Description, Staging and Quantification of Pulmonary Artery Angiophagy in a Large Animal Model of Chronic Thromboembolic Pulmonary Hypertension
Abstract
1. Introduction
2. Methods
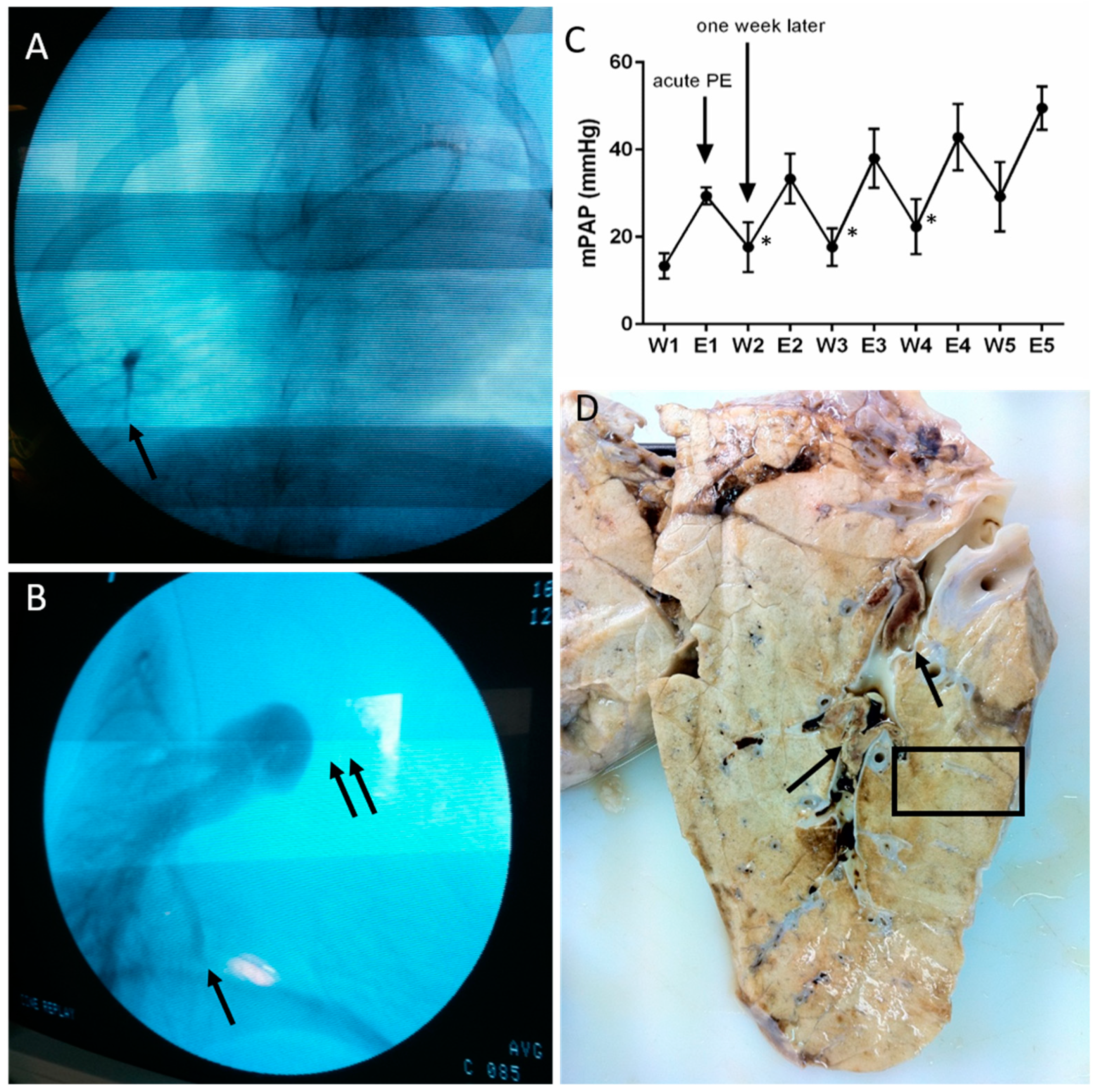
2.1. Animal Model
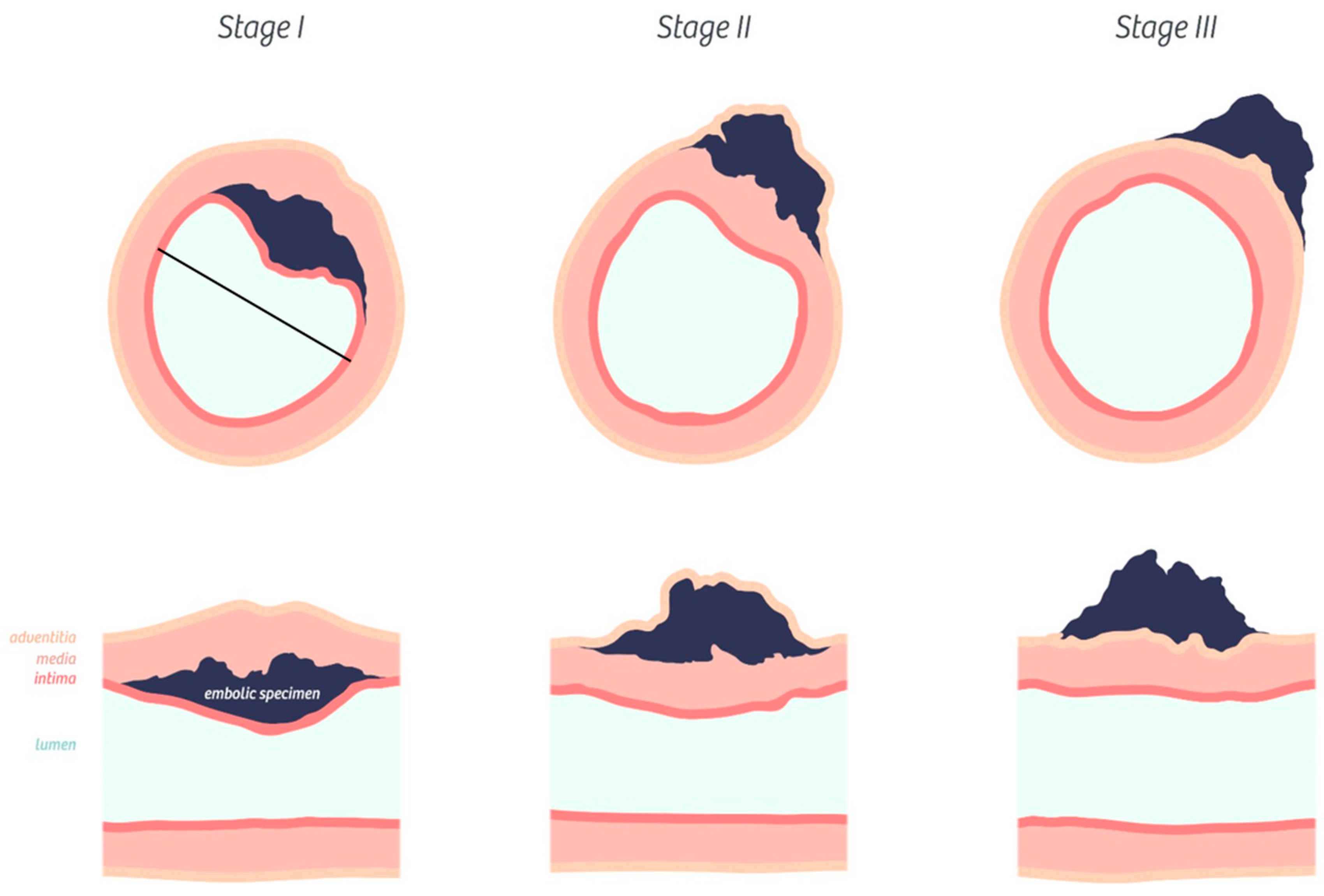
2.2. Histopathological Analysis of Pulmonary Artery Angiophagy in Right Lower Lobe of the CTEPH Model
2.3. Human Lung Sample Analysis
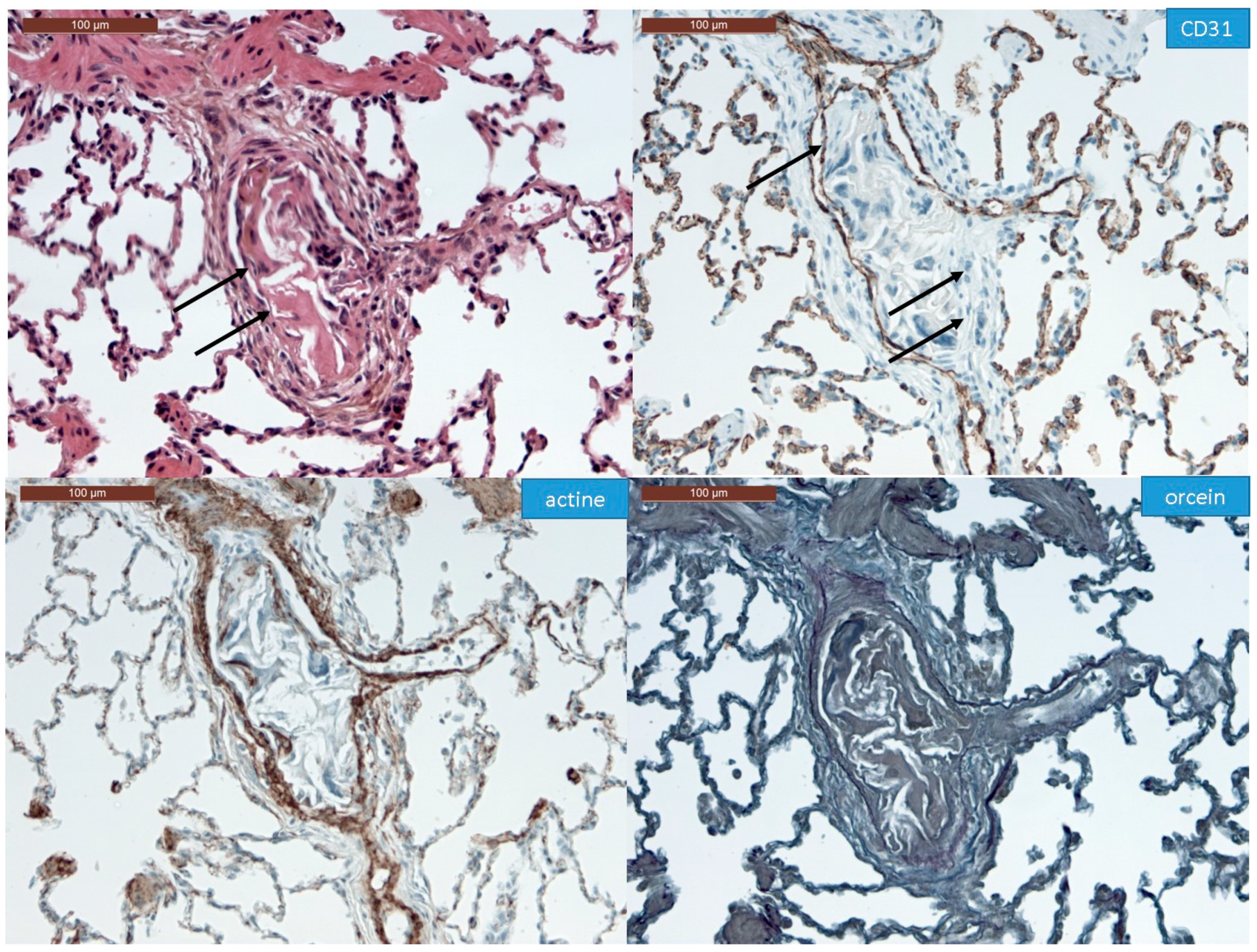
2.4. Immunohistochemistry
2.5. Statistical Analysis
3. Results
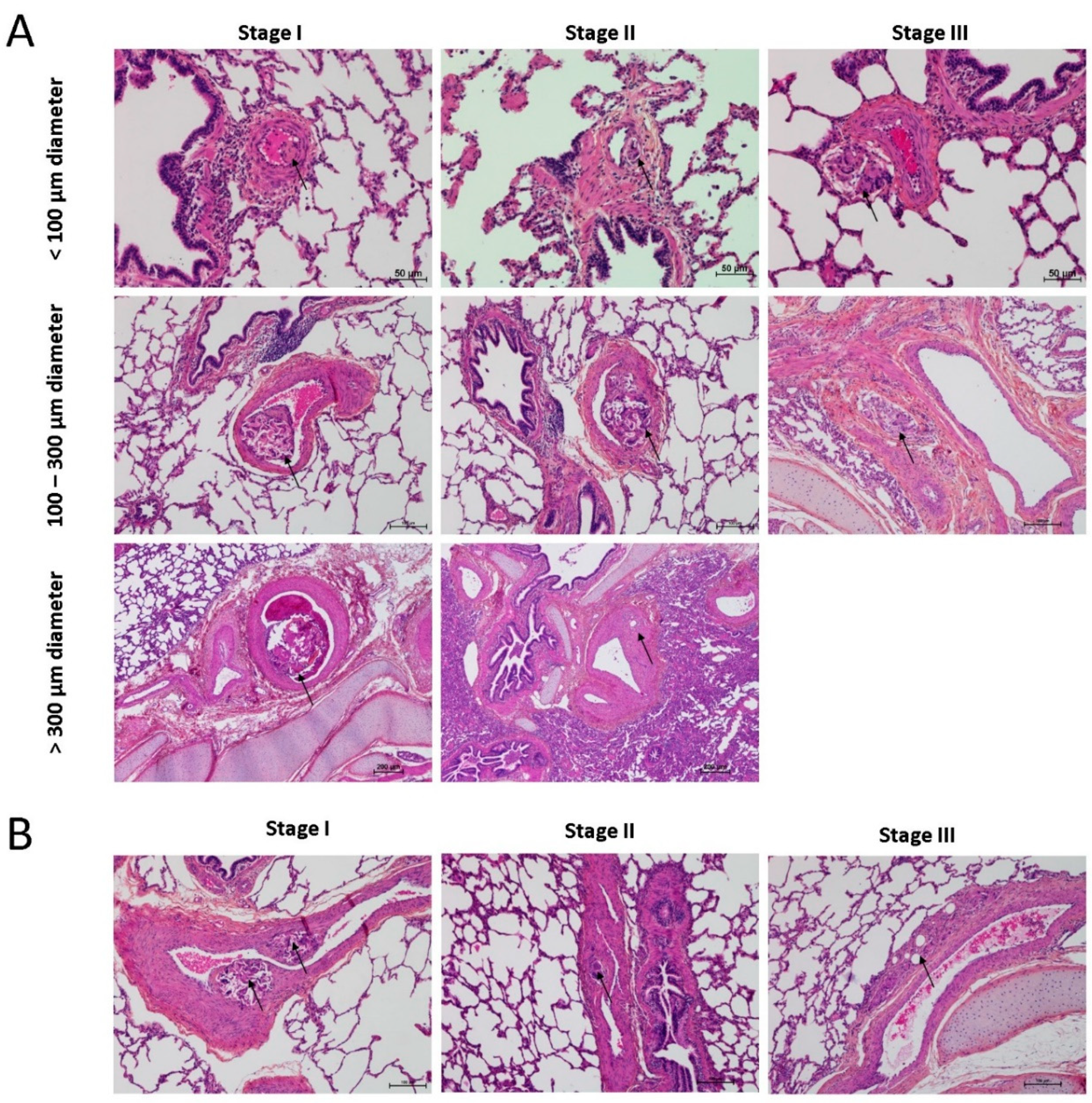
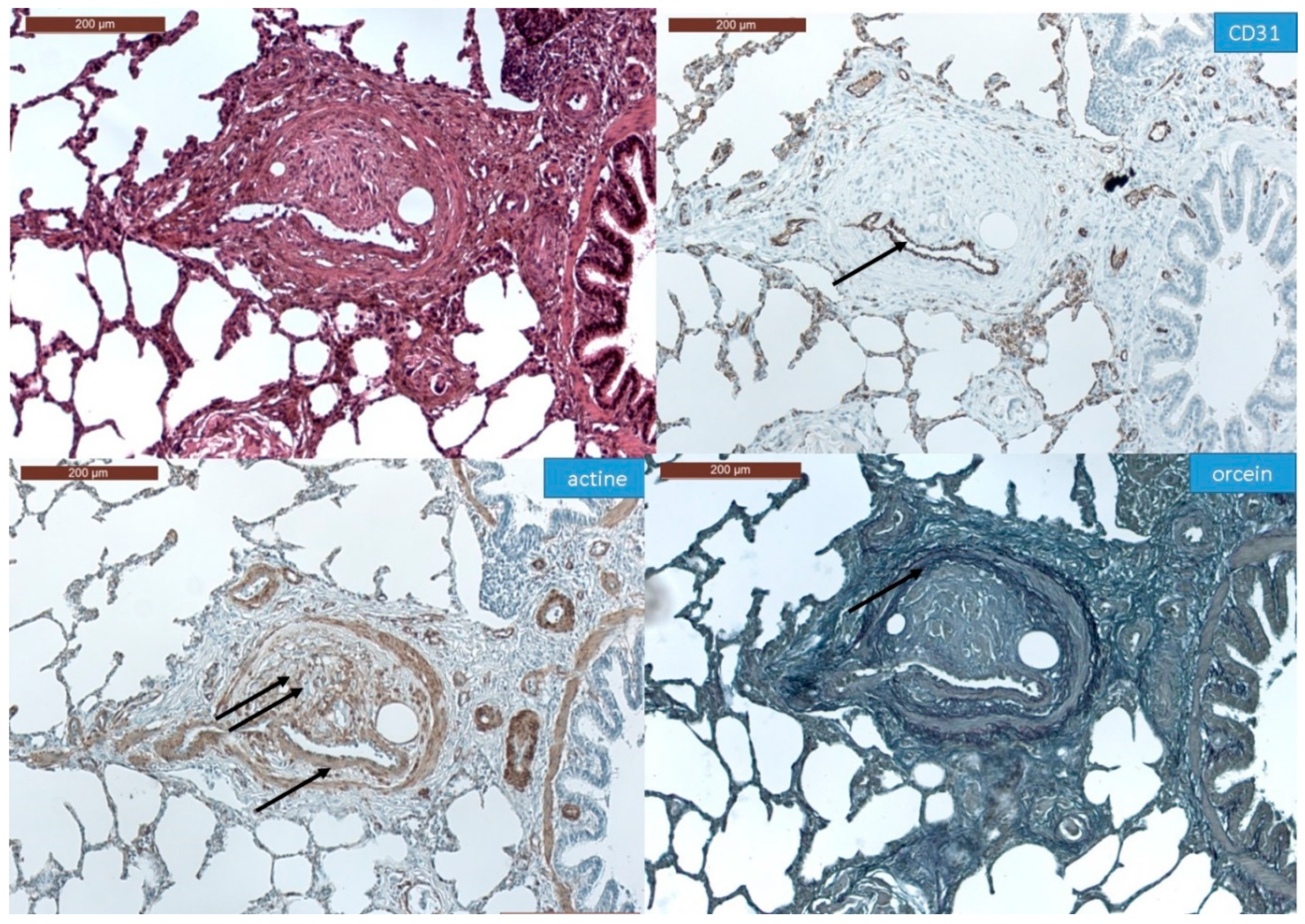
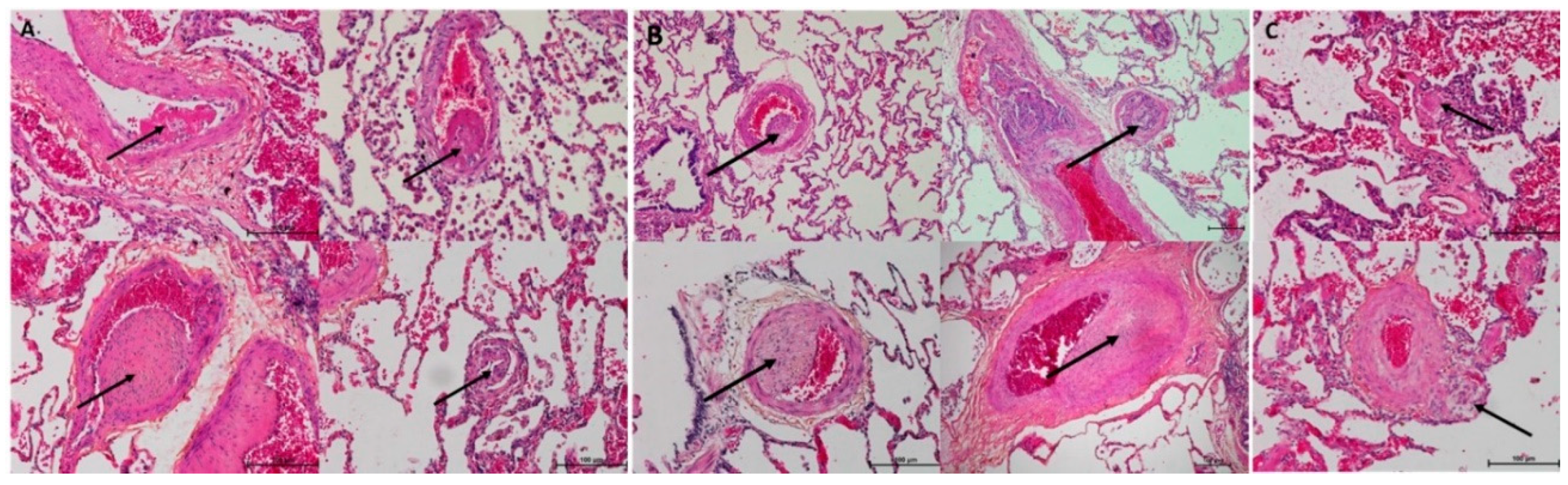
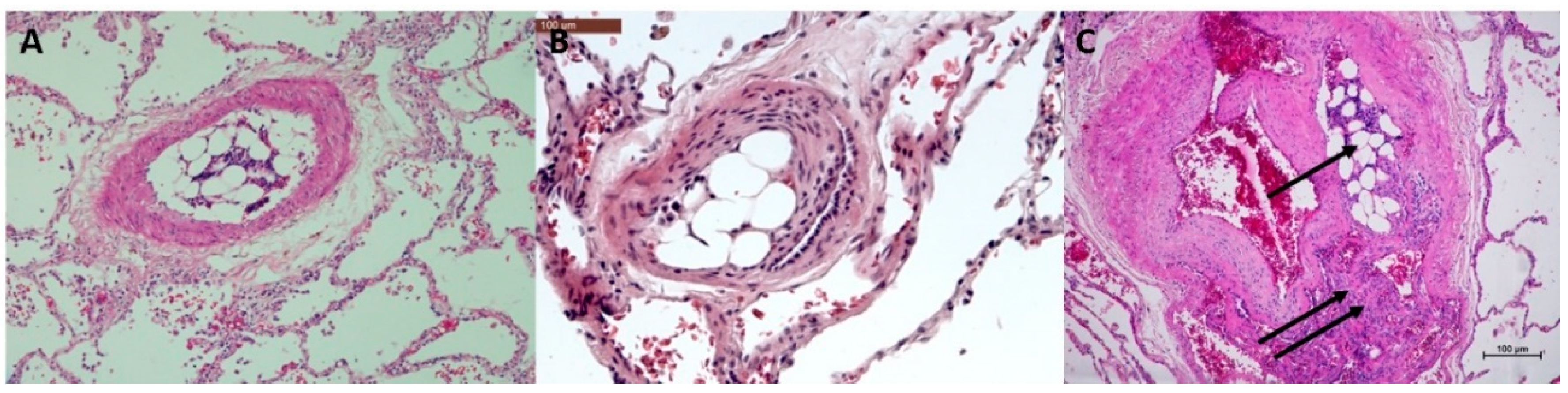
3.1. Histopathological Assessment of Angiophagy in Right Lower Lobes of the CTEPH Animal Model
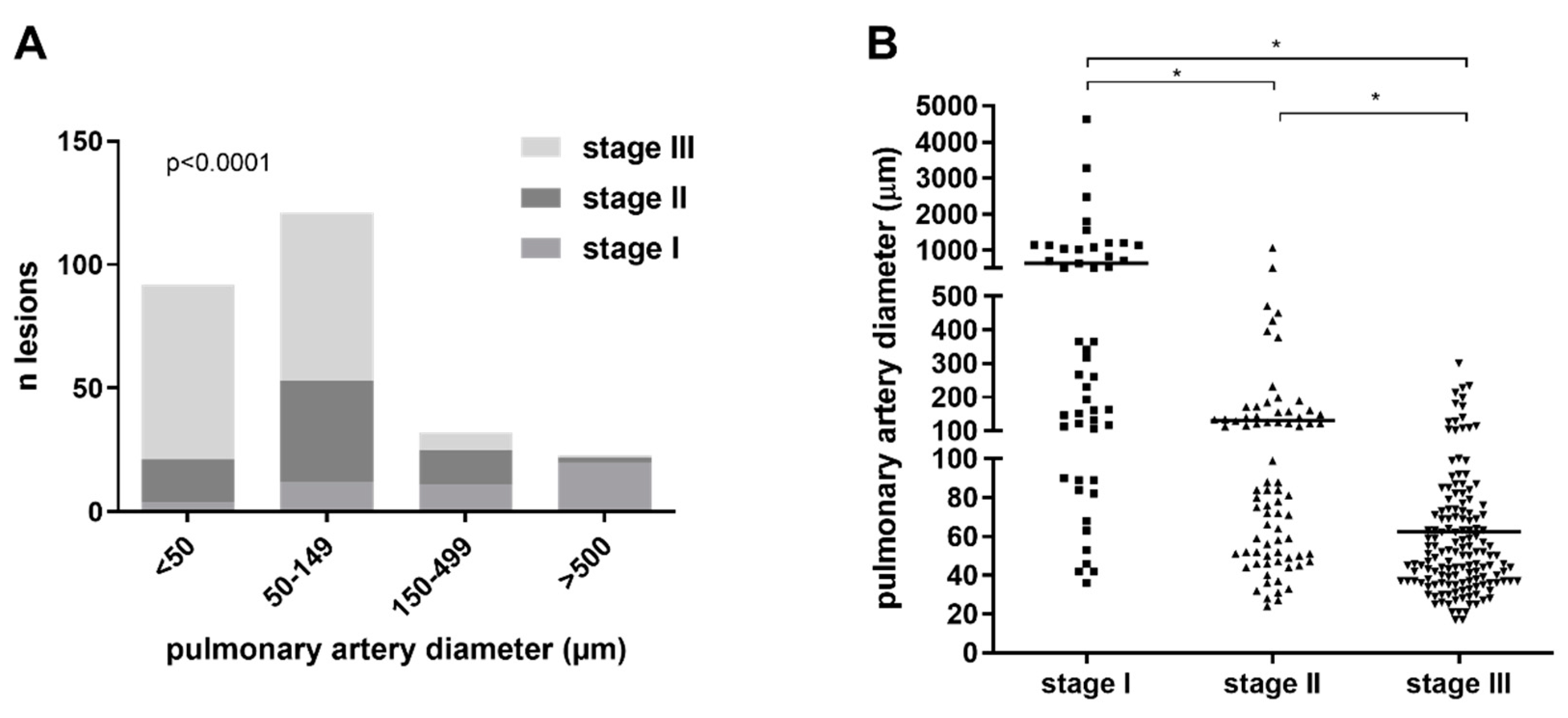
3.2. Quantitative Morphological Analysis of Angiophagy in Right Lower Lobes of the CTEPH Animal Model
3.3. Histopathology of Pulmonary Angiophagy in Human Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nijkeuter, M.; Hovens, M.M.; Davidson, B.L.; Huisman, M.V. Resolution of Thromboemboli in Patients With Acute Pulmonary Embolism. Chest 2006, 129, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Tomashefski, J.F.; Hirsch, C.S. The pulmonary vascular lesions of intravenous drug abuse. Hum. Pathol. 1980, 11, 133–145. [Google Scholar] [CrossRef]
- Tomashefski, J.; Felo, J. The pulmonary pathology of illicit drug and substance abuse. Curr. Diagn. Pathol. 2004, 10, 413–426. [Google Scholar] [CrossRef]
- Alias, S.; Lang, I.M. Coagulation and the Vessel Wall in Pulmonary Embolism. Pulm. Circ. 2013, 3, 728–738. [Google Scholar] [CrossRef]
- Moser, K.M.; Guisan, M.; Bartimmo, E.E.; Longo, A.M.; Harsanyi, P.G.; Chiorazzi, N. In Vivoand Post Mortem Dissolution Rates of Pulmonary Emboli and Venous Thrombi in the Dog. Circulation 1973, 48, 170–178. [Google Scholar] [CrossRef]
- Grutzendler, J. Angiophagy: Mechanism of Microvascular Recanalization Independent of the Fibrinolytic System. Stroke 2013, 44. [Google Scholar] [CrossRef]
- Rothman, A.; Wiencek, R.G.; Davidson, S.; Evans, W.N.; Restrepo, H.; Sarukhanov, V.; Mann, D. Challenges in the development of chronic pulmonary hypertension models in large animals. Pulm. Circ. 2017, 7, 156–166. [Google Scholar] [CrossRef][Green Version]
- Agüero, J.; Ishikawa, K.; Fish, K.M.; Hammoudi, N.; Hadri, L.; García-Alvarez, A.; Ibañez, B.; Fuster, V.; Hajjar, R.J.; Leopold, J.A. Combination Proximal Pulmonary Artery Coiling and Distal Embolization Induces Chronic Elevations in Pulmonary Artery Pressure in Swine. PLoS ONE 2015, 10, e0124526. [Google Scholar] [CrossRef]
- Grutzendler, J.; Murikinati, S.; Hiner, B.; Ji, L.; Lam, C.K.; Yoo, T.; Gupta, S.; Hafler, B.P.; Adelman, R.A.; Yuan, P.; et al. Angiophagy Prevents Early Embolus Washout But Recanalizes Microvessels Through Embolus Extravasation. Sci. Transl. Med. 2014, 6, 226ra31. [Google Scholar] [CrossRef]
- Mercier, O.; Tivane, A.; Dorfmuller, P.; De Perrot, M.; Raoux, F.; Decante, B.; Eddahibi, S.; Dartevelle, P.; Fadel, E. Piglet model of chronic pulmonary hypertension. Pulm. Circ. 2013, 3, 908–915. [Google Scholar] [CrossRef]
- Dorfmuller, P.; Günther, S.; Ghigna, M.-R.; De Montpréville, V.T.; Boulate, D.; Paul, J.-F.; Jaïs, X.; Decante, B.; Simonneau, G.; Dartevelle, P.; et al. Microvascular disease in chronic thromboembolic pulmonary hypertension: A role for pulmonary veins and systemic vasculature. Eur. Respir. J. 2014, 44, 1275–1288. [Google Scholar] [CrossRef]
- Boulate, D.; Perros, F.; Dorfmuller, P.; Arthur-Ataam, J.; Guihaire, J.; Lamrani, L.; Decante, B.; Humbert, M.; Eddahibi, S.; Dartevelle, P.; et al. Pulmonary microvascular lesions regress in reperfused chronic thromboembolic pulmonary hypertension. J. Hear. Lung Transplant. 2015, 34, 457–467. [Google Scholar] [CrossRef]
- Kim, H.; Yung, G.; Marsh, J.; Konopka, R.; Pedersen, C.; Chiles, P.; Morris, T.; Channick, R. Endothelin mediates pulmonary vascular remodelling in a canine model of chronic embolic pulmonary hypertension. Eur. Respir. J. 2000, 15, 640–648. [Google Scholar] [CrossRef]
- Weimann, J.; Zink, W.; Schnabel, P.A.; Jakob, H.; Gebhard, M.M.; Martin, E.; Motsch, J. Selective vasodilatation by nitric oxide inhalation during sustained pulmonary hypertension following recurrent microembolism in pigs. J. Crit. Care 1999, 14, 133–140. [Google Scholar] [CrossRef]
- Shelub, I.; Van Grondelle, A.; McCullough, R.; Hofmeister, S.; Reeves, J.T. A model of embolic chronic pulmonary hypertension in the dog. J. Appl. Physiol. 1984, 56, 810–815. [Google Scholar] [CrossRef]
- Tomashefski, J.F.; Cohen, A.M.; Doershuk, C.F. Longterm histopathologic follow-up of bronchial arteries after therapeutic embolization with polyvinyl alcohol (Ivalon) in patients with cystic fibrosis. Hum. Pathol. 1988, 19, 555–561. [Google Scholar] [CrossRef]
- Moser, K.M.; Bioor, C.M. Pulmonary Vascular Lesions Occurring in Patients With Chronic Major Vessel Thromboembolic Pulmonary Hypertension. Chest 1993, 103, 685–692. [Google Scholar] [CrossRef]
- Gopal, T.V. Gene transfer method for transient gene expression, stable transformation, and cotransformation of suspension cell cultures. Mol. Cell. Biol. 1985, 5, 1188–1190. [Google Scholar] [CrossRef][Green Version]
- Wake, K.; Kawai, Y.; Smedsrød, B. Re-evaluation of the reticulo-endothelial system. Ital. J. Anat. Embryol. 2001, 106, 261–269. [Google Scholar]
- Xie, R.; Gao, C.; Li, W.; Zhu, J.; Novakovic, V.; Wang, J.; Ma, R.; Zhou, J.; Gilbert, G.E.; Shi, J. Phagocytosis by macrophages and endothelial cells inhibits procoagulant and fibrinolytic activity of acute promyelocytic leukemia cells. Blood 2012, 119, 2325–2334. [Google Scholar] [CrossRef]
- Chrastina, A.; Massey, K.A.; Schnitzer, J.E. Overcoming in vivo barriers to targeted nanodelivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2011, 3, 421–437. [Google Scholar] [CrossRef]
- Fens, M.H.; Storm, G.; Pelgrim, R.C.; Ultee, A.; Byrne, A.T.; Gaillard, C.A.; Van Solinge, W.W.; Schiffelers, R.M. Erythrophagocytosis by angiogenic endothelial cells is enhanced by loss of erythrocyte deformability. Exp. Hematol. 2010, 38, 282–291. [Google Scholar] [CrossRef]
- Rengarajan, M.; Hayer, A.; Theriot, J.A. Endothelial Cells Use a Formin-Dependent Phagocytosis-Like Process to Internalize the Bacterium Listeria monocytogenes. PLoS Pathog. 2016, 12, e1005603. [Google Scholar] [CrossRef]
- Jones, D.; Park, D.-Y.; Anghelina, M.; Pécot, T.; Machiraju, R.; Xue, R.; Lannutti, J.J.; Thomas, J.; Cole, S.L.; Moldovan, L.; et al. Actin grips: Circular actin-rich cytoskeletal structures that mediate the wrapping of polymeric microfibers by endothelial cells. Biomaterial 2015, 52, 395–406. [Google Scholar] [CrossRef]
- Hart, S.P.; Smith, J.; Dransfield, I. Phagocytosis of opsonized apoptotic cells: Roles for ’old-fashioned’ receptors for antibody and complement. Clin. Exp. Immunol. 2004, 135, 181–185. [Google Scholar] [CrossRef]
- Liu, S.; Cao, F.; Liu, Y.; Ma, R.; Si, Y.; Liu, Y.; Bi, Y.; Gilbert, G.E.; Gao, C.; Xie, R.; et al. Endothelial cell phagocytosis of senescent neutrophils decreases procoagulant activity. Thromb. Haemost. 2013, 109, 1079–1090. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perros, F.; Ghigna, M.-R.; Loisel, F.; Chemla, D.; Decante, B.; de Montpreville, V.; Montani, D.; Humbert, M.; Fadel, E.; Mercier, O.; et al. Description, Staging and Quantification of Pulmonary Artery Angiophagy in a Large Animal Model of Chronic Thromboembolic Pulmonary Hypertension. Biomedicines 2020, 8, 493. https://doi.org/10.3390/biomedicines8110493
Perros F, Ghigna M-R, Loisel F, Chemla D, Decante B, de Montpreville V, Montani D, Humbert M, Fadel E, Mercier O, et al. Description, Staging and Quantification of Pulmonary Artery Angiophagy in a Large Animal Model of Chronic Thromboembolic Pulmonary Hypertension. Biomedicines. 2020; 8(11):493. https://doi.org/10.3390/biomedicines8110493
Chicago/Turabian StylePerros, Frédéric, Maria-Rosa Ghigna, Fanny Loisel, Denis Chemla, Benoit Decante, Vincent de Montpreville, David Montani, Marc Humbert, Elie Fadel, Olaf Mercier, and et al. 2020. "Description, Staging and Quantification of Pulmonary Artery Angiophagy in a Large Animal Model of Chronic Thromboembolic Pulmonary Hypertension" Biomedicines 8, no. 11: 493. https://doi.org/10.3390/biomedicines8110493
APA StylePerros, F., Ghigna, M.-R., Loisel, F., Chemla, D., Decante, B., de Montpreville, V., Montani, D., Humbert, M., Fadel, E., Mercier, O., & Boulate, D. (2020). Description, Staging and Quantification of Pulmonary Artery Angiophagy in a Large Animal Model of Chronic Thromboembolic Pulmonary Hypertension. Biomedicines, 8(11), 493. https://doi.org/10.3390/biomedicines8110493






