Fetal Undernutrition Induces Resistance Artery Remodeling and Stiffness in Male and Female Rats Independent of Hypertension
Abstract
1. Introduction
2. Experimental Section
2.1. Maternal Undernutrition (MUN) Model and Experimental Design
2.2. Hemodynamic Parameters
2.2.1. Intra-Arterial Measurements
2.2.2. Tail-Cuff Plethysmography
2.3. Pressure Myography
2.4. Confocal Microscopy
2.5. Zymography
2.6. Statistical Analysis
3. Results
3.1. Body Weight
3.2. Hemodynamic Parameters
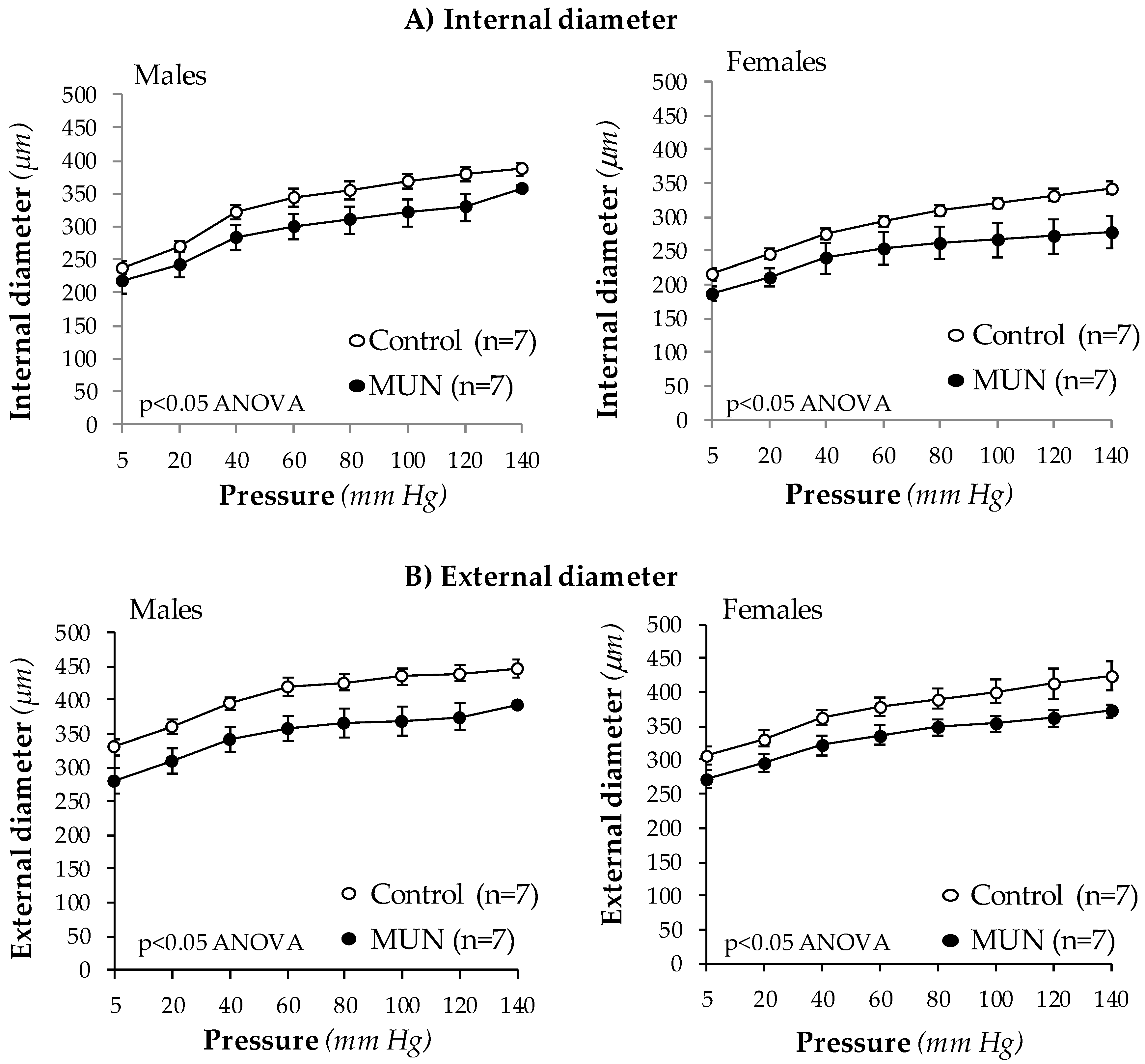
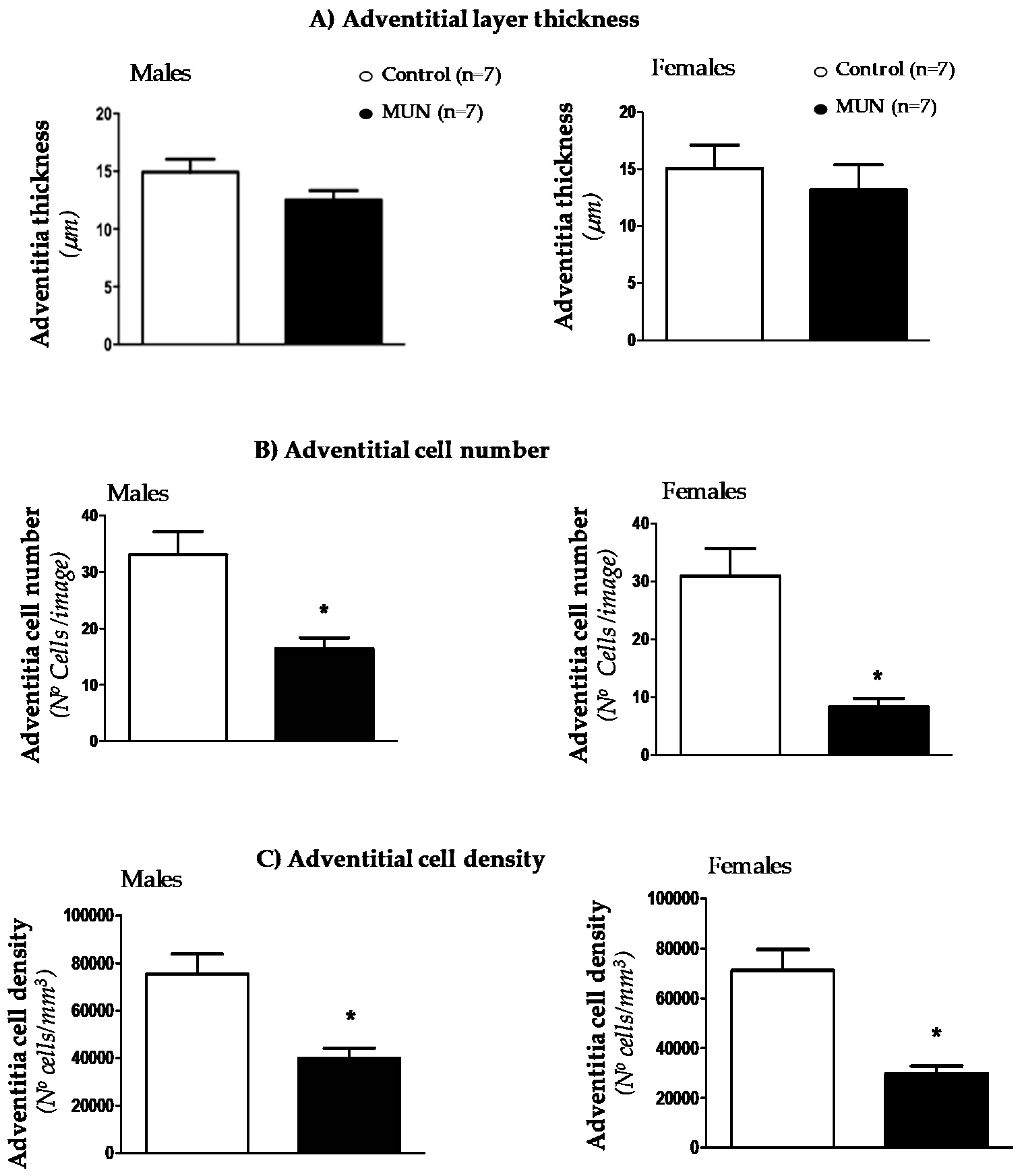
3.3. Mesenteric Resistance Artery (MRA) Structure
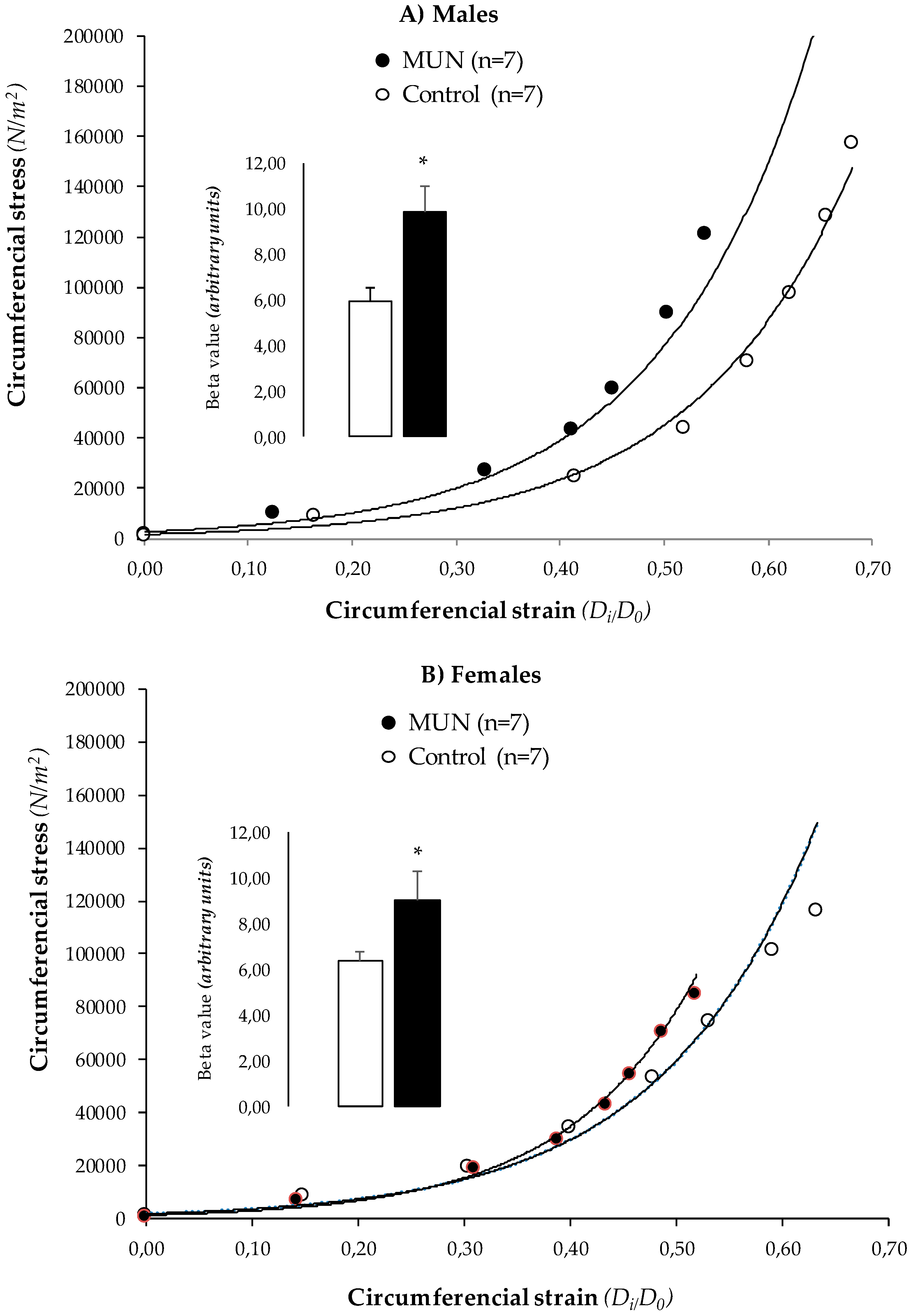
3.4. MRA Mechanical Properties
3.5. Internal Elastic Lamina (IEL)
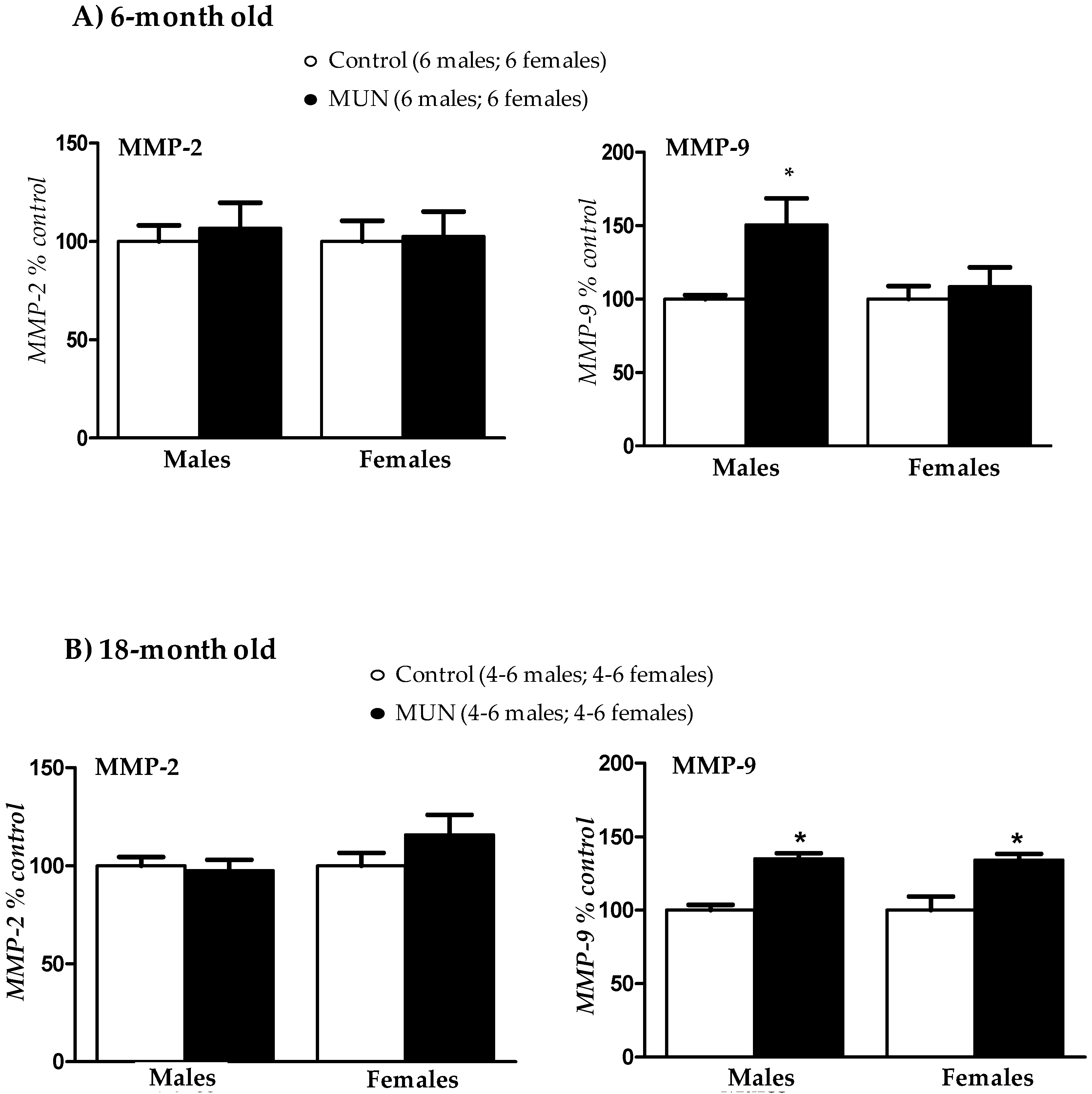
3.6. Plasma Metalloprotease (MMP) Activity
4. Discussion
4.1. Hypertension Development in Males and Females
4.2. Resistance Artery Remodeling and Hypertension
4.3. Characteristics of MRA Remodeling
4.4. Role of MMPs
5. Conclusions
- Fetal undernutrition induces MRA inward eutrophic remodeling in males and females, independent of blood pressure level.
- MRA remodeling in MUN rats is associated with cellular and ECM alterations and stiffening.
- MUN females develop hypertension in ageing, and MRA remodeling may be a contributing factor.
- Increased plasma MMP-9 activity is associated with hypertension.
- Resistance artery remodeling induced by fetal undernutrition may contribute to organ damage and development of CVD.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barker, D.J.; Osmond, C.; Golding, J.; Kuh, D.; Wadsworth, M.E. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ 1989, 298, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Dasinger, J.H.; Alexander, B.T. Gender differences in developmental programming of cardiovascular diseases. Clin. Sci. 2016, 130, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Crispi, F.; Miranda, J.; Gratacos, E. Long-term cardiovascular consequences of fetal growth restriction: Biology, clinical implications, and opportunities for prevention of adult disease. Am. J. Obs. Gynecol. 2018, 218, S869–S879. [Google Scholar] [CrossRef] [PubMed]
- Edwards, L.J.; Coulter, C.L.; Symonds, M.E.; McMillen, I.C. Prenatal undernutrition, glucocorticoids and the programming of adult hypertension. Clin. Exp. Pharm. Physiol. 2001, 28, 938–941. [Google Scholar] [CrossRef] [PubMed]
- Martyn, C.N.; Greenwald, S.E. Impaired synthesis of elastin in walls of aorta and large conduit arteries during early development as an initiating event in pathogenesis of systemic hypertension. Lancet 1997, 350, 953–955. [Google Scholar] [CrossRef]
- Skilton, M.R.; Evans, N.; Griffiths, K.A.; Harmer, J.A.; Celermajer, D.S. Aortic wall thickness in newborns with intrauterine growth restriction. Lancet 2005, 365, 1484–1486. [Google Scholar] [CrossRef]
- Brodszki, J.; Lanne, T.; Marsal, K.; Ley, D. Impaired vascular growth in late adolescence after intrauterine growth restriction. Circulation 2005, 111, 2623–2628. [Google Scholar] [CrossRef][Green Version]
- Gutierrez-Arzapalo, P.Y.; Rodriguez-Rodriguez, P.; Ramiro-Cortijo, D.; Lopez de Pablo, A.L.; Lopez-Gimenez, M.R.; Condezo-Hoyos, L.; Greenwald, S.E.; Gonzalez, M.D.C.; Arribas, S.M. Role of fetal nutrient restriction and postnatal catch-up growth on structural and mechanical alterations of rat aorta. J. Physiol. 2018, 596, 5791–5806. [Google Scholar] [CrossRef]
- Bund, S.J.; Lee, R.M. Arterial structural changes in hypertension: A consideration of methodology, terminology and functional consequence. J. Vasc. Res. 2003, 40, 547–557. [Google Scholar] [CrossRef]
- Mulvany, M.J. Small artery remodelling in hypertension. Basic Clin. Pharm. Toxicol. 2012, 110, 49–55. [Google Scholar] [CrossRef]
- Agabiti-Rosei, E.; Rizzoni, D. Microvascular structure as a prognostically relevant endpoint. J. Hypertens 2017, 35, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Khalil, R.A. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv. Pharm. 2018, 81, 241–330. [Google Scholar] [CrossRef]
- Khorram, O.; Momeni, M.; Desai, M.; Ross, M.G. Nutrient restriction in utero induces remodeling of the vascular extracellular matrix in rat offspring. Reprod. Sci. 2007, 14, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Sesso, R.; Franco, M.C. Abnormalities in metalloproteinase pathways and IGF-I axis: A link between birth weight, hypertension, and vascular damage in childhood. Am. J. Hypertens 2010, 23, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Massaro, M.; Scoditti, E.; Carluccio, M.A.; De Caterina, R. Oxidative stress and vascular stiffness in hypertension: A renewed interest for antioxidant therapies? Vasc. Pharm. 2019, 116, 45–50. [Google Scholar] [CrossRef]
- Ozaki, T.; Nishina, H.; Hanson, M.A.; Poston, L. Dietary restriction in pregnant rats causes gender-related hypertension and vascular dysfunction in offspring. J. Physiol. 2001, 530, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rodriguez, P.; de Pablo, A.L.; Condezo-Hoyos, L.; Martin-Cabrejas, M.A.; Aguilera, Y.; Ruiz-Hurtado, G.; Gutierrez-Arzapalo, P.Y.; Ramiro-Cortijo, D.; Fernandez-Alfonso, M.S.; Gonzalez Mdel, C.; et al. Fetal undernutrition is associated with perinatal sex-dependent alterations in oxidative status. J. Nutr. Biochem. 2015, 26, 1650–1659. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, P.; Ramiro-Cortijo, D.; Reyes-Hernandez, C.G.; Lopez de Pablo, A.L.; Gonzalez, M.C.; Arribas, S.M. Implication of Oxidative Stress in Fetal Programming of Cardiovascular Disease. Front. Physiol. 2018, 9, 602. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rodriguez, P.; Lopez de Pablo, A.L.; Garcia-Prieto, C.F.; Somoza, B.; Quintana-Villamandos, B.; Gomez de Diego, J.J.; Gutierrez-Arzapalo, P.Y.; Ramiro-Cortijo, D.; Gonzalez, M.C.; Arribas, S.M. Long term effects of fetal undernutrition on rat heart. Role of hypertension and oxidative stress. PLoS ONE 2017, 12, e0171544. [Google Scholar] [CrossRef]
- Vieira-Rocha, M.S.; Rodriguez-Rodriguez, P.; Sousa, J.B.; Gonzalez, M.C.; Arribas, S.M.; Lopez de Pablo, A.L.; Diniz, C. Vascular angiotensin AT1 receptor neuromodulation in fetal programming of hypertension. Vasc. Pharm. 2019, 117, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Briones, A.M.; Gonzalez, J.M.; Somoza, B.; Giraldo, J.; Daly, C.J.; Vila, E.; Gonzalez, M.C.; McGrath, J.C.; Arribas, S.M. Role of elastin in spontaneously hypertensive rat small mesenteric artery remodelling. J. Physiol. 2003, 552, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.T.; Dasinger, J.H.; Intapad, S. Fetal programming and cardiovascular pathology. Compr. Physiol. 2015, 5, 997–1025. [Google Scholar] [CrossRef] [PubMed]
- Vieira-Rocha, M.S.; Sousa, J.B.; Rodriguez-Rodriguez, P.; Morato, M.; Arribas, S.M.; Diniz, C. Insights into sympathetic nervous system and GPCR interplay in fetal programming of hypertension: A bridge for new pharmacological strategies. Drug Discov. Today 2020, 25, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Maranon, R.O.; Lima, R.; Mathbout, M.; do Carmo, J.M.; Hall, J.E.; Roman, R.J.; Reckelhoff, J.F. Postmenopausal hypertension: Role of the sympathetic nervous system in an animal model. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 306, R248–R256. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dodson, R.B.; Rozance, P.J.; Petrash, C.C.; Hunter, K.S.; Ferguson, V.L. Thoracic and abdominal aortas stiffen through unique extracellular matrix changes in intrauterine growth restricted fetal sheep. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H429–H437. [Google Scholar] [CrossRef]
- Herrera, E.A.; Camm, E.J.; Cross, C.M.; Mullender, J.L.; Wooding, F.B.; Giussani, D.A. Morphological and functional alterations in the aorta of the chronically hypoxic fetal rat. J. Vasc. Res. 2012, 49, 50–58. [Google Scholar] [CrossRef]
- Zanardo, V.; Fanelli, T.; Weiner, G.; Fanos, V.; Zaninotto, M.; Visentin, S.; Cavallin, F.; Trevisanuto, D.; Cosmi, E. Intrauterine growth restriction is associated with persistent aortic wall thickening and glomerular proteinuria during infancy. Kidney Int. 2011, 80, 119–123. [Google Scholar] [CrossRef]
- Litwin, M.; Niemirska, A. Intima-media thickness measurements in children with cardiovascular risk factors. Pediatr. Nephrol. 2009, 24, 707–719. [Google Scholar] [CrossRef]
- Grigore, D.; Ojeda, N.B.; Alexander, B.T. Sex differences in the fetal programming of hypertension. Gend. Med. 2008, 5 (Suppl. A), S121–S132. [Google Scholar] [CrossRef]
- Brawley, L.; Poston, L.; Hanson, M.A. Mechanisms underlying the programming of small artery dysfunction: Review of the model using low protein diet in pregnancy in the rat. Arch. Physiol. Biochem. 2003, 111, 23–35. [Google Scholar] [CrossRef]
- Mendes Garrido Abregu, F.; Gobetto, M.N.; Juriol, L.V.; Caniffi, C.; Elesgaray, R.; Tomat, A.L.; Arranz, C. Developmental programming of vascular dysfunction by prenatal and postnatal zinc deficiency in male and female rats. J. Nutr. Biochem. 2018, 56, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M.; Alves-Lopes, R.; Rios, F.J.; Camargo, L.L.; Anagnostopoulou, A.; Arner, A.; Montezano, A.C. Vascular smooth muscle contraction in hypertension. Cardiovasc. Res. 2018, 114, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Durrant, L.M.; Khorram, O.; Buchholz, J.N.; Pearce, W.J. Maternal food restriction modulates cerebrovascular structure and contractility in adult rat offspring: Effects of metyrapone. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 306, R401–R410. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Siljee, J.E.; Wortelboer, E.J.; Koster, M.P.; Imholz, S.; Rodenburg, W.; Visser, G.H.; de Vries, A.; Schielen, P.C.; Pennings, J.L. Identification of interleukin-1 beta, but no other inflammatory proteins, as an early onset pre-eclampsia biomarker in first trimester serum by bead-based multiplexed immunoassays. Prenat. Diagn. 2013, 33, 1183–1188. [Google Scholar] [CrossRef]
- Rizzoni, D.; Porteri, E.; Boari, G.E.; De Ciuceis, C.; Sleiman, I.; Muiesan, M.L.; Castellano, M.; Miclini, M.; Agabiti-Rosei, E. Prognostic significance of small-artery structure in hypertension. Circulation 2003, 108, 2230–2235. [Google Scholar] [CrossRef] [PubMed]
- Intengan, H.D.; Thibault, G.; Li, J.S.; Schiffrin, E.L. Resistance artery mechanics, structure, and extracellular components in spontaneously hypertensive rats: Effects of angiotensin receptor antagonism and converting enzyme inhibition. Circulation 1999, 100, 2267–2275. [Google Scholar] [CrossRef]
- Christie, M.J.; Romano, T.; Murphy, R.M.; Posterino, G.S. The effect of intrauterine growth restriction on Ca(2+) -activated force and contractile protein expression in the mesenteric artery of adult (6-month-old) male and female Wistar-Kyoto rats. Physiol. Rep. 2018, 6, e13954. [Google Scholar] [CrossRef]
- Arribas, S.M.; Hinek, A.; Gonzalez, M.C. Elastic fibres and vascular structure in hypertension. Pharm. Ther. 2006, 111, 771–791. [Google Scholar] [CrossRef]
- Sehgal, A.; Doctor, T.; Menahem, S. Cardiac function and arterial biophysical properties in small for gestational age infants: Postnatal manifestations of fetal programming. J. Pediatr. 2013, 163, 1296–1300. [Google Scholar] [CrossRef]
- Tauzin, L.; Rossi, P.; Giusano, B.; Gaudart, J.; Boussuges, A.; Fraisse, A.; Simeoni, U. Characteristics of arterial stiffness in very low birth weight premature infants. Pediatr. Res. 2006, 60, 592–596. [Google Scholar] [CrossRef]
- Rehan, V.K.; Sakurai, R.; Li, Y.; Karadag, A.; Corral, J.; Bellusci, S.; Xue, Y.Y.; Belperio, J.; Torday, J.S. Effects of maternal food restriction on offspring lung extracellular matrix deposition and long term pulmonary function in an experimental rat model. Pediatr. Pulmonol. 2012, 47, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.M.; Rizzi, E.; Prado, C.M.; Rossi, M.A.; Tanus-Santos, J.E.; Gerlach, R.F. Imbalance between matrix metalloproteinases and tissue inhibitor of metalloproteinases in hypertensive vascular remodeling. Matrix Biol. 2010, 29, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Mathiassen, O.N.; Buus, N.H.; Sihm, I.; Thybo, N.K.; Morn, B.; Schroeder, A.P.; Thygesen, K.; Aalkjaer, C.; Lederballe, O.; Mulvany, M.J.; et al. Small artery structure is an independent predictor of cardiovascular events in essential hypertension. J. Hypertens 2007, 25, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Schiffrin, E.L. Mechanisms of remodelling of small arteries, antihypertensive therapy and the immune system in hypertension. Clin. Investig. Med. 2015, 38, E394–E402. [Google Scholar] [CrossRef]


| Males | Females | |||
|---|---|---|---|---|
| Control (n = 7) | MUN (n = 7) | Control (n = 7) | MUN (n = 7) | |
| SBP (mm Hg) | 125.6 ± 5.1 | 157.4 ± 2.9 * | 134.2 ± 3.4 | 135.5 ± 4.0 # |
| DBP (mm Hg) | 68.7 ± 4.1 | 95.8 ± 4.3 * | 64.2 ± 3.6 | 76.8 ± 4.1 # |
| HR (beats/min) | 258.4 ± 8.4 | 258.3 ± 10.3 | 250.1 ± 9.6 | 234.2 ± 9.7 |
| Males | Females | |||
|---|---|---|---|---|
| Control | MUN | Control | MUN | |
| 6-month-old | 147.7 ± 3.1 (n = 9) | 163.1 ± 2.8 * (n = 10) | 136.9 ± 2.3 (n = 9) | 145.0 ± 4.5 # (n = 10) |
| 18-month-old | 137.7 ± 3.2 (n = 10) | 162.2 ± 3.2 * (n = 9) | 131.0 ± 4.7 * (n = 7) | 152.7 ± 5.2 * (n = 7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-Arzapalo, P.Y.; Rodríguez-Rodríguez, P.; Ramiro-Cortijo, D.; Gil-Ortega, M.; Somoza, B.; de Pablo, Á.L.L.; González, M.d.C.; Arribas, S.M. Fetal Undernutrition Induces Resistance Artery Remodeling and Stiffness in Male and Female Rats Independent of Hypertension. Biomedicines 2020, 8, 424. https://doi.org/10.3390/biomedicines8100424
Gutiérrez-Arzapalo PY, Rodríguez-Rodríguez P, Ramiro-Cortijo D, Gil-Ortega M, Somoza B, de Pablo ÁLL, González MdC, Arribas SM. Fetal Undernutrition Induces Resistance Artery Remodeling and Stiffness in Male and Female Rats Independent of Hypertension. Biomedicines. 2020; 8(10):424. https://doi.org/10.3390/biomedicines8100424
Chicago/Turabian StyleGutiérrez-Arzapalo, Perla Y., Pilar Rodríguez-Rodríguez, David Ramiro-Cortijo, Marta Gil-Ortega, Beatriz Somoza, Ángel Luis López de Pablo, Maria del Carmen González, and Silvia M. Arribas. 2020. "Fetal Undernutrition Induces Resistance Artery Remodeling and Stiffness in Male and Female Rats Independent of Hypertension" Biomedicines 8, no. 10: 424. https://doi.org/10.3390/biomedicines8100424
APA StyleGutiérrez-Arzapalo, P. Y., Rodríguez-Rodríguez, P., Ramiro-Cortijo, D., Gil-Ortega, M., Somoza, B., de Pablo, Á. L. L., González, M. d. C., & Arribas, S. M. (2020). Fetal Undernutrition Induces Resistance Artery Remodeling and Stiffness in Male and Female Rats Independent of Hypertension. Biomedicines, 8(10), 424. https://doi.org/10.3390/biomedicines8100424







