, 1125–1132. [Google Scholar] [CrossRef]
- LIU, W.; ZHANG, X. Receptor activator of nuclear factor-κB ligand (RANKL)/RANK/osteoprotegerin system in bone and other tissues (Review). Mol. Med. Rep. 2015, 11, 3212–3218. [Google Scholar] [CrossRef]
- van Hamburg, J.P.; Tas, S.W. Molecular mechanisms underpinning T helper 17 cell heterogeneity and functions in rheumatoid arthritis. J. Autoimmun. 2018, 87, 69–81. [Google Scholar] [CrossRef]
- DeLay, M.L.; Turner, M.J.; Klenk, E.I.; Smith, J.A.; Sowders, D.P.; Colbert, R.A. HLA-B27 misfolding and the unfolded protein response augment interleukin-23 production and are associated with Th17 activation in transgenic rats. Arthritis Rheum. 2009, 60, 2633–2643. [Google Scholar] [CrossRef]
- Kenna, T.J.; Davidson, S.I.; Duan, R.; Bradbury, L.A.; McFarlane, J.; Smith, M.; Weedon, H.; Street, S.; Thomas, R.; Thomas, G.P.; et al. Enrichment of circulating interleukin-17-secreting interleukin-23 receptor-positive γ/δ T cells in patients with active ankylosing spondylitis. Arthritis Rheum. 2012, 64, 1420–1429. [Google Scholar] [CrossRef]
- Sato, K.; Suematsu, A.; Okamoto, K.; Yamaguchi, A.; Morishita, Y.; Kadono, Y.; Tanaka, S.; Kodama, T.; Akira, S.; Iwakura, Y.; et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J. Exp. Med. 2006, 203, 2673–2682. [Google Scholar] [CrossRef]
- Schett, G.; Lories, R.J.; D’Agostino, M.-A.; Elewaut, D.; Kirkham, B.; Soriano, E.R.; McGonagle, D. Enthesitis: From pathophysiology to treatment. Nat. Rev. Rheumatol. 2017, 13, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Appel, H.; Maier, R.; Wu, P.; Scheer, R.; Hempfing, A.; Kayser, R.; Thiel, A.; Radbruch, A.; Loddenkemper, C.; Sieper, J. Analysis of IL-17+ cells in facet joints of patients with spondyloarthritis suggests that the innate immune pathway might be of greater relevance than the Th17-mediated adaptive immune response. Arthritis Res. Ther. 2011, 13, R95. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.; Baraliakos, X.; Deodhar, A.; Baeten, D.; Sieper, J.; Emery, P.; Readie, A.; Martin, R.; Mpofu, S.; Richards, H.B. Effect of secukinumab on clinical and radiographic outcomes in ankylosing spondylitis: 2-year results from the randomised phase III MEASURE 1 study. Ann. Rheum. Dis. 2017, 76, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Choy, E. Understanding the dynamics: Pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology 2012, 51, v3–v11. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, P.C.; Behrmann, I.; Haan, S.; Hermanns, H.M.; Müller-Newen, G.; Schaper, F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003, 374, 1–20. [Google Scholar] [CrossRef]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta Mol. Cell Res. 2011, 1813, 878–888. [Google Scholar] [CrossRef]
- Taga, T.; Kishimoto, T. gp130 and the interleukin-6 family of cytokines. Annu. Rev. Immunol. 1997, 15, 797–819. [Google Scholar] [CrossRef]
- Jostock, T.; Müllberg, J.; Özbek, S.; Atreya, R.; Blinn, G.; Voltz, N.; Fischer, M.; Neurath, M.F.; Rose-John, S. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur. J. Biochem. 2001, 268, 160–167. [Google Scholar] [CrossRef]
- Kishimoto, T. The biology of interleukin-6. Blood 1989, 74, 1–10. [Google Scholar]
- Heinrich, P.C.; Castell, J.V.; Andus, T. Interleukin-6 and the acute phase response. Biochem. J. 1990, 265, 621–636. [Google Scholar] [CrossRef]
- Wu, Q.; Zhou, X.; Huang, D.; JI, Y.; Kang, F. IL-6 Enhances osteocyte-mediated osteoclastogenesis by promoting JAK2 and RANKL activity in vitro. Cell. Physiol. Biochem. 2017, 41, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- McGregor, N.E.; Murat, M.; Elango, J.; Poulton, I.J.; Walker, E.C.; Crimeen-Irwin, B.; Ho, P.W.M.; Gooi, J.H.; Martin, T.J.; Sims, N.A. IL-6 exhibits both cis- and trans-signaling in osteocytes and osteoblasts, but only trans -signaling promotes bone formation and osteoclastogenesis. J. Biol. Chem. 2019, 294, 7850–7863. [Google Scholar] [CrossRef] [PubMed]
- Karateev, D.E.; Luchikhina, E.L. New possibilities of drug therapy for rheumatoid arthritis: Focus at sarilumab. Alm. Clin. Med. 2019, 47, 461–469. [Google Scholar] [CrossRef]
- Hirota, M.; Murakami, I.; Ishikawa, Y.; Suzuki, T.; Sumida, S.I.; Ibaragi, S.; Kasai, H.; Horai, N.; Drolet, D.W.; Gupta, S.; et al. Chemically modified interleukin-6 aptamer inhibits development of collagen-induced arthritis in cynomolgus monkeys. Nucleic Acid Ther. 2016, 26, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Kruspe, S.; Meyer, C.; Hahn, U. Chlorin e6 conjugated Interleukin-6 receptor aptamers selectively kill target cells upon irradiation. Mol. Ther. Nucleic Acids 2014, 3, e143. [Google Scholar] [CrossRef] [PubMed]
- Prisner, L.; Bohn, N.; Hahn, U.; Mews, A. Size dependent targeted delivery of gold nanoparticles modified with the IL-6R-specific aptamer AIR-3A to IL-6R-carrying cells. Nanoscale 2017, 9, 14486–14498. [Google Scholar] [CrossRef]
- Hahn, U. Charomers—Interleukin-6 receptor specific aptamers for cellular internalization and targeted drug delivery. Int. J. Mol. Sci. 2017, 18, 2641. [Google Scholar] [CrossRef]
- Novikov, A.A.; Aleksandrova, E.N.; Diatroptova, M.A.; Nasonov, E.L. Role of cytokines in the pathogenesis of rheumatoid arthritis. Rheumatol. Sci. Pract. 2010, 71. [Google Scholar] [CrossRef]
- Tan, Z.Y.; Bealgey, K.W.; Fang, Y.; Gong, Y.M.; Bao, S. Interleukin-23: Immunological roles and clinical implications. Int. J. Biochem. Cell Biol. 2009, 41, 733–735. [Google Scholar] [CrossRef]
- Lee, Y.; Awasthi, A.; Yosef, N.; Quintana, F.J.; Xiao, S.; Peters, A.; Wu, C.; Kleinewietfeld, M.; Kunder, S.; Hafler, D.A.; et al. Induction and molecular signature of pathogenic TH17 cells. Nat. Immunol. 2012, 13, 991–999. [Google Scholar] [CrossRef]
- Tang, C.; Chen, S.; Qian, H.; Huang, W. Interleukin-23: As a drug target for autoimmune inflammatory diseases. Immunology 2012, 135, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Giese, T.; Ludwig, B.; Mueller-Molaian, I.; Marth, T.; Zeuzem, S.; Meuer, S.C.; Stallmach, A. Expression of interleukin-12-related cytokine transcripts in inflammatory bowel disease: Elevated interleukin-23p19 and interleukin-27p28 in Crohn’s disease but not in ulcerative colitis. Inflamm. Bowel Dis. 2005, 11, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Cici, D.; Corrado, A.; Rotondo, C.; Cantatore, F.P. Wnt Signaling and biological therapy in rheumatoid arthritis and spondyloarthritis. Int. J. Mol. Sci. 2019, 20, 5552. [Google Scholar] [CrossRef]
- Saito-Diaz, K.; Chen, T.W.; Wang, X.; Thorne, C.A.; Wallace, H.A.; Page-McCaw, A.; Lee, E. The way Wnt works: Components and mechanism. Growth Factors 2013, 31, 1–31. [Google Scholar] [CrossRef]
- Maruotti, N.; Corrado, A.; Neve, A.; Cantatore, F.P. Systemic effects of Wnt signaling. J. Cell. Physiol. 2013, 228, 1428–1432. [Google Scholar] [CrossRef]
- Uluçkan, Ö.; Jimenez, M.; Karbach, S.; Jeschke, A.; Graña, O.; Keller, J.; Busse, B.; Croxford, A.L.; Finzel, S.; Koenders, M.; et al. Chronic skin inflammation leads to bone loss by IL-17–mediated inhibition of Wnt signaling in osteoblasts. Sci. Transl. Med. 2016, 8, 330ra37. [Google Scholar] [CrossRef]
- Heiland, G.R.; Zwerina, K.; Baum, W.; Kireva, T.; Distler, J.H.; Grisanti, M.; Asuncion, F.; Li, X.; Ominsky, M.; Richards, W.; et al. Neutralisation of Dkk-1 protects from systemic bone loss during inflammation and reduces sclerostin expression. Ann. Rheum. Dis. 2010, 69, 2152–2159. [Google Scholar] [CrossRef]
- Lim, S.Y.; Bolster, M. Profile of romosozumab and its potential in the management of osteoporosis. Drug Des. Devel. Ther. 2017, 11, 1221–1231. [Google Scholar] [CrossRef]
- McClung, M.R.; Grauer, A.; Boonen, S.; Bolognese, M.A.; Brown, J.P.; Diez-Perez, A.; Langdahl, B.L.; Reginster, J.-Y.; Zanchetta, J.R.; Wasserman, S.M.; et al. Romosozumab in postmenopausal women with low bone mineral density. N. Engl. J. Med. 2014, 370, 412–420. [Google Scholar] [CrossRef]
- Cosman, F.; Crittenden, D.B.; Ferrari, S.; Khan, A.; Lane, N.E.; Lippuner, K.; Matsumoto, T.; Milmont, C.E.; Libanati, C.; Grauer, A. FRAME Study: The foundation effect of building bone with 1 year of romosozumab leads to continued lower fracture risk after transition to denosumab. J. Bone Miner. Res. 2018, 33, 1219–1226. [Google Scholar] [CrossRef]
- McClung, M.R.; Brown, J.P.; Diez-Perez, A.; Resch, H.; Caminis, J.; Meisner, P.; Bolognese, M.A.; Goemaere, S.; Bone, H.G.; Zanchetta, J.R.; et al. Effects of 24 months of treatment with romosozumab followed by 12 months of denosumab or placebo in postmenopausal women with low bone mineral density: A randomized, double-blind, phase 2, parallel group study. J. Bone Miner. Res. 2018, 33, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Grebennikova, T.A.; Belaya, Z.E.; Rozhinskaya, L.Y.; Melnichenko, G.A. The canonical Wnt/β-catenin pathway: From the history of its discovery to clinical application. Ter. Arkh. 2016, 88, 74. [Google Scholar] [CrossRef] [PubMed]
- Pietrzyk, B.; Smertka, M.; Chudek, J. Sclerostin: Intracellular mechanisms of action and its role in the pathogenesis of skeletal and vascular disorders. Adv. Clin. Exp. Med. 2017, 26, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Poole, K.E.S.; Van Bezooijen, R.L.; Loveridge, N.; Hamersma, H.; Papapoulos, S.E.; Löwik, C.W.; Reeve, J. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005, 19, 1842–1844. [Google Scholar] [CrossRef]
- Xiong, J.; Piemontese, M.; Onal, M.; Campbell, J.; Goellner, J.J.; Dusevich, V.; Bonewald, L.; Manolagas, S.C.; O’Brien, C.A. Osteocytes, not osteoblasts or lining cells, are the main source of the RANKL required for osteoclast formation in remodeling bone. PLoS ONE 2015, 10, e0138189. [Google Scholar] [CrossRef]
- Singh, A.; Gupta, M.K.; Mishra, S.P. Study of correlation of level of expression of Wnt signaling pathway inhibitors sclerostin and dickkopf-1 with disease activity and severity in rheumatoid arthritis patients. Drug Discov. Ther. 2019, 13, 22–27. [Google Scholar] [CrossRef]
- Brandenburg, V.M.; D’Haese, P.; Deck, A.; Mekahli, D.; Meijers, B.; Neven, E.; Evenepoel, P. From skeletal to cardiovascular disease in 12 steps—The evolution of sclerostin as a major player in CKD-MBD. Pediatr. Nephrol. 2016, 31, 195–206. [Google Scholar] [CrossRef]
- Diarra, D.; Stolina, M.; Polzer, K.; Zwerina, J.; Ominsky, M.S.; Dwyer, D.; Korb, A.; Smolen, J.; Hoffmann, M.; Scheinecker, C.; et al. Dickkopf-1 is a master regulator of joint remodeling. Nat. Med. 2007, 13, 156–163. [Google Scholar] [CrossRef]
- Daoussis, D.; Liossis, S.N.C.; Solomou, E.E.; Tsanaktsi, A.; Bounia, K.; Karampetsou, M.; Yiannopoulos, G.; Andonopoulos, A.P. Evidence that Dkk-1 is dysfunctional in ankylosing spondylitis. Arthritis Rheum. 2010, 62, 150–158. [Google Scholar] [CrossRef]
- Klingberg, E.; Nurkkala, M.; Carlsten, H.; Forsblad-D’Elia, H. Biomarkers of bone metabolism in ankylosing spondylitis in relation to osteoproliferation and osteoporosis. J. Rheumatol. 2014, 41, 1349–1356. [Google Scholar] [CrossRef]
- Gatti, D.; Viapiana, O.; Idolazzi, L.; Fracassi, E.; Ionescu, C.; Dartizio, C.; Troplini, S.; Kunnathully, V.; Adami, S.; Rossini, M. Distinct effect of zoledronate and clodronate on circulating levels of DKK1 and sclerostin in women with postmenopausal osteoporosis. Bone 2014, 67, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Gatti, D.; Viapiana, O.; Fracassi, E.; Idolazzi, L.; Dartizio, C.; Povino, M.R.; Adami, S.; Rossini, M. Sclerostin and DKK1 in postmenopausal osteoporosis treated with denosumab. J. Bone Miner. Res. 2012, 27, 2259–2263. [Google Scholar] [CrossRef] [PubMed]
- Tai, N.; Inoue, D. Anti-Dickkopf1 (Dkk1) antibody as a bone anabolic agent for the treatment of osteoporosis. Clin. Calcium 2014, 24, 75–83. [Google Scholar] [PubMed]
- Szentpétery, Á.; Horváth, Á.; Gulyás, K.; Pethö, Z.; Bhattoa, H.P.; Szántó, S.; Szücs, G.; FitzGerald, O.; Schett, G.; Szekanecz, Z. Effects of targeted therapies on the bone in arthritides. Autoimmun. Rev. 2017, 16, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Ramazani, Y.; Knops, N.; Elmonem, M.A.; Nguyen, T.Q.; Arcolino, F.O.; van den Heuvel, L.; Levtchenko, E.; Kuypers, D.; Goldschmeding, R. Connective tissue growth factor (CTGF) from basics to clinics. Matrix Biol. 2018, 68–69, 44–66. [Google Scholar] [CrossRef]
- Rosenbloom, J.; Macarak, E.; Piera-Velazquez, S.; Jimenez, S.A. Human fibrotic diseases: Current challenges in fibrosis research. In Methods in Molecular Biology; Fibrosis, R.L., Ed.; Humana Press: New York, NY, USA, 2017; Volume 1627. [Google Scholar] [CrossRef]
- Varga, J.; Trojanowska, M.; Kuwana, M. Pathogenesis of systemic sclerosis: Recent insights of molecular and cellular mechanisms and therapeutic opportunities. J. Scleroderma Relat. Disord. 2017, 2, 137–152. [Google Scholar] [CrossRef]
- Makino, K.; Makino, T.; Stawski, L.; Lipson, K.E.; Leask, A.; Trojanowska, M. Anti-connective tissue growth factor (CTGF/CCN2) monoclonal antibody attenuates skin fibrosis in mice models of systemic sclerosis. Arthritis Res. Ther. 2017, 19, 134. [Google Scholar] [CrossRef]
- Parapuram, S.K.; Shi-wen, X.; Elliott, C.; Welch, I.D.; Jones, H.; Baron, M.; Denton, C.P.; Abraham, D.J.; Leask, A. Loss of PTEN expression by dermal fibroblasts causes skin fibrosis. J. Invest. Dermatol. 2011, 131, 1996–2003. [Google Scholar] [CrossRef]
- Agnholt, J.; Kelsen, J.; Schack, L.; Hvas, C.L.; Dahlerup, J.F.; Sørensen, E.S. Osteopontin, a protein with cytokine-like properties, is associated with inflammation in Crohn’s disease. Scand. J. Immunol. 2007, 65, 453–460. [Google Scholar] [CrossRef]
- Sodek, J.; Ganss, B.; McKee, M.D. Osteopontin. Crit. Rev. Oral Biol. Med. 2000, 11, 279–303. [Google Scholar] [CrossRef]
- Ishijima, M.; Rittling, S.R.; Yamashita, T.; Tsuji, K.; Kurosawa, H.; Nifuji, A.; Denhardt, D.T.; Noda, M. Enhancement of osteoclastic bone resorption and suppression of osteoblastic bone formation in response to reduced mechanical stress do not occur in the absence of osteopontin. J. Exp. Med. 2001, 193, 399–404. [Google Scholar] [CrossRef]
- Thurner, P.J.; Chen, C.G.; Ionova-Martin, S.; Sun, L.; Harman, A.; Porter, A.; Ager, J.W.; Ritchie, R.O.; Alliston, T. Osteopontin deficiency increases bone fragility but preserves bone mass. Bone 2010, 46, 1564–1573. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; He, R.; Zou, L.; Meng, J. [Clinical value of biomarkers in diagnosis and treatment of idiopathic pulmonary fibrosis]. Nan Fang Yi Ke Da Xue Xue Bao 2020, 40, 1062–1065. [Google Scholar] [CrossRef]
- Wu, P.-M.; Lin, C.-H.; Lee, H.-T.; Shih, H.-I.; Huang, C.-C.; Tu, Y.-F. Early blood biomarkers distinguish inflammation from neonatal hypoxic-ischemia encephalopathy. Neurochem. Res. 2020. [Google Scholar] [CrossRef]
- Yazici, O.; Dogan, M.; Ozal, G.; Aktas, S.H.; Demirkazik, A.; Utkan, G.; Senler, F.C.; Icli, F.; Akbulut, H. Osteopontin is a prognostic factor in patients with advanced gastric cancer. Comb. Chem. High. Throughput Screen. 2020, 23. [Google Scholar] [CrossRef]
- Smith, E.A.; Krumpelbeck, E.F.; Jegga, A.G.; Prell, M.; Matrka, M.M.; Kappes, F.; Greis, K.D.; Ali, A.M.; Meetei, A.R.; Wells, S.I. The nuclear DEK interactome supports multi-functionality. Proteins Struct. Funct. Bioinforma. 2018, 86, 88–97. [Google Scholar] [CrossRef]
- Dong, X.; Wang, J.; Kabir, F.N.; Shaw, M.; Reed, A.M.; Stein, L.; Andrade, L.E.C.; Trevisani, V.F.M.; Miller, M.L.; Fujii, T.; et al. Autoantibodies to DEK oncoprotein in human inflammatory disease. Arthritis Rheum. 2000, 43, 85–93. [Google Scholar] [CrossRef]
- Audrito, V.; Messana, V.G.; Deaglio, S. NAMPT and NAPRT: Two metabolic enzymes with key roles in inflammation. Front. Oncol. 2020, 10, 1–17. [Google Scholar] [CrossRef]
- Franco-Trepat, E.; Alonso-Pérez, A.; Guillán-Fresco, M.; Jorge-Mora, A.; Gualillo, O.; Gómez-Reino, J.J.; Gómez Bahamonde, R. Visfatin as a therapeutic target for rheumatoid arthritis. Expert Opin. Ther. Targets 2019, 23, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Vandooren, J.; Van den Steen, P.E.; Opdenakker, G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): The next decade. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 222–272. [Google Scholar] [CrossRef] [PubMed]
- Ram, M.; Sherer, Y.; Shoenfeld, Y. Matrix metalloproteinase-9 and autoimmune diseases. J. Clin. Immunol. 2006, 26, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.P.M.; Bager, C.; Platt, A.; Karsdal, M.; Bay-Jensen, A.-C. Identification of pathological RA endotypes using blood-based biomarkers reflecting tissue metabolism. A retrospective and explorative analysis of two phase III RA studies. PLoS ONE 2019, 14, e0219980. [Google Scholar] [CrossRef]
- Park, S.G.; Jeong, S.U.; Lee, J.H.; Ryu, S.H.; Jeong, H.J.; Sim, Y.J.; Kim, D.K.; Kim, G.C. The changes of ctx, dpd, osteocalcin, and bone mineral density during the postmenopausal period. Ann. Rehabil. Med. 2018, 42, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Banshchikova, N.Y.; Letyagina, Y.A.; Omelchenko, V.O.; Korolev, M.A. Antiresorptive activity of denosumab in the treatment of osteoporosis in patients with rheumatoid arthritis. Osteoporos. Bone Dis. 2018, 21, 4–11. [Google Scholar] [CrossRef]
- Bay-Jensen, A.C.; Platt, A.; Siebuhr, A.S.; Christiansen, C.; Byrjalsen, I.; Karsdal, M.A. Early changes in blood-based joint tissue destruction biomarkers are predictive of response to tocilizumab in the LITHE study. Arthritis Res. Ther. 2016, 18, 13. [Google Scholar] [CrossRef] [PubMed]
- Segal, A.W. How neutrophils kill microbes. Annu. Rev. Immunol. 2005, 23, 197–223. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, B.; Hajjar, E.; Kalupov, T.; Reuter, N.; Brillard-Bourdet, M.; Moreau, T.; Juliano, L.; Gauthier, F. Influence of Charge Distribution at the Active Site Surface on the Substrate Specificity of Human Neutrophil Protease 3 and Elastase. J. Biol. Chem. 2007, 282, 1989–1997. [Google Scholar] [CrossRef]
- Reumaux, D.; Duthilleul, P.; Roos, D. Pathogenesis of diseases associated with antineutrophil cytoplasm autoantibodies. Hum. Immunol. 2004, 65, 1–12. [Google Scholar] [CrossRef]
- Kallenberg, C.G.M. Pathogenesis of PR3-ANCA associated vasculitis. J. Autoimmun. 2008, 30, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Kallenberg, C.G.; Heeringa, P.; Stegeman, C.A. Mechanisms of Disease: Pathogenesis and treatment of ANCA-associated vasculitides. Nat. Clin. Pract. Rheumatol. 2006, 2, 661–670. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Krumbholz, M.; Schönermarck, U.; Back, W.; Gross, W.L.; Werb, Z.; Gröne, H.-J.; Brinkmann, V.; Jenne, D.E. Netting neutrophils in autoimmune small-vessel vasculitis. Nat. Med. 2009, 15, 623–625. [Google Scholar] [CrossRef] [PubMed]
- Petrini, I. Biology of MET: A double life between normal tissue repair and tumor progression. Ann. Transl. Med. 2015, 3. [Google Scholar] [CrossRef]
- Grano, M.; Galimi, F.; Zambonin, G.; Colucci, S.; Cottone, E.; Zallone, A.Z.; Comoglio, P.M. Hepatocyte growth factor is a coupling factor for osteoclasts and osteoblasts in vitro. Proc. Natl. Acad. Sci. USA 1996, 93, 7644–7648. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, M.; Hasegawa, J.; Kato, K.; Yamazaki, J.; Nishigai, K.; Ishiwata, T.; Asano, G.; Yoshino, S. Hepatocyte growth factor (HGF), HGF activator, and c-Met in synovial tissues in rheumatoid arthritis and osteoarthritis. J. Rheumatol. 2001, 28, 1772–1778. [Google Scholar] [PubMed]
- Sugiura, T.; Kawaguchi, Y.; Soejima, M.; Katsumata, Y.; Gono, T.; Baba, S.; Kawamoto, M.; Murakawa, Y.; Yamanaka, H.; Hara, M. Increased HGF and c-Met in muscle tissues of polymyositis and dermatomyositis patients: Beneficial roles of HGF in muscle regeneration. Clin. Immunol. 2010, 136, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Torres, L.; Klingberg, E.; Nurkkala, M.; Carlsten, H.; Forsblad-d’Elia, H. Hepatocyte growth factor is a potential biomarker for osteoproliferation and osteoporosis in ankylosing spondylitis. Osteoporos. Int. 2019, 30, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Navarini, L.; Margiotta, D.P.E.; Vadacca, M.; Afeltra, A. Leptin in autoimmune mechanisms of systemic rheumatic diseases. Cancer Lett. 2018, 423, 139–146. [Google Scholar] [CrossRef]
- Diaz-Rizo, V.; Bonilla-Lara, D.; Gonzalez-Lopez, L.; Sanchez-Mosco, D.; Fajardo-Robledo, N.S.; Perez-Guerrero, E.E.; Rodriguez-Jimenez, N.A.; Saldaña-Cruz, A.M.; Vazquez-Villegas, M.L.; Gomez-Bañuelos, E.; et al. Serum levels of adiponectin and leptin as biomarkers of proteinuria in lupus nephritis. PLoS ONE 2017, 12, e0184056. [Google Scholar] [CrossRef]
- Batún-Garrido, J.A. de J.; Salas-Magaña, M.; Juárez-Rojop, I.E.; Hernández-Núñez, E.; Olán, F. Relación entre las concentraciones de la leptina y la actividad de la enfermedad en pacientes con artritis reumatoide. Med. Clin. 2018, 150, 341–344. [Google Scholar] [CrossRef]
- Abella, V.; Scotece, M.; Conde, J.; Pino, J.; Gonzalez-Gay, M.A.; Gómez-Reino, J.J.; Mera, A.; Lago, F.; Gómez, R.; Gualillo, O. Leptin in the interplay of inflammation, metabolism and immune system disorders. Nat. Rev. Rheumatol. 2017, 13, 100–109. [Google Scholar] [CrossRef]
- Schäffler, A.; Ehling, A.; Neumann, E.; Herfarth, H.; Tarner, I.; Schölmerich, J.; Müller-Ladner, U.; Gay, S. Adipocytokines in synovial fluid. JAMA 2003, 290, 1709–1710. [Google Scholar] [CrossRef] [PubMed]
- Sglunda, O.; Mann, H.; Hulejová, H.; Kuklová, M.; Pecha, O.; Pleštilová, L.; Filková, M.; Pavelka, K.; Vencovský, J.; Šenolt, L. Decreased circulating visfatin is associated with improved disease activity in early rheumatoid arthritis: Data from the PERAC cohort. PLoS ONE 2014, 9, e103495. [Google Scholar] [CrossRef]
- Batún-Garrido, J.A.D.J.; Salas-Magaña, M.; Juárez-Rojop, I.E. Association between leptin and IL-6 concentrations with cardiovascular risk in patients with rheumatoid arthritis. Clin. Rheumatol. 2018, 37, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Stawski, L.; Trojanowska, M. Oncostatin M and its role in fibrosis. Connect. Tissue Res. 2019, 60, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Rajashekhar, G.; Willuweit, A.; Patterson, C.E.; Sun, P.; Hilbig, A.; Breier, G.; Helisch, A.; Clauss, M. Continuous endothelial cell activation increases angiogenesis: Evidence for the direct role of endothelium linking angiogenesis and inflammation. J. Vasc. Res. 2006, 43, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Hermanns, H.M. Oncostatin M and interleukin-31: Cytokines, receptors, signal transduction and physiology. Cytokine Growth Factor Rev. 2015, 26, 545–558. [Google Scholar] [CrossRef]
- West, N.R.; Hegazy, A.N.; Owens, B.M.J.; Bullers, S.J.; Linggi, B.; Buonocore, S.; Coccia, M.; Görtz, D.; This, S.; Stockenhuber, K.; et al. Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor–neutralizing therapy in patients with inflammatory bowel disease. Nat. Med. 2017, 23, 579–589. [Google Scholar] [CrossRef]
- McGarry, T.; Orr, C.; Wade, S.; Biniecka, M.; Wade, S.; Gallagher, L.; Low, C.; Veale, D.J.; Fearon, U. JAK/STAT Blockade alters synovial bioenergetics, mitochondrial function, and proinflammatory mediators in rheumatoid arthritis. Arthritis Rheumatol. 2018, 70, 1959–1970. [Google Scholar] [CrossRef]
- Fearon, U.; Mullan, R.; Markham, T.; Connolly, M.; Sullivan, S.; Poole, A.R.; FitzGerald, O.; Bresnihan, B.; Veale, D.J. Oncostatin M induces angiogenesis and cartilage degradation in rheumatoid arthritis synovial tissue and human cartilage cocultures. Arthritis Rheum. 2006, 54, 3152–3162. [Google Scholar] [CrossRef]
- Langdon, C.; Kerr, C.; Hassen, M.; Hara, T.; Arsenault, A.L.; Richards, C.D. Murine Oncostatin M stimulates mouse synovial fibroblasts in vitro and induces inflammation and destruction in mouse joints in vivo. Am. J. Pathol. 2000, 157, 1187–1196. [Google Scholar] [CrossRef]
- Hanlon, M.M.; Rakovich, T.; Cunningham, C.C.; Ansboro, S.; Veale, D.J.; Fearon, U.; McGarry, T. STAT3 mediates the differential effects of oncostatin M and TNFα on RA synovial fibroblast and endothelial cell function. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Ochsner, U.A.; Green, L.S.; Gold, L.; Janjic, N. Systematic selection of modified aptamer pairs for diagnostic sandwich assays. Biotechniques 2014, 56, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhou, J.; Shao, Z.; Liu, J.; Song, J.; Wang, R.; Li, J.; Tan, W. Aptamers as versatile molecular tools for antibody production monitoring and quality control. J. Am. Chem. Soc. 2020, 142, 12079–12086. [Google Scholar] [CrossRef] [PubMed]
- Wildner, S.; Huber, S.; Regl, C.; Huber, C.G.; Lohrig, U.; Gadermaier, G. Aptamers as quality control tool for production, storage and biosimilarity of the anti-CD20 biopharmaceutical rituximab. Sci. Rep. 2019, 9, 1111. [Google Scholar] [CrossRef] [PubMed]
- Kohlberger, M.; Wildner, S.; Regl, C.; Huber, C.G.; Gadermaier, G. Rituximab-specific DNA aptamers are able to selectively recognize heat-treated antibodies. PLoS ONE 2020, 15, e0241560. [Google Scholar] [CrossRef]

| Target | Aptamer, Type, Length | Sequence, 5′-> 3′ | Binding Affinity (KD) | Ref. |
|---|---|---|---|---|
| CRP | RNA 44-mer (RNA, 44 nt) | GCCUGUAAGGUGGUCGGUGUGGCGAGUGUGUUAGGAGAGAUUGC | - | [73] |
| CRP1-1 RNA, 104 nt | GGGCGAAUUCGGGACUUCGAUCCGUAGUACCCACCAGGCAUACACCAGCACGCGGAGCCAAGGAAAAAUAGUAAACUAGCACUCAGUGCUCGUAUGCGGAAGCU | 2.3 nM | [74] | |
| Clone 1 DNA, 71 nt | GGCAGGAAGACAAACACGATGGGGGGTATGATTTGATGTGGTTGTTGCATGATCGTGGTCTGTGGTGCTGT | 3.51 nM | [75] | |
| 6th-62-40, DNA, 52 nt | CGAAGGGGATTCGAGGGGTGATTGCGTGCTCCATTTGGTGTTTTTTTTTTTT | 16.2 nM | [76] | |
| CRP-80-17 DNA, 79 nt | AGCAGCACAGAGGTCAGATGCCCCGCGGGTCGGCTTGCCGTTCCGTTCGGCGCTTCCCCCCTATGCGTGCTACCGTGAA | 3.9 nM | [77] | |
| TNFα | aptTNF-α DNA, 41 nt | GCGCCACTACAGGGGAGCTGCCATTCGAATAGGTGGGCCGC | 8 nM | [78] |
| VR11 DNA, 25 nt | TGGTGGATGGCGCAGTCGGCGACAA | 7 nM | [79] | |
| T3.11.7, 2′-NH2- RNA, 28 nt | GGAGUAUCUGAUGACAAUUCGGAGCUCC | - | [80] | |
| T1-4, DNA, 49 nt | TCCGATCGGTATATCCGTCGGATTTTTTTTTTGGTCACTGCATGTGACC | 67 nM | [81] | |
| VEGF | VEGF Apt 1 DNA, 24 nt | GTGGGGGTGGACGGGCCGGGTAGA | - | [82] |
| VEGF Apt 2 DNA, 26 nt | CAATTGGGCCCGTCCGTATGGTGGGT | - | [82] | |
| RANK | apt1 2′-F-RNA, 46 nt | ACGGAUUCGUCGUAUGGGUGGGAUCGGGAAGGGCUACGAACGCCGU | 0.6 μM | [83] |
| IL-17RA | RA10-6 DNA, 30 nt | CTTGGATCACCATAGTCGCTAGTCGAGGCT | 1.2 nM | [84] |
| IL-17A | Apt21-2 2′-F-RNA, 33 nt | GGUCUAGCCGGAGGAGUCAGUAAUCGGUAGACC | 48.5 nM | [85] |
| IL-17A/F | AptAF42-dope1 2′-F-RNA, 68 nt | GGGCUAGCUGAUCGUACCAGUAGCGUGGCCUGGGGGGCCUAGUCGUGCGAUACUAACAGCUAACACCC | - | [86] |
| IL-6 | SL1025 SOMAmer, 31 nt | GGCAGBnBnPeGGNapABnBnAACACGBnBnAAGBnCGBnGG | 0.19 nM | [87] |
| IL-6R | AIR-3A RNA, 19 nt, | GGGGAGGCUGUGGUGAGGG | 60 nM | [88] |
| FAIR-6 2′-F-RNA, 50 nt | GUAAGUAGUGUAGGCUGUGGGAGUUAUAGGGGUGGAUGUGGAGUGGGGUG | 41 nM | [89] | |
| RAID3 2′-F-RNA, 34 nt | GGGAGAACUGUGGGAGUGGAGGGUGGAUGGUUCU | 43 nM | [90] | |
| IL-23 | RNA (mRfY) 60 nt | AGGGAAAUCAGGCUUUAUCGGCGCCGCUCCCUGUGCCAUCGUCCGAGAGUAGGUAGUCUG | - | [91] |
| IL-32 | AC3-3 RNA, 90 nt | GGGUUCACUGCAGACUUGACGAAGCUUCCGGAGAGAAGGGUCAAAGUUGUGCGGGAGUGUGUUGUGGAAUGGAUCCACAUCUACGAAUUC | 78 nM | [92] |
| IL-8 | 8A-3 52′-F-RNA, 35 nt | GGGGGCUUAUCAUUCCAUUUAGUGUUAUGAUAACC | 1.72 pM | [93] |
| IL-1α | SL1067 SOMAmer, 22 nt | CGNapGAGNapNapANapGGGNapNapAGAGNapCG | 7.3 nM | [94] |
| DKK1 | TD10 DNA, 39 nt | CATATGATTAGGCTGTAACGGGGCTAGGCGGGGATCATT | 25 nM | [95] |
| Sclerostin | Scl DNA, 30 nt | TTGCGCGTTAATTGGGGGGGTGGGTGGGTT | 0.67 μM | [96] |
| CTGF | APT1M6T DNA | not reported | 1.1 nM | [97] |
| C-ap11 DNA, 39 nt | GGACAAGAATCACCGCTCCCCGTACAGGAGGCATACAGA | 7.4 nM | [98] | |
| Osteopontin | OPN-R3 2′-F-RNA, 40 nt | CGGCCACAGAAUGAAAAACCUCAUCGAUGUUGCAUAGUUG | 18 nM | [99] |
| DEK | DTA 64 DNA, 41 nt | GGGGTTAAATATTCCCACATTGCCTGCGCCAGTACAAATAG | - | [100] |
| Visfatin | apt№19 DNA, 75 nt | ATACCAGCTTATTCAATTGGGCAGGACAGGTGTCGGCTTGATAGGCTGGGTGTGTGTAGATAGTAAGTGCAATCT | 72 nM | [101] |
| MMP9 | F3Bomf 2′-F-RNA, 36 nt | UGCCAAACGCGUCCCCUUUGCCCGGCCUCCGCCGCA | 20 nM | [102] |
| 8F14A, DNA, 30 nt | TCGTATGGCACGGGGTTGGTGTTGGGTTGG | - | [103] | |
| CTxI | CTx 2R-2h DNA, 72 nt | ATCCGTCACACCTGCTCTAGACGAATATTGTATCCTCATTAGATCAAAAACGGGTGGTGTTGGCTCCCGTAT | - | [104] |
| HNE | DNA I DNA, 44 nt | TAGCGATACTGCGTGGGTTGGGGCGGGTAGGGCCAGCAGTCTCG | 17 nM | [105] |
| HGF | H38-15 DNA, 59 nt | GCGCCAGCTTTGCTGATGGGTGGCCACCCTTGCCCTGGGTTTGAATTTCGATCCTATCG | 19 nM | [106] |
| Leptin | Lep3 DNA, 40 nt | GTTAATGGGGGATCTCGCGGCCGTTCTTGTTGCTTATACA | 0.3 μM | [107] |
| Oncostatin M | ADR58 2′-F-RNA, 33 nt | GAACCGGCCCAGCAGACUGCUGACGGCACGAUC | 7 nM | [108] |
| Target | Sensor Type | Working Range | Samples | Ref. |
|---|---|---|---|---|
| CRP | SPR | 500–1000 ng/mL | Buffer solution | [73] |
| Square-wave voltammetry | 25–250 pg/mL | 10% spiked serum | [110] | |
| Fluorescent | 10 ng/mL–100 μg/mL | 1% spiked serum | [111] | |
| Electrochemical sandwich assay | 0.1–50 μg/mL | 10% spiked serum | [114] | |
| Fluorescent sandwich-assay | 0.4–10 μg/mL | 1% spiked serum | [116] | |
| Square-wave voltammetry | 0.005–125 ng/mL | 0.2% clinical and spiked serum | [115] | |
| non-Faradaic impedance spectroscopy | 100–500 pg/mL | Buffer solution | [109] | |
| Isotachophoresis with fluorescent detection | - | 5% spiked serum | [117] | |
| Luminescent sandwich-assay | 0.0125–10 μg/mL | Buffer solution | [75] | |
| Field-effect-transistor | 0.625–10 μg/mL | Buffer solution | [118] | |
| SPR | 0.25 ng/mL–2.5 μg/mL | 1% spiked serum | [76] | |
| Fluorescent | 12.5 ng/mL–5 μg/mL | Buffer solution | [119] | |
| Lossy mode resonance | - | Buffer solution | [120] | |
| TNFα | Differential pulse voltammetry | 10 pg/mL–40 μg/mL | 10% clinical serum | [121] |
| Quantum dots-based photoluminescence | 1.7–400 ng/mL | 10% spiked serum | [122] | |
| Aptameric graphene field-effect transistor | - | Buffer solution | [123] | |
| Alternating current voltammetry | 1.75 ng/mL–8.75 μg/mL | Diluted saliva and urine samples | [124] | |
| Square-wave voltammetry | 10–100 ng/mL | Diluted spiked blood | [125] | |
| VEGF | Colorimetric | 100–1 × 105 pg/mL | Clinical serum samples | [126] |
| Chemiluminescent sandwich assay | 1–20 ng/mL | Cell culture medium | [82] | |
| Colorimetric | 0.5–225 pg/mL | 12.5% spiked serum | [127] | |
| Colorimetric | 3.7–148 pg/mL | Buffer solution | [128] | |
| Colorimetric, aptazyme-based | 0.1–40 nM | 1% spiked serum | [129] | |
| Chemiluminescent | - | 10% spiked serum | [130] | |
| pH-Meter based | 0.8–480 pg/mL | 1% serum, centrifuged | [131] | |
| Glucose meter based | 3–100 pg/mL | 10% clinical serum | [132] | |
| IL-17RA | Impedimetric | 10–10,000 pg/mL | 10% spiked serum | [133] |
| IL-6 | Aptameric graphene field-effect transistor | - | Buffer solution | [134] |
| Impedimetric | 5 pg/mL–100 ng/mL | 50% patients’ serum | [135] | |
| Au-NP aptamer-based sandwich-assay | 3.3–125 μg/mL | Buffer solution | [136] | |
| sIL-2Rα | Au-NP colorimetric | 25 ng/mL–2.5 μg/mL | 10% spiked serum | [137] |
| IL-8 | On-chip rolling cycle amplification | 7.5–120 pg/mL | Buffer solution | [138] |
| DKK1 | Aptamer-based ELISA | 62.5–4000 pg/mL | 10% clinical serum | [95] |
| CTGF | Aptamer-based biolayer interferometry ELISA | 1.1–112 ng/mL | 10% spiked serum | [97] |
| Osteopontin | Lateral flow | 10–500 ng/mL | 10% spiked serum | [139] |
| Visfatin | non-Faradaic impedance spectroscopy | 1–50 ng/mL | 20% filtered spiked serum | [101] |
| MMP-9 | Quartz crystal microbalance | 92 pg/mL–230 ng/mL | 2–0.25% spiked serum | [103] |
| CTxI | Fluorescent | - | Buffer solution | [104] |
| HNE | Fluorescent | 1.3 ng/mL–2 μg/mL | Buffer solution | [140] |
| Colorimetric | 31.2 ng/mL–3.1 μg/mL | Buffer solution | [141] | |
| Capillary electrophoresis coupled with laser-induced fluorescence | 15.6 ng/mL–15.6 μg/mL | 1% spiked serum | [142] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shatunova, E.A.; Korolev, M.A.; Omelchenko, V.O.; Kurochkina, Y.D.; Davydova, A.S.; Venyaminova, A.G.; Vorobyeva, M.A. Aptamers for Proteins Associated with Rheumatic Diseases: Progress, Challenges, and Prospects of Diagnostic and Therapeutic Applications. Biomedicines 2020, 8, 527. https://doi.org/10.3390/biomedicines8110527
Shatunova EA, Korolev MA, Omelchenko VO, Kurochkina YD, Davydova AS, Venyaminova AG, Vorobyeva MA. Aptamers for Proteins Associated with Rheumatic Diseases: Progress, Challenges, and Prospects of Diagnostic and Therapeutic Applications. Biomedicines. 2020; 8(11):527. https://doi.org/10.3390/biomedicines8110527
Chicago/Turabian StyleShatunova, Elizaveta A., Maksim A. Korolev, Vitaly O. Omelchenko, Yuliya D. Kurochkina, Anna S. Davydova, Alya G. Venyaminova, and Mariya A. Vorobyeva. 2020. "Aptamers for Proteins Associated with Rheumatic Diseases: Progress, Challenges, and Prospects of Diagnostic and Therapeutic Applications" Biomedicines 8, no. 11: 527. https://doi.org/10.3390/biomedicines8110527
APA StyleShatunova, E. A., Korolev, M. A., Omelchenko, V. O., Kurochkina, Y. D., Davydova, A. S., Venyaminova, A. G., & Vorobyeva, M. A. (2020). Aptamers for Proteins Associated with Rheumatic Diseases: Progress, Challenges, and Prospects of Diagnostic and Therapeutic Applications. Biomedicines, 8(11), 527. https://doi.org/10.3390/biomedicines8110527





