Abstract
Temozolomide, a DNA methylating drug, is currently being used first-line in glioblastoma therapy. Although the mode of action of this so-called SN1 alkylating agent is well described, including the types of induced DNA damage triggering the DNA damage response and survival and death pathways, some researchers expressed doubt that data mostly obtained by in vitro models can be translated into the in vivo situation. In experimental settings, high doses of the agent are often used, which are likely to activate responses triggered by base N-alkylations instead of O6-methylguanine (O6MeG), which is the primary cytotoxic lesion induced by low doses of temozolomide and other methylating drugs in O6-methylguanine-DNA methyltransferase (MGMT) repair incompetent cells. However, numerous studies provided compelling evidence that O6MeG is not only a mutagenic, but also a powerful toxic lesion inducing DNA double-strand breaks, apoptosis, autophagy and cellular senescence. MGMT, repairing the lesion through methyl group transfer, is a key node in protecting cells against all these effects and has a significant impact on patient’s survival following temozolomide therapy, supporting the notion that findings obtained on a molecular and cellular level can be translated to the therapeutic setting in vivo. This comment summarizes the current knowledge on O6MeG-triggered pathways, including dose dependence and the question of thresholds, and comes up with the conclusion that data obtained on cell lines using low dose protocols are relevant and apoptosis, autophagy and senescence are therapeutically important endpoints.
In a recent review in Biomedicines, Strobel et al. discussed the mode of action of temozolomide, which is being used first-line in glioblastoma therapy [1]. Temozolomide (TMZ, Temodal®) is a spontaneously decomposing methylating agent, which acts similarly to dacarbazine (DTIC) that needs metabolic activation. TMZ was also frequently used in malignant melanoma therapy, but has been replaced in recent years by checkpoint inhibitors [2] and immunomodulators [3]. Although the authors provide a solid overview on the metabolism of methylating anticancer drugs, their reaction with the DNA, adduct formation and the role of membrane permeability, they largely neglected the cell biological effects of these SN1 methylating agents, for which a significant body of data is available (reviewed in [4,5]). Thus, they came to the conclusion that “…the only consistently shown effect of TMZ on cells is the increase of DNA content.” Furthermore, it was stated that “While TMZ has been shown to induce cell death, this is usually only produced in experimental systems with unphysiologically high concentrations, often in the range of 100 μM TMZ (ref) up to 1000 and 4000 μM (ref) while models predict a peak concentration in the tumor in the range of 14.95–34.54 μM (ref).” It was further concluded that “TMZ should be considered primarily cytostatic and senescence-inducing and not cytotoxic and apoptosis-inducing, potentially preventing cancer cells from G2 to M phase transition when tumor cells are most sensitive for mitotic cell death.” [1]. In my opinion, these are misleading and unsubstantiated statements that need comment.
First, there is solid data to show that TMZ and other SN1 methylating agents induce cell death by apoptosis [6,7], and the pathways activated by the critical lesion O6-methylguanine (O6MeG) are well described [4]. Thus, it has been shown that TMZ induces via processing of O6MeG/T mismatches DNA double-strand breaks (DSBs) in the post-treatment cell cycle [8,9] and triggers activation of the DNA damage kinases ATR and, as a secondary event, ATM [10], which in turn activate CHK1/CHK2-p53-driven apototic pathways [11]. It has also been shown that in p53 mutant glioma cells, the endogenous (mitochondrial) apoptosis pathway becomes activated, which is, however, less effective in triggering apoptosis than the exogenous p53 driven pathway [12]. Furthermore, it has been shown that TMZ induces autophagy and cellular senescence, which are important responses triggered by the O6MeG lesion [13,14]. It is important to note that these observed responses were evoked in glioblastoma cell lines with TMZ doses below and up to 50 µM [12,13].
In a recent study, the dose responses of these critical endpoints were assessed and it was shown that apoptosis, senescence (SA-βGal) and autophagy increased linearly with dose. Thus, even very low doses (between 5 and 25 µM), which are in the range of what is achieved systemically in the therapeutic setting [15,16,17], elicited toxic effects [18]. Interestingly, using the LN-229 and LN-308 (p53 wild-type) cell models, thresholds for apoptosis were not observed. This finding is compatible with the observation that the amount of γH2AX foci, which are an accepted marker of DSBs that trigger apoptosis, increases linearly with the dose of TMZ [18] and other O6-methylating agents (unpublished data). It should also be noted that O6MeG-derived secondary lesions activate upon treatment of glioblastoma cells with doses ≤50 µM TMZ the ATR/ATM-CHK1/2-SIAH1/HIPK2-p53Ser46 axis [19], which is considered to trigger the apoptosis pathway [20]. Furthermore, also the Jun kinase pathway, forcing both receptor-mediated and mitochondrial apoptosis via Fas ligand (FASL) and BIM, is activated following TMZ [21]. The pathways activated by O6MeG are outlined in Figure 1. Taken together, the available data show undoubtedly that the TMZ-induced DNA adduct O6MeG is a highly cytotoxic, genotoxic, recombinogenic and DNA damage response (DDR)-activating lesion. The data demonstrate at the same time that a single DNA repair protein, MGMT, is highly efficient in protecting against all these effects [22]. Importantly, already at low dose levels, O6MeG is a powerful activator of the apoptosis pathway [6,7], which can be explained by its MMR-mediated conversion into DSBs that trigger efficiently apoptosis without the involvement of PARP1 activation that stimulates necrosis [23].
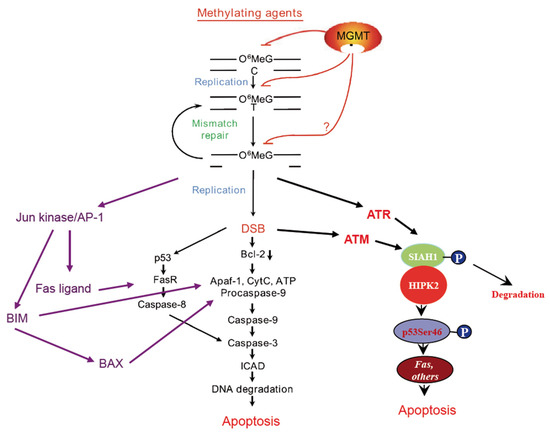
Figure 1.
Cell death pathways triggered by the temozolomide-induced DNA lesion O6-methylguanine. Although TMZ, like other SN1 alkylators, induces more than a dozen DNA adducts, O6MeG is the key cytotoxic lesion and MGMT the primary node of drug resistance (reviewed in Reference [22]). The damage is converted into DSBs and, in p53 mutated cells, stimulates mitochondrial apoptosis, a hallmark of which is Bcl-2 decline [8]. In p53 wild-type cells, apoptosis is additionally driven by death receptor triggered caspase-8 activation, which requires upregulation of the Fas receptor controlled by p53 [12], and the Fas ligand, which is under control of AP-1. Additionally, the AP-1 dependent BIM/BAX apoptosis pathway [21], as well as the SIAH1/HIPK2-p53Ser46 pathway become activated [19] thus contributing to cell death executed by apoptosis. The model is based on experiments with TMZ doses of ≤50 µM, which were used for glioblastoma cell treatment in the cited works.
Interestingly, O6MeG is also a powerful trigger of the senescence pathway [13]. Comparing the responses under identical treatment conditions, we observed with a dose of 15 µM TMZ yields of about 20% apoptosis (annexin V positive) and 42% senescence (C12FDG positive) in LN-229 glioblastoma cells [18]. Thus, it appears that senescence is a major pathway activated by O6MeG lesions, which does not mean that apoptosis is not induced and irrelevant for explaining the cytotoxic effects. Of note, we do not know whether senescent cells die by apoptosis or by another process at a later stage. In view of the bulk of published data (for an extended list of references see [5]) the statement that “TMZ should be considered primarily cytostatic and senescence-inducing and not cytotoxic and apoptosis-inducing” is not tenable.
I do agree with the authors that, in some publications, unusually high concentrations of TMZ were used, which are even in the mM range. These high doses are required to elicit cytotoxic and genotoxic effects in MGMT expressing, TMZ-resistant cells and are brought about by N-alkylations and other adducts repaired by base excision repair (BER) and ALKBH2 [5]. At these high dose levels, the cellular BER and ALKBH2 repair capacity appears to be saturated and, therefore, these adducts become the preponderant toxic insults. It is true that these effects elicited at very high doses of TMZ cannot be achieved in vivo, unless the tumor is impaired in BER and/or ALKBH2. Therefore, responses observed in MGMT expressing (MGMT promoter unmethylated) cell models and high TMZ doses (>100 µM) should be taken with caution.
It should also be taken into consideration that in the therapeutic setting TMZ is administered repeatedly with daily doses of 50 to 130 mg/m2 or even higher [24,25]. Under these conditions, a huge accumulation of O6MeG in the DNA is expected to occur in MGMT lacking (MGMT promoter methylation positive) tumors, strongly enhancing the DNA damage response, signal activation and activation of apoptosis and senescence pathways. Therefore, the biologically effective dose in a therapeutic setting is even likely higher than what is achieved in vitro in most of the experimental settings, when single doses of up to 100 µM are used.
TMZ is clearly a cytotoxic drug, as cells following treatment die by apoptosis even at low dose levels [18,19]. Since TMZ induces, like all genotoxic agents, DNA synthesis inhibition and cell cycle arrest as well as cellular senescence, it may also be considered a cytostatic drug (i.e., cells are just inhibited in proliferation). However, measuring these endpoints, it remains unclear whether cells blocked in particular cell cycle positions or the senescent state are irreversibly arrested or only transiently, and finally enter a death pathway (or continue to proliferate). S-phase inhibition and cell cycle delay following DNA damage are well-known transient phenomena, which should not be considered as a cytostatic activity. It should also be noted that cell cycle inhibition and senescence are regulated, at least in part, by the same upstream DNA damage response players that regulate apoptosis. For these reasons, TMZ should be considered a cytotoxic agent rather than a cytostatic drug.
To the best of my knowledge, neither apoptosis nor senescence has been demonstrated in human glioblastoma specimens obtained after resection upon the first therapy cycle. However, this lack of data should not be taken as an argument that glioblastoma cells do not die by apoptosis following therapy. If apoptotic markers are not detectable following therapy, this may result from the clearance of apoptotic cells in the recurrent tumor in the period between the end of TMZ treatment and resection. The important role of MGMT, which was first discovered in bacteria [26], intensively assessed as a key defense [27] and drug resistance mechanism in cancer cell lines in vitro [28,29] and then translated to the tumor response [30], is an impressive example for demonstrating that processes occurring on a molecular level can be translated to cancer cells that grow in a much more complex tumor environment.
Funding
Most of the author’s studies have been supported by the Deutsche Forschungsgemeinschaft (DFG KA727).
Conflicts of Interest
The author declares no conflict of interest.
References
- Strobel, H.; Baisch, T.; Fitzel, R.; Schilberg, K.; Siegelin, M.D.; Karpel-Massler, G.; Debatin, K.-M.; Westhoff, M.-A. Temozolomide and Other Alkylating Agents in Glioblastoma Therapy. Biomedicines 2019, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M.; et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011, 364, 2507–2516. [Google Scholar] [CrossRef] [PubMed]
- Hogan, S.A.; Levesque, M.P.; Cheng, P.F. Melanoma Immunotherapy: Next-Generation Biomarkers. Front. Oncol. 2018, 8, 178. [Google Scholar] [CrossRef] [PubMed]
- Roos, W.P.; Kaina, B. DNA damage-induced cell death: From specific DNA lesions to the DNA damage response and apoptosis. Cancer Lett. 2013, 332, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Kaina, B.; Christmann, M. DNA repair in personalized brain cancer therapy with temozolomide and nitrosoureas. DNA Repair 2019, 78, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Kaina, B.; Ziouta, A.; Ochs, K.; Coquerelle, T. Chromosomal instability, reproductive cell death and apoptosis induced by O6-methylguanine in Mex−, Mex+ and methylation-tolerant mismatch repair compromised cells: Facts and models. Mutat. Res. 1997, 381, 227–241. [Google Scholar] [CrossRef]
- Meikrantz, W. O6-alkylguanine DNA lesions trigger apoptosis. Carcinogenesis 1998, 19, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Ochs, K.; Kaina, B. Apoptosis induced by DNA damage O6-methylguanine is Bcl-2 and caspase-9/3 regulated and Fas/caspase-8 independent. Cancer Res. 2000, 60, 5815–5824. [Google Scholar] [PubMed]
- Quiros, S.; Roos, W.P.; Kaina, B. Processing of O6-methylguanine into DNA double-strand breaks requires two rounds of replication whereas apoptosis is also induced in subsequent cell cycles. Cell Cycle 2010, 9, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Eich, M.; Roos, W.; Nikolova, T.; Kaina, B. Contribution of ATM and ATR to the Resistance of Glioblastoma and Malignant Melanoma Cells to the Methylating Anticancer Drug Temozolomide. Mol. Cancer Ther. 2013, 12, 2529–2540. [Google Scholar] [CrossRef] [PubMed]
- Roos, W.P.; Thomas, A.D.; Kaina, B. DNA damage and the balance between survival and death in cancer biology. Nat. Rev. Cancer 2016, 16, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Roos, W.P.; Batista, L.F.Z.; Naumann, S.C.; Wick, W.; Weller, M.; Menck, C.F.M.; Kaina, B. Apoptosis in malignant glioma cells triggered by the temozolomide-induced DNA lesion O6-methylguanine. Oncogene 2007, 26, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Knizhnik, A.V.; Roos, W.P.; Nikolova, T.; Quirós, S.; Tomaszowski, K.-H.; Christmann, M.; Kaina, B. Survival and Death Strategies in Glioma Cells: Autophagy, Senescence and Apoptosis Triggered by a Single Type of Temozolomide-Induced DNA Damage. PLoS ONE 2013, 8, e55665. [Google Scholar] [CrossRef] [PubMed]
- Aasland, D.; Götzinger, L.; Hauck, L.; Berte, N.; Meyer, J.; Effenberger, M.; Schneider, S.; Reuber, E.E.; Roos, W.P.; Tomicic, M.T.; et al. Temozolomide Induces Senescence and Repression of DNA Repair Pathways in Glioblastoma Cells via Activation of ATR-CHK1, p21, and NF-kappaB. Cancer Res. 2019, 79, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Marzolini, C.; Shen, F.; Bauer, J.; Buclin, T.; Decosterd, L.A.; Lejeune, F.; Gander, M.; Leyvraz, S. Pharmacokinetics of temozolomide in association with fotemustine in malignant melanoma and malignant glioma patients: Comparison of oral, intravenous, and hepatic intra-arterial administration. Cancer Chemother. Pharmacol. 1998, 42, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Portnow, J.; Badie, B.; Chen, M.; Liu, A.; Blanchard, S.; Synold, T.W. The neuropharmacokinetics of temozolomide in patients with resectable brain tumors: Potential implications for the current approach to chemoradiation. Clin. Cancer Res. 2009, 15, 7092–7098. [Google Scholar] [CrossRef] [PubMed]
- Ostermann, S.; Csajka, C.; Buclin, T.; Leyvraz, S.; Lejeune, F.; Decosterd, L.A.; Stupp, R. Plasma and Cerebrospinal Fluid Population Pharmacokinetics of Temozolomide in Malignant Glioma Patients. Clin. Cancer Res. 2004, 10, 3728–3736. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Kaina, B. Are There Thresholds in Glioblastoma Cell Death Responses Triggered by Temozolomide? Int. J. Mol. Sci. 2019, 20, 1562. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Roos, W.P.; Wu, Q.; Hofmann, T.G.; Kaina, B. The SIAH1–HIPK2–p53ser46 Damage Response Pathway is Involved in Temozolomide-Induced Glioblastoma Cell Death. Mol. Cancer Res. 2019, 17, 1129–1141. [Google Scholar] [CrossRef] [PubMed]
- Matt, S.; Hofmann, T.G. The DNA damage-induced cell death response: A roadmap to kill cancer cells. Cell. Mol. Life Sci. 2016, 73, 2829–2850. [Google Scholar] [CrossRef] [PubMed]
- Tomicic, M.T.; Meise, R.; Aasland, D.; Berte, N.; Kitzinger, R.; Krämer, O.H.; Kaina, B.; Christmann, M. Apoptosis induced by temozolomide and nimustine in glioblastoma cells is supported by JNK/c-Jun-mediated induction of the BH3-only protein BIM. Oncotarget 2015, 6, 33755–33768. [Google Scholar] [CrossRef] [PubMed]
- Kaina, B.; Christmann, M.; Naumann, S.; Roos, W.P. MGMT: Key node in the battle against genotoxicity, carcinogenicity and apoptosis induced by alkylating agents. DNA Repair 2007, 6, 1079–1099. [Google Scholar] [CrossRef] [PubMed]
- Lips, J.; Kaina, B. DNA double-strand breaks trigger apoptosis in p53-deficient fibroblasts. Carcinogenesis 2001, 22, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; Bent, M.J.V.D. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Buhk, J.-H.; Wrede, A.; Hoffmann, A.L.; Bock, H.C.; Kaina, B.; Strik, H.M.; Christmann, M. Rechallenge with temozolomide with different scheduling is effective in recurrent malignant gliomas. Mol. Med. Rep. 2008, 1, 863–867. [Google Scholar]
- Samson, L.; Cairns, J. A new pathway for DNA repair in Escherichia coli. Nat. 1977, 267, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Bernd, K.; Fritz, G.; Mitra, S.; Coquerelle, T. Transfection and expression of human O6-methylguanine-DNA methyltransferase (MGMT) cDNA in Chinese hamster cells: The role of MGMT in protection against the genotoxic effects of alkylating agents. Carcinogenesis 1991, 12, 1857–1867. [Google Scholar] [CrossRef] [PubMed]
- Kaina, B.; Christmann, M. DNA repair in resistance to alkylating anticancer drugs. Int. J. Clin. Pharmacol. Ther. 2002, 40, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Hermisson, M.; Klumpp, A.; Wick, W.; Wischhusen, J.; Nagel, G.; Roos, W.; Kaina, B.; Weller, M. O6-methylguanine DNA methyltransferase and p53 status predict temozolomide sensitivity in human malignant glioma cells. J. Neurochem. 2006, 96, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Hegi, M.E.; Diserens, A.C.; Gorlia, T.; Hamou, M.F.; De Tribolet, N.; Weller, M.; Kros, J.M.; Hainfellner, J.A.; Mason, W.; Mariani, L.; et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005, 352, 997–1003. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).