Genistein Loaded Nanofibers Protect Spinal Cord Tissue Following Experimental Injury in Rats
Abstract
1. Introduction
2. Results
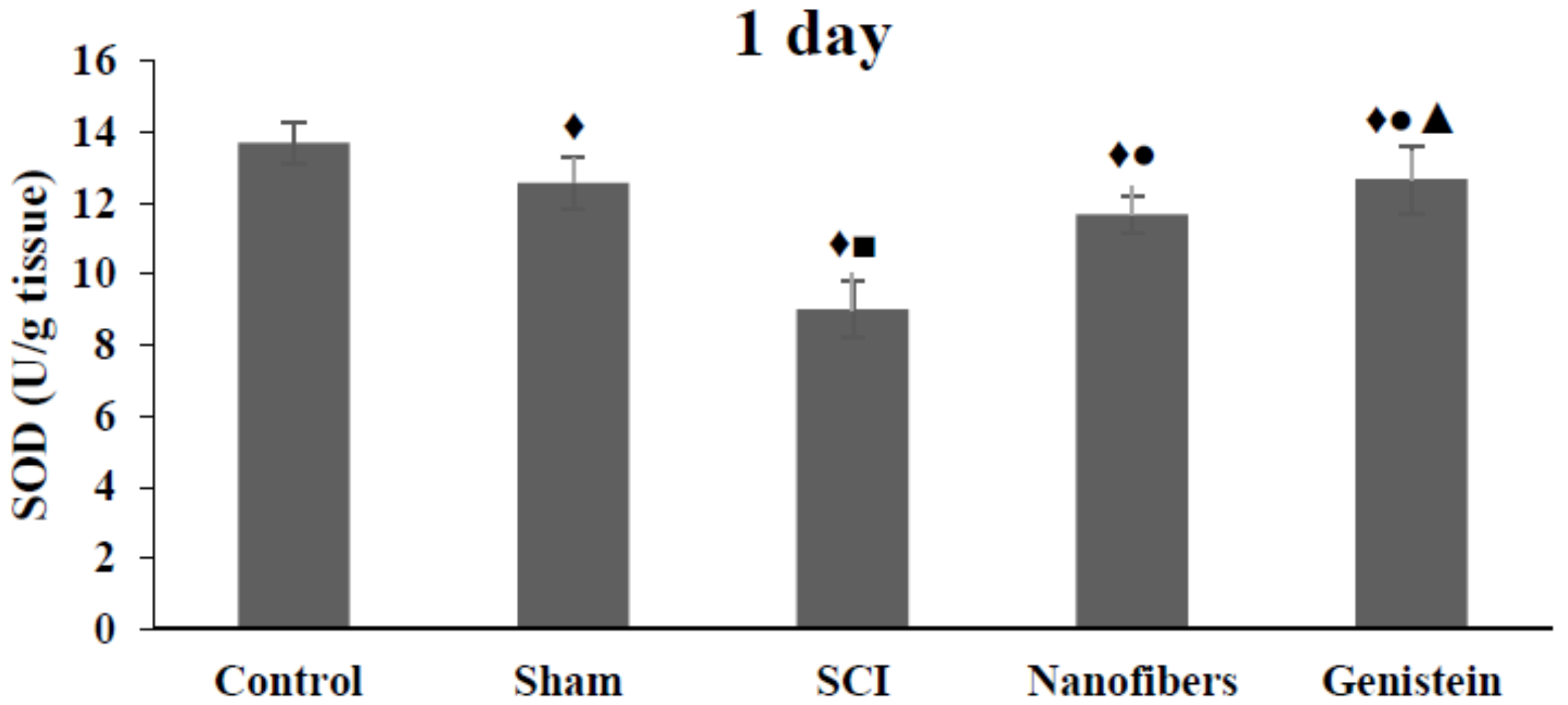
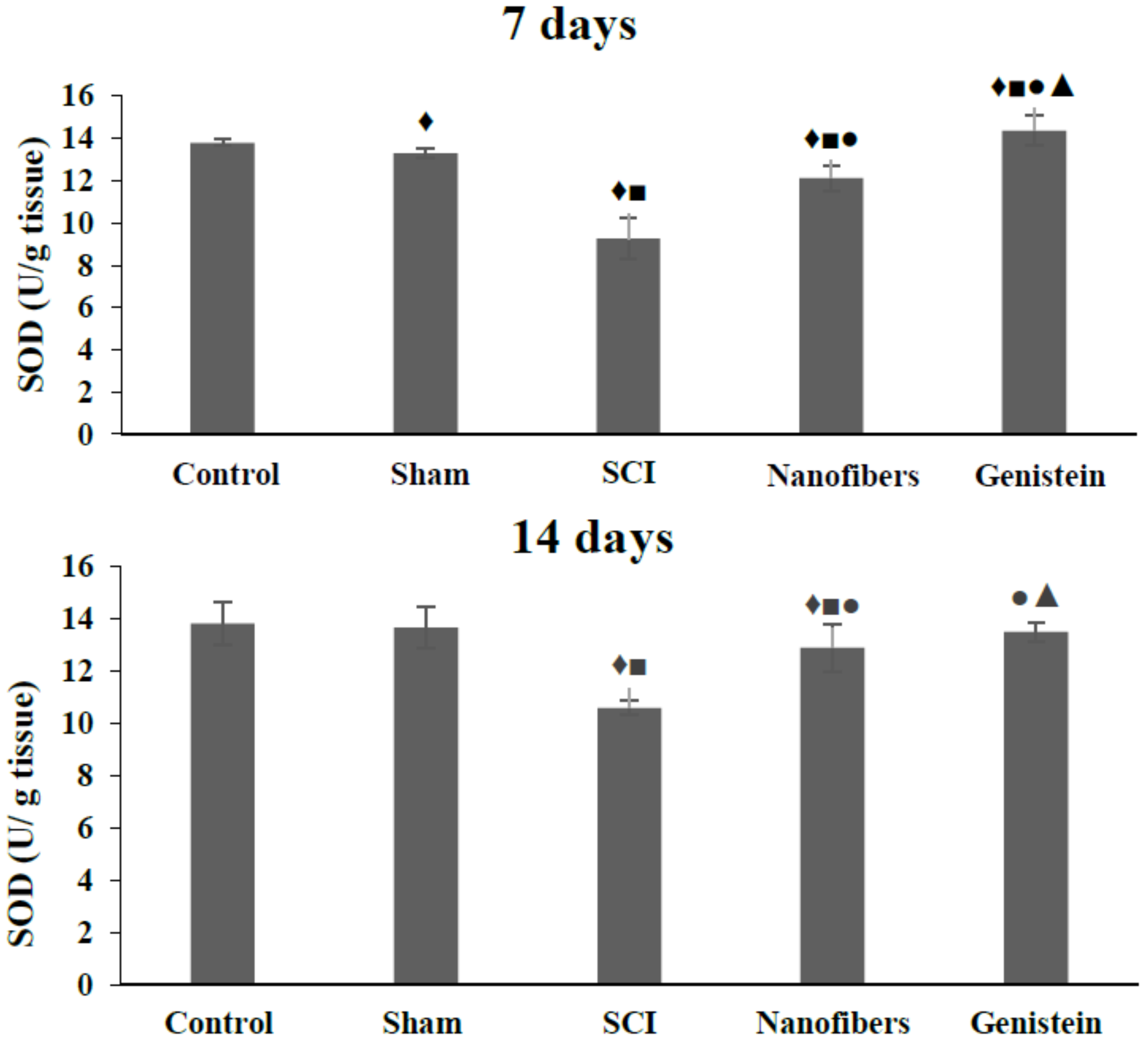
2.1. Genistein and Nanofibers Increase Super Oxide Dismutase (SOD) Activity in Spinal Cord Tissue
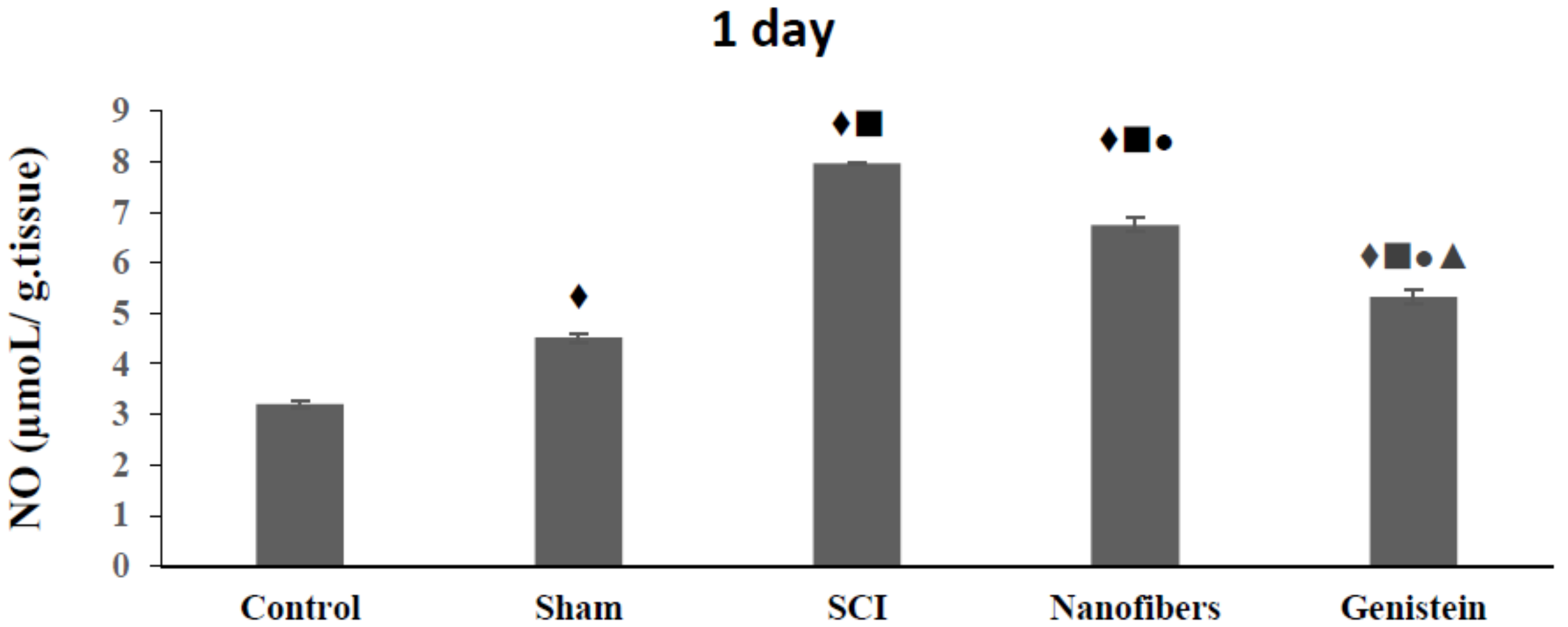
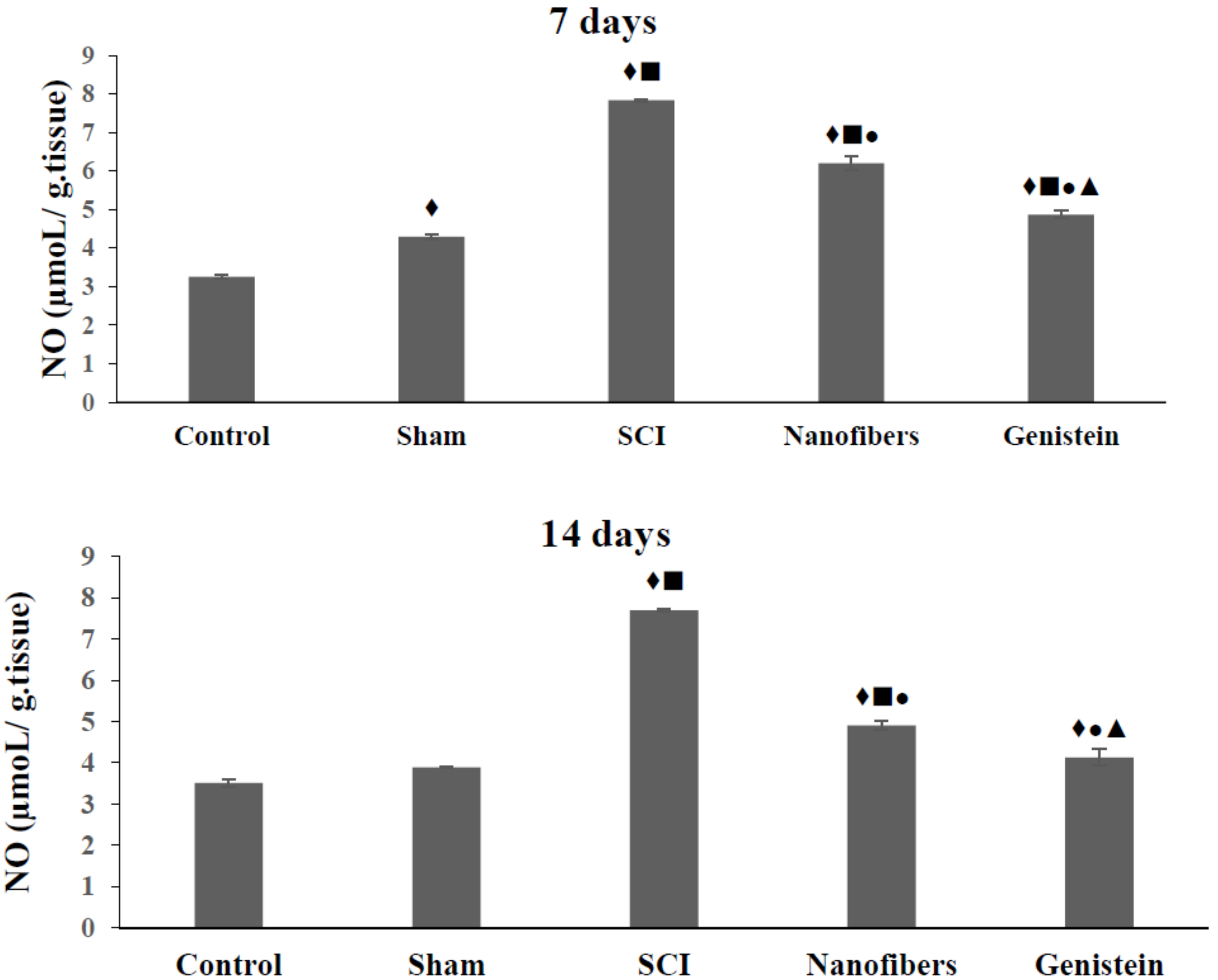
2.2. Genistein Decreases Nitrous Oxide (NO) Concentration in Spinal Cord Tissue
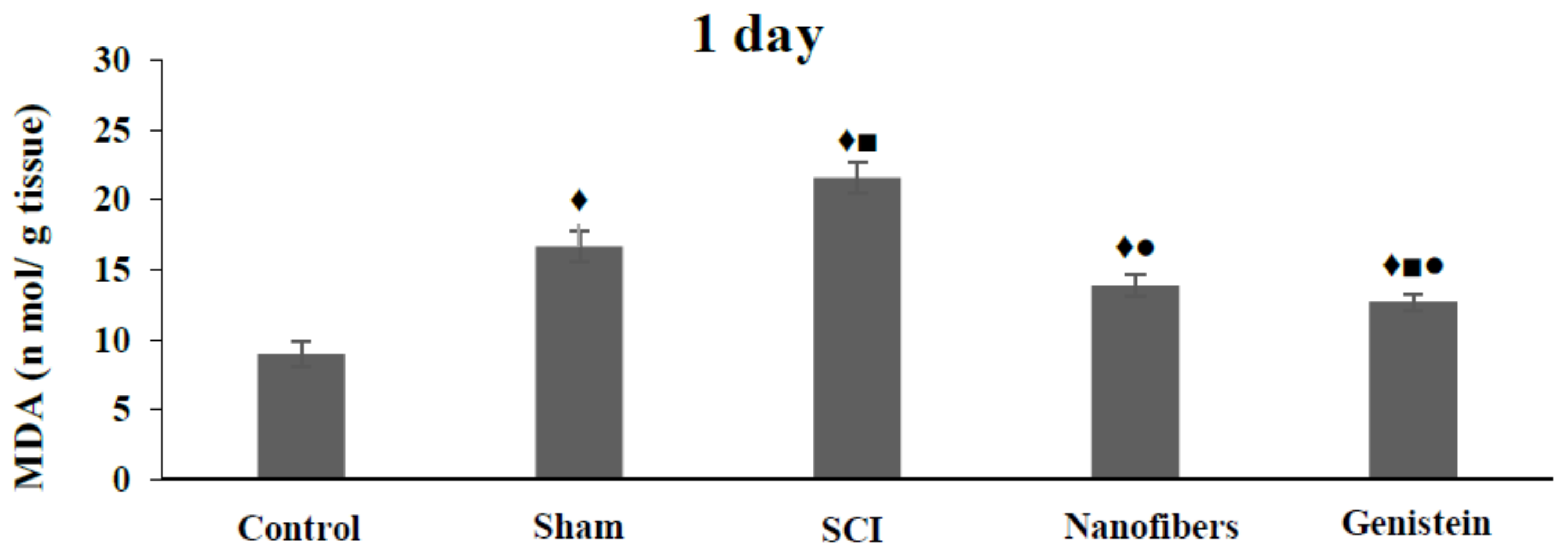
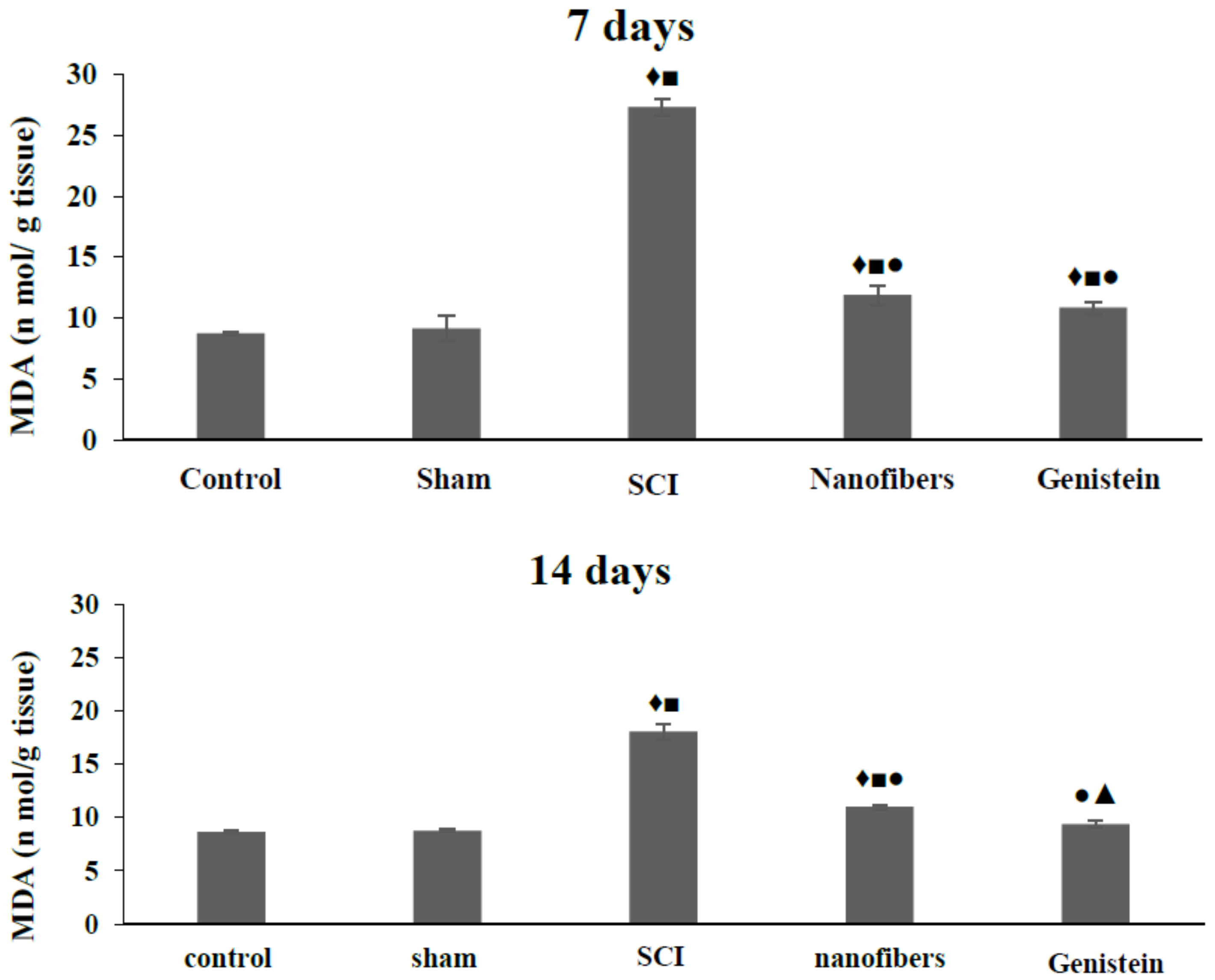
2.3. Genistein Decreased Lipid Peroxidation and Tissue Damage in Spinal Cord Tissue
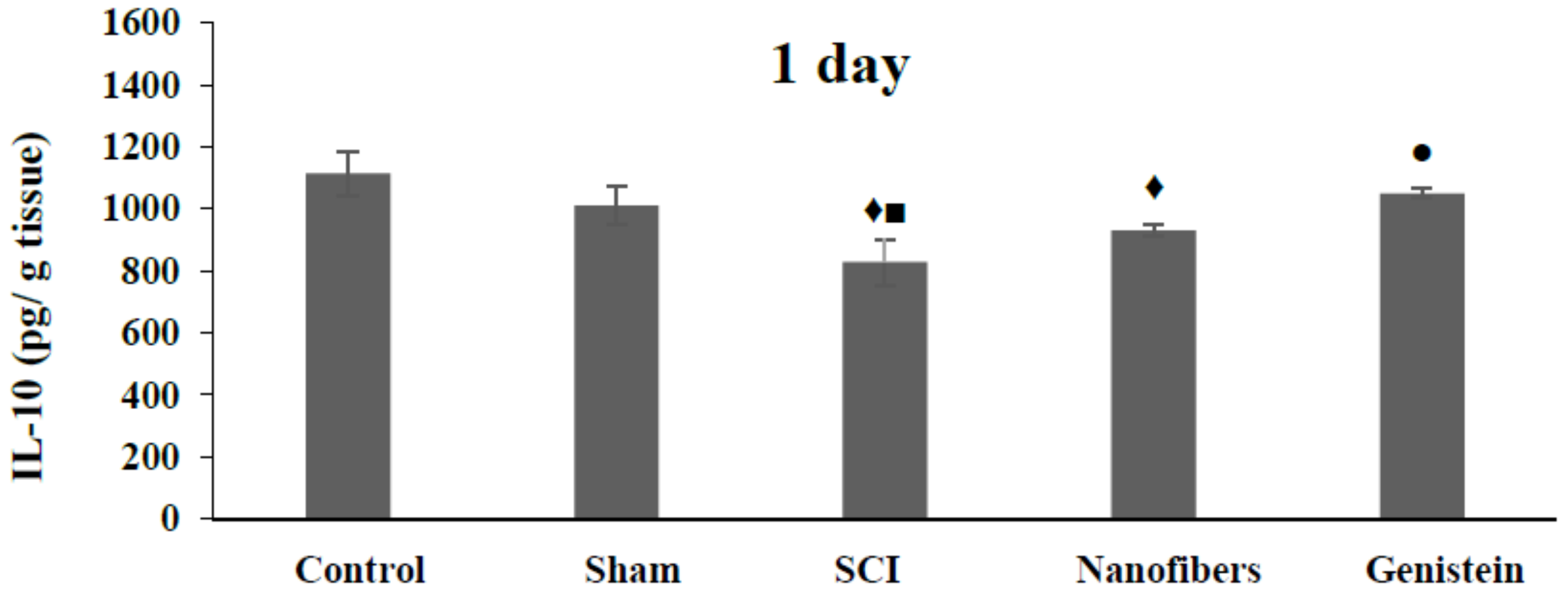
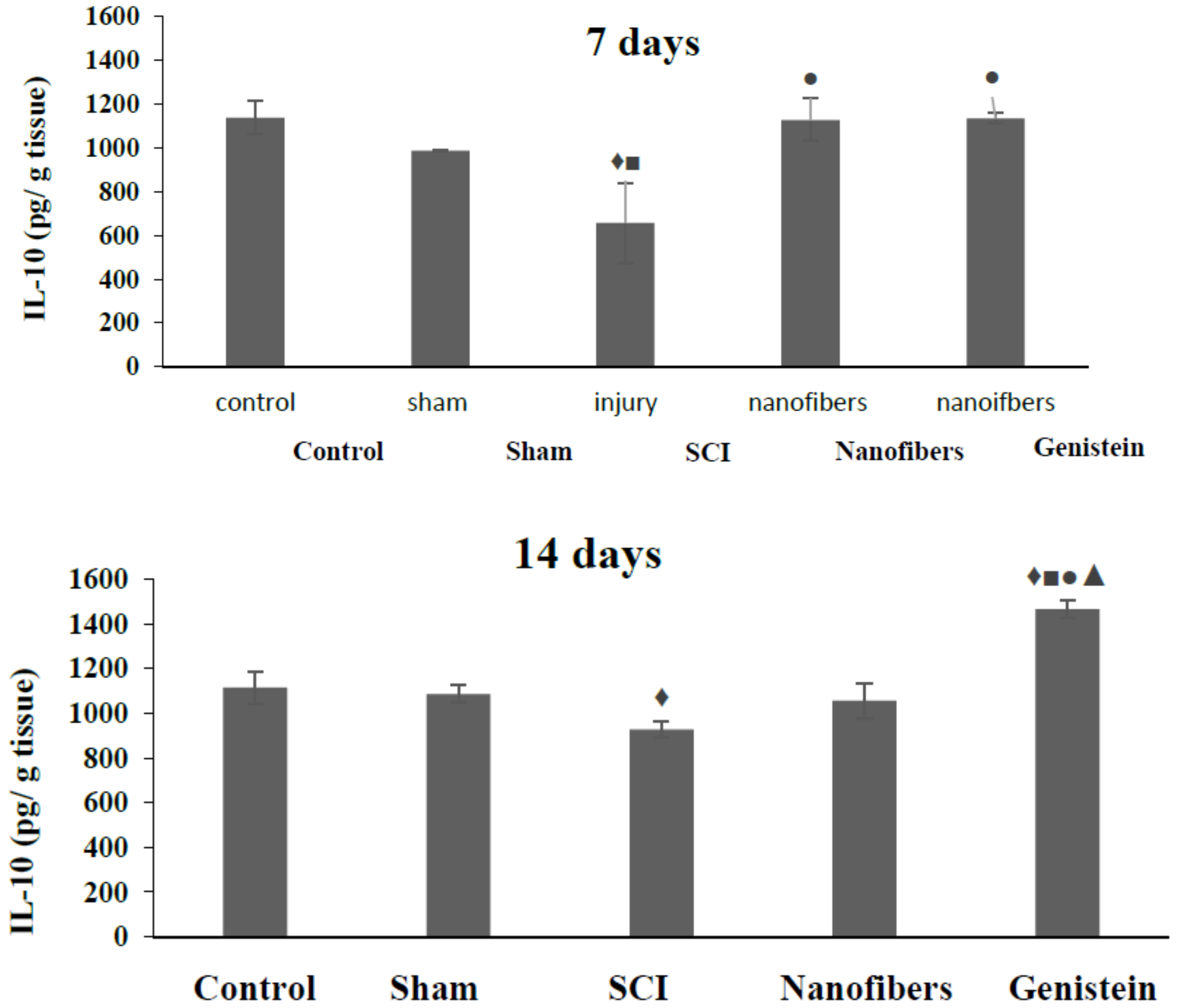
2.4. Interleukin 10 (IL-10) Level in Spinal Cord Tissue
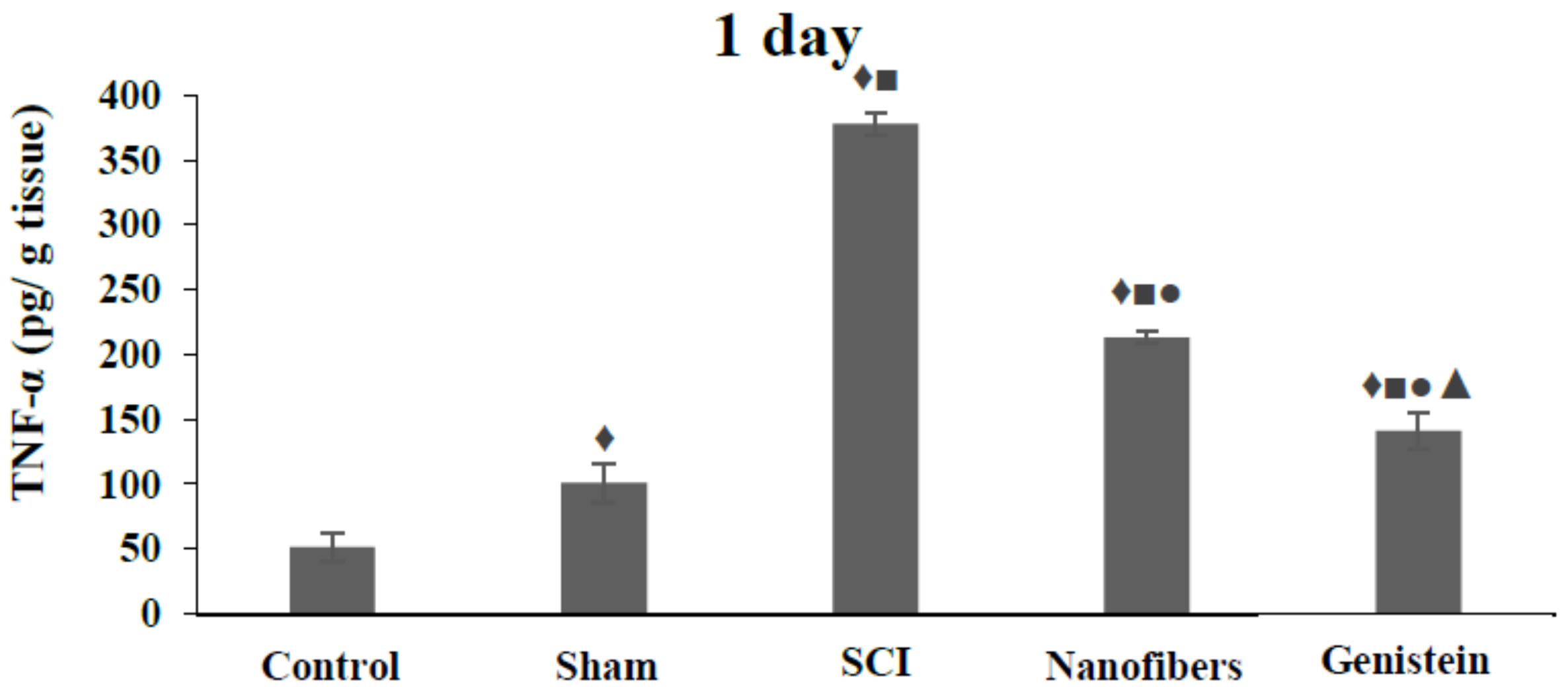
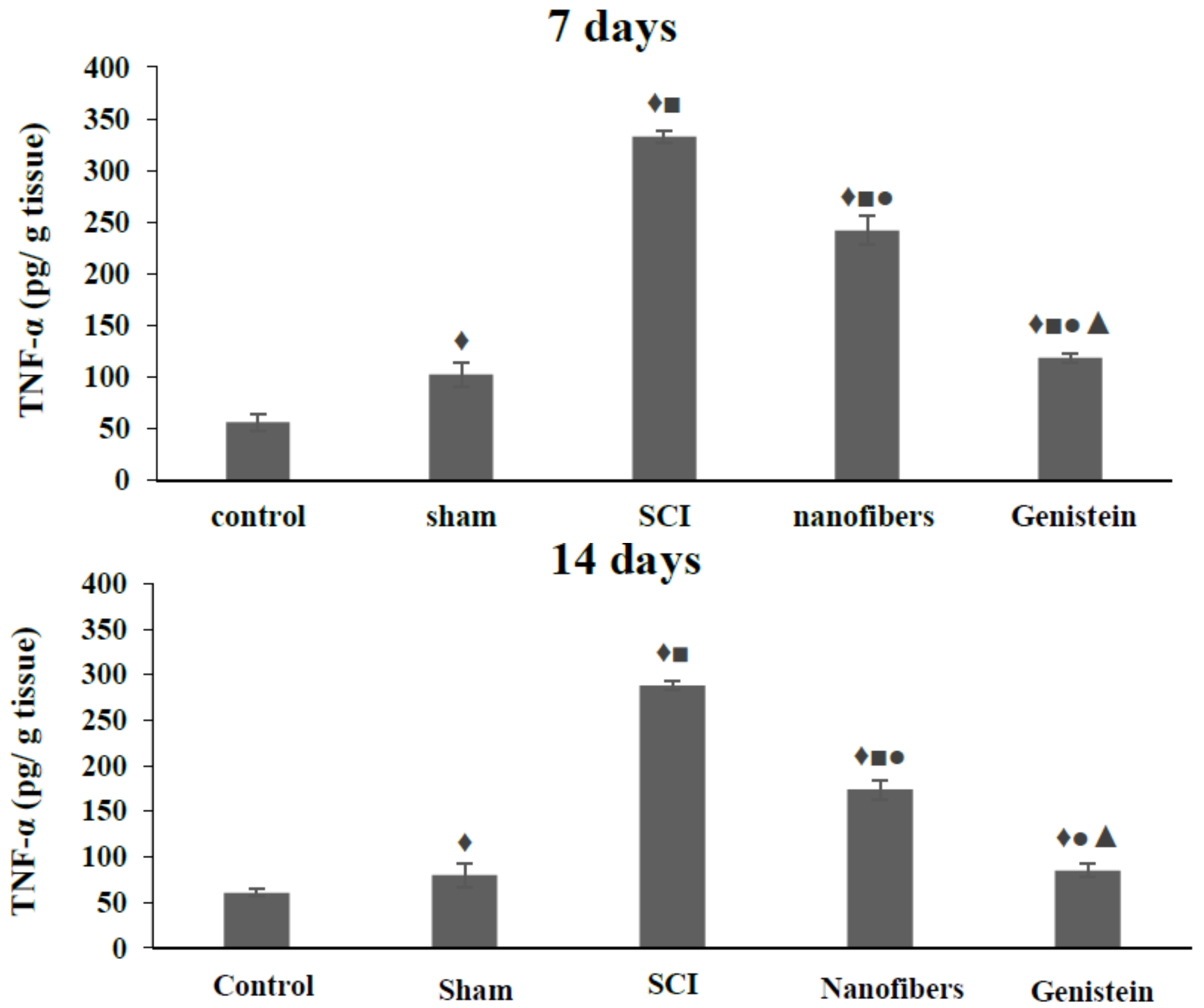
2.5. Tumor Necrosis Factor—α (TNF-α) Levels in Spinal Cord Tissue
3. Discussion
4. Materials and Methods
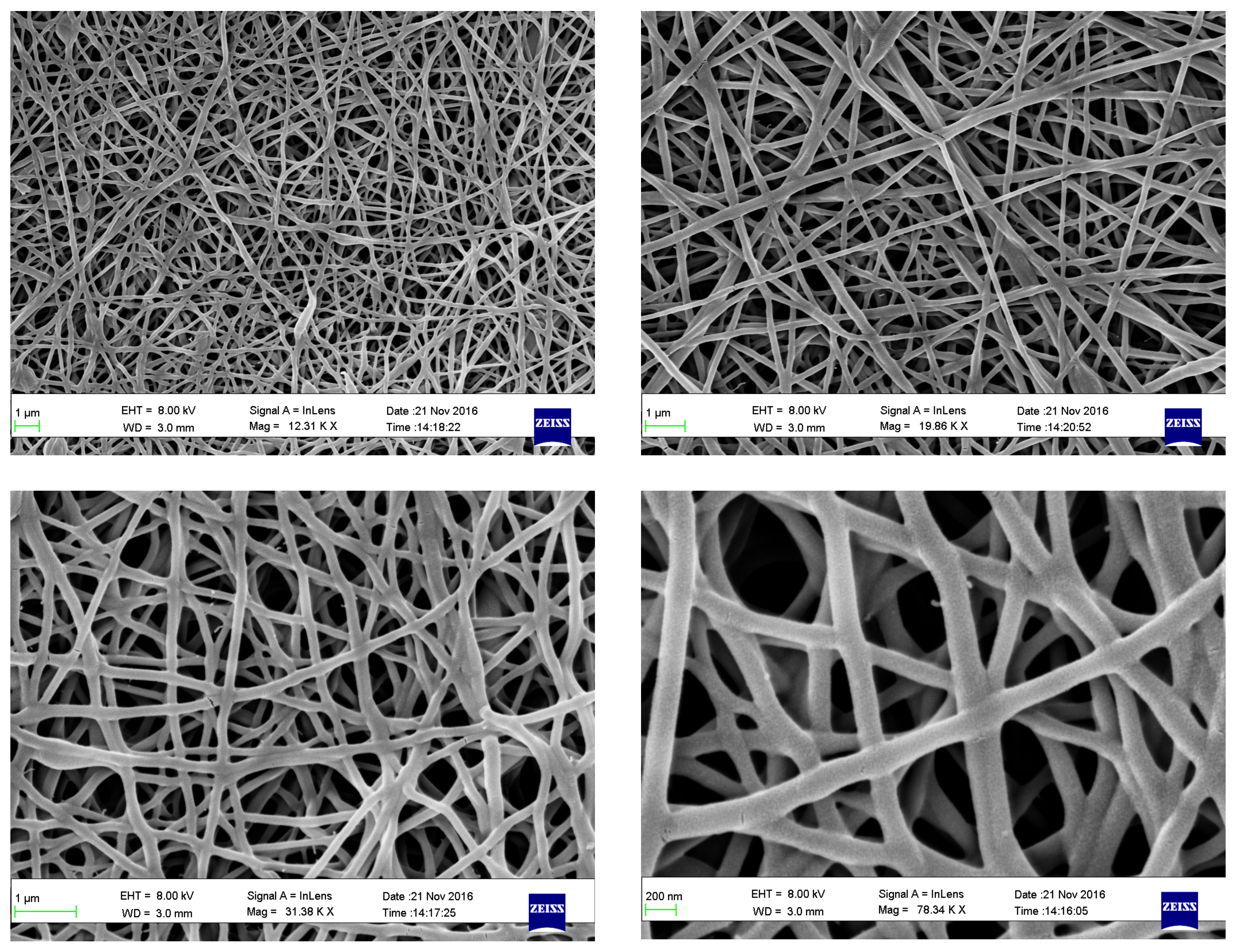
4.1. Preparation of CS/PVA Nanofibers
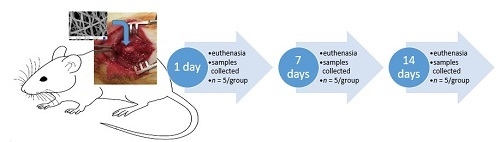
4.2. Experimental Animals
4.2.1. Handling of Animals
4.2.2. Surgical Procedure of Spinal Cord Injury
4.3. Biochemical Analyses
4.3.1. Determination of Super Oxide Dismutase (SOD) Activity
4.3.2. Determination of Nitrous Oxide (NO) Concentration
4.3.3. Determination of MDA Concentration
4.3.4. Quantitative Determination of IL-10 and TNF-α by Enzyme-Linked Immunosorbent Assay (ELISA)
4.3.5. Statistical Analyses
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| BBB | Blood brain barrier |
| CS | Chitosan |
| CNS | Central nervous system |
| eNOS | endothelial nitric oxide synthase |
| iNOS | inducible nitric oxide synthase |
| IACUC | Institutional Animal Care and Use Committee |
| IL-10 | interleukin 10 |
| IP | intraperitoneal |
| KV | Kilo volt |
| LSD | Least Significant Difference |
| MDA | Malondialdehyde |
| NIH | National institute of health |
| NO | Nitrous oxide |
| PBS | phosphate buffer solution |
| PVA | Polyvinyl alcohol |
| ROS | reactive oxygen species |
| SCI | Spinal cord injury |
| S.E.M | standard error of mean |
| SOD | superoxide dismutase |
| TCD | tip-to-collector distance |
| TNF-α | tumor necrosis factor-α |
References
- Willerth, S.M.; Sakiyama-Elbert, S.E. Combining Stem Cells and Biomaterial Scaffolds for Constructing Tissues and Cell Delivery; StemBook: Cambridge, MA, USA, 2008. [Google Scholar]
- David, S.; Lacroix, S. Molecular approaches to spinal cord repair. Annu. Rev. Neurosci. 2003, 26, 411–440. [Google Scholar] [CrossRef] [PubMed]
- Sekhon, L.H.; Fehlings, M.G. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine (Phila Pa 1976) 2001, 26, S2–S12. [Google Scholar] [CrossRef]
- Pineau, I.; Lacroix, S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: Multiphasic expression pattern and identification of the cell types involved. J. Comp. Neurol. 2007, 500, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Fatima, G.; Sharma, V.P.; Das, S.K.; Mahdi, A.A. Oxidative stress and antioxidative parameters in patients with spinal cord injury: Implications in the pathogenesis of disease. Spinal Cord 2015, 53, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Sribnick, E.A.; Samantaray, S.; Das, A.; Smith, J.; Matzelle, D.D.; Ray, S.K.; Banik, N.L. Postinjury estrogen treatment of chronic spinal cord injury improves locomotor function in rats. J. Neurosci. Res. 2010, 88, 1738–1750. [Google Scholar] [CrossRef] [PubMed]
- Kachadroka, S.; Hall, A.M.; Niedzielko, T.L.; Chongthammakun, S.; Floyd, C.L. Effect of endogenous androgens on 17beta-estradiol-mediated protection after spinal cord injury in male rats. J. Neurotrauma 2010, 27, 611–626. [Google Scholar] [CrossRef] [PubMed]
- Ritz, M.F.; Hausmann, O.N. Effect of 17beta-estradiol on functional outcome, release of cytokines, astrocyte reactivity and inflammatory spreading after spinal cord injury in male rats. Brain Res. 2008, 1203, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Morrow, A.L.; Biggio, G.; Serra, M.; Becker, H.C.; Lopez, M.F.; Porcu, P.; Alward, S.E.; O’Buckley, T.K. The role of neuroactive steroids in ethanol/stress interactions: Proceedings of symposium VII at the Volterra conference on alcohol and stress, May 2008. Alcohol 2009, 43, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.G.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; van der Saag, P.T.; van der Burg, B.; Gustafsson, J.A. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, M.; Rezakhani, A.; Nyström, S.; Turkina, M.V.; Roghani, M.; Hammarström, P.; Mohseni, S. Amyloid beta(1-40)-induced astrogliosis and the effect of genistein treatment in rat: A three-dimensional confocal morphometric and proteomic study. PLoS ONE 2013, 8, e76526. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Wu, H.; Li, W.; Gao, P. Protective effects of genistein in homocysteine-induced endothelial cell inflammatory injury. Mol. Cell. Biochem. 2015, 403, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Zhang, Y.; Yang, Q.; Cheng, S.; Hao, J.; Zhao, X.; Jiang, Z. Genistein suppresses LPS-induced inflammatory response through inhibiting NF-kappaB following AMP kinase activation in RAW 264.7 macrophages. PLoS ONE 2012, 7, e53101. [Google Scholar] [CrossRef] [PubMed]
- Caccamo, D.; Campisi, A.; Currò, M.; Bramanti, V.; Tringali, M.; Li Volti, G.; Vanella, A.; Ientile, R. Antioxidant treatment inhibited glutamate-evoked NF-kappaB activation in primary astroglial cell cultures. Neurotoxicology 2005, 26, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, B.; Aviram, M. Flavonoids protect LDL from oxidation and attenuate atherosclerosis. Curr. Opin. Lipidol. 2001, 12, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Ellis-Behnke, R. Nano neurology and the four P’s of central nervous system regeneration: Preserve, permit, promote, plasticity. Med. Clin. North Am. 2007, 91, 937–962. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, J.D.; Schauer, C.L. Cross-linking chitosan nanofibers. Biomacromolecules 2007, 8, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A.; Greco, F.; Busilacchi, A.; Sollazzo, V.; Gigante, A. Chitosan, hyaluronan and chondroitin sulfate in tissue engineering for cartilage regeneration: A review. Carbohydr. Polym. 2012, 89, 723–739. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, K.; Ouchi, T.; Ohya, Y. Biodegradable nanogels prepared by self-assembly of poly(L-lactide)-grafted dextran: Entrapment and release of proteins. Macromol. Biosci. 2008, 8, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.B.; Vitorino, P.M.; Bogyo, M. Activity-based protein profiling: Applications to biomarker discovery, in vivo imaging and drug discovery. Am. J. Pharmacogenomics 2004, 4, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Rivest, S. Anti-inflammatory effects of prostaglandin E2 in the central nervous system in response to brain injury and circulating lipopolysaccharide. J. Neurochem. 2001, 76, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; MacEwan, M.R.; Schwartz, A.G.; Xia, Y. Electrospun nanofibers for neural tissue engineering. Nanoscale 2010, 2, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Li, F.; Li, J.; Wang, L.; Li, Y.; Zhang, C.; Xu, J.; Chen, S. A new biodegradable sisal fiber-starch packing composite with nest structure. Carbohydr. Polym. 2018, 189, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Tao, Y.; Pan, Y.; Qu, W.; Cheng, G.; Huang, L.; Chen, D.; Wang, X.; Liu, Z.; Yuan, Z. Biodegradable nanoparticles for intracellular delivery of antimicrobial agents. J. Control. Release 2014, 187, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Lin, H.Y. Crosslinked chitosan: Its physical properties and the effects of matrix stiffness on chondrocyte cell morphology and proliferation. J. Biomed. Mater. Res. A 2005, 75, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C.P.; Pemble, C.W.; Brand, D.D.; Simpson, D.G.; Bowlin, G.L. Cross-linking electrospun type II collagen tissue engineering scaffolds with carbodiimide in ethanol. Tissue Eng. 2007, 13, 1593–1605. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C.P.; Sell, S.A.; Boland, E.D.; Simpson, D.G.; Bowlin, G.L. Nanofiber technology: Designing the next generation of tissue engineering scaffolds. Adv. Drug Deliv. Rev. 2007, 59, 1413–1433. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Zhu, Y.; Wu, L.; You, B.; Zi, J. Fabrication of robust crystal balls from the electrospray of soft polymer spheres/silica dispersion. Langmuir 2010, 26, 6604–6609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.N.; Xiao, Z.P.; Ding, H.; Ge, H.M.; Xu, C.; Zhu, H.L.; Tan, R.X. Synthesis and cytotoxic evaluation of novel 7-O-modified genistein derivatives. Chem. Biodivers. 2007, 4, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W. Neurotoxicology of the brain barrier system: New implications. J. Toxicol. Clin. Toxicol. 2001, 39, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Faden, A.I. Naloxone after spinal cord injury. Neurosurgery 1987, 21, 429–431. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dyck, R.; Cynader, M. The correlation between cortical neuron maturation and neurofilament phosphorylation: A developmental study of phosphorylated 200 kDa neurofilament protein in cat visual cortex. Brain Res. Dev. Brain Res. 1994, 81, 151–161. [Google Scholar] [CrossRef]
- Guo, T.L.; Wang, Y.; Xiong, T.; Ling, X.; Zheng, J. Genistein modulation of streptozotocin diabetes in male B6C3F1 mice can be induced by diet. Toxicol. Appl. Pharmacol. 2014, 280, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.L.; Germolec, D.R.; Zheng, J.F.; Kooistra, L.; Auttachoat, W.; Smith, M.J.; White, K.L.; Elmore, S.A. Genistein protects female nonobese diabetic mice from developing type 1 diabetes when fed a soy- and alfalfa-free diet. Toxicol. Pathol. 2015, 43, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.S.; Pu, Z.C.; Hao, Z.H. Carvacrol protects against spinal cord injury in rats via suppressing oxidative stress and the endothelial nitric oxide synthase pathway. Mol. Med. Rep. 2015, 12, 5349–5354. [Google Scholar] [CrossRef] [PubMed]
- Kalayci, M.; Coskun, O.; Cagavi, F.; Kanter, M.; Armutcu, F.; Gul, S.; Acikgoz, B. Neuroprotective effects of ebselen on experimental spinal cord injury in rats. Neurochem. Res. 2005, 30, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Varija, D.; Kumar, K.P.; Reddy, K.P.; Reddy, V.K. Prolonged constriction of sciatic nerve affecting oxidative stressors & antioxidant enzymes in rat. Indian J. Med. Res. 2009, 129, 587–592. [Google Scholar] [PubMed]
- Vural, M.; Arslantas, A.; Yazihan, N.; Köken, T.; Uzuner, K.; Arslantaş, D.; Ozbek, Z. NMDA receptor blockage with 2-amino-5-phosphonovaleric acid improves oxidative stress after spinal cord trauma in rats. Spinal Cord 2010, 48, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.H.; Lanaro, C.; Leiria, L.O.; Rodrigues, R.L.; Davel, A.P.; Claudino, M.A.; Toque, H.A.; Antunes, E. Oxidative stress associated with middle aging leads to sympathetic hyperactivity and downregulation of soluble guanylyl cyclase in corpus cavernosum. Am. J. Physiol. Heart Circ. Physiol. 2014, 307, H1393–H1400. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhao, P.; Zhu, K. Fabrication and characterization of zein-based nanofibrous scaffolds by an electrospinning method. Macromol. Biosci. 2007, 7, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Murugan, R.; Huang, Z.M.; Yang, F.; Ramakrishna, S. Nanofibrous scaffold engineering using electrospinning. J. Nanosci. Nanotechnol. 2007, 7, 4595–4603. [Google Scholar] [PubMed]
- Murugan, R.; Ramakrishna, S. Design strategies of tissue engineering scaffolds with controlled fiber orientation. Tissue Eng. 2007, 13, 1845–1866. [Google Scholar] [CrossRef] [PubMed]
- Sangsanoh, P.; Supaphol, P. Stability improvement of electrospun chitosan nanofibrous membranes in neutral or weak basic aqueous solutions. Biomacromolecules 2006, 7, 2710–2714. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Bellamkonda, R.V. Biomaterials for the central nervous system. J. R. Soc. Interface 2008, 5, 957–975. [Google Scholar] [CrossRef] [PubMed]
- Garreta, E.; Genove, E.; Borrós, S.; Semino, C.E. Osteogenic differentiation of mouse embryonic stem cells and mouse embryonic fibroblasts in a three-dimensional self-assembling peptide scaffold. Tissue Eng. 2006, 12, 2215–2227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.J.; Yan, Y.J.; Xiang, W.S. Comparative studies on the interaction of genistein, 8-chlorogenistein, and 3′,8-dichlorogenistein with bovine serum albumin. J. Agric. Food Chem. 2011, 59, 7506–7513. [Google Scholar] [CrossRef] [PubMed]
- Kousidou, O.; Tzanakakis, G.N.; Karamanos, N.K. Effects of the natural isoflavonoid genistein on growth, signaling pathways and gene expression of matrix macromolecules by breast cancer cells. Mini Rev. Med. Chem. 2006, 6, 331–337. [Google Scholar] [CrossRef] [PubMed]
- McClain, R.M.; Wolz, E.; Davidovich, A.; Edwards, J.; Bausch, J. Reproductive safety studies with genistein in rats. Food Chem. Toxicol. 2007, 45, 1319–1332. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Tzagarakis-Foster, C.; Scharschmidt, T.C.; Lomri, N.; Leitman, D.C. Estrogen receptor beta-selective transcriptional activity and recruitment of coregulators by phytoestrogens. J. Biol. Chem. 2001, 276, 17808–17814. [Google Scholar] [CrossRef] [PubMed]
- Duffy, C.; Perez, K.; Partridge, A. Implications of phytoestrogen intake for breast cancer. CA Cancer J. Clin. 2007, 57, 260–277. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, K.; Wen, G.; Guo, H.; Li, S.; Wu, X.; Dai, R.; Sheng, Z.; Liao, E. Comparison of the effects of genistein and zoledronic acid on the bone loss in OPG-deficient mice. Bone 2008, 42, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Groyer, G.; Eychenne, B.; Girard, C.; Rajkowski, K.; Schumacher, M.; Cadepond, F. Expression and functional state of the corticosteroid receptors and 11 beta-hydroxysteroid dehydrogenase type 2 in Schwann cells. Endocrinology 2006, 147, 4339–4350. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.X.; Chen, W.F.; Xie, J.X.; Wong, M.S. Neuroprotective effects of genistein on dopaminergic neurons in the mice model of Parkinson’s disease. Neurosci. Res. 2008, 60, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Straub, R.H. The complex role of estrogens in inflammation. Endocr. Rev. 2007, 28, 521–574. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.S.H.; EL-Rafei, A.M.; Allam, N.K. Improved genistein loading and release on electrospun chitosan nanofiber blends. J. Mol. Liquids 2016, 223, 1056–1061. [Google Scholar] [CrossRef]
- Nishikimi, M.; Appaji, N.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Montgomery, J.A.; Johnston, T.P.; Gallagher, A.; Stringfellow, C.R.; Schabel, F.M., Jr. A comparative study of the anticancer activity of some S-substituted derivatives of 6-mercaptopurine and their ribonucleosides. J. Med. Pharm. Chem. 1960, 3, 265–288. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismail, M.; Ibrahim, S.; El-Amir, A.; EL-Rafei, A.M.; Allam, N.K.; Abdellatif, A. Genistein Loaded Nanofibers Protect Spinal Cord Tissue Following Experimental Injury in Rats. Biomedicines 2018, 6, 96. https://doi.org/10.3390/biomedicines6040096
Ismail M, Ibrahim S, El-Amir A, EL-Rafei AM, Allam NK, Abdellatif A. Genistein Loaded Nanofibers Protect Spinal Cord Tissue Following Experimental Injury in Rats. Biomedicines. 2018; 6(4):96. https://doi.org/10.3390/biomedicines6040096
Chicago/Turabian StyleIsmail, Mohamed, Sara Ibrahim, Azza El-Amir, Amira M. EL-Rafei, Nageh K. Allam, and Ahmed Abdellatif. 2018. "Genistein Loaded Nanofibers Protect Spinal Cord Tissue Following Experimental Injury in Rats" Biomedicines 6, no. 4: 96. https://doi.org/10.3390/biomedicines6040096
APA StyleIsmail, M., Ibrahim, S., El-Amir, A., EL-Rafei, A. M., Allam, N. K., & Abdellatif, A. (2018). Genistein Loaded Nanofibers Protect Spinal Cord Tissue Following Experimental Injury in Rats. Biomedicines, 6(4), 96. https://doi.org/10.3390/biomedicines6040096