Babam2 Regulates Cell Cycle Progression and Pluripotency in Mouse Embryonic Stem Cells as Revealed by Induced DNA Damage
Abstract
:1. Introduction
2. Experimental Section
2.1. Generation of BABAM2 Knockout mESCs
2.2. Cell Proliferation Assay
2.3. Induction of DNA Damage
2.4. Western Blot Analysis
2.5. Cell Cycle Profiling
2.6. Apoptosis Assays
2.7. Immunofluorescence Staining
2.8. Reverse-Transcript Quantitative PCR
2.9. Alkaline Phosphatase Staining
2.10. Co-Immunoprecipitation
2.11. Ubiquitination Assay
2.12. Statistical Analysis
3. Results
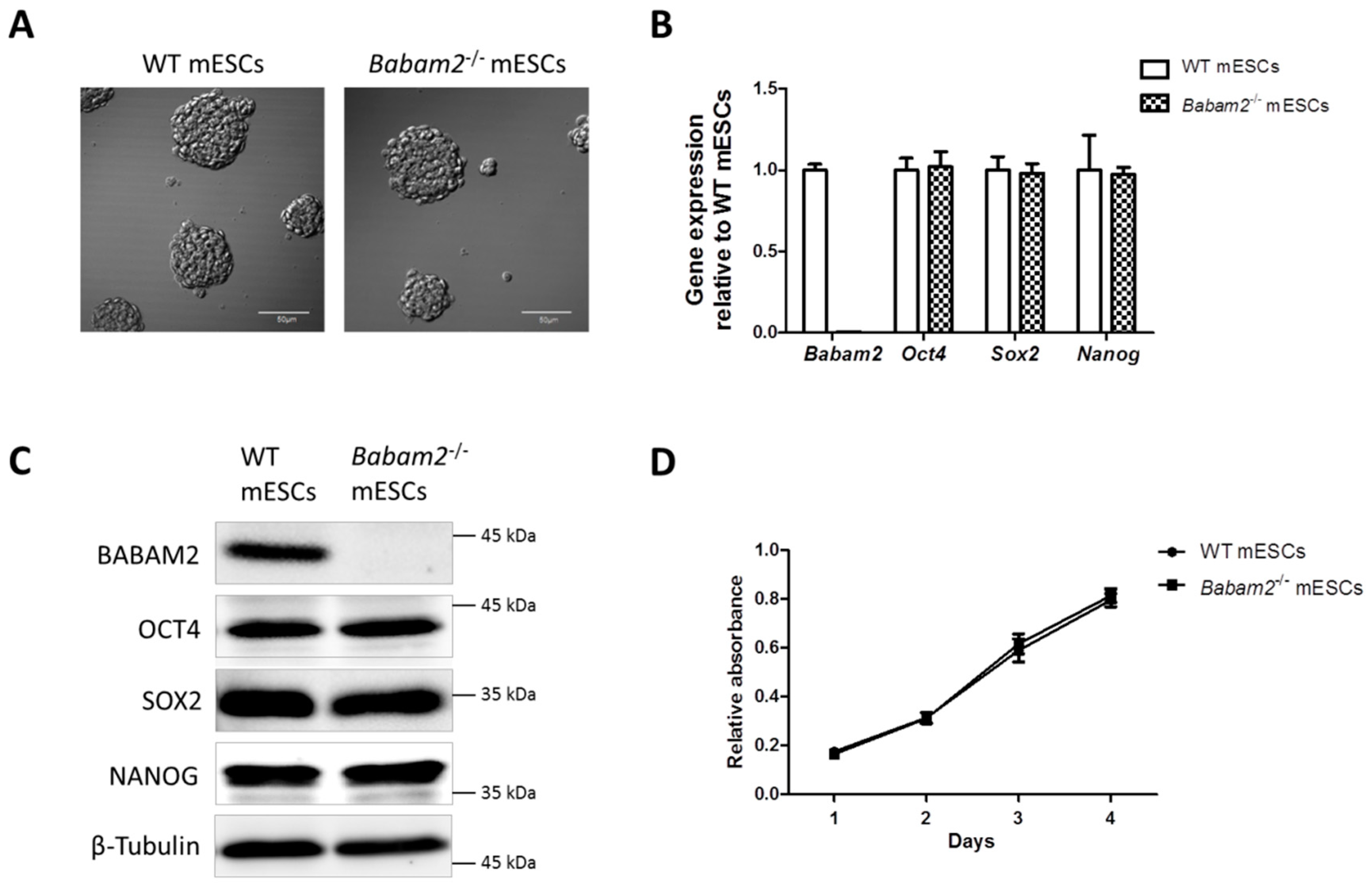
3.1. Absence of Babam2 Expressions Does Not Affect mESCs’ Basal Pluripotency and Proliferation
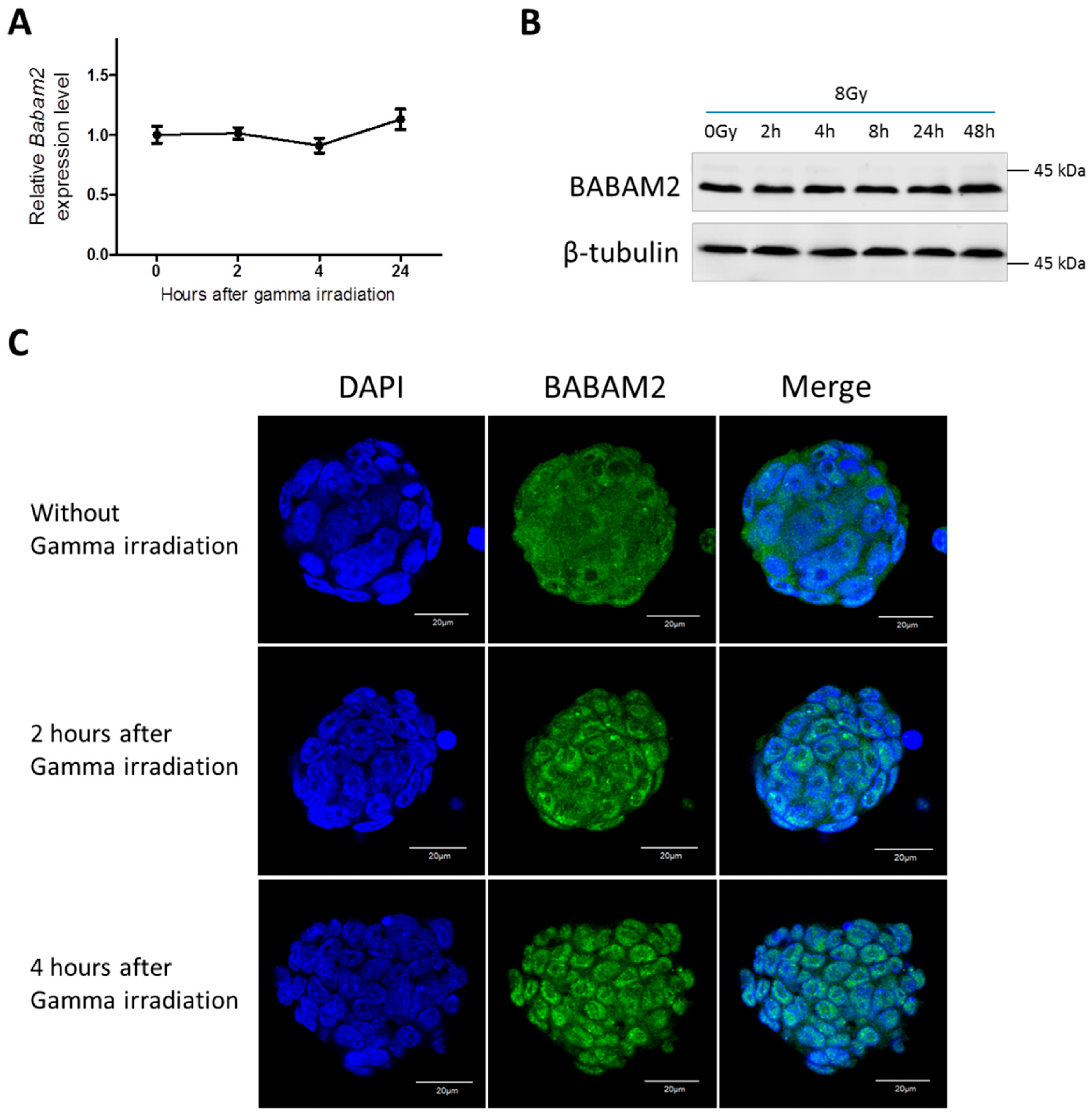
3.2. Gamma Irradiation Treatment Induces Cyto-Nuclear Translocation of BABAM2 in WT mESCs
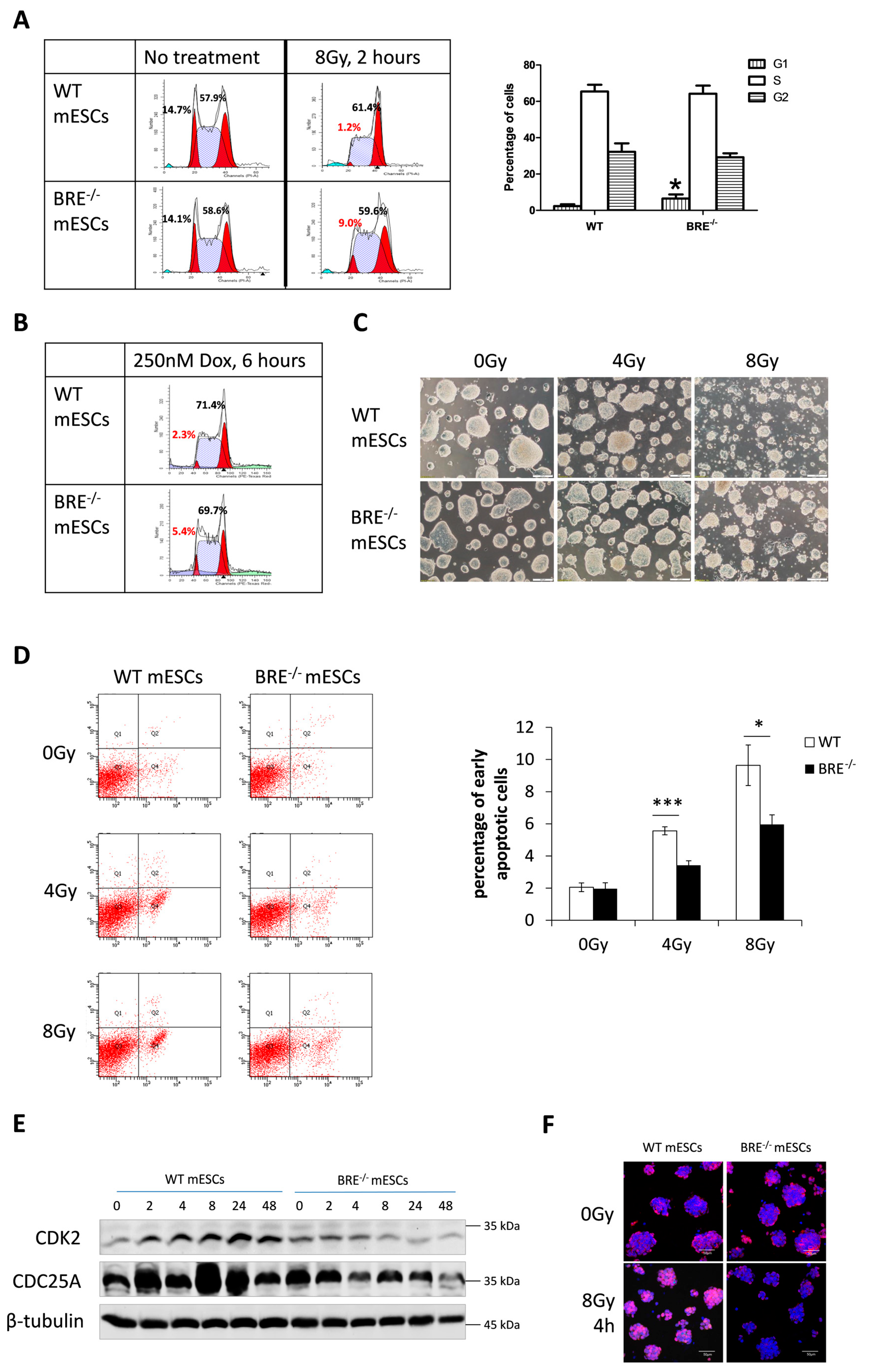
3.3. Prolonged G1 Phase in Babam2−/− mESCs That Attenuates Gamma Irradiation-Induced Apoptotic Response
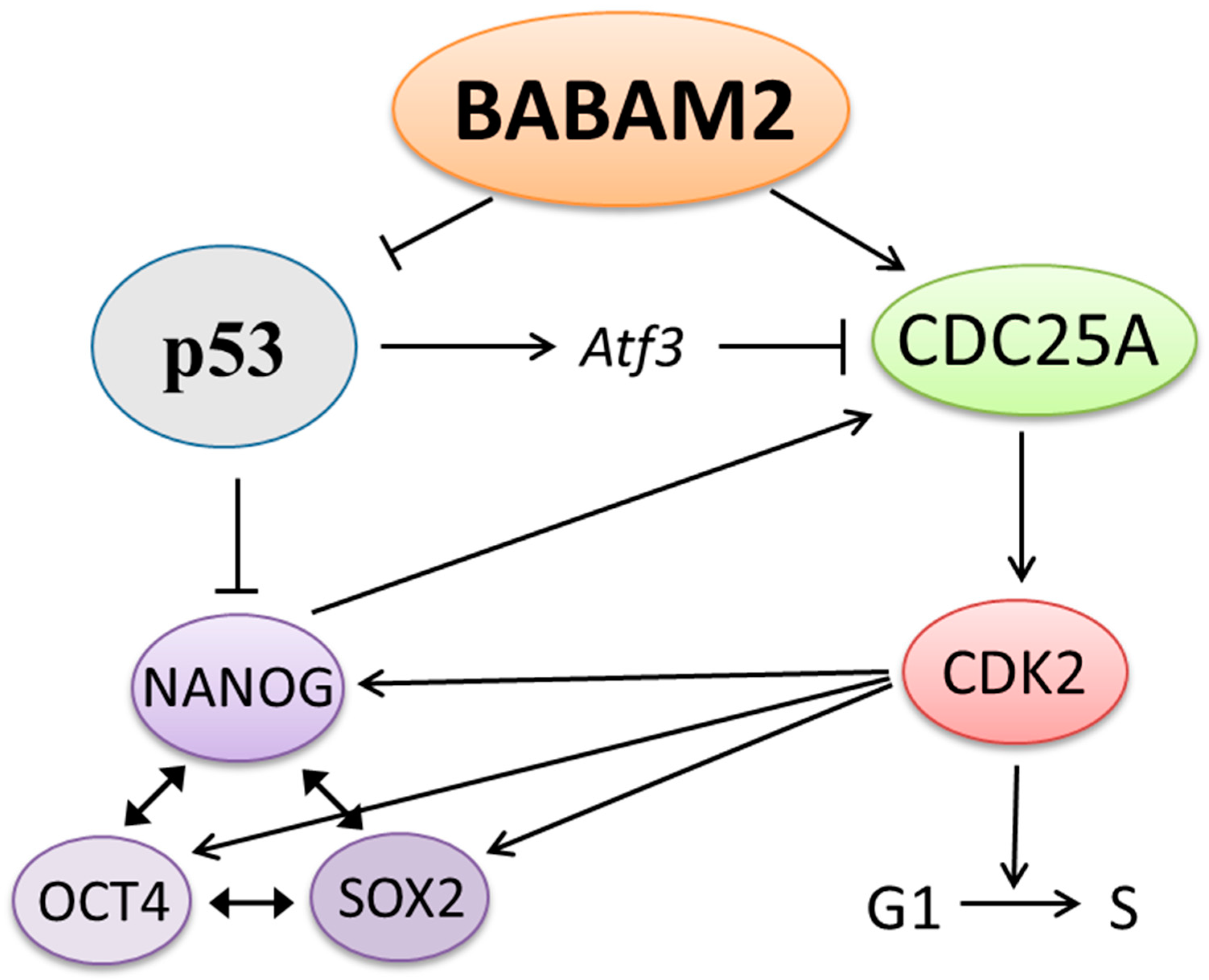
3.4. Decreased CDK2 and CDC25A Expressions in Babam2−/− mESCs Inhibits G1 Phase Progression
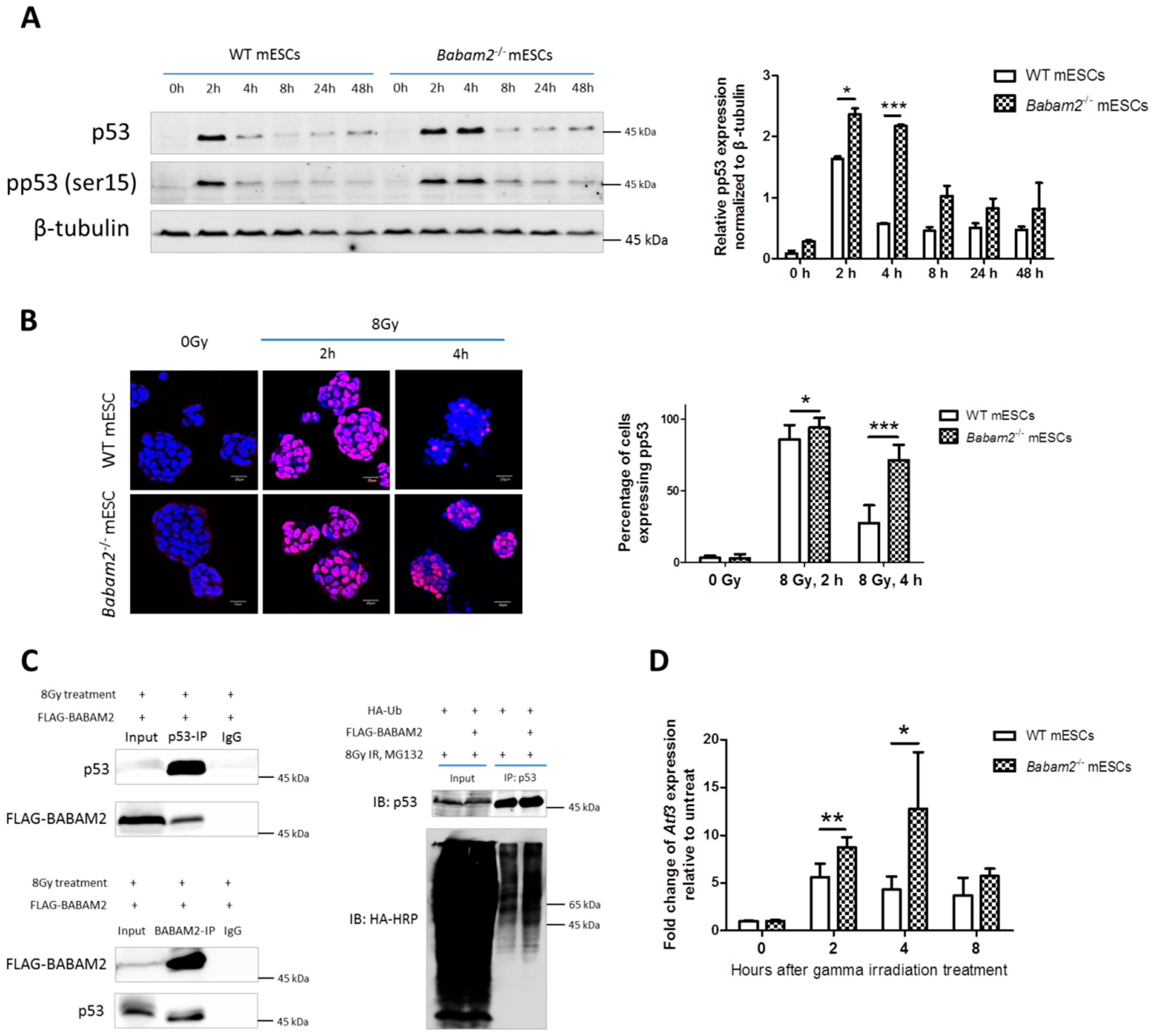
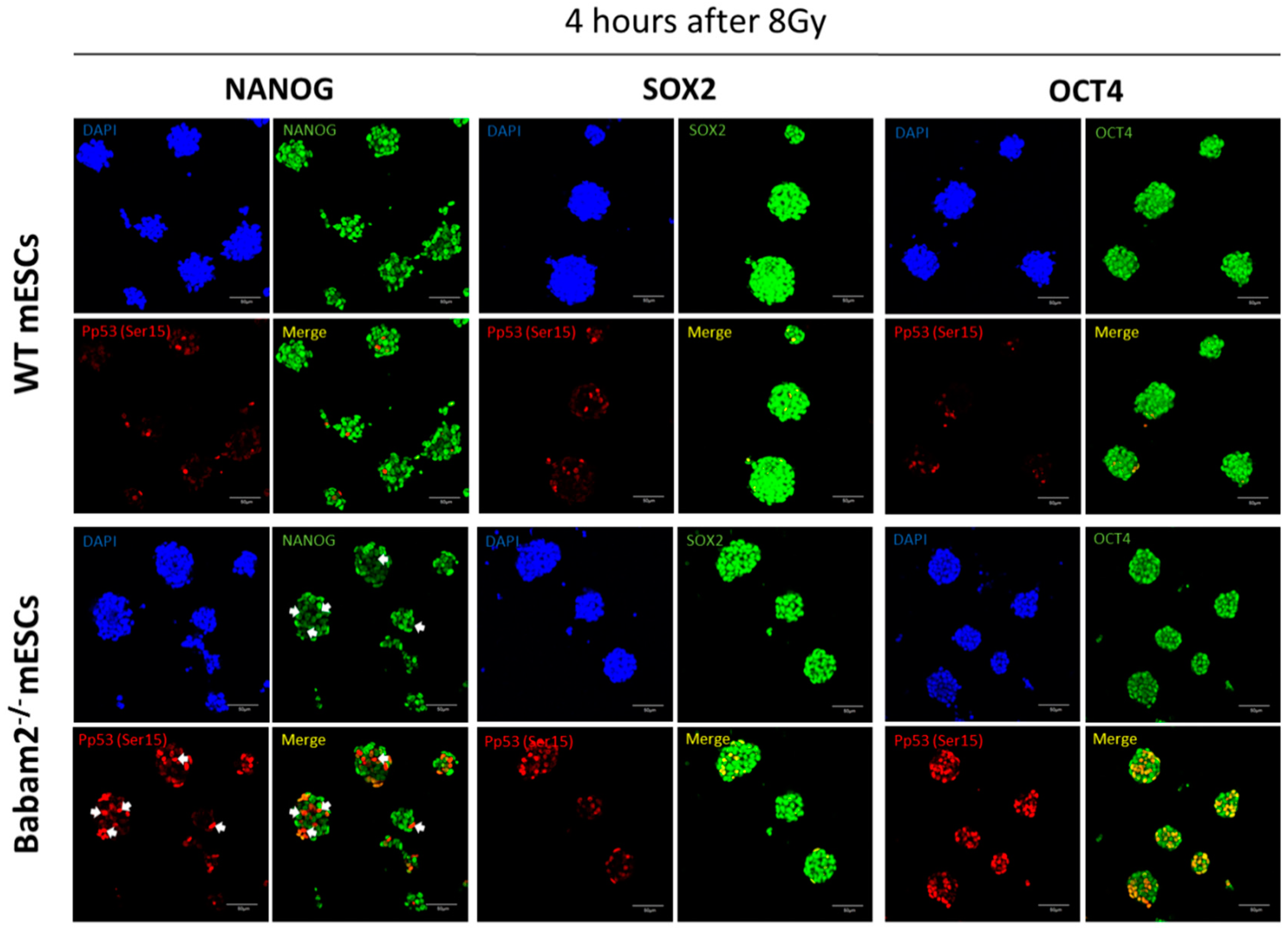
3.5. High and Prolonged Expression of p53 in Gamma-Irradiated Babam2−/− mESCs Inhibits NANOG Expression
3.6. BABAM2 Is Essential for Maintaining Pluripotency in mESCs after Gamma Irradiation Treatment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Babam2 | BRISC and BRCA1-A complex member 2 |
| BRE | Brain and reproductive organ-expressed |
| WT | Wild-type |
| mESCs | Mouse embryonic stem cells |
| CDC25A | Cell division cycle 25 A |
| CDK2 | Cyclin-dependent kinase 2 |
| UEV-like | Ubiquitin-conjugating enzyme family-like |
| USP7 | Ubiquitin-specific-processing protease 7 |
| Mdm2 | Mouse double minute 2 |
| OCT4 | Octamer-binding transcription factor 4 |
| SOX2 | Sex-determining region Y-box 2 |
| NANOG | Nanog homeobox |
| p53 | Protein 53 |
| p21 | Cyclin-dependent kinase inhibitor 1A |
| ATF3 | Activating transcription factor 3 |
| Gata4 | GATA binding protein 4 |
| GSC | Goosecoid homeobox |
| Fgf5 | Fibroblast growth factor 5 |
| Otx2 | Orthodenticle homeobox 2 |
| HA | Hemaglutinin antigen |
| RT-qPCR | Reverse transcription-quantitative polymerase chain reaction |
| cDNA | Complementary DNA |
| PBS | Phosphate buffered saline |
| PBST | 0.1% Tween-20 in PBS |
| PMSF | Phenylmethylsulfonyl fluoride |
| DTT | Dithiothreitol |
| SD | Standard deviation |
References
- Li, L.; Yoo, H.; Becker, F.F.; Ali-Osman, F.; Chan, J.Y. Identification of a brain- and reproductive-organs-specific gene responsive to DNA damage and retinoic acid. Biochem. Biophys. Res. Commun. 1995, 206, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Ching, A.K.; Li, P.S.; Li, Q.; Chan, B.C.; Chan, J.Y.; Lim, P.L.; Pang, J.C.; Chui, Y.L. Expression of human BRE in multiple isoforms. Biochem. Biophys. Res. Commun. 2001, 288, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.; Tang, M.K.; Yao, Y.; Yau, W.W.; Lo, L.M.; Yang, X.; Chui, Y.L.; Chan, J.; Lee, K.K. Silencing BRE expression in human umbilical cord perivascular (HUCPV) progenitor cells accelerates osteogenic and chondrogenic differentiation. PLoS ONE 2013, 8, e67896. [Google Scholar] [CrossRef] [Green Version]
- Shi, W.; Tang, M.K.; Yao, Y.; Tang, C.; Chui, Y.L.; Lee, K.K. BRE plays an essential role in preventing replicative and DNA damage-induced premature senescence. Sci. Rep. 2016, 6, 23506. [Google Scholar] [CrossRef] [PubMed]
- Biswas, K.; Philip, S.; Yadav, A.; Martin, B.K.; Burkett, S.; Singh, V.; Babbar, A.; North, S.L.; Chang, S.; Sharan, S.K. BRE/BRCC45 regulates CDC25A stability by recruiting USP7 in response to DNA damage. Nat. Commun. 2018, 9, 537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, F.; Wang, Y.; Wang, X.; Wu, Y.; Wang, X.; Liu, Q.; Zhu, Y.; Liu, E.; Fan, J.; Wang, Y. Bre enhances osteoblastic differentiation by promoting the mdm2-mediated degradation of p53. Stem Cells 2017, 35, 1760–1772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, B.C.; Ching, A.K.; To, K.F.; Leung, J.C.; Chen, S.; Li, Q.; Lai, P.B.; Tang, N.L.; Shaw, P.C.; Chan, J.Y.; et al. BRE is an antiapoptotic protein in vivo and overexpressed in human hepatocellular carcinoma. Oncogene 2008, 27, 1208–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.B.; Pan, K.; Tang, M.K.; Chui, Y.L.; Chen, L.; Su, Z.J.; Shen, Z.Y.; Li, E.M.; Xie, W.; Lee, K.K. Comparative proteomic analysis reveals differentially expressed proteins regulated by a potential tumor promoter, BRE, in human esophageal carcinoma cells. Biochem. Cell Biol. 2008, 86, 302–311. [Google Scholar] [CrossRef]
- Chui, Y.L.; Ching, A.K.; Chen, S.; Yip, F.P.; Rowlands, D.K.; James, A.E.; Lee, K.K.; Chan, J.Y. BRE over-expression promotes growth of hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 2010, 391, 1522–1525. [Google Scholar] [CrossRef]
- White, J.; Dalton, S. Cell cycle control of embryonic stem cells. Stem Cell Rev. 2005, 1, 131–138. [Google Scholar] [CrossRef]
- Stead, E.; White, J.; Faast, R.; Conn, S.; Goldstone, S.; Rathjen, J.; Dhingra, U.; Rathjen, P.; Walker, D.; Dalton, S. Pluripotent cell division cycles are driven by ectopic Cdk2, cyclin A/E and E2F activities. Oncogene 2002, 21, 8320–8333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suvorova, I.I.; Grigorash, B.B.; Chuykin, I.A.; Pospelova, T.V.; Pospelov, V.A. G1 checkpoint is compromised in mouse ESCs due to functional uncoupling of p53–p21Waf1 signaling. Cell Cycle 2016, 15, 52–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, Y.; Cervantes, R.B.; Tichy, E.; Tischfield, J.A.; Stambrook, P.J. Protecting genomic integrity in somatic cells and embryonic stem cells. Mutat. Res. 2007, 614, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Koledova, Z.; Kafkova, L.R.; Calabkova, L.; Krystof, V.; Dolezel, P.; Divoky, V. Cdk2 inhibition prolongs G1 phase progression in mouse embryonic stem cells. Stem Cells Dev. 2010, 19, 181–194. [Google Scholar] [CrossRef]
- Hong, Y.; Stambrook, P.J. Restoration of an absent G1 arrest and protection from apoptosis in embryonic stem cells after ionizing radiation. Proc. Natl. Acad. Sci. USA 2004, 101, 14443–14448. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Michowski, W.; Inuzuka, H.; Shimizu, K.; Nihira, N.T.; Chick, J.M.; Li, N.; Geng, Y.; Meng, A.Y.; Ordureau, A.; et al. G1 cyclins link proliferation, pluripotency and differentiation of embryonic stem cells. Nat. Cell Biol. 2017, 19, 177–188. [Google Scholar] [CrossRef]
- Ouyang, J.; Yu, W.; Liu, J.; Zhang, N.; Florens, L.; Chen, J.; Liu, H.; Washburn, M.; Pei, D.; Xie, T. Cyclin-dependent kinase-mediated Sox2 phosphorylation enhances the ability of Sox2 to establish the pluripotent state. J. Biol. Chem. 2015, 290, 22782–22794. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.; Chao, C.; Saito, S.; Mazur, S.J.; Murphy, M.E.; Appella, E.; Xu, Y. p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression. Nat. Cell Biol. 2005, 7, 165–171. [Google Scholar] [CrossRef]
- Solozobova, V.; Rolletschek, A.; Blattner, C. Nuclear accumulation and activation of p53 in embryonic stem cells after DNA damage. BMC Cell Biol. 2009, 10, 46. [Google Scholar] [CrossRef] [Green Version]
- Karimian, A.; Ahmadi, Y.; Yousefi, B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair 2016, 42, 63–71. [Google Scholar] [CrossRef]
- Rother, K.; Kirschner, R.; Sanger, K.; Bohlig, L.; Mossner, J.; Engeland, K. p53 downregulates expression of the G1/S cell cycle phosphatase Cdc25A. Oncogene 2007, 26, 1949–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demidova, A.R.; Aau, M.Y.; Zhuang, L.; Yu, Q. Dual regulation of Cdc25A by Chk1 and p53-ATF3 in DNA replication checkpoint control. J. Biol. Chem. 2009, 284, 4132–4139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, T.; Huang, S. The role of Cdc25A in the regulation of cell proliferation and apoptosis. Anti-Cancer Agents Med. Chem. 2012, 12, 631–639. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Wang, C.; Dai, Q.; Li, F.; Bergholz, J.; Li, Z.; Li, Q.; Xiao, Z.X. p53 and p73 regulate apoptosis but not cell-cycle progression in mouse embryonic stem cells upon DNA damage and differentiation. Stem Cell Rep. 2016, 7, 1087–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aladjem, M.I.; Spike, B.T.; Rodewald, L.W.; Hope, T.J.; Klemm, M.; Jaenisch, R.; Wahl, G.M. ES cells do not activate p53-dependent stress responses and undergo p53-independent apoptosis in response to DNA damage. Curr. Biol. 1998, 8, 145–155. [Google Scholar] [CrossRef] [Green Version]
- Corbet, S.W.; Clarke, A.R.; Gledhill, S.; Wyllie, A.H. P53-dependent and -independent links between DNA-damage, apoptosis and mutation frequency in ES cells. Oncogene 1999, 18, 1537–1544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, L.; Lee, K.K. BRE facilitates skeletal muscle regeneration by promoting satellite cell motility and differentiation. Biol. Open 2016, 5, 100–111. [Google Scholar] [CrossRef] [Green Version]
- Koledova, Z.; Kafkova, L.R.; Kramer, A.; Divoky, V. DNA damage-induced degradation of Cdc25A does not lead to inhibition of Cdk2 activity in mouse embryonic stem cells. Stem Cells 2010, 28, 450–461. [Google Scholar] [CrossRef]
- Gubanova, E.; Issaeva, N.; Gokturk, C.; Djureinovic, T.; Helleday, T. SMG-1 suppresses CDK2 and tumor growth by regulating both the p53 and Cdc25A signaling pathways. Cell Cycle 2013, 12, 3770–3780. [Google Scholar] [CrossRef] [Green Version]
- Stefkova, K.; Prochazkova, J.; Pachernik, J. Alkaline phosphatase in stem cells. Stem Cells Int. 2015, 2015, 628368. [Google Scholar] [CrossRef] [Green Version]
- Yeo, J.C.; Ng, H.H. The transcriptional regulation of pluripotency. Cell Res. 2013, 23, 20–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olariu, V.; Lovkvist, C.; Sneppen, K. Nanog, Oct4 and Tet1 interplay in establishing pluripotency. Sci. Rep. 2016, 6, 25438. [Google Scholar] [CrossRef] [PubMed]
- Chambers, I.; Colby, D.; Robertson, M.; Nichols, J.; Lee, S.; Tweedie, S.; Smith, A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell 2003, 113, 643–655. [Google Scholar] [CrossRef] [Green Version]
- Mitsui, K.; Tokuzawa, Y.; Itoh, H.; Segawa, K.; Murakami, M.; Takahashi, K.; Maruyama, M.; Maeda, M.; Yamanaka, S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell 2003, 113, 631–642. [Google Scholar] [CrossRef] [Green Version]
- Loh, Y.H.; Wu, Q.; Chew, J.L.; Vega, V.B.; Zhang, W.; Chen, X.; Bourque, G.; George, J.; Leong, B.; Liu, J.; et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat. Genet. 2006, 38, 431–440. [Google Scholar] [CrossRef]
- Zhang, X.; Neganova, I.; Przyborski, S.; Yang, C.; Cooke, M.; Atkinson, S.P.; Anyfantis, G.; Fenyk, S.; Keith, W.N.; Hoare, S.F.; et al. A role for NANOG in G1 to S transition in human embryonic stem cells through direct binding of CDK6 and CDC25A. J. Cell Biol. 2009, 184, 67–82. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, C.Y.T.; Lo, P.H.Y.; Lee, K.K.H. Babam2 Regulates Cell Cycle Progression and Pluripotency in Mouse Embryonic Stem Cells as Revealed by Induced DNA Damage. Biomedicines 2020, 8, 397. https://doi.org/10.3390/biomedicines8100397
Chung CYT, Lo PHY, Lee KKH. Babam2 Regulates Cell Cycle Progression and Pluripotency in Mouse Embryonic Stem Cells as Revealed by Induced DNA Damage. Biomedicines. 2020; 8(10):397. https://doi.org/10.3390/biomedicines8100397
Chicago/Turabian StyleChung, Cheuk Yiu Tenny, Paulisally Hau Yi Lo, and Kenneth Ka Ho Lee. 2020. "Babam2 Regulates Cell Cycle Progression and Pluripotency in Mouse Embryonic Stem Cells as Revealed by Induced DNA Damage" Biomedicines 8, no. 10: 397. https://doi.org/10.3390/biomedicines8100397
APA StyleChung, C. Y. T., Lo, P. H. Y., & Lee, K. K. H. (2020). Babam2 Regulates Cell Cycle Progression and Pluripotency in Mouse Embryonic Stem Cells as Revealed by Induced DNA Damage. Biomedicines, 8(10), 397. https://doi.org/10.3390/biomedicines8100397





