Vav1 Down-Modulates Akt2 Expression in Cells from Pancreatic Ductal Adenocarcinoma: Nuclear Vav1 as a Potential Regulator of Akt Related Malignancy in Pancreatic Cancer
Abstract
1. Introduction
2. Experimental Section
2.1. Cell Lines and Tissue Samples
2.2. Modulation of Vav1 Expression and Immunochemical Analysis
2.3. Real-Time Cell Assays of Migration and Invasion
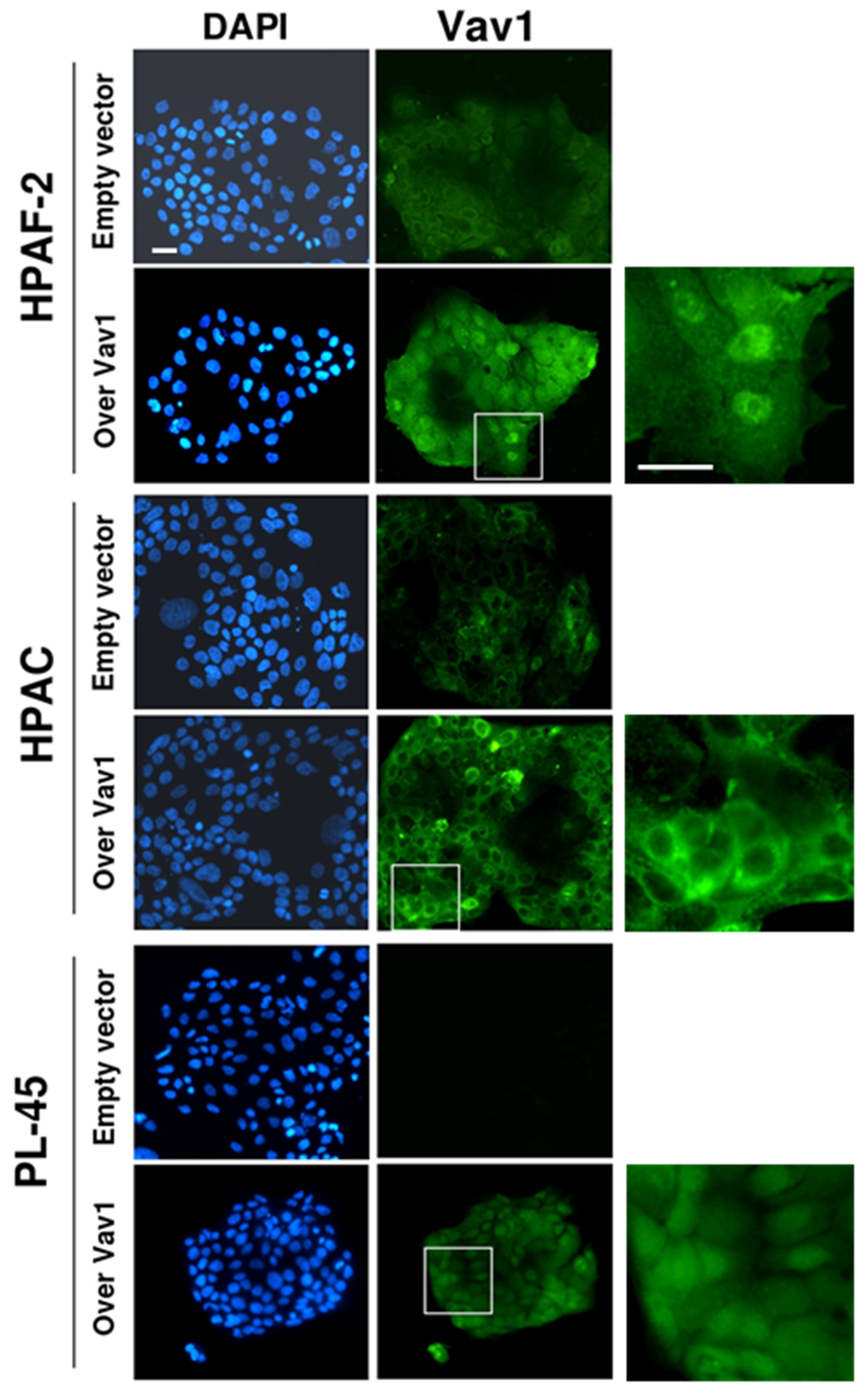
2.4. Immunocytochemical and Confocal Analysis
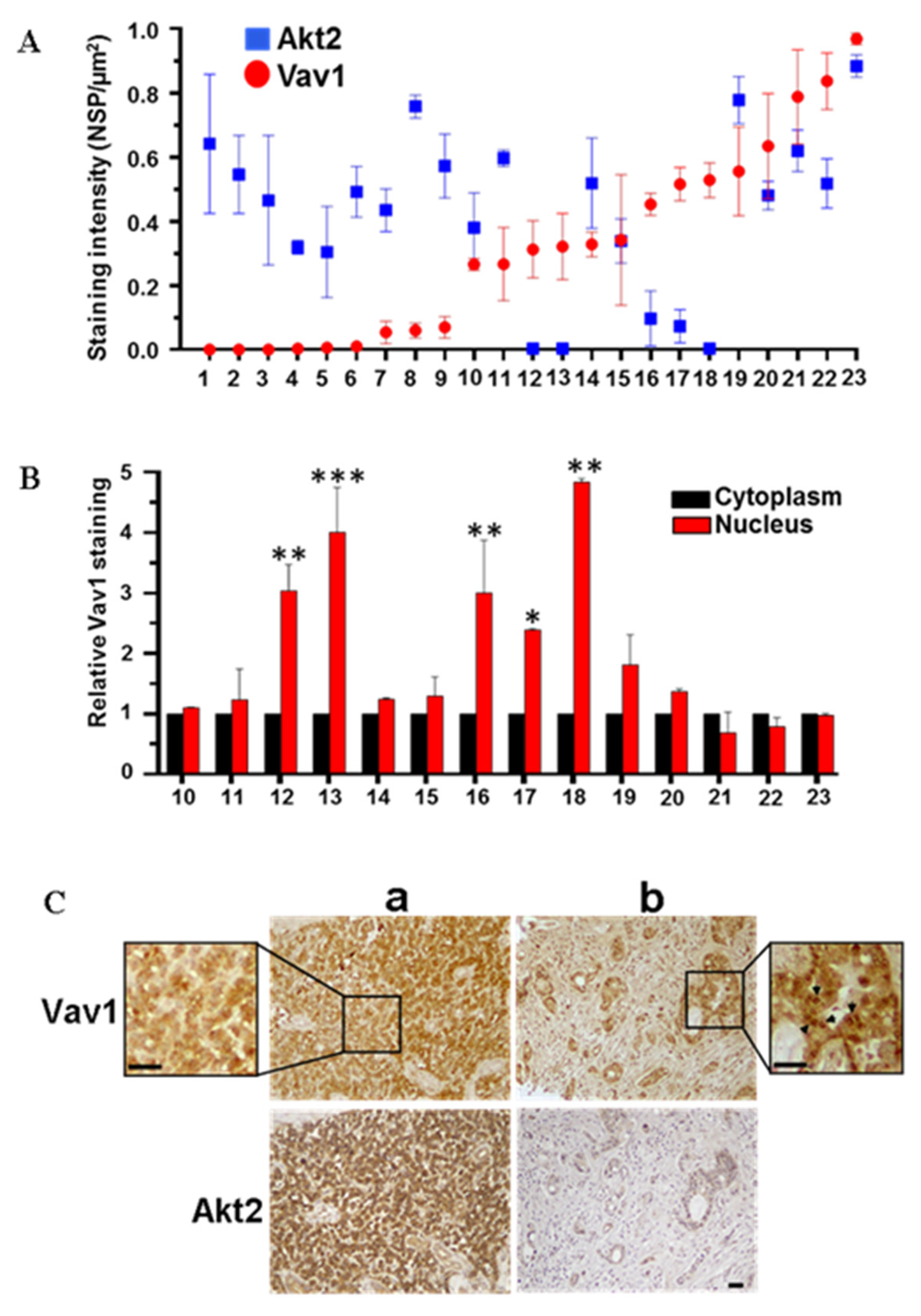
2.5. Immunohistochemical Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Vav1 Down-Modulates Motility and Akt2 Expression in PDAC-Derived Cell Lines
3.2. Akt2 Expression Negatively Correlates with Nuclear Vav1 in PDAC-Derived Cell Lines and Tissues
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Elaileh, A.; Saharia, A.; Potter, L.; Baio, F.; Ghafel, A.; Abdelrahim, M.; Heyne, K. Promising new treatments for pancreatic cancer in the era of targeted and immune therapies. Am. J. Cancer Res. 2019, 9, 1871–1888. [Google Scholar] [PubMed]
- Schlieman, M.G.; Fahy, B.N.; Ramsamooj, R.; Beckett, L.; Bold, R.J. Incidence, mechanism and prognostic value of activated AKT in pancreas cancer. Br. J. Cancer 2003, 89, 2110–2115. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Tomita, Y.; Hoshida, Y.; Morooka, T.; Nagano, H.; Dono, K.; Umeshita, K.; Sakon, M.; Ishikawa, O.; Ohigashi, H.; et al. Prognostic significance of activated Akt expression in pancreatic ductal adenocarcinoma. Clin. Cancer Res. 2004, 10, 2846–2850. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, S.; Hosseini, M.; Shahidsales, S.; Maftouh, M.; Ferns, G.A.; Ghayour-Mobarhan, M.; Hassanian, S.M.; Avan, A. Targeting the Akt/PI3K Signaling Pathway as a Potential Therapeutic Strategy for the Treatment of Pancreatic Cancer. Curr. Med. Chem. 2017, 24, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Banno, E.; Togashi, Y.; de Velasco, M.A.; Mizukami, T.; Nakamura, Y.; Terashima, M.; Sakai, K.; Fujita, Y.; Kamata, K.; Kitano, M.; et al. Clinical significance of Akt2 in advanced pancreatic cancer treated with erlotinib. Int. J. Oncol. 2017, 50, 2049–2058. [Google Scholar] [CrossRef] [PubMed]
- Grassilli, S.; Brugnoli, F.; Lattanzio, R.; Marchisio, M.; Perracchio, L.; Piantelli, M.; Bavelloni, A.; Capitani, S.; Bertagnolo, V. Vav1 downmodulates Akt in different breast cancer subtypes: A new promising chance to improve breast cancer outcome. Mol. Oncol. 2018, 12, 1012–1025. [Google Scholar] [CrossRef]
- Tybulewicz, V.L. Vav-family proteins in T-cell signalling. Curr. Opin Immunol. 2005, 17, 267–274. [Google Scholar] [CrossRef]
- Farago, M.; Yarnitzky, T.; Shalom, B.; Katzav, S. Vav1 mutations: What makes them oncogenic? Cell Signal 2020, 65, 109438. [Google Scholar] [CrossRef]
- Fernandez-Zapico, M.E.; Gonzalez-Paz, N.C.; Weiss, E.; Savoy, D.N.; Molina, J.R.; Fonseca, R.; Smyrk, T.C.; Chari, S.T.; Urrutia, R.; Billadeau, D.D. Ectopic expression of VAV1 reveals an unexpected role in pancreatic cancer tumorigenesis. Cancer Cell 2005, 7, 39–49. [Google Scholar] [CrossRef]
- Razidlo, G.L.; Magnine, C.; Sletten, A.C.; Hurley, R.M.; Almada, L.L.; Fernandez-Zapico, M.E.; Ji, B.; McNiven, M.A. Targeting pancreatic cancer metastasis by inhibition of Vav1, a driver of tumor cell invasion. Cancer Res. 2015, 75, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.H.; Lu, P.J.; Ding, L.Y.; Chu, P.C.; Hsu, W.Y.; Chen, C.S.; Tsao, C.C.; Chen, B.H.; Lee, C.T.; Shan, Y.S.; et al. TGFbeta promotes mesenchymal phenotype of pancreatic cancer cells, in part, through epigenetic activation of VAV1. Oncogene 2017, 36, 2202–2214. [Google Scholar] [CrossRef] [PubMed]
- Grassilli, S.; Brugnoli, F.; Lattanzio, R.; Rossi, C.; Perracchio, L.; Mottolese, M.; Marchisio, M.; Palomba, M.; Nika, E.; Natali, P.G.; et al. High nuclear level of Vav1 is a positive prognostic factor in early invasive breast tumors: A role in modulating genes related to the efficiency of metastatic process. Oncotarget 2014, 5, 4320–4336. [Google Scholar] [CrossRef] [PubMed]
- Bertagnolo, V.; Brugnoli, F.; Grassilli, S.; Nika, E.; Capitani, S. Vav1 in differentiation of tumoral promyelocytes. Cell Signal 2012, 24, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Vezzali, F.; Grassilli, S.; Lambertini, E.; Brugnoli, F.; Patergnani, S.; Nika, E.; Piva, R.; Pinton, P.; Capitani, S.; Bertagnolo, V. Vav1 is necessary for PU.1 mediated upmodulation of miR-29b in acute myeloid leukaemia-derived cells. J. Cell Mol. Med. 2018, 22, 3149–3158. [Google Scholar] [CrossRef] [PubMed]
- Al-Qassab, Y.; Grassilli, S.; Brugnoli, F.; Vezzali, F.; Capitani, S.; Bertagnolo, V. Protective role of all-trans retinoic acid (ATRA) against hypoxia-induced malignant potential of non-invasive breast tumor derived cells. BMC Cancer 2018, 18, 1194. [Google Scholar] [CrossRef]
- LoRusso, P.M. Inhibition of the PI3K/AKT/mTOR Pathway in Solid Tumors. J. Clin. Oncol. 2016, 34, 3803–3815. [Google Scholar] [CrossRef] [PubMed]
- Nitulescu, G.M.; Van De Venter, M.; Nitulescu, G.; Ungurianu, A.; Juzenas, P.; Peng, Q.; Olaru, O.T.; Gradinaru, D.; Tsatsakis, A.; Tsoukalas, D.; et al. The Akt pathway in oncology therapy and beyond (Review). Int. J. Oncol. 2018, 53, 2319–2331. [Google Scholar] [CrossRef]
- Iida, M.; Harari, P.M.; Wheeler, D.L.; Toulany, M. Targeting AKT/PKB to improve treatment outcomes for solid tumors. Mutat. Res. 2020, 819–820, 111690. [Google Scholar] [CrossRef]
- Dillon, R.L.; Marcotte, R.; Hennessy, B.T.; Woodgett, J.R.; Mills, G.B.; Muller, W.J. Akt1 and akt2 play distinct roles in the initiation and metastatic phases of mammary tumor progression. Cancer Res. 2009, 69, 5057–5064. [Google Scholar] [CrossRef]
- Honardoost, M.; Rad, S. Triangle of AKT2, miRNA, and Tumorigenesis in Different Cancers. Appl. Biochem. Biotechnol. 2018, 185, 524–540. [Google Scholar] [CrossRef] [PubMed]
- Hinz, N.; Jucker, M. Distinct functions of AKT isoforms in breast cancer: A comprehensive review. Cell Commun. Signal. 2019, 17, 154. [Google Scholar] [CrossRef] [PubMed]
- Altomare, D.A.; Tanno, S.; De Rienzo, A.; Klein-Szanto, A.J.; Tanno, S.; Skele, K.L.; Hoffman, J.P.; Testa, J.R. Frequent activation of AKT2 kinase in human pancreatic carcinomas. J. Cell Biochem. 2002, 87, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Katzav, S. Vav1: A Dr. Jekyll and Mr. Hyde protein—Good for the hematopoietic system, bad for cancer. Oncotarget 2015, 6, 28731–28742. [Google Scholar] [CrossRef] [PubMed]
- Sebban, S.; Farago, M.; Gashai, D.; Ilan, L.; Pikarsky, E.; Ben-Porath, I.; Katzav, S. Vav1 fine tunes p53 control of apoptosis versus proliferation in breast cancer. PLoS ONE 2013, 8, e54321. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, T.; Wang, C.; Jin, X.; Jia, C.; Yu, S.; Chen, J. MiRNA-615-5p functions as a tumor suppressor in pancreatic ductal adenocarcinoma by targeting AKT2. PLoS ONE 2015, 10, e0119783. [Google Scholar]
- Shankar, J.; Messenberg, A.; Chan, J.; Underhill, T.M.; Foster, L.J.; Nabi, I.R. Pseudopodial actin dynamics control epithelial-mesenchymal transition in metastatic cancer cells. Cancer Res. 2010, 70, 3780–3790. [Google Scholar] [CrossRef]
- Bertagnolo, V.; Grassilli, S.; Petretto, A.; Lambertini, E.; Astati, L.; Bruschi, M.; Brugnoli, F.; Nika, E.; Candiano, G.; Piva, R.; et al. Nuclear proteome analysis reveals a role of Vav1 in modulating RNA processing during maturation of tumoral promyelocytes. J. Proteom. 2011, 75, 398–409. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grassilli, S.; Brugnoli, F.; Lattanzio, R.; Buglioni, S.; Bertagnolo, V. Vav1 Down-Modulates Akt2 Expression in Cells from Pancreatic Ductal Adenocarcinoma: Nuclear Vav1 as a Potential Regulator of Akt Related Malignancy in Pancreatic Cancer. Biomedicines 2020, 8, 379. https://doi.org/10.3390/biomedicines8100379
Grassilli S, Brugnoli F, Lattanzio R, Buglioni S, Bertagnolo V. Vav1 Down-Modulates Akt2 Expression in Cells from Pancreatic Ductal Adenocarcinoma: Nuclear Vav1 as a Potential Regulator of Akt Related Malignancy in Pancreatic Cancer. Biomedicines. 2020; 8(10):379. https://doi.org/10.3390/biomedicines8100379
Chicago/Turabian StyleGrassilli, Silvia, Federica Brugnoli, Rossano Lattanzio, Simonetta Buglioni, and Valeria Bertagnolo. 2020. "Vav1 Down-Modulates Akt2 Expression in Cells from Pancreatic Ductal Adenocarcinoma: Nuclear Vav1 as a Potential Regulator of Akt Related Malignancy in Pancreatic Cancer" Biomedicines 8, no. 10: 379. https://doi.org/10.3390/biomedicines8100379
APA StyleGrassilli, S., Brugnoli, F., Lattanzio, R., Buglioni, S., & Bertagnolo, V. (2020). Vav1 Down-Modulates Akt2 Expression in Cells from Pancreatic Ductal Adenocarcinoma: Nuclear Vav1 as a Potential Regulator of Akt Related Malignancy in Pancreatic Cancer. Biomedicines, 8(10), 379. https://doi.org/10.3390/biomedicines8100379






