Comparative Evaluation of the Angiogenic Potential of Hypoxia Preconditioned Blood-Derived Secretomes and Platelet-Rich Plasma: An In Vitro Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Platelet-Derived Secretomes—Platelet Rich Plasma (PRP)
2.2. Preparation of Hypoxia Preconditioned Secretomes—Hypoxia Preconditioned Serum (HPS)/Plasma (HPP)
2.3. Preparation of Blood-Derived Secretomes
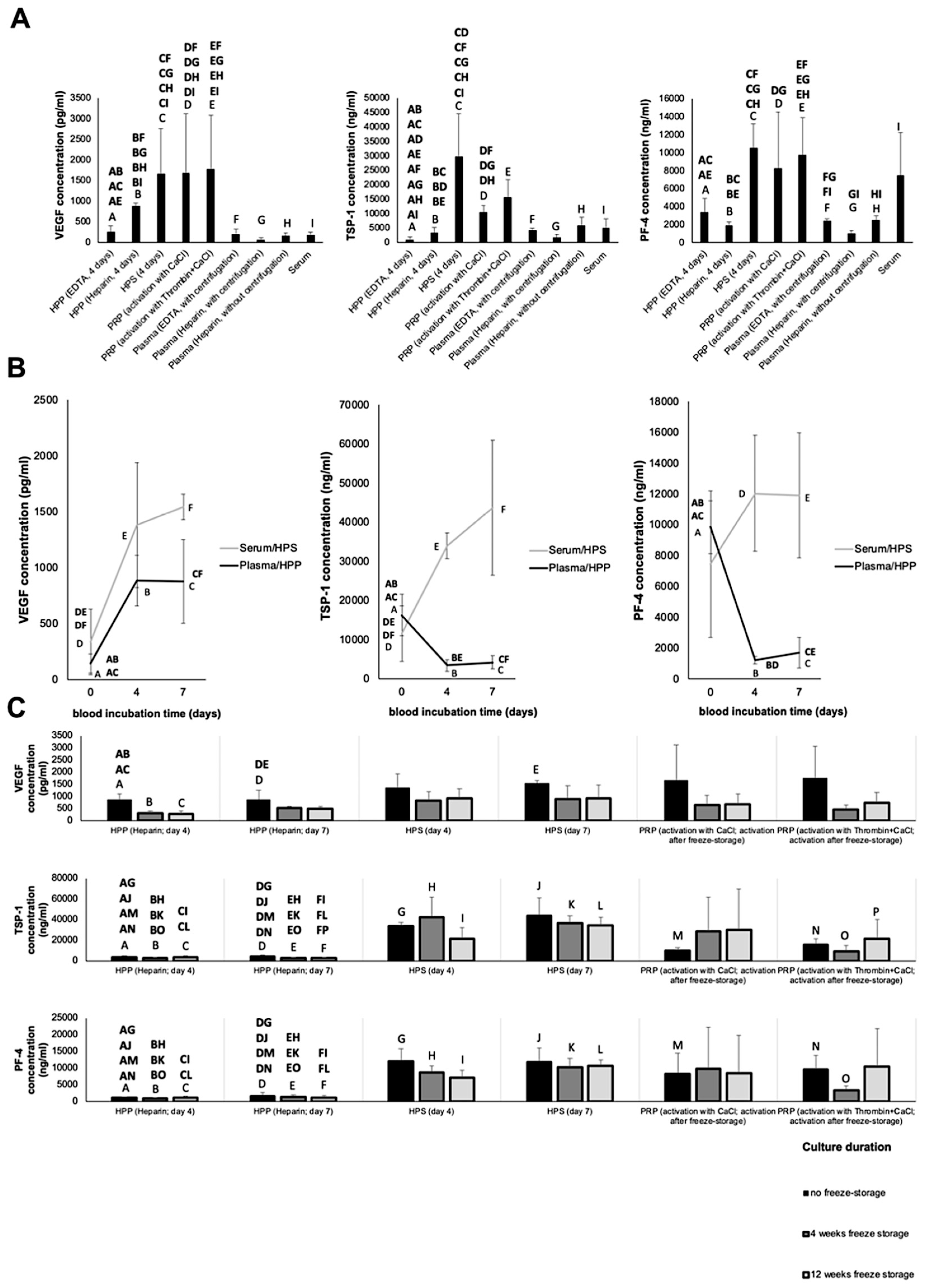
2.4. Quantitative Analysis of VEGF, TSP-1 and PF-4 Concentration in Blood-Derived Secretomes
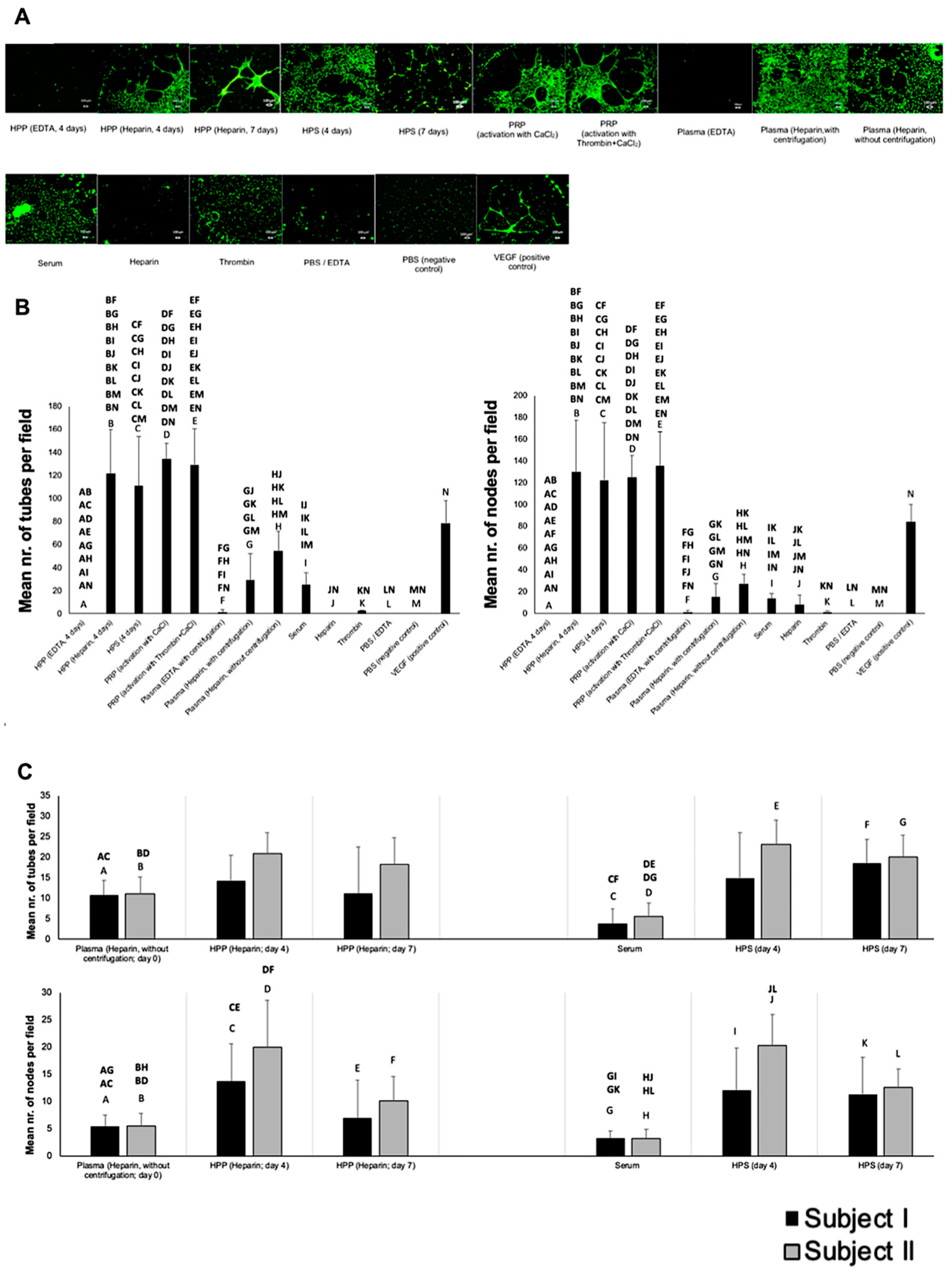
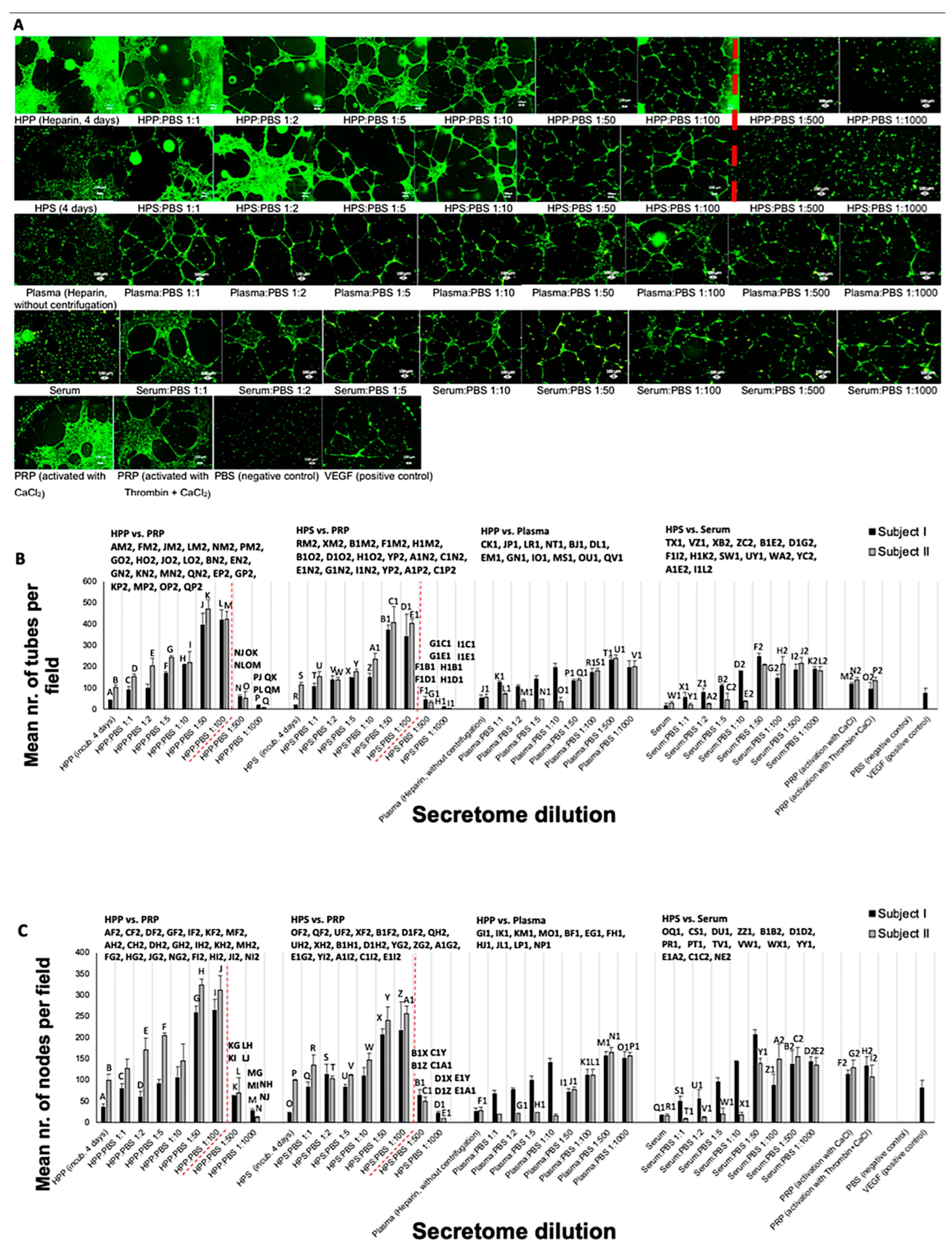
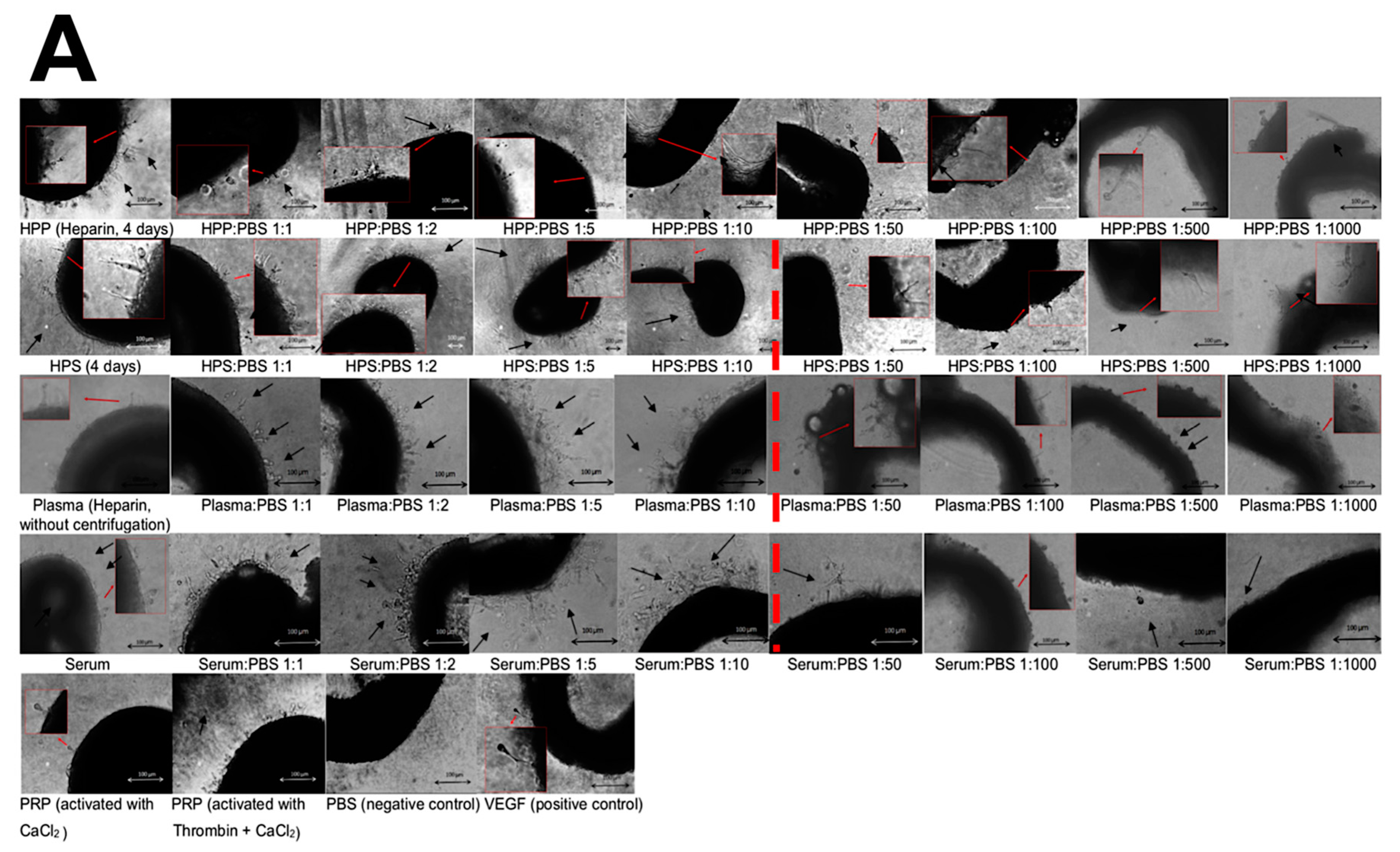
2.5. Analysis of the Effect of Blood-Derived Secretomes on In Vitro Microvessel Formation
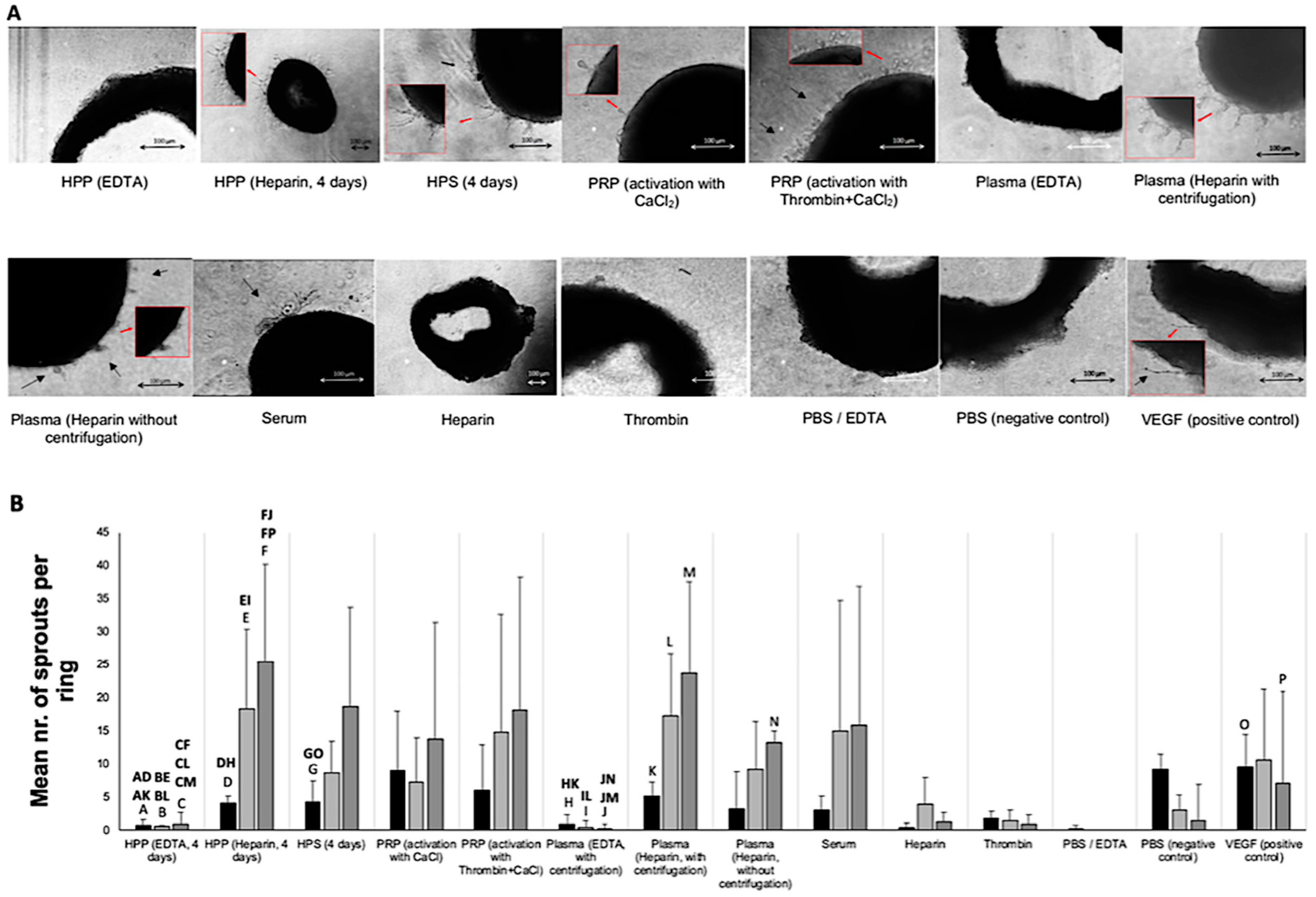
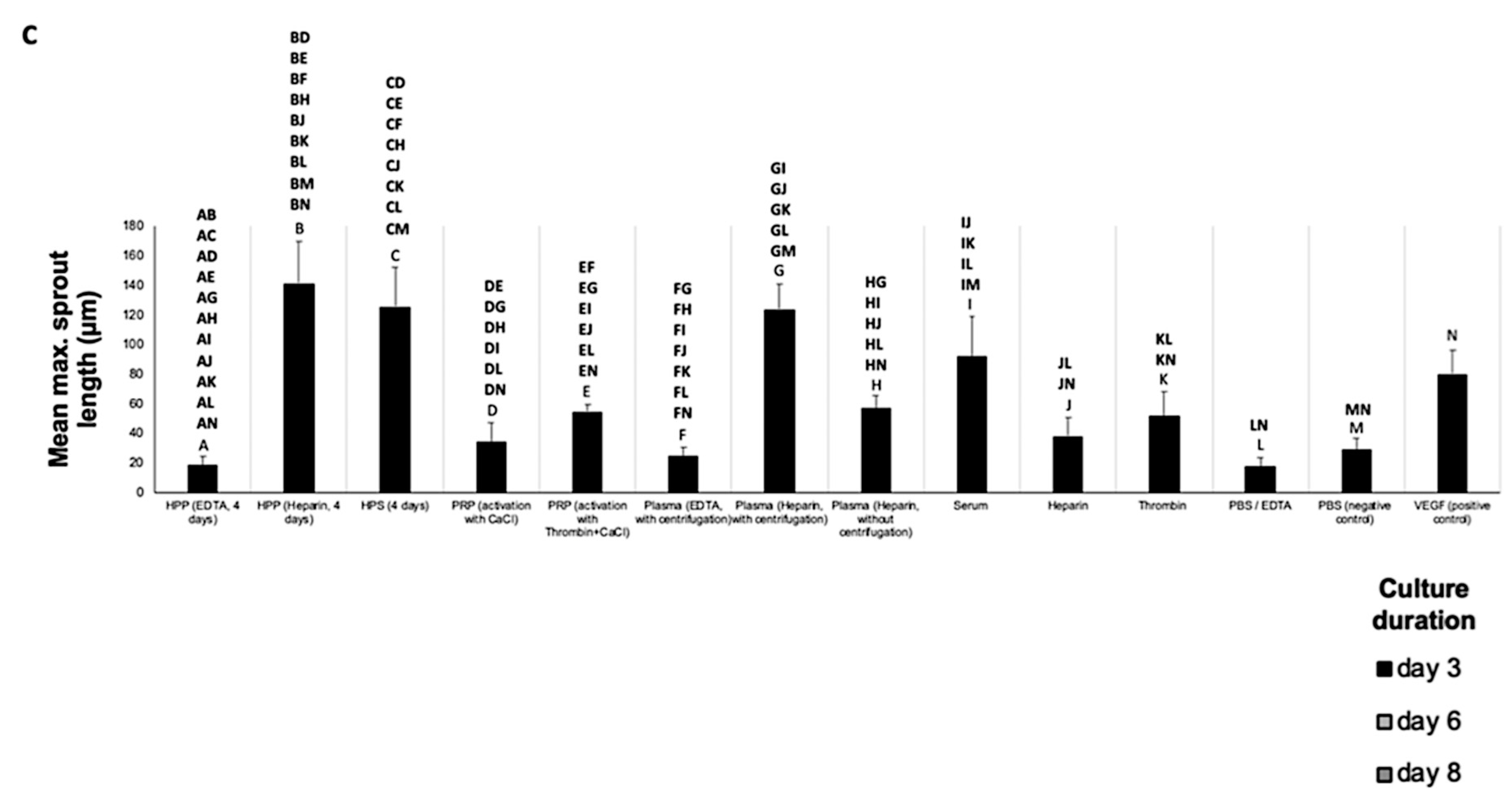
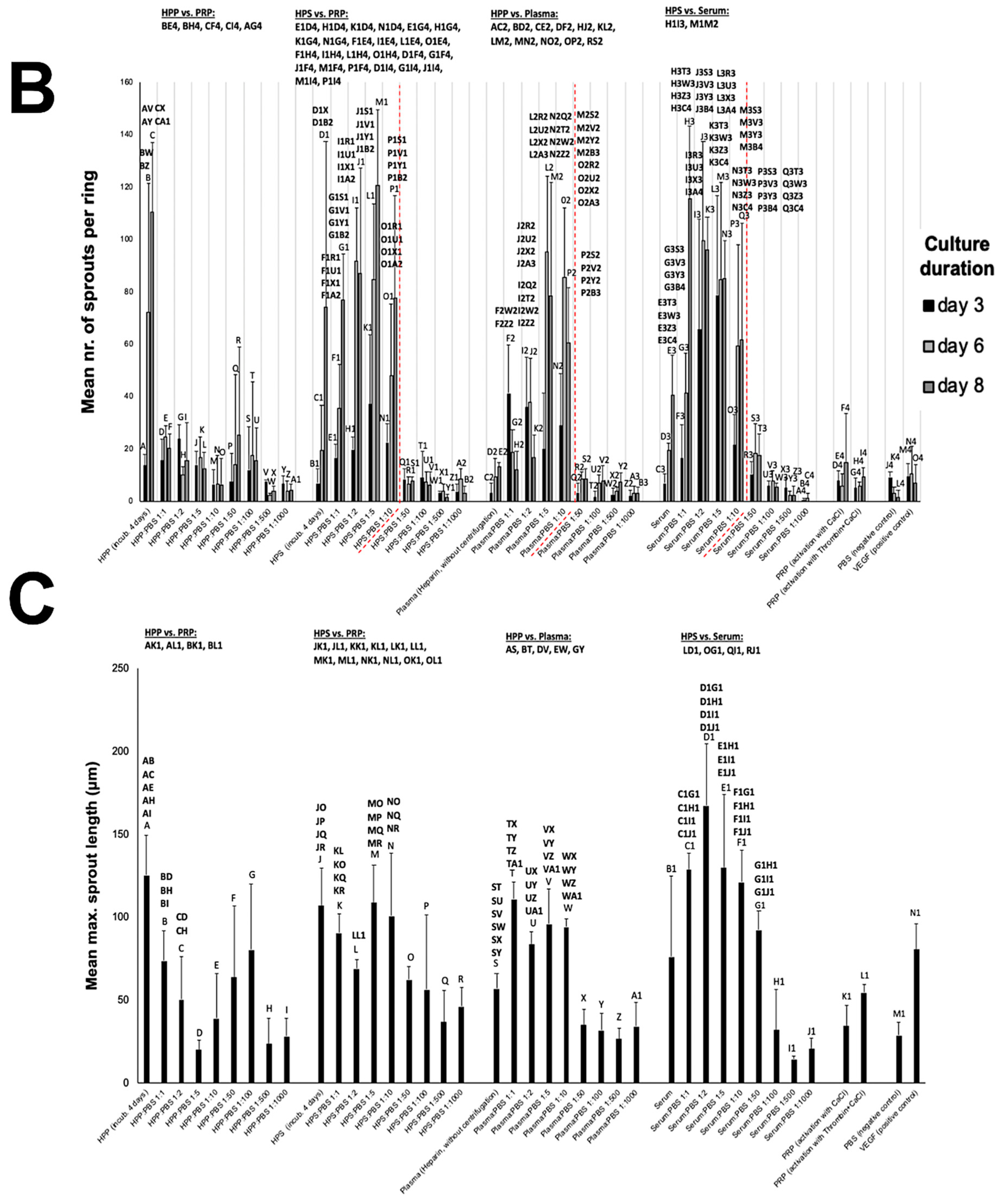
2.6. Analysis of the Effect of Blood-Derived Secretomes on In Vitro Microvessel Sprouting
2.7. Statistical Analysis
3. Results
3.1. Quantitative Analysis of Pro- (VEGF) and Anti-Angiogenic (TSP-1, PF-4) Growth Factor Concentration in Blood-Derived Secretomes
3.2. Analysis of the Ability of Blood-Derived Secretomes to Induce Microvessel Formation In Vitro
3.3. Analysis of the Ability of Blood-Derived Secretomes to Induce Microvessel Sprouting In Vitro
3.4. Effect of Hypoxia Preconditioned Secretome Dilution on Angiogenic Activity
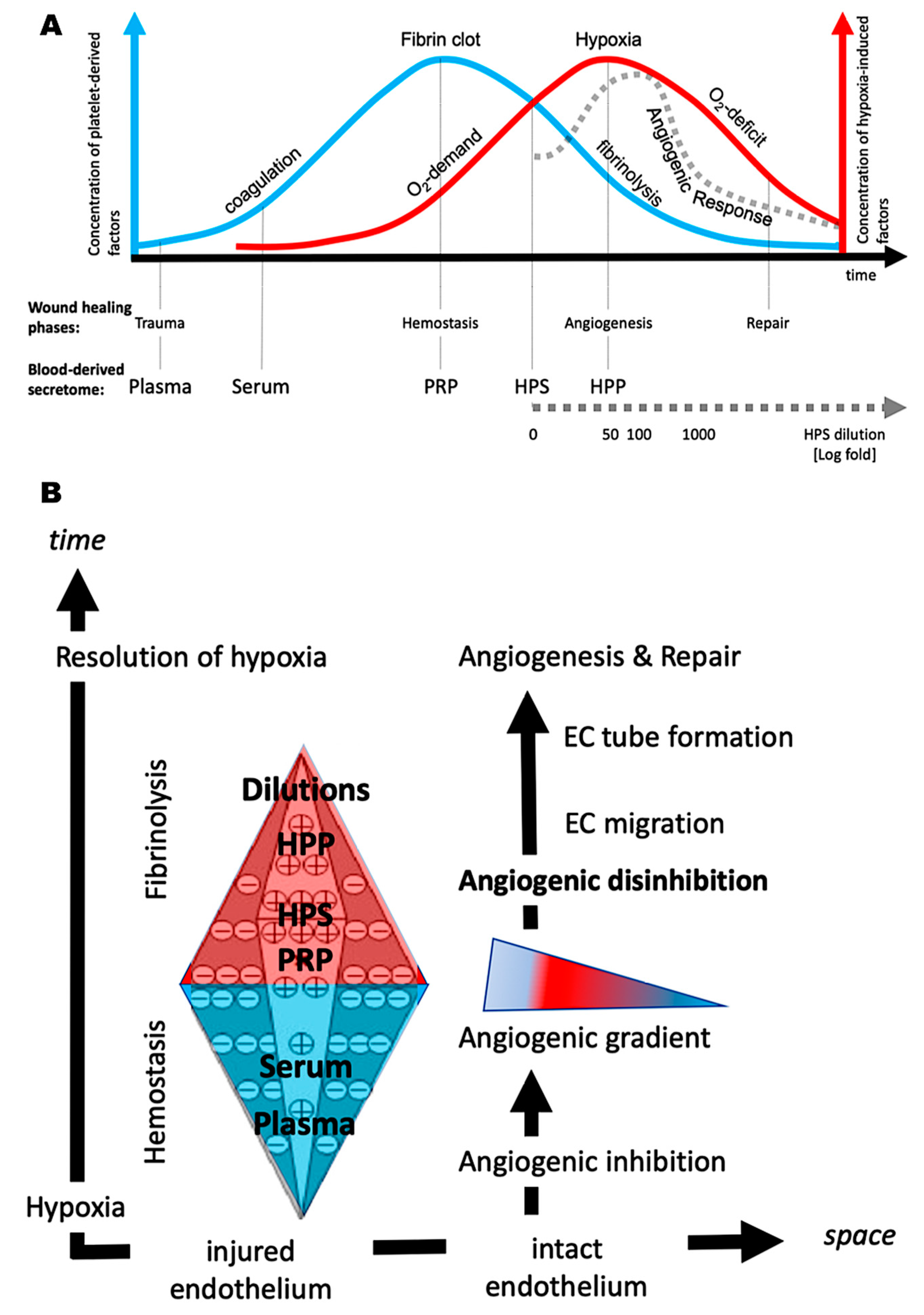
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PBC | peripheral blood cells |
| HPS | hypoxia preconditioned serum |
| HPP | hypoxia preconditioned plasma |
| PRP | platelet-rich plasma |
| PRFM | platelet-rich fibrin matrix |
| VEGF | vascular endothelial growth factor |
| TSP-1 | thrombospondin-1 |
| PF-4 | platelet factor-4 |
| IL-8 | interleukin-8 |
| FGF | fibroblast growth factor |
| MMP-9 | matrix metalloproteinase-9 |
| PBS | phosphate buffered saline |
References
- Hesseler, M.J.; Shyam, N. Platelet-rich plasma and its utility in medical dermatology: A systematic review. J. Am. Acad. Dermatol. 2019, 81, 834–846. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanayi, E.; Moog, P.; Bekeran, S.; Berezhnoi, A.; Aguir, J.; Bauer, A.T.; Kükrek, H.; Schmauss, D.; Isenburg, S.; Ntziachristos, V.; et al. In vitro characterization of hypoxia preconditioned serum (HPS)-Fibrin hydrogels: Basis for an injectable biomimetic tissue regeneration therapy. J. Funct. Biomater. 2019, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Sampson, S.; Gerhardt, M.; Mandelbaum, B. Platelet rich plasma injection grafts for musculoskeletal injuries: A review. Curr. Rev. Musculoskelet. Med. 2008, 1, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.J.; Fylling, C.; Parnell, L.K. Use of platelet rich plasma gel on wound healing: A systematic review and meta-analysis. Eplasty 2011, 11, e38. [Google Scholar]
- Hadjipanayi, E.; Bauer, A.T.; Moog, P.; Salgin, B.; Kükrek, H.; Fersch, B.; Hopfner, U.; Meissner, T.; Schlüter, A.; Ninkovic, M.; et al. Cell-free Carrier System for Localised Delivery of Peripheral Blood Cell-Derived Engineered Factor Signaling: Towards Development of a One-Step Device for Autologous Angiogenic Therapy. J. Control. Release 2013, 169, 91–102. [Google Scholar] [CrossRef]
- Reinke, J.M.; Sorg, H. Wound repair and regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef]
- Stadelmann, W.K.; Digenis, A.; Tobin, G.R. Physiology and healing dynamics of chronic cutaneous wounds. Am. J. Surg. 1998, 176, 26S–38S. [Google Scholar] [CrossRef]
- Chicharro-Alcántara, D.; Rubio-Zaragoza, M.; Damiá-Giménez, E.; Carrillo-Poveda, J.M.; Cuervo-Serrato, B.; Peláez-Gorrea, P.; Sopena-Juncosa, J.J. Platelet Rich Plasma: New Insights for Cutaneous Wound Healing Management. J. Funct. Biomater. 2018, 9, 10. [Google Scholar] [CrossRef]
- Golebiewska, E.M.; Poole, A. Platelet secretion: From haemostasis to wound healing and beyond. Blood Rev. 2015, 29, 153–162. [Google Scholar] [CrossRef]
- Italiano, J.E.; Richardson, J.L.; Patel-Hett, S.; Battinelli, E.; Zaslavsky, A.; Short, S. Angiogenesis is regulated by a novel mechanism: Pro-and antiangiogenic proteins are organized into separate platelet α granules and differentially released. Blood 2008, 111, 1227–1233. [Google Scholar] [CrossRef]
- Masoudi, E.; Ribas, J.; Kaushik, G.; Leijten, J.; Khademhosseini, A. Platelet-Rich Blood Derivatives for Stem Cell-Based Tissue Engineering and Regeneration. Curr. Stem Cell Rep. 2016, 2, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Tonnesen, M.G.; Feng, X.; Clark, R.A. Angiogenesis in wound healing. J. Investig. Dermatol. Symp. Proc. 2000, 5, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanayi, E.; Kuhn, P.H.; Moog, P.; Bauer, A.T.; Kuekrek, H.; Mirzoyan, L.; Hummel, A.; Kirchhoff, K.; Salgin, B.; Isenburg, S.; et al. The Fibrin Matrix regulates Angiogenic Responses within the Hemostatic Microenvironment through Biochemical Control. PLoS ONE 2015, 10, e0135618. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Tonnesen, M.G.; Mousa, S.A.; Clark, R.A. Fibrin and collagen differentially but synergistically regu- late sprout angiogenesis of human dermal microvascular endothelial cells in 3-dimensional matrix. Int. J. Cell Biol. 2013, 2013, 231279. [Google Scholar] [CrossRef]
- Bogdanovski, D.A.; DiFazio, L.T.; Bogdanovski, A.K.; Csóka, B.; Jordan, G.B.; Paul, E.R.; Antonioli, L.; Pilip, S.A.; Nemeth, Z.H. Hypoxia-inducible-factor-1 in trauma and critical care. J. Crit. Care 2017, 42, 207–212. [Google Scholar] [CrossRef]
- Hadjipanayi, E.; Schilling, A.F. Hypoxia- based strategies for angiogenic induction. Organogenesis 2013, 9, 261–272. [Google Scholar] [CrossRef]
- Hickey, M.M.; Simon, M.C. Regulation of angiogenesis by hypoxia and hypoxia-inducible factors. Curr. Top. Dev. Biol. 2006, 76, 217–257. [Google Scholar]
- Simon, M.C.; Keith, B. The role of oxygen availability in embryonic development and stem cell function. Nat. Rev. Mol. Cell Biol. 2008, 9, 285–296. [Google Scholar] [CrossRef]
- Hadjipanayi, E.; Schilling, A.F. Regeneration through autologous hypoxia preconditioned plasma. Organogenesis 2014, 10, 164–169. [Google Scholar] [CrossRef]
- Muñoz, M.; García-Vallejo, J.; Ruiz, M.D.; Romero, R.; Olalla, E.; Sebastián, C. Transfusion of post-operative shed blood: Laboratory characteristics and clinical utility. Eur. Spine J. 2004, 1, 107–113. [Google Scholar] [CrossRef]
- Sikorski, R.A.; Rizkalla, N.A.; Yang, W.W.; Frank, S.M. Autologous blood salvage in the era of patient blood management. Vox Sang. 2017, 112, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Eppley, B.L.; William, S.; Pietrzak, M.B. Platelet- rich plasma:a review of biology and Applications in Plastic Surgery. Plast. Reconstr. Surg. 2006, 118, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.E. Platelet-rich plasma (PRP): What ist PRP and what is not PRP? Implant Dent. 2001, 10, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Akhundov, K.; Pietramaggiori, G.; Waselle, L.; Darwiche, S.; Guerid, S.; Scaletta, C.; Hirt-Burri, N.; Applegate, L.A.; Raffoul, W.V. Development of a cost- effective method for platelet- rich plasma (PRP) preparation for topical wound healing. Ann. Burns Fire Dis. 2012, 25, 207–213. [Google Scholar]
- Abu-Ghname, A.; Perdanasari, A.T.; Davis, M.J.; Reece, E.M. Platelet-Rich Plasma: Principles and Applications in Plastic Surgery. Semin Plast. Surg. 2019, 33, 155–161. [Google Scholar] [CrossRef]
- Marx, R.E. Platelet-rich plasma: Evidence to support its use. J. Oral Maxillofac. Surg. 2004, 62, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Sclafani, A.P.; McCormick, S.A. Induction of dermal collagenesis, angiogenesis, and adipogenesis in human skin by injection of platelet-rich fibrin matrix. Arch. Facial Plast. Surg. 2012, 14, 132–136. [Google Scholar]
- Raeissadat, S.A.; Rayegani, S.; Hassanabadi, H.; Fathi, M.; Ghorbani, E.; Babaee, M.; Azma, K. Knee Osteoarthritis Injection Choices: Platelet- Rich Plasma (PRP) Versus Hyaluronic Acid (A one-year randomized clinical trial). Clin. Med. Insights Arthritis Musculoskelet. Disord. 2015, 8, 1–8. [Google Scholar] [CrossRef]
- Amable, P.R.; Caias, R.; Teixeira, M.V.T.; da Cruz Pacheco, I.; Corrêa do Amaral, R.J.F.; Granjeiro, J.M.; Borojevic, R. Platelet-rich plasma preparation for regenerative medicine: Optimization and quantification of cytokines and growth factors. Stem Cell Res. Ther. 2013, 4, 67. [Google Scholar] [CrossRef]
- Pachito, D.V.; Latorraca, C.O.C.; Riera, R. Efficacy of platelet-rich plasma for non-transfusion use: Overview of systematic reviews. Int. J. Clin. Pract. 2019, 13, e13402. [Google Scholar] [CrossRef]
- Sclafani, A.P. Applications of platelet-rich fibrin matrix in facial plastic surgery. Facial Plast. Surg. 2009, 25, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Hom, D.B. New Developments in wound healing relevant to facial plastic surgery. Arch. Facial Plast. Surg. 2008, 10, 402–406. [Google Scholar] [CrossRef] [PubMed]
- van den Dolder, J.; Mooren, R.; Vloon, A.P.; Stoelinga, P.J.; Jansen, J.A. Platelet-Rich Plasma: Quantification of Growth Factor Levels and the Effect on Growth and Differentiation of Rat Bone Marrow Cells. Tissue Eng. 2006, 12, 3067–3073. [Google Scholar] [CrossRef] [PubMed]
- Aghaloo, T.L.; Moy, P.K.; Freymiller, D.E.G. Investigation of platelet-rich plasma in rabbit cranial defects: A pilot study. J. Oral Maxillofac. Surg. 2002, 60, 1176–1181. [Google Scholar] [CrossRef]
- Kuffler, D.P. Platelet-Rich Plasma Promotes Axon Regeneration, Wound Healing, and Pain Reduction: Fact or Fiction. Mol. Neurobiol. 2015, 52, 990–1014. [Google Scholar] [CrossRef]
- Cervelli, V.; Gentile, P.; Scioli, M.G.; Grimaldi, M.; Casciani, C.U.; Spagnoli, L.G.; Orlandi, A. Application of platelet-rich plasma in plastic surgery: Clinical and in vitro evaluation. Tissue Eng. Part C Methods 2009, 15, 625–634. [Google Scholar] [CrossRef]
- Cho, E.B.; Park, G.S.; Park, S.S.; Jang, Y.J.; Kim, K.H.; Kim, K.J.; Park, E.J. Effect of platelet-rich plasma on proliferation and migration in human dermal fibroblasts. J. Cosmet. Dermatol. 2019, 18, 1105–1112. [Google Scholar] [CrossRef]
- Martinez, C.E.; Smith, P.; Palma Alvarado, V.A. The influence of platelet-derived products on angiogenesis and tissue repair: A concise update. Front. Physiol. 2015, 6, 290. [Google Scholar] [CrossRef]
- Kim, D.H.; Je, Y.J.; Kim, C.D.; Lee, Y.H.; Seo, Y.J.; Lee, J.H.; Lee, Y. Can Platelet-rich Plasma Be Used for Skin Rejuvenation? Evaluation of Effects of Platelet-rich Plasma on Human Dermal Fibroblast. Ann. Dermatol. 2011, 23, 424–431. [Google Scholar] [CrossRef]
- Martinez-Zapata, M.J.; Martí-Carvajal, A.J.; Sola, I.; Expósito, J.A.; Bolibar, I.; Rodriguez, L.; Garcia, J.; Zaror, C. Autologous platelet-rich plasma for treating chronic wounds. AR Cochrane Database Syst. Rev. 2016, 25, 1–56. [Google Scholar] [CrossRef]
- Kimmel, H.M.; Grant, A.; Ditata, J. The Presence of Oxygen in Wound Healing. Wounds 2016, 28, 264–270. [Google Scholar]
- Carmeliet, P. Angiogenesis in life, disease and medicine. Nature 2005, 174, 2336–2342. [Google Scholar] [CrossRef]
- Ribatti, D.; Folkman, J. A pioneer in the study of angiogenesis. Angiogenesis 2008, 11, 3–10. [Google Scholar] [CrossRef]
- Hadjipanayi, E.; Bekeran, S.; Moog, P. Extracorporeal Wound Simulation as a Foundation for Tissue Repair und Regeneration Therapies. Int. J. Transp. Plast. Surg. 2018, 2, 1–10. [Google Scholar]
- Burke, B.; Giannoudis, A.; Corke, K.P.; Gill, D.; Wells, M.; Ziegler-Heitbrock, L.; Lewis, C.E. Hypoxia-induced gene expression in human macrophages: Implications for ischemic tissues and hypoxia-regulated gene therapy. Am. J. Pathol. 2003, 163, 1233–1243. [Google Scholar] [CrossRef]
- Panutsopulos, D.; Zafiropoulos, A.; Krambovitis, E.; Kochiadakis, G.E.; Igoumenidis, N.E.; Spandidos, D.A. Peripheral monocytes from diabetic patients with coronary artery disease display increased bFGF and VEGF mRNA expression. J. Transl. Med. 2003, 1, 6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lichtenauer, M.; Mildner, M.; Hoetzenecker, K.; Zimmermann, M.; Podesser, B.K.; Sipos, W.; Berényi, E.; Dworschak, M.; Tschachler, E.; Gyöngyösi, M.; et al. Secretome of apoptotic peripheral blood cells (APOSEC) confers cytoprotection to cardiomyocytes and inhibits tissue remodelling after acute myocardial infarction: A preclinical study. Basic Res. Cardiol. 2011, 106, 1283–1297. [Google Scholar] [CrossRef] [PubMed]
- Reher, P.; Doan, N.; Bradnock, B.; Meghji, S.; Harris, M. Effect of ultrasound on the production of IL-8, basic FGF and VEGF. Cytokine 1999, 11, 416–423. [Google Scholar] [CrossRef]
- Nagata, M.J.; Messora, M.R.; Furlaneto, F.A.; Fucini, S.E.; Bosco, A.F.; Garcia, V.G.; Deliberador, T.M.; de Melo, L.G. Effectiveness of two methods for preparation of autologous platelet-rich plasma: An experimental study in rabbits. Eur. J. Dent. 2010, 4, 395–402. [Google Scholar] [CrossRef]
- Baker, M.; Robinson, S.D.; Lechertier, T.; Barber, P.R.; Tavora, B.; D’Amico, G.; Jones, D.T.; Vojnovic, B.; Hodivala-Dilke, K. Use of the mouse aortic ring assay to study angiogenesis. Nat. Protoc. 2011, 7, 89–104. [Google Scholar] [CrossRef]
- Wahl, S.M.; Wong, H.; McCartney-Francis, N. Role of growth factors in inflammation and repair. J. Cell Biochem. 1989, 40, 193–199. [Google Scholar] [CrossRef]
- Samadi, P.; Sheykhhasan, M.; Khoshinani, H.M. The Use of Platelet-Rich Plasma in Aesthetic and Regenerative Medicine: A Comprehensive Review. Aesth. Plast. Surg. 2019, 43, 803–814. [Google Scholar] [CrossRef]
- Cañedo-Dorantes, L.; Cañedo-Ayala, M. Skin Acute Wound Healing: A Comprehensive Review. Int. J. Inflam. 2019, 2019, 3706315. [Google Scholar] [CrossRef]
- Guo, S.; DiPietro, L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Alser, O.H.; Goutos, I. The evidence behind the use of platelet-rich plasma (PRP) in scar management: A literature review. Scars Burn Heal. 2018, 4, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Malairaman, U.; Dandapani, K.; Katyal, A. Effect of Ca2EDTA on Zinc Mediated Inflammation and Neuronal Apoptosis in Hippocampus of an In Vivo Mouse Model of Hypobaric Hypoxia. PLoS ONE 2014, 9, e110253. [Google Scholar] [CrossRef] [PubMed]
- Senzel, L.; Gnatenko, D.; Bahou, W.F. The Platelet Proteome. Curr. Opin. Hematol. 2009, 16, 329–333. [Google Scholar] [CrossRef]
- Go, R.S.; Ritman, E.L.; Owen, W.G. Angiogenesis in rat aortic rings stimulated by very low concentrations of serum and plasma. Angiogenesis 2003, 6, 25–29. [Google Scholar] [CrossRef]
- Nowak-Sliwinska, P.; Alitalo, K.; Allen, E.; Anisimov, A.; Aplin, A.C.; Auerbach, R.; Augustin, H.G.; Bates, D.O.; van Beijnum, J.R.; Bender, R.H.; et al. Consensus guidelines for the use and interpretation of angiogenesis assays. Angiogenesis 2018, 21, 425–532. [Google Scholar] [CrossRef]
- Hang, T.C.; Tedford, N.C.; Reddy, R.J.; Rimchala, T.; Wells, A.; White, F.M.; Kamm, R.D.; Lauffenburger, D.A. Vascular Endothelial Growth Factor (VEGF) and Platelet (PF-4) Factor 4 Inputs Modulate Human Microvascular Endothelial Signaling in a Three-Dimensional Matrix Migration Context. Mol. Cell Proteom. 2013, 12, 3704–3718. [Google Scholar] [CrossRef]
- El-Sharkawy, H.; Kantarci, A.; Deady, J.; Hasturk, H.; Liu, H.; Alshahat, M.; Van Dyke, T.E. Platelet-rich plasma: Growth factors and pro- and anti-inflammatory properties. J. Periodontol. 2007, 78, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Kubo, M.; Li, T.S.; Suzuki, R.; Shirasawa, B.; Morikage, N.; Ohshima, M.; Qin, S.L.; Hamano, K. Hypoxic preconditioning increases survival and angiogenic potency of peripheral blood mononuclear cells via oxidative stress resistance. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H590–H595. [Google Scholar] [CrossRef] [PubMed]
- Lelkes, P.I.; Hahn, K.L.; Sukovich, D.A.; Karmiol SSchmidt, D.H. On the possible role of reactive oxygen species in angiogenesis. Adv. Exp. Med. Biol. 1998, 454, 295–310. [Google Scholar] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moog, P.; Kirchhoff, K.; Bekeran, S.; Bauer, A.-T.; von Isenburg, S.; Dornseifer, U.; Machens, H.-G.; Schilling, A.F.; Hadjipanayi, E. Comparative Evaluation of the Angiogenic Potential of Hypoxia Preconditioned Blood-Derived Secretomes and Platelet-Rich Plasma: An In Vitro Analysis. Biomedicines 2020, 8, 16. https://doi.org/10.3390/biomedicines8010016
Moog P, Kirchhoff K, Bekeran S, Bauer A-T, von Isenburg S, Dornseifer U, Machens H-G, Schilling AF, Hadjipanayi E. Comparative Evaluation of the Angiogenic Potential of Hypoxia Preconditioned Blood-Derived Secretomes and Platelet-Rich Plasma: An In Vitro Analysis. Biomedicines. 2020; 8(1):16. https://doi.org/10.3390/biomedicines8010016
Chicago/Turabian StyleMoog, Philipp, Katharina Kirchhoff, Sanjar Bekeran, Anna-Theresa Bauer, Sarah von Isenburg, Ulf Dornseifer, Hans-Günther Machens, Arndt F. Schilling, and Ektoras Hadjipanayi. 2020. "Comparative Evaluation of the Angiogenic Potential of Hypoxia Preconditioned Blood-Derived Secretomes and Platelet-Rich Plasma: An In Vitro Analysis" Biomedicines 8, no. 1: 16. https://doi.org/10.3390/biomedicines8010016
APA StyleMoog, P., Kirchhoff, K., Bekeran, S., Bauer, A.-T., von Isenburg, S., Dornseifer, U., Machens, H.-G., Schilling, A. F., & Hadjipanayi, E. (2020). Comparative Evaluation of the Angiogenic Potential of Hypoxia Preconditioned Blood-Derived Secretomes and Platelet-Rich Plasma: An In Vitro Analysis. Biomedicines, 8(1), 16. https://doi.org/10.3390/biomedicines8010016





