The Acute Phase Reaction and Its Prognostic Impact in Patients with Head and Neck Squamous Cell Carcinoma: Single Biomarkers Including C-Reactive Protein Versus Biomarker Profiles
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants in the Study
2.2. Laboratory Analyses
2.3. Statistical and Bioinformatical Analyses
3. Results
3.1. Patients Included in the Study
3.2. The Acute Phase Profile of the Cancer Patients Differs from Healthy Controls
3.3. The Acute Phase Reaction Differs between Patients with Head and Neck Squamous Cell Carcinoma
3.4. Two Main Patient Subsets Were Identified Based on the Plasma Profile of IL6 Family Mediators
3.5. Two Main Patient Subsets are Also Identified Based on an Extended Acute Phase Cytokine Profile
3.6. HPV-Positive Patients Show a Similar Variation in Their Systemic Mediator Profile but Differ in Survival Compared with HPV-Negative Patients
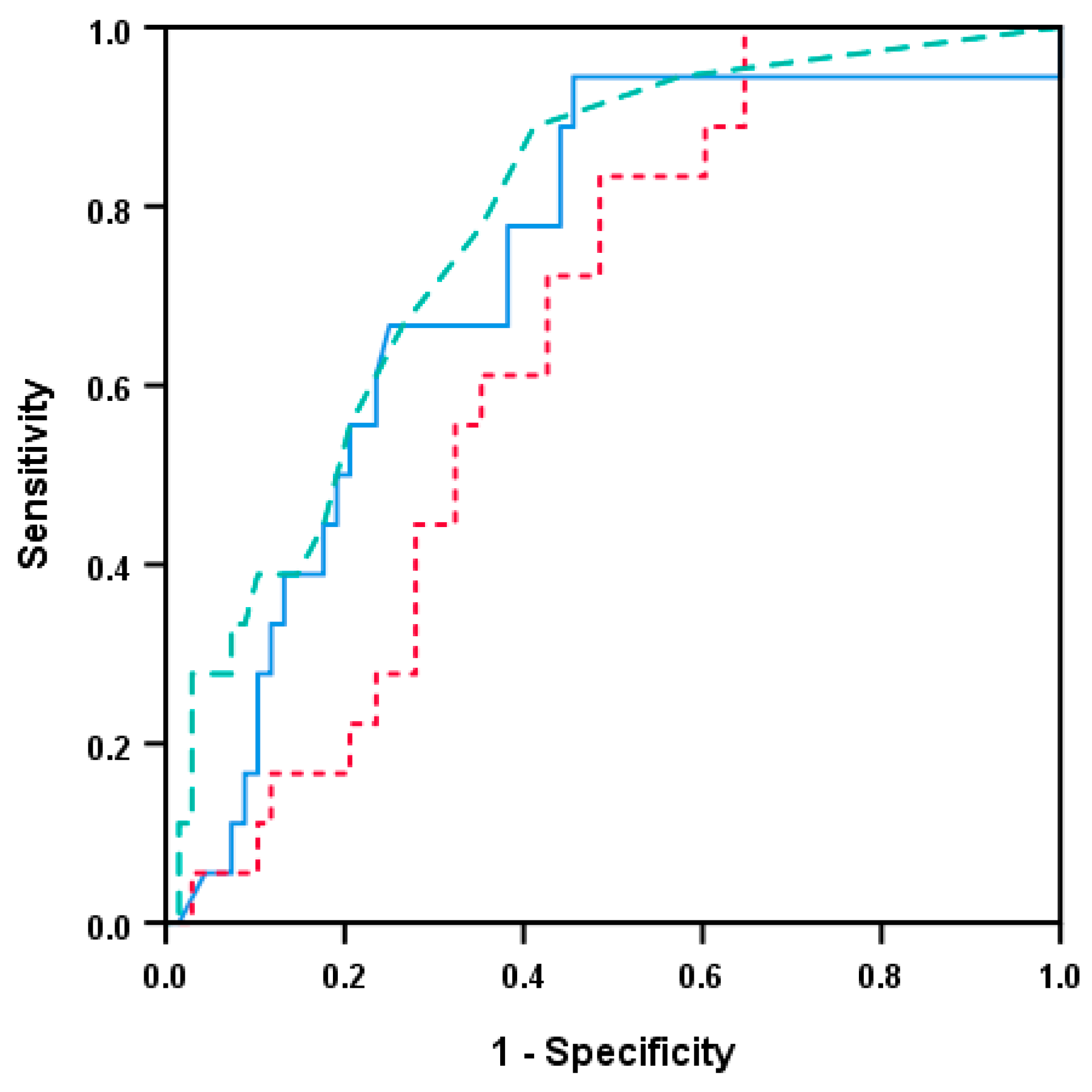
3.7. Single Acute Phase Cytokines Are Associated with Prognosis in Head and Neck Squamous Cell Carcinoma Even Though the Overall Cytokine Profile Shows No Such Associations
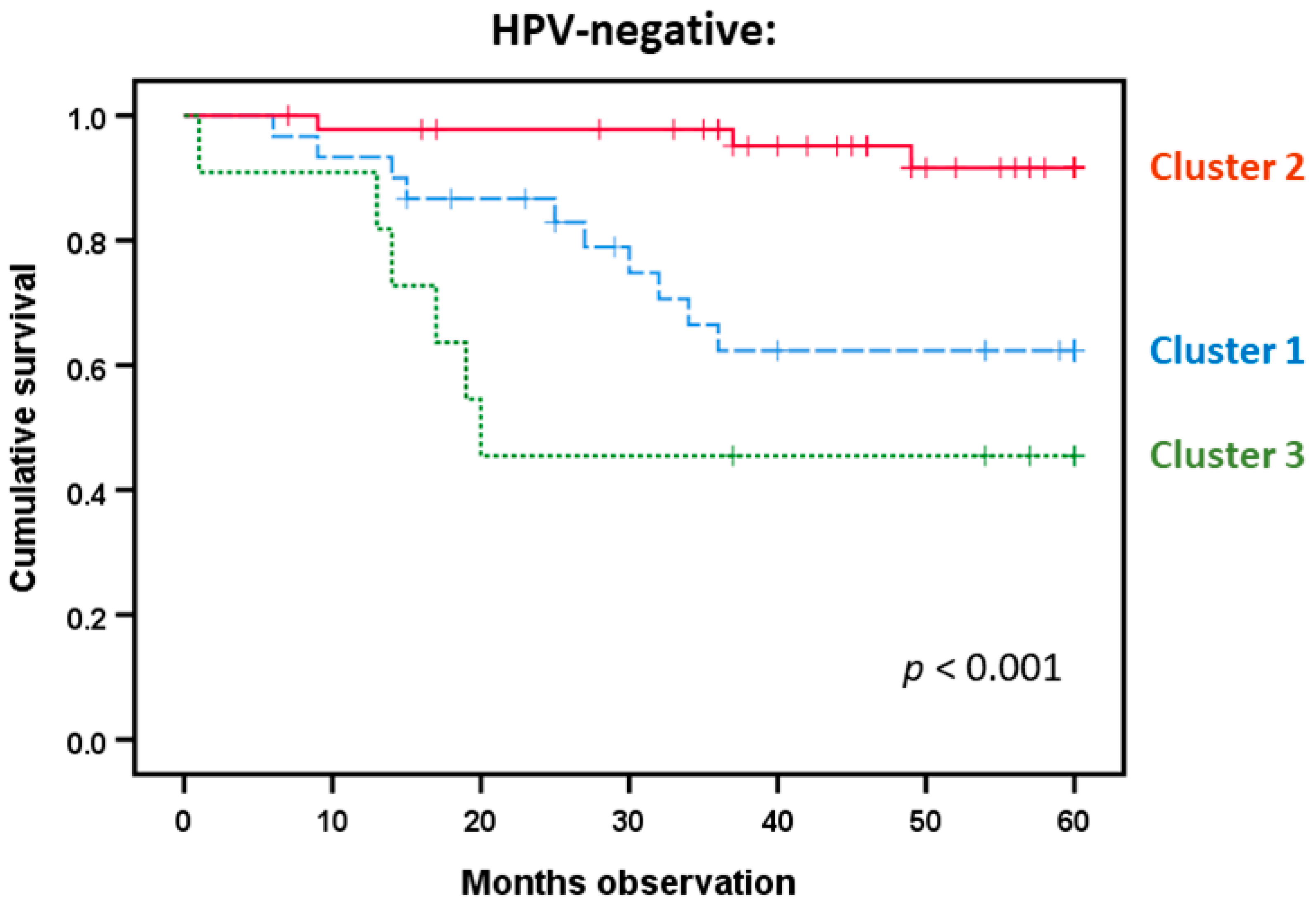
3.8. HPV-Negative Patients Differ in Survival Based on a Modified Acute Phase Profile from Indicated Predictions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Bertsch, T.; Triebel, J.; Bollheimer, C.; Christ, M.; Sieber, C.; Fassbender, K.; Heppner, H.J. C-reactive protein and the acute phase reaction in geriatric patients. Zeitschrift Fur Gerontologie Und Geriatrie 2015, 48, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Kushner, I. The phenomenon of the acute phase response. Ann. N. Y. Acad. Sci. 1982, 389, 39–48. [Google Scholar] [CrossRef]
- Liew, F.Y. Il-33: A janus cytokine. Ann. Rheum. Dis. 2012, 71 (Suppl. S2), i101–i104. [Google Scholar] [CrossRef] [PubMed]
- Srinagesh, H.K.; Levine, J.E.; Ferrara, J.L.M. Biomarkers in acute graft-versus-host disease: New insights. Ther. Adv. Hematol. 2019, 10, 2040620719891358. [Google Scholar] [CrossRef] [PubMed]
- Willems, S.; Hoefer, I.; Pasterkamp, G. The role of the interleukin 1 receptor-like 1 (st2) and interleukin-33 pathway in cardiovascular disease and cardiovascular risk assessment. Minerva Med. 2012, 103, 513–524. [Google Scholar]
- Zhao, J.; Zhao, Y. Interleukin-33 and its receptor in pulmonary inflammatory diseases. Crit. Rev. Immunol. 2015, 35, 451–461. [Google Scholar] [CrossRef]
- Knittelfelder, O.; Delago, D.; Jakse, G.; Lukasiak, K.; Thurner, E.M.; Thurnher, D.; Pichler, M.; Renner, W.; Stranzl-Lawatsch, H.; Langsenlehner, T. The pre-treatment c-reactive protein represents a prognostic factor in patients with oral and oropharyngeal cancer treated with radiotherapy. Cancers 2020, 12, 626. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.Y.; Kuo, Y.B.; Wu, T.L.; Liao, C.T.; Sun, Y.C.; Yen, T.C.; Chan, E.C. Association and prognostic value of serum inflammation markers in patients with leukoplakia and oral cavity cancer. Clin. Chem. Lab. Med. 2013, 51, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhang, L.; Luo, M.; Hu, G.; Mei, Q.; Liu, D.; Long, G.; Hu, G. Pretreatment hematologic markers as prognostic factors in patients with nasopharyngeal carcinoma: Neutrophil-lymphocyte ratio and platelet-lymphocyte ratio. Head Neck 2016, 38 (Suppl. S1), E1332–E1340. [Google Scholar] [CrossRef]
- Moskovitz, J.; Moy, J.; Ferris, R.L. Immunotherapy for head and neck squamous cell carcinoma. Curr. Oncol. Rep. 2018, 20, 22. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Karin, M. Il-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin. Immunol. 2014, 26, 54–74. [Google Scholar] [CrossRef] [PubMed]
- Mihara, M.; Hashizume, M.; Yoshida, H.; Suzuki, M.; Shiina, M. Il-6/il-6 receptor system and its role in physiological and pathological conditions. Clin. Sci. 2012, 122, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Scheller, J.; Garbers, C.; Rose-John, S. Interleukin-6: From basic biology to selective blockade of pro-inflammatory activities. Semin. Immunol. 2014, 26, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Bansal, Y.; Kumar, R.; Bansal, G. A panoramic review of il-6: Structure, pathophysiological roles and inhibitors. Bioorganic Med. Chem. 2020, 28, 115327. [Google Scholar] [CrossRef]
- Garbers, C.; Heink, S.; Korn, T.; Rose-John, S. Interleukin-6: Designing specific therapeutics for a complex cytokine. Nat. Rev. Drug Discov. 2018, 17, 395–412. [Google Scholar] [CrossRef]
- Blay, J.Y.; Negrier, S.; Combaret, V.; Attali, S.; Goillot, E.; Merrouche, Y.; Mercatello, A.; Ravault, A.; Tourani, J.M.; Moskovtchenko, J.F.; et al. Serum level of interleukin 6 as a prognosis factor in metastatic renal cell carcinoma. Cancer Res. 1992, 52, 3317–3322. [Google Scholar]
- Ataie-Kachoie, P.; Pourgholami, M.H.; Richardson, D.R.; Morris, D.L. Gene of the month: Interleukin 6 (il-6). J. Clin. Pathol. 2014, 67, 932–937. [Google Scholar] [CrossRef]
- Yao, X.; Huang, J.; Zhong, H.; Shen, N.; Faggioni, R.; Fung, M.; Yao, Y. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol. Ther. 2014, 141, 125–139. [Google Scholar] [CrossRef]
- Lippitz, B.E.; Harris, R.A. Cytokine patterns in cancer patients: A review of the correlation between interleukin 6 and prognosis. Oncoimmunology 2016, 5, e1093722. [Google Scholar] [CrossRef]
- Duffy, S.A.; Taylor, J.M.; Terrell, J.E.; Islam, M.; Li, Y.; Fowler, K.E.; Wolf, G.T.; Teknos, T.N. Interleukin-6 predicts recurrence and survival among head and neck cancer patients. Cancer 2008, 113, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Zhu, Y.; Zhou, H. Prognostic value of interleukin-6 and interleukin-8 in laryngeal squamous cell cancer. Medical Oncol. 2013, 30, 333. [Google Scholar] [CrossRef] [PubMed]
- Ke, L.; Xiang, Y.; Xia, W.; Yang, J.; Yu, Y.; Ye, Y.; Liang, H.; Guo, X.; Lv, X. A prognostic model predicts the risk of distant metastasis and death for patients with nasopharyngeal carcinoma based on pre-treatment interleukin 6 and clinical stage. Clin. Immunol. 2016, 164, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhao, S.; Halstensen, T.S. Increased interleukin-6 expression is associated with poor prognosis and acquired cisplatin resistance in head and neck squamous cell carcinoma. Oncol. Rep. 2016, 35, 3265–3274. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tvedt, T.H.A.; Ersvaer, E.; Tveita, A.A.; Bruserud, O. Interleukin-6 in allogeneic stem cell transplantation: Its possible importance for immunoregulation and as a therapeutic target. Front. Immunol. 2017, 8, 667. [Google Scholar] [CrossRef] [PubMed]
- Silver, J.S.; Hunter, C.A. Gp130 at the nexus of inflammation, autoimmunity, and cancer. J. Leukoc. Biol. 2010, 88, 1145–1156. [Google Scholar] [CrossRef]
- Lamertz, L.; Rummel, F.; Polz, R.; Baran, P.; Hansen, S.; Waetzig, G.H.; Moll, J.M.; Floss, D.M.; Scheller, J. Soluble gp130 prevents interleukin-6 and interleukin-11 cluster signaling but not intracellular autocrine responses. Sci. Signal. 2018, 11, eaar7388. [Google Scholar] [CrossRef] [PubMed]
- Rose-John, S. Interleukin-6 family cytokines. Cold Spring Harb. Perspect. Biol. 2018, 10, a028415. [Google Scholar] [CrossRef]
- Jones, S.A.; Jenkins, B.J. Recent insights into targeting the il-6 cytokine family in inflammatory diseases and cancer. Nat. Rev. Immunol. 2018, 18, 773–789. [Google Scholar] [CrossRef]
- Dinarello, C.A.; Netea, M.G. The interleukin-1 family: Role in inflammation and disease. In Cytokine Frontiers: Regulation of Immune Responses in Health and Disease; Yoshimoto, T., Ed.; Springer: Tokyo, Japan, 2014; pp. 3–51. [Google Scholar]
- Pusceddu, I.; Dieplinger, B.; Mueller, T. St2 and the st2/il-33 signalling pathway-biochemistry and pathophysiology in animal models and humans. Clin. Chim. Acta Int. J. Clin. Chem. 2019, 495, 493–500. [Google Scholar] [CrossRef]
- Griesenauer, B.; Jiang, H.; Yang, J.; Zhang, J.; Ramadan, A.M.; Egbosiuba, J.; Campa, K.; Paczesny, S. St2/myd88 deficiency protects mice against acute graft-versus-host disease and spares regulatory t cells. J. Immunol. 2019, 202, 3053–3064. [Google Scholar] [CrossRef] [PubMed]
- Reichenbach, D.K.; Schwarze, V.; Matta, B.M.; Tkachev, V.; Lieberknecht, E.; Liu, Q.; Koehn, B.H.; Pfeifer, D.; Taylor, P.A.; Prinz, G.; et al. The il-33/st2 axis augments effector t-cell responses during acute gvhd. Blood 2015, 125, 3183–3192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ramadan, A.M.; Griesenauer, B.; Li, W.; Turner, M.J.; Liu, C.; Kapur, R.; Hanenberg, H.; Blazar, B.R.; Tawara, I.; et al. St2 blockade reduces sst2-producing t cells while maintaining protective mst2-expressing t cells during graft-versus-host disease. Sci. Transl. Med. 2015, 7, 308ra160. [Google Scholar] [CrossRef] [PubMed]
- Stremska, M.E.; Jose, S.; Sabapathy, V.; Huang, L.; Bajwa, A.; Kinsey, G.R.; Sharma, P.R.; Mohammad, S.; Rosin, D.L.; Okusa, M.D.; et al. Il233, a novel il-2 and il-33 hybrid cytokine, ameliorates renal injury. J. Am. Soc. Nephrol. 2017, 28, 2681–2693. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.F.; Nieh, S.; Jao, S.W.; Wu, M.Z.; Liu, C.L.; Chang, Y.C.; Lin, Y.S. The paracrine effect of cancer-associated fibroblast-induced interleukin-33 regulates the invasiveness of head and neck squamous cell carcinoma. J. Pathol. 2013, 231, 180–189. [Google Scholar] [CrossRef]
- Ishikawa, K.; Yagi-Nakanishi, S.; Nakanishi, Y.; Kondo, S.; Tsuji, A.; Endo, K.; Wakisaka, N.; Murono, S.; Yoshizaki, T. Expression of interleukin-33 is correlated with poor prognosis of patients with squamous cell carcinoma of the tongue. Auris Nasus Larynx 2014, 41, 552–557. [Google Scholar] [CrossRef]
- Ding, L.; Ren, J.; Zhang, D.; Li, Y.; Huang, X.; Hu, Q.; Wang, H.; Song, Y.; Ni, Y.; Hou, Y. A novel stromal lncrna signature reprograms fibroblasts to promote the growth of oral squamous cell carcinoma via lncrna-caf/interleukin-33. Carcinogenesis 2018, 39, 397–406. [Google Scholar] [CrossRef]
- Wen, Y.H.; Lin, H.Q.; Li, H.; Zhao, Y.; Lui, V.W.Y.; Chen, L.; Wu, X.M.; Sun, W.; Wen, W.P. Stromal interleukin-33 promotes regulatory t cell-mediated immunosuppression in head and neck squamous cell carcinoma and correlates with poor prognosis. Cancer Immunol. Immunother. 2019, 68, 221–232. [Google Scholar] [CrossRef]
- Zhang, W.; Borcherding, N.; Kolb, R. Il-1 signaling in tumor microenvironment. Adv. Exp. Med. Biol. 2020, 1240, 1–23. [Google Scholar]
- Gillison, M.L.; Chaturvedi, A.K.; Anderson, W.F.; Fakhry, C. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J. Clin. Oncol. 2015, 33, 3235–3242. [Google Scholar] [CrossRef]
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. The molecular landscape of head and neck cancer. Nat. Rev. Cancer 2018, 18, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Mork, J.; Lie, A.K.; Glattre, E.; Hallmans, G.; Jellum, E.; Koskela, P.; Møller, B.; Pukkala, E.; Schiller, J.T.; Youngman, L.; et al. Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2001, 344, 1125–1131. [Google Scholar] [CrossRef]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef]
- Mehanna, H.; Robinson, M.; Hartley, A.; Kong, A.; Foran, B.; Fulton-Lieuw, T.; Dalby, M.; Mistry, P.; Sen, M.; O’Toole, L.; et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (de-escalate hpv): An open-label randomised controlled phase 3 trial. Lancet 2019, 393, 51–60. [Google Scholar] [CrossRef]
- Saloura, V.; Izumchenko, E.; Zuo, Z.; Bao, R.; Korzinkin, M.; Ozerov, I.; Zhavoronkov, A.; Sidransky, D.; Bedi, A.; Hoque, M.O.; et al. Immune profiles in primary squamous cell carcinoma of the head and neck. Oral Oncol. 2019, 96, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Xu, C.; Wu, P.; Zhang, L.H.; Li, D.W.; Sun, J.H.; Li, W.F.; Liao, Z.S. Prognostic role of c-reactive protein in patients with nasopharyngeal carcinoma: A meta-analysis and literature review. Medicine 2017, 96, e8463. [Google Scholar] [CrossRef]
- Kawasaki, T.; Wasano, K.; Yamamoto, S.; Tomisato, S.; Ogawa, K. Utility of clinico-biological data for long-term prognosis of head and neck terminal cancer. Acta Oto-Laryngol. 2017, 137, 895–898. [Google Scholar] [CrossRef]
- Li, J.; Chen, S.; Peng, S.; Liu, Y.; Xing, S.; He, X.; Chen, H. Prognostic nomogram for patients with nasopharyngeal carcinoma incorporating hematological biomarkers and clinical characteristics. Int. J. Biol. Sci. 2018, 14, 549–556. [Google Scholar] [CrossRef]
- De Paz, D.; Young, C.K.; Chien, H.T.; Tsao, C.K.; Fok, C.C.; Fan, K.H.; Liao, C.T.; Wang, H.M.; Kang, C.J.; Chang, J.T.; et al. Prognostic roles of scc antigen, crp and cyfra 21-1 in oral cavity squamous cell carcinoma. Anticancer Res. 2019, 39, 2025–2033. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Chen, C.; Xia, Y.; Bi, X.; Liu, P.; Zhang, F.; Yang, H.; An, X.; Jiang, W.; Wang, F. The ratio of c-reactive protein/albumin is a novel inflammatory predictor of overall survival in cisplatin-based treated patients with metastatic nasopharyngeal carcinoma. Dis. Markers 2017, 2017, 6570808. [Google Scholar] [CrossRef]
- Adel, M.; Tsao, C.K.; Wei, F.C.; Chien, H.T.; Lai, C.H.; Liao, C.T.; Wang, H.M.; Fan, K.H.; Kang, C.J.; Chang, J.T.; et al. Preoperative scc antigen, crp serum levels, and lymph node density in oral squamous cell carcinoma. Medicine 2016, 95, e3149. [Google Scholar] [CrossRef]
- Schiegnitz, E.; Kammerer, P.W.; Schon, H.; Blatt, S.; Berres, M.; Sagheb, K.; Al-Nawas, B. Proinflammatory cytokines as serum biomarker in oral carcinoma-a prospective multi-biomarker approach. J. Oral Pathol. Med. 2018, 47, 268–274. [Google Scholar] [CrossRef]
- Zergoun, A.A.; Zebboudj, A.; Sellam, S.L.; Kariche, N.; Djennaoui, D.; Ouraghi, S.; Kerboua, E.; Amir-Tidadini, Z.C.; Chilla, D.; Asselah, F.; et al. Il-6/nos2 inflammatory signals regulate mmp-9 and mmp-2 activity and disease outcome in nasopharyngeal carcinoma patients. Tumour Biol. 2016, 37, 3505–3514. [Google Scholar] [CrossRef]
- Arduino, P.G.; Menegatti, E.; Cappello, N.; Martina, E.; Gardino, N.; Tanteri, C.; Cavallo, F.; Scully, C.; Broccoletti, R. Possible role for interleukins as biomarkers for mortality and recurrence in oral cancer. Int. J. Biol. Markers 2015, 30, e262–e266. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.P.; Kao, H.K.; Wu, C.C.; Fang, K.H.; Chang, Y.L.; Huang, Y.C.; Liu, S.C.; Cheng, M.H. Pretreatment interleukin-6 serum levels are associated with patient survival for oral cavity squamous cell carcinoma. Otolaryngol. Neck Surg. 2013, 148, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Sung, W.W.; Lin, Y.M.; Chen, M.K.; Lee, C.H.; Lee, H.; Yeh, K.T.; Ko, J.L. Gender difference in the prognostic role of interleukin 6 in oral squamous cell carcinoma. PLoS ONE 2012, 7, e50104. [Google Scholar] [CrossRef] [PubMed]
- Ojesina, A.I.; Lichtenstein, L.; Freeman, S.S.; Pedamallu, C.S.; Imaz-Rosshandler, I.; Pugh, T.J.; Cherniack, A.D.; Ambrogio, L.; Cibulskis, K.; Bertelsen, B.; et al. Landscape of genomic alterations in cervical carcinomas. Nature 2014, 506, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Aarstad, H.H.; Vintermyr, O.K.; Ulvestad, E.; Kross, K.; Heimdal, J.H.; Aarstad, H.J. In vitro-stimulated il-6 monocyte secretion and in vivo peripheral blood t lymphocyte activation uniquely predicted 15-year survival in patients with head and neck squamous cell carcinoma. PLoS ONE 2015, 10, e0129724. [Google Scholar] [CrossRef]
- Stavrum, A.K.; Petersen, K.; Jonassen, I.; Dysvik, B. Analysis of gene-expression data using j-express. Curr. Protoc. Bioinform. 2008, 7, 7.3. [Google Scholar] [CrossRef] [PubMed]
- Haeggblom, L.; Ramqvist, T.; Tommasino, M.; Dalianis, T.; Nasman, A. Time to change perspectives on hpv in oropharyngeal cancer. A systematic review of hpv prevalence per oropharyngeal sub-site the last 3 years. Papillomavirus Res. 2017, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bruserud, O.; Aarstad, H.H.; Tvedt, T.H.A. Combined crp and novel inflammatory parameters as a predictor in cancer—What can we learn from the hematological experience? Cancers 2020, 12, 1966. [Google Scholar] [CrossRef] [PubMed]
- Rose-John, S. The biology of interleukin-6 in the 21st century. Semin. Immunol. 2014, 26, 1. [Google Scholar] [CrossRef]
- Garbers, C.; Aparicio-Siegmund, S.; Rose-John, S. The il-6/gp130/stat3 signaling axis: Recent advances towards specific inhibition. Curr. Opin. Immunol. 2015, 34, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, P.C.; Behrmann, I.; Haan, S.; Hermanns, H.M.; Müller-Newen, G.; Schaper, F. Principles of interleukin (il)-6-type cytokine signalling and its regulation. Biochem. J. 2003, 374, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Larsen, K.M.; Minaya, M.K.; Vaish, V.; Pena, M.M.O. The role of il-33/st2 pathway in tumorigenesis. Int. J. Mol. Sci. 2018, 19, 2676. [Google Scholar] [CrossRef] [PubMed]
- Fields, J.K.; Günther, S.; Sundberg, E.J. Structural basis of il-1 family cytokine signaling. Front. Immunol. 2019, 10, 1412. [Google Scholar] [CrossRef]
- Balkwill, F. Tumour necrosis factor and cancer. Nat. Rev. Cancer 2009, 9, 361–371. [Google Scholar] [CrossRef]
- Wajant, H.; Pfizenmaier, K.; Scheurich, P. Tumor necrosis factor signaling. Cell Death Differ. 2003, 10, 45–65. [Google Scholar] [CrossRef]
- Rundgren, I.M.; Ryningen, A.; Tvedt, T.H.A.; Bruserud, O.; Ersvaer, E. Immunomodulatory drugs alter the metabolism and the extracellular release of soluble mediators by normal monocytes. Molecules 2020, 25, 367. [Google Scholar] [CrossRef]
- Aarstad, H.H.; Guðbrandsdottir, G.; Hjelle, K.M.; Bostad, L.; Bruserud, Ø.; Tvedt, T.H.A.; Beisland, C. The biological context of c-reactive protein as a prognostic marker in renal cell carcinoma: Studies on the acute phase cytokine profile. Cancers 2020, 12, 1961. [Google Scholar] [CrossRef]
- Arantes, L.; De Carvalho, A.C.; Melendez, M.E.; Carvalho, A.L. Serum, plasma and saliva biomarkers for head and neck cancer. Expert Rev. Mol. Diagn. 2018, 18, 85–112. [Google Scholar] [CrossRef]
- Nakayama, M.; Gosho, M.; Hirose, Y.; Nishimura, B.; Tanaka, S.; Tabuchi, K.; Okubo, H.; Wada, T.; Hara, A. Modified combination of platelet count and neutrophil “to” lymphocyte ratio as a prognostic factor in patients with advanced head and neck cancer. Head Neck 2018, 40, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, Y.; Oya, R.; Kitamiura, T.; Ashida, N.; Shimizu, K.; Takemura, K.; Yamamoto, Y.; Uno, A. Platelet count and platelet-lymphocyte ratio as prognostic markers for head and neck squamous cell carcinoma: Meta-analysis. Head Neck 2018, 40, 2714–2723. [Google Scholar] [CrossRef] [PubMed]
- Bardash, Y.; Olson, C.; Herman, W.; Khaymovich, J.; Costantino, P.; Tham, T. Platelet-lymphocyte ratio as a predictor of prognosis in head and neck cancer: A systematic review and meta-analysis. Oncol. Res. Treat. 2019, 42, 665–677. [Google Scholar] [CrossRef]
- Chiba, Y.; Mizoguchi, I.; Hasegawa, H.; Ohashi, M.; Orii, N.; Nagai, T.; Sugahara, M.; Miyamoto, Y.; Xu, M.; Owaki, T.; et al. Regulation of myelopoiesis by proinflammatory cytokines in infectious diseases. Cell. Mol. Life Sci. 2018, 75, 1363–1376. [Google Scholar] [CrossRef]
- Bruserud, O.; Foss, B.; Petersen, H. Hematopoietic growth factors in patients receiving intensive chemotherapy for malignant disorders: Studies of granulocyte-colony stimulating factor (g-csf), granulocyte-macrophage colony stimulating factor (gm-csf), interleukin-3 (il-3) and flt-3 ligand (flt3l). Eur. Cytokine Netw. 2001, 12, 231–238. [Google Scholar]
- Kobayashi, K.; Hisamatsu, K.; Suzui, N.; Hara, A.; Tomita, H.; Miyazaki, T. A review of hpv-related head and neck cancer. J. Clin. Med. 2018, 7, 241. [Google Scholar] [CrossRef] [PubMed]
- Haave, H.; Gulati, S.; Brekke, J.; Lybak, S.; Vintermyr, O.K.; Aarstad, H.J. Tumor stromal desmoplasia and inflammatory response uniquely predict survival with and without stratification for hpv tumor infection in opscc patients. Acta Oto-Laryngol. 2018, 138, 1035–1042. [Google Scholar] [CrossRef]
- Sabatini, M.E.; Chiocca, S. Human papillomavirus as a driver of head and neck cancers. Br. J. Cancer 2020, 122, 306–314. [Google Scholar] [CrossRef]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Gupta, M.; Stenson, M.; O’Byrne, M.; Maurer, M.J.; Habermann, T.; Cerhan, J.R.; Weiner, G.W.; Witzig, T.E. Comprehensive serum cytokine analysis identifies il-1ra and soluble il-2rα as predictors of event-free survival in t-cell lymphoma. Ann. Oncol. 2016, 27, 165–172. [Google Scholar] [CrossRef]
- Rutkowski, P.; Kamińska, J.; Kowalska, M.; Ruka, W.; Steffen, J. Cytokine and cytokine receptor serum levels in adult bone sarcoma patients: Correlations with local tumor extent and prognosis. J. Surg. Oncol. 2003, 84, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Miki, C. Profile of circulating levels of interleukin-1 receptor antagonist and interleukin-6 in colorectal cancer patients. Scand. J. Gastroenterol. 1999, 34, 1139–1143. [Google Scholar] [PubMed]
- Kamińska, J.; Kowalska, M.M.; Nowacki, M.P.; Chwaliński, M.G.; Rysińska, A.; Fuksiewicz, M. Crp, tnf-alpha, il-1ra, il-6, il-8 and il-10 in blood serum of colorectal cancer patients. Pathol. Oncol. Res. POR 2000, 6, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Niedźwiecki, S.; Stepień, T.; Kuzdak, K.; Stepień, H.; Krupiński, R.; Seehofer, D.; Rayes, N.; Ulrich, F. Serum levels of interleukin-1 receptor antagonist (il-1ra) in thyroid cancer patients. Langenbeck’s Arch. Surg. 2008, 393, 275–280. [Google Scholar] [CrossRef]
- Allen, T.L.; Matthews, V.B.; Febbraio, M.A. Overcoming insulin resistance with ciliary neurotrophic factor. Handb. Exp. Pharmacol. 2011, 179–199. [Google Scholar] [CrossRef]
- Richardson, P.M. Ciliary neurotrophic factor: A review. Pharmacol. Ther. 1994, 63, 187–198. [Google Scholar] [CrossRef]
- Sims, N.A.; Walsh, N.C. Gp130 cytokines and bone remodelling in health and disease. BMB Rep. 2010, 43, 513–523. [Google Scholar] [CrossRef]
- Omokehinde, T.; Johnson, R.W. Gp130 cytokines in breast cancer and bone. Cancers 2020, 12, 326. [Google Scholar] [CrossRef]
- Espat, N.J.; Auffenberg, T.; Rosenberg, J.J.; Rogy, M.; Martin, D.; Fang, C.H.; Hasselgren, P.O.; Copeland, E.M.; Moldawer, L.L. Ciliary neurotrophic factor is catabolic and shares with il-6 the capacity to induce an acute phase response. Am. J. Physiol. 1996, 271, R185–R190. [Google Scholar] [CrossRef]
- Perugini, J.; Di Mercurio, E.; Tossetta, G.; Severi, I.; Monaco, F.; Reguzzoni, M.; Tomasetti, M.; Dani, C.; Cinti, S.; Giordano, A. Biological effects of ciliary neurotrophic factor on hmads adipocytes. Front. Endocrinol. 2019, 10, 768. [Google Scholar] [CrossRef]
- Aziz, N.; Detels, R.; Quint, J.J.; Li, Q.; Gjertson, D.; Butch, A.W. Stability of cytokines, chemokines and soluble activation markers in unprocessed blood stored under different conditions. Cytokine 2016, 84, 17–24. [Google Scholar] [CrossRef]
- Schneider, K.; Marbaix, E.; Bouzin, C.; Hamoir, M.; Mahy, P.; Bol, V.; Grégoire, V. Immune cell infiltration in head and neck squamous cell carcinoma and patient outcome: A retrospective study. Acta Oncol. 2018, 57, 1165–1172. [Google Scholar] [CrossRef]
- Lechner, A.; Schlößer, H.; Rothschild, S.I.; Thelen, M.; Reuter, S.; Zentis, P.; Shimabukuro-Vornhagen, A.; Theurich, S.; Wennhold, K.; Garcia-Marquez, M.; et al. Characterization of tumor-associated t-lymphocyte subsets and immune checkpoint molecules in head and neck squamous cell carcinoma. Oncotarget 2017, 8, 44418–44433. [Google Scholar] [CrossRef]
- Mandal, R.; Şenbabaoğlu, Y.; Desrichard, A.; Havel, J.J.; Dalin, M.G.; Riaz, N.; Lee, K.W.; Ganly, I.; Hakimi, A.A.; Chan, T.A.; et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight 2016, 1, e89829. [Google Scholar] [CrossRef] [PubMed]
- de Ruiter, E.J.; Ooft, M.L.; Devriese, L.A.; Willems, S.M. The prognostic role of tumor infiltrating t-lymphocytes in squamous cell carcinoma of the head and neck: A systematic review and meta-analysis. Oncoimmunology 2017, 6, e1356148. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.; Bellile, E.; Thomas, D.; McHugh, J.; Rozek, L.; Virani, S.; Peterson, L.; Carey, T.E.; Walline, H.; Moyer, J.; et al. Tumor infiltrating lymphocytes and survival in patients with head and neck squamous cell carcinoma. Head Neck 2016, 38, 1074–1084. [Google Scholar] [CrossRef]
- Sakakura, K.; Takahashi, H.; Kaira, K.; Toyoda, M.; Murata, T.; Ohnishi, H.; Oyama, T.; Chikamatsu, K. Relationship between tumor-associated macrophage subsets and cd47 expression in squamous cell carcinoma of the head and neck in the tumor microenvironment. Lab. Investig. 2016, 96, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.F.; Wong, M.C.M.; Thomson, P.J.; Li, K.Y.; Su, Y.X. The prognostic role of pd-l1 expression for survival in head and neck squamous cell carcinoma: A systematic review and meta-analysis. Oral Oncol. 2018, 86, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Aarstad, H.J.; Vintermyr, O.K.; Ulvestad, E.; Aarstad, H.H.; Kross, K.W.; Heimdal, J.H. Peripheral blood monocyte and t-lymphocyte activation levels at diagnosis predict long-term survival in head and neck squamous cell carcinoma patients. APMIS 2015, 123, 305–314. [Google Scholar] [CrossRef]
- Aarstad, H.J.; Heimdal, J.H.; Klementsen, B.; Olofsson, J.; Ulvestad, E. Presence of activated t lymphocytes in peripheral blood of head and neck squamous cell carcinoma patients predicts impaired prognosis. Acta Oto-Laryngol. 2006, 126, 1326–1333. [Google Scholar] [CrossRef] [PubMed]
- Kaidar-Person, O.; Gil, Z.; Billan, S. Precision medicine in head and neck cancer. Drug Resist. Updat. 2018, 40, 13–16. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |






| PARAMETER | HPV-Negative or Unknown (n = 87) 1 | HPV-Positive (n = 57) | p-Value (Chi-Square Test) |
|---|---|---|---|
| Age at diagnosis (median and range, years) | 66.0 (37–82) | 56.0 (38–78) | 0.062 |
| Sex (males/females, numbers) | 59/28 | 46/11 | 0.089 |
| Blood values (median and range) 2 | |||
| Hemoglobin (g/100 mL) | 14.5 (9.7–17.7) | 14.9 (11.5–17.0) | 0.782 |
| Leukocytes (×109/L) | 7.4 (4.0–21.1) | 6.7 (4.1–15.2) | 0.497 |
| Thrombocytes (×109/L) | 255 (112–405) | 254 (157–759) | 0.421 |
| CRP (mg/L) | 3 (1–150) | 3 (1–54) | 0.698 |
| Erythrocyte sedimentation rate (mm/h) | 14 (2–106) | 13 (1–83) | 0.309 |
| Serum-glucose (mM) | 5.6 (3.5–14.1) | 5.9 (4.0–15.2) | 0.261 |
| Serum-creatinine (μM) | 76 (47–167) | 79 (48–135) | 0.273 |
| Tumor localization | < 0.001 | ||
| Tonsils, tongue base, head and neck cancer origo inserta | 5 (5.7) | 50 (87.8) | |
| Other oropharyngeal sites | 12 (13.8) | 4 (7.0) | |
| Oral cavity | 61 (70.1) | 1 (1.8) | |
| Other (nasopharynx, hypopharynx, glottis) | 9 (10.3) | 2 (3.5) | |
| Cancer characteristic | |||
| T stage (number and percentage) | 0.431 | ||
| T0/unknown primary | 3 (3.4) | 4 (7.0) | |
| T1 | 29 (33.3) | 13 (22.8) | |
| T2 | 34 (39.1) | 29 (50.9) | |
| T3 | 8 (9.2) | 5 (8.8) | |
| T4 | 13 (14.9) | 6 (10.5) | |
| N stage (number and percentage) 3 | < 0.001 | ||
| N0 | 57 (65.5) | 11 (19.3) | |
| N1 | 9 (10.3) | 13 (22.8) | |
| N2 | 20 (23.0) | 31 (54.4) | |
| N3 | 1 (1.1) | 2 (3.5) | |
| M stage | All patients M0 | All patients M0 | - |
| Primary treatment | |||
| Surgery including neck dissection, numbers Primary, primary surgery, Neck, neck dissection) 4 | Primary 64 Neck 34 | Primary 10 Neck 8 | Primary < 0.001 Neck 0.001 |
| Radio-/chemotherapy 5, numbers | 62/23 | 56/46 | <0.001 |
| Mediator | Upper/Left Main Cluster (n = 99) | Lower/Right Main Cluster (n = 45) | p-Value |
|---|---|---|---|
| gp130 (pg/mL) | 81,943 (25,184–108,957) | 83,253 (41,057–101,998) | 0.399 |
| IL6Rα (pg/mL) | 32,289 (5119–44,259) | ↑ 34,839 (21,527–47,290) | 0.039 |
| IL6 (pg/mL) | 1.66 (0.20–9.41) | ↑ 2.50 (0.83–45.03) | <0.001 |
| IL27 (pg/mL) | 457 (116–1096) | 463 (116–882) | 0.402 |
| IL31 (pg/mL) | 62.5 (19.4–132.2) | ↑ 78.5 (23.8–123.0) | 0.003 |
| OSM (pg/mL) | 4642 (2487–5799) | 4531 (3165–5747) | 0.887 |
| CNTF (pg/mL) | 1113 (293–13,595) | ↑ 206 (33.8–1980) | <0.001 |
| IL33Rα (pg/mL) | 16,950 (75.9–107,876) | 18,588 (8434–113,011) | 0.109 |
| IL1RA (pg/mL) | 356 (119–3185) | ↑ 468 (194–1409) | 0.027 |
| TNFα (pg/mL) | 15.8 (2.6–28.6) | ↑ 17.5 (2.6–28.6) | 0.016 |
| CRP (mg/L) | 2 (1–55) | ↑ 4 (1–150) | 0.003 |
| Hemoglobin (g/100 mL) | 14.6 (10.9–17.7) | 14.8 (9.7–17.0) | 0.545 |
| Leukocytes (×109/L) | 6.7 (4.0–15.2) | 7.4 (4.0–21.1) | 0.276 |
| Thrombocytes (×109/L) | 252 (112–441) | 261 (128–759) | 0.458 |
| Mediator, Median (Range) | Upper Main Cluster (n = 66) | Lower Main Cluster (n = 77) | p-Value |
|---|---|---|---|
| gp130 (pg/mL) | 76,526 (40,175–108,957) | ↑ 83,538 (25,184–107,124) | 0.023 |
| IL6Rα (pg/mL) | 31,413 (5119–44,523) | ↑ 34,288 (21,943–47,290) | 0.004 |
| IL6 (pg/mL) | 2.10 (0.20–45.03) | 1.80 (0.36–10.00) | 0.217 |
| IL27 (pg/mL) | 415 (116–1096) | ↑ 485 (116–882) | 0.007 |
| IL31 (pg/mL) | 62.5 (19.4–132.2) | ↑ 75.7 (23.8–123.0) | 0.001 |
| OSM (pg/mL) | 4647 (2487–5799) | 4603 (3165–5747) | 0.786 |
| CNTF (pg/mL) | 1980 (293–13,595) | ↓ 312 (33.8–881.5) | <0.001 |
| IL33Rα (pg/mL) | 17,359 (75.9–113,011) | 18,202 (7462–64,650) | 0.565 |
| IL1RA (pg/mL) | 400 (119–3185) | 361 (194–1152) | 0.111 |
| TNFα (pg/mL) | 14.4 (2.6–26.2) | ↑ 18.2 (2.6–28.6) | <0.001 |
| CRP (mg/L) | 3 (1–150) | 2 (1–39) | 0.128 |
| Hemoglobin (g/100 mL) | 14.6 (11.4–17.0) | 14.8 (9.7–17.7) | 0.502 |
| Leukocyte counts (×109/L) | 7.0 (4.2–21.1) | 6.9 (4.0–16.1) | 0.140 |
| Thrombocyte counts (×109/L) | 257 (112–759) | 250 (128–396) | 0.870 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aarstad, H.H.; Moe, S.E.E.; Bruserud, Ø.; Lybak, S.; Aarstad, H.J.; Tvedt, T.H.A. The Acute Phase Reaction and Its Prognostic Impact in Patients with Head and Neck Squamous Cell Carcinoma: Single Biomarkers Including C-Reactive Protein Versus Biomarker Profiles. Biomedicines 2020, 8, 418. https://doi.org/10.3390/biomedicines8100418
Aarstad HH, Moe SEE, Bruserud Ø, Lybak S, Aarstad HJ, Tvedt THA. The Acute Phase Reaction and Its Prognostic Impact in Patients with Head and Neck Squamous Cell Carcinoma: Single Biomarkers Including C-Reactive Protein Versus Biomarker Profiles. Biomedicines. 2020; 8(10):418. https://doi.org/10.3390/biomedicines8100418
Chicago/Turabian StyleAarstad, Helene Hersvik, Svein Erik Emblem Moe, Øystein Bruserud, Stein Lybak, Hans Jørgen Aarstad, and Tor Henrik Anderson Tvedt. 2020. "The Acute Phase Reaction and Its Prognostic Impact in Patients with Head and Neck Squamous Cell Carcinoma: Single Biomarkers Including C-Reactive Protein Versus Biomarker Profiles" Biomedicines 8, no. 10: 418. https://doi.org/10.3390/biomedicines8100418
APA StyleAarstad, H. H., Moe, S. E. E., Bruserud, Ø., Lybak, S., Aarstad, H. J., & Tvedt, T. H. A. (2020). The Acute Phase Reaction and Its Prognostic Impact in Patients with Head and Neck Squamous Cell Carcinoma: Single Biomarkers Including C-Reactive Protein Versus Biomarker Profiles. Biomedicines, 8(10), 418. https://doi.org/10.3390/biomedicines8100418





