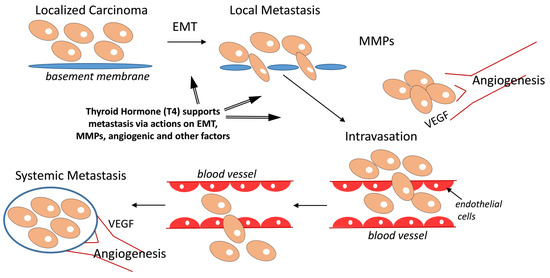
Contributions of Thyroid Hormone to Cancer Metastasis
Abstract
1. Introduction
2. Cancer Metastasis-Relevant Molecular Mechanisms of Thyroid Hormone Action
2.1. Matrix Metalloproteinase (MMP) Gene Expression and Metastasis
2.2. Angiogenesis and Metastasis
2.3. microRNAs (miRs) and Metastasis
2.4. Epithelial–Mesenchymal Transition (EMT) and Mechanism of Metastasis
2.5. Transforming Growth Factor β (TGFβ) and Metastasis
2.6. EGF Receptor (EGFR), Metastasis and Angiogenesis
2.7. Tumor-Associated Macrophages (TAMs) and Metastasis
3. Selected Cancer Driver Genes, Thyroid Hormone Analogues and Metastases
4. Fibronectin and Metastasis
5. Discussion
6. Conclusions
Funding
Conflicts of Interest
References
- Pinto, M.; Soares, P.; Ribatti, D. Thyroid hormone as a regulator of tumor induced angiogenesis. Cancer Lett. 2011, 301, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.J.; Sudha, T.; Lin, H.Y.; Mousa, S.A. Thyroid hormone, hormone analogs, and angiogenesis. Compr. Physiol. 2015, 6, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Cremaschi, G.A.; Cayrol, F.; Sterle, H.A.; Diaz Flaque, M.C.; Barreiro Arcos, M.L. Thyroid hormones and their membrane receptors as therapeutic targets for T cell lymphomas. Pharmacol. Res. 2016, 109, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Chin, Y.T.; Yang, Y.C.; Lai, H.Y.; Wang-Peng, J.; Liu, L.F.; Tang, H.Y.; Davis, P.J. Thyroid hormone, cancer, and apoptosis. Compr. Physiol. 2016, 6, 1221–1237. [Google Scholar] [CrossRef] [PubMed]
- Shinderman-Maman, E.; Cohen, K.; Weingarten, C.; Nabriski, D.; Twito, O.; Baraf, L.; Hercbergs, A.; Davis, P.J.; Werner, H.; Ellis, M.; et al. The thyroid hormone-αvβ3 integrin axis in ovarian cancer: Regulation of gene transcription and MAPK-dependent proliferation. Oncogene 2016, 35, 1977–1987. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.T.; Wei, P.L.; Ho, Y.; Nana, A.W.; Changou, C.A.; Chen, Y.R.; Yang, Y.S.; Hsieh, M.T.; Hercbergs, A.; Davis, P.J.; et al. Thyroxine inhibits resveratrol-caused apoptosis by PD-L1 in ovarian cancer cells. Endocr. Relat. Cancer 2018, 25, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Schmidinger, M.; Vogl, U.M.; Bojic, M.; Lamm, W.; Heinzl, H.; Haitel, A.; Clodi, M.; Kramer, G.; Zielinski, C.C. Hypothyroidism in patients with renal cell carcinoma: Blessing or curse? Cancer 2011, 117, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Bailey, E.B.; Tantravahi, S.K.; Poole, A.; Agarwal, A.M.; Straubhar, A.M.; Batten, J.A.; Patel, S.B.; Wells, C.E.; Stenehjem, D.D.; Agarwal, N. Correlation of degree of hypothyroidism with survival outcomes in patients with metastatic renal cell carcinoma receiving vascular endothelial growth factor receptor tyrosine kinase inhibitors. Clin. Genitourin. Cancer 2015, 13, e131–e137. [Google Scholar] [CrossRef] [PubMed]
- Cristofanilli, M.; Yamamura, Y.; Kau, S.W.; Bevers, T.; Strom, S.; Patangan, M.; Hsu, L.; Krishnamurthy, S.; Theriault, R.L.; Hortobagyi, G.N. Thyroid hormone and breast carcinoma. Primary hypothyroidism is associated with a reduced incidence of primary breast carcinoma. Cancer 2005, 103, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.; Hercbergs, A.; Rybicki, L.; Strome, M. Association between development of hypothyroidism and improved survival in patients with head and neck cancer. Arch. Otolaryngol. Head Neck Surg. 2006, 132, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.Y.; Leonard, J.L.; Davis, P.J. Molecular aspects of thyroid hormone actions. Endocr. Rev. 2010, 31, 139–170. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.J.; Goglia, F.; Leonard, J.L. Nongenomic actions of thyroid hormone. Nat. Rev. Endocrinol. 2016, 12, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.J.; Glinsky, G.V.; Lin, H.-Y.; Leith, J.T.; Hercbergs, A.; Tang, H.-Y.; Ashur-Fabian, O.; Incerpi, S.; Mousa, S.A. Cancer cell gene expression modulated from plasma membrane integrin αvβ3 by thyroid hormone and nanoparticulate tetrac. Front. Endocrinol. 2014, 5, 240. [Google Scholar] [CrossRef]
- Lin, H.Y.; Landersdorfer, C.B.; London, D.; Meng, R.; Lim, C.U.; Lin, C.; Lin, S.; Tang, H.Y.; Brown, D.; Van Scoy, B.; et al. Pharmacodynamic modeling of anti-cancer activity of tetraiodothyroacetic acid in a perfused cell culture system. PLoS Comput. Biol. 2011, 7, e1001073. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Sun, M.; Tang, H.Y.; Lin, C.; Luidens, M.K.; Mousa, S.A.; Incerpi, S.; Drusano, G.L.; Davis, F.B.; Davis, P.J. l-thyroxine vs. 3,5,3’-triiodo-l-thyronine and cell proliferation: Activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase. Am. J. Physiol. Cell Physiol. 2009, 296, C980–C991. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Zhou, M.; Li, X.; Hu, M.; Li, C.; Li, M.; Sheng, F.; Li, Z.; Wu, G.; Luo, M.; et al. Synergistic active targeting of dually integrin αvβ3/CD44-targeted nanoparticles to B16F10 tumors located at different sites of mouse bodies. J. Control. Release 2016, 235, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Weingarten, C.; Jenudi, Y.; Tshuva, R.Y.; Moskovich, D.; Alfandari, A.; Hercbergs, A.; Davis, P.J.; Ellis, M.; Ashur-Fabian, O. The interplay between epithelial-mesenchymal transition (EMT) and the thyroid hormones-αvβ3 axis in ovarian cancer. Horm. Cancer 2018, 9, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Chen, L.; Song, Y.; Li, X.; Sun, Y.; Xiao, Y.; Xing, Y. Tetraiodothyroacetic acid and transthyretin silencing inhibit pro-metastatic effect of l-thyroxin in anoikis-resistant prostate cancer cells through regulation of MAPK/ERK pathway. Exp. Cell Res. 2016, 347, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Mousa, S.A.; Davis, P.J. Pro- and anti-metastatic properties of specific thyroid hormone analogues. manuscript in preparation.
- Tauro, M.; Lynch, C.C. Cutting to the chase: How matrix metalloproteinase-2 activity controls breast-cancer-to-bone metastasis. Cancers 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Cheng, H.; Song, L.; Wang, W.; Wang, Q.; Xu, D.; Xing, W. Wogonin suppresses the activity of matrix metalloproteinase-9 and inhibits migration and invasion in human hepatocellular carcinoma. Molecules 2018, 23, 384. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Zhu, H.; Li, Y. Pkcz, MMP-2 and MMP-9 expression in lung adenocarcinoma and association with a metastatic phenotype. Mol. Med. Rep. 2017, 16, 8301–8306. [Google Scholar] [CrossRef] [PubMed]
- Cohen, K.; Flint, N.; Shalev, S.; Erez, D.; Baharal, T.; Davis, P.J.; Hercbergs, A.; Ellis, M.; Ashur-Fabian, O. Thyroid hormone regulates adhesion, migration and matrix metalloproteinase 9 activity via αvβ3 integrin in myeloma cells. Oncotarget 2014, 5, 6312–6322. [Google Scholar] [CrossRef] [PubMed]
- Davis, F.B.; Mousa, S.A.; O’Connor, L.; Mohamed, S.; Lin, H.Y.; Cao, H.J.; Davis, P.J. Proangiogenic action of thyroid hormone is fibroblast growth factor-dependent and is initiated at the cell surface. Circ. Res. 2004, 94, 1500–1506. [Google Scholar] [CrossRef] [PubMed]
- Mousa, S.S.; Mousa, S.S.; Mousa, S.A. Effect of resveratrol on angiogenesis and platelet/fibrin-accelerated tumor growth in the chick chorioallantoic membrane model. Nutr. Cancer 2005, 52, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ortmeier, S.B.; Savinova, O.V.; Nareddy, V.B.; Beyer, A.J.; Wang, D.; Gerdes, A.M. Thyroid hormone induces sprouting angiogenesis in adult heart of hypothyroid mice through the PDGF-Akt pathway. J. Cell. Mol. Med. 2012, 16, 2726–2735. [Google Scholar] [CrossRef] [PubMed]
- Mousa, S.A.; Lin, H.Y.; Tang, H.Y.; Hercbergs, A.; Luidens, M.K.; Davis, P.J. Modulation of angiogenesis by thyroid hormone and hormone analogues: Implications for cancer management. Angiogenesis 2014, 17, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.R.; Gomes, C.C.; Santos, M.F. Role of microRNAs in endocrine cancer metastasis. Mol. Cell. Endocrinol. 2017, 456, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Leng, J.; Song, Q.; Zhao, Y.; Wang, Z. Mi-R15a represses cancer cell migration and invasion under conditions of hypoxia by targeting and downregulating Bcl-2 expression in human osteosarcoma cells. Int. J. Oncol. 2018, 52, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Chen, F.; Wang, K.; Song, Y.; Fei, X.; Wu, B. miR-15a/miR-16 cluster inhibits invasion of prostate cancer cells by suppressing TGF-β signaling pathway. Biomed. Pharmacother. 2018, 104, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, S.R.; Yang, C.H.; Pfeffer, L.M. The role of miR-21 in cancer. Drug Dev. Res. 2015, 76, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Wang, H.; Liu, J.; Wang, Z.X. MicroRNA-21 (miR-21) expression promotes growth, metastasis, and chemo- or radioresistance in non-small cell lung cancer cells by targeting PTEN. Mol. Cell. Biochem. 2013, 372, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Seyfried, T.N.; Huysentruyt, L.C. On the origin of cancer metastasis. Crit. Rev. Oncog. 2013, 18, 43–73. [Google Scholar] [CrossRef] [PubMed]
- Koli, K.; Keski-Oja, J. Transforming growth factor-β system and its regulation by members of the steroid-thyroid hormone superfamily. Adv. Cancer Res. 1996, 70, 63–94. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, B.G.; Naeimi, S.; Bos, I.S.; Menzen, M.H.; Halayko, A.J.; Hashjin, G.S.; Meurs, H. l-thyroxine promotes a proliferative airway smooth muscle phenotype in the presence of TGF-β1. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L301–L306. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Merino, E.; Martin Orozco, R.; Ruiz-Llorente, L.; Martinez-Iglesias, O.A.; Velasco-Martin, J.P.; Montero-Pedrazuela, A.; Fanjul-Rodriguez, L.; Contreras-Jurado, C.; Regadera, J.; Aranda, A. Thyroid hormones inhibit TGF-β signaling and attenuate fibrotic responses. Proc. Natl. Acad. Sci. USA 2016, 113, E3451–E3460. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, C.; Caja, L.; Moustakas, A. Transforming growth factor β as regulator of cancer stemness and metastasis. Br. J. Cancer 2016, 115, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Candelora, C. In vitro enzyme kinetics analysis of EGFR. Methods Mol. Biol. 2017, 1487, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sjolinder, M.; Wang, X.; Altenbacher, G.; Hagner, M.; Berglund, P.; Gao, Y.; Lu, T.; Jonsson, A.B.; Sjolinder, H. Thyroid hormone enhances nitric oxide-mediated bacterial clearance and promotes survival after meningococcal infection. PLoS ONE 2012, 7, e41445. [Google Scholar] [CrossRef] [PubMed]
- De Vito, P.; Balducci, V.; Leone, S.; Percario, Z.; Mangino, G.; Davis, P.J.; Davis, F.B.; Affabris, E.; Luly, P.; Pedersen, J.Z.; et al. Nongenomic effects of thyroid hormones on the immune system cells: New targets, old players. Steroids 2012, 77, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Duquette, M.; Sadow, P.M.; Husain, A.; Sims, J.N.; Antonello, Z.A.; Fischer, A.H.; Song, C.; Castellanos-Rizaldos, E.; Makrigiorgos, G.M.; Kurebayashi, J.; et al. Metastasis-associated MCL1 and P16 copy number alterations dictate resistance to vemurafenib in a BRAFV600E patient-derived papillary thyroid carcinoma preclinical model. Oncotarget 2015, 6, 42445–42467. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Park, Y.L.; Kim, N.; Oh, H.H.; Son, D.J.; Kim, M.Y.; Oak, C.Y.; Chung, C.Y.; Park, H.C.; Kim, J.S.; et al. Myeloid cell leukemia-1 regulates the cell growth and predicts prognosis in gastric cancer. Int. J. Oncol. 2015, 46, 2154–2162. [Google Scholar] [CrossRef] [PubMed]
- Glinskii, A.B.; Glinsky, G.V.; Lin, H.Y.; Tang, H.Y.; Sun, M.; Davis, F.B.; Luidens, M.K.; Mousa, S.A.; Hercbergs, A.H.; Davis, P.J. Modification of survival pathway gene expression in human breast cancer cells by tetraiodothyroacetic acid (tetrac). Cell Cycle 2009, 8, 3562–3570. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, M.S.; Toraih, E.A.; Ibrahiem, A.; Abdeldayem, H.; Mohamed, A.O.; Abdel-Daim, M.M. Evaluation of miRNA-196a2 and apoptosis-related target genes: ANXA1, DFFA and PDCD4 expression in gastrointestinal cancer patients: A pilot study. PLoS ONE 2017, 12, e0187310. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, D.; Ueda, H.; Shimizu, R.; Kato, R.; Otoshi, T.; Kawamura, T.; Tamai, K.; Shibata, Y.; Matsumoto, T.; Nagata, K.; et al. Features and prognostic impact of distant metastasis in patients with stage IV lung adenocarcinoma harboring EGFR mutations: Importance of bone metastasis. Clin. Exp. Metastasis 2014, 31, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Glinksy, G.V.; Davis, P.J. Differential cancer driver gene expression by P-bi-TAT, a PEGylated modification of the thyroid hormone analogue, tetraiodothyroacetic acid (tetrac). manuscript in preparation.
- Fuchs, E.M.; Kostler, W.J.; Horvat, R.; Hudelist, G.; Kubista, E.; Attems, J.; Zielinski, C.C.; Singer, C.F. High-level ERBB2 gene amplification is associated with a particularly short time-to-metastasis, but results in a high rate of complete response once trastuzumab-based therapy is offered in the metastatic setting. Int. J. Cancer 2014, 135, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Donovan, C.A.; Pommier, R.F.; Schillace, R.; O’Neill, S.; Muller, P.; Alabran, J.L.; Hansen, J.E.; Murphy, J.A.; Naik, A.M.; Vetto, J.T.; et al. Correlation of breast cancer axillary lymph node metastases with stem cell mutations. JAMA Surg. 2013, 148, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.T.; Nian, X.X.; Wang, S.Y.; Jiao, H.L.; Wang, Y.X.; Xiao, Z.Y.; Yang, R.W.; Ding, Y.Q.; Ye, Y.P.; Liao, W.T. MiR-422a inhibits cell proliferation in colorectal cancer by targeting AKT1 and MAPK1. Cancer Cell Int. 2017, 17, 91. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Chen, Y.; Zhou, Y.; Huang, X.; Li, G.; Zeng, J.; Ruan, Z.; Xie, X.; Zhang, J. Delineating the underlying molecular mechanisms and key genes involved in metastasis of colorectal cancer via bioinformatics analysis. Oncol. Rep. 2018, 39, 2297–2305. [Google Scholar] [CrossRef] [PubMed]
- Sahlberg, S.H.; Mortensen, A.C.; Haglof, J.; Engskog, M.K.R.; Arvidsson, T.; Pettersson, C.; Glimelius, B.; Stenerlow, B.; Nestor, M. Different functions of AKT1 and AKT2 in molecular pathways, cell migration and metabolism in colon cancer cells. Int. J. Oncol. 2017, 50, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Xiao, X.; Liang, Y.; Peng, L.; Guo, Z.; Li, W.; Yu, W. MiR-19a-mediated downregulation of RhoB inhibits the dephosphorylation of AKT1 and induces osteosarcoma cell metastasis. Cancer Lett. 2018, 428, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xu, W.W.; Lam, A.K.Y.; Wang, Y.; Hu, H.F.; Guan, X.Y.; Qin, Y.R.; Saremi, N.; Tsao, S.W.; He, Q.Y.; et al. Significance of PI3K/AKT signaling pathway in metastasis of esophageal squamous cell carcinoma and its potential as a target for anti-metastasis therapy. Oncotarget 2017, 8, 38755–38766. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Li, L.; Liu, J.; Wang, L.; Zhou, Y. Mir-495 inhibits esophageal squamous cell carcinoma progression by targeting Akt1. Oncotarget 2016, 7, 51223–51236. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.Y.; Wang, W.; Yan, W.X.; Chen, D.; Ding, X.Q.; Wang, A.X. Dysregulation of AKT1, a miR-138 target gene, is involved in the migration and invasion of tongue squamous cell carcinoma. J. Oral. Pathol. Med. 2017, 46, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Riggio, M.; Perrone, M.C.; Polo, M.L.; Rodriguez, M.J.; May, M.; Abba, M.; Lanari, C.; Novaro, V. AKT1 and AKT2 isoforms play distinct roles during breast cancer progression through the regulation of specific downstream proteins. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, E.; Robb, C.M.; Smith, L.M.; Brattain, M.G.; Wang, J.; Black, J.D.; Chowdhury, S. Role of Akt2 in regulation of metastasis suppressor 1 expression and colorectal cancer metastasis. Oncogene 2017, 36, 3104–3118. [Google Scholar] [CrossRef] [PubMed]
- Honardoost, M.; Rad, S. Triangle of AKT2, miRNA, and tumorigenesis in different cancers. Appl. Biochem. Biotechnol. 2018, 185, 524–540. [Google Scholar] [CrossRef] [PubMed]
- Tome-Garcia, J.; Li, D.; Ghazaryan, S.; Shu, L.; Wu, L. ERBB2 increases metastatic potentials specifically in androgen-insensitive prostate cancer cells. PLoS ONE 2014, 9, e99525. [Google Scholar] [CrossRef] [PubMed]
- Ferracin, M.; Bassi, C.; Pedriali, M.; Pagotto, S.; D’Abundo, L.; Zagatti, B.; Corra, F.; Musa, G.; Callegari, E.; Lupini, L.; et al. MiR-125b targets erythropoietin and its receptor and their expression correlates with metastatic potential and ERBB2/HER2 expression. Mol. Cancer 2013, 12. [Google Scholar] [CrossRef] [PubMed]
- Worzfeld, T.; Swiercz, J.M.; Looso, M.; Straub, B.K.; Sivaraj, K.K.; Offermanns, S. ErbB-2 signals through Plexin-B1 to promote breast cancer metastasis. J. Clin. Investig. 2012, 122, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Bayrak, M.; Olmez, O.F.; Kurt, E.; Cubukcu, E.; Evrensel, T.; Kanat, O.; Manavoglu, O. Prognostic significance of c-erbB2 overexpression in patients with metastatic gastric cancer. Clin. Transl. Oncol. 2013, 15, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.K.; Parachoniak, C.A.; Ghanta, K.S.; Fitamant, J.; Ross, K.N.; Najem, M.S.; Gurumurthy, S.; Akbay, E.A.; Sia, D.; Cornella, H.; et al. Mutant IDH inhibits HNF-4α to block hepatocyte differentiation and promote biliary cancer. Nature 2014, 513, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.R.; Li, Z.H.; Qi, B.W.; Ernest, M.E.; Hu, X.; Yu, A.X. Downregulation of IDH2 exacerbates the malignant progression of osteosarcoma cells via increased NF-κB and MMP-9 activation. Oncol. Rep. 2016, 35, 2277–2285. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.Y.; Zang, S.F.; Wang, L.; Luo, Y.; Shi, J.P.; Lou, G.Q. Isocitrate dehydrogenase 2 suppresses the invasion of hepatocellular carcinoma cells via matrix metalloproteinase 9. Cell. Physiol. Biochem. 2015, 37, 2405–2414. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zheng, S.; Zheng, Y.; Huang, R.; An, N.; Liang, A.; Hu, C. Glioma derived isocitrate dehydrogenase-2 mutations induced up-regulation of HIF-1α and β-catenin signaling: Possible impact on glioma cell metastasis and chemo-resistance. Int. J. Biochem. Cell Biol. 2012, 44, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, S.; Yashiro, M.; Takashima, T.; Aomatsu, N.; Kawajiri, H.; Ogawa, Y.; Onoda, N.; Ishikawa, T.; Wakasa, K.; Hirakawa, K. c-Kit expression as a prognostic molecular marker in patients with basal-like breast cancer. Br. J. Surg. 2013, 100, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Jansson, S.; Bendahl, P.O.; Grabau, D.A.; Falck, A.K.; Ferno, M.; Aaltonen, K.; Ryden, L. The three receptor tyrosine kinases c-KIT, VEGFR2 and PDGFRα, closely spaced at 4q12, show increased protein expression in triple-negative breast cancer. PLoS ONE 2014, 9, e102176. [Google Scholar] [CrossRef] [PubMed]
- Kuonen, F.; Laurent, J.; Secondini, C.; Lorusso, G.; Stehle, J.C.; Rausch, T.; Faes-Van’t Hull, E.; Bieler, G.; Alghisi, G.C.; Schwendener, R.; et al. Inhibition of the Kit ligand/c-Kit axis attenuates metastasis in a mouse model mimicking local breast cancer relapse after radiotherapy. Clin. Cancer Res. 2012, 18, 4365–4374. [Google Scholar] [CrossRef] [PubMed]
- Mainetti, L.E.; Zhe, X.; Diedrich, J.; Saliganan, A.D.; Cho, W.J.; Cher, M.L.; Heath, E.; Fridman, R.; Kim, H.R.; Bonfil, R.D. Bone-induced c-kit expression in prostate cancer: A driver of intraosseous tumor growth. Int. J. Cancer 2015, 136, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Spahn, M.; Kneitz, S.; Scholz, C.J.; Stenger, N.; Rudiger, T.; Strobel, P.; Riedmiller, H.; Kneitz, B. Expression of microRNA-221 is progressively reduced in aggressive prostate cancer and metastasis and predicts clinical recurrence. Int. J. Cancer 2010, 127, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, C.; Nabha, S.M.; Dos Santos, E.B.; Yamamoto, H.; Meng, H.; Melchior, S.W.; Bittinger, F.; Thuroff, J.W.; Vessella, R.L.; Cher, M.; et al. C-kit and its ligand stem cell factor: Potential contribution to prostate cancer bone metastasis. Neoplasia 2008, 10, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Yang, S.; Shen, P.; Sun, H.; Xiao, J.; Wang, Y.; Wu, B.; Ji, F.; Yan, J.; Xue, H.; et al. C-kit signaling promotes proliferation and invasion of colorectal mucinous adenocarcinoma in a murine model. Oncotarget 2015, 6, 27037–27048. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Guo, J.; Yang, W.; Goncalves, C.; Rzymski, T.; Dreas, A.; Zylkiewicz, E.; Mikulski, M.; Brzozka, K.; Golas, A.; et al. MNK1/2 inhibition limits oncogenicity and metastasis of KIT-mutant melanoma. J. Clin. Investig. 2017, 127, 4179–4192. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Hidalgo, J.M.; Duran-Martinez, M.; Molero-Payan, R.; Rufian-Pena, S.; Arjona-Sanchez, A.; Casado-Adam, A.; Cosano-Alvarez, A.; Briceno-Delgado, J. Gastrointestinal stromal tumors: A multidisciplinary challenge. World J. Gastroenterol. 2018, 24, 1925–1941. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.M.; Yang, L.; Lu, X.X.; Chen, J.S.; Wu, D.; Wei, Y.F.; Nong, Q.Q.; Zhang, L.S.; Fang, W.X.; Chen, X.L.; et al. The MKK7 p.Glu116Lys rare variant serves as a predictor for lung cancer risk and prognosis in Chinese. PLoS Genet. 2016, 12. [Google Scholar] [CrossRef] [PubMed]
- Sakai, H.; Sato, A.; Aihara, Y.; Ikarashi, Y.; Midorikawa, Y.; Kracht, M.; Nakagama, H.; Okamoto, K. MKK7 mediates miR-493-dependent suppression of liver metastasis of colon cancer cells. Cancer Sci. 2014, 105, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Naito, Y.; Cope, L.; Naranjo-Suarez, S.; Saunders, T.; Hong, S.M.; Goggins, M.G.; Herman, J.M.; Wolfgang, C.L.; Iacobuzio-Donahue, C.A. Functional p38 MAPK identified by biomarker profiling of pancreatic cancer restrains growth through JNK inhibition and correlates with improved survival. Clin. Cancer Res. 2014, 20, 6200–6211. [Google Scholar] [CrossRef] [PubMed]
- Venur, V.A.; Leone, J.P. Targeted therapies for brain metastases from breast cancer. Int. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Park, K.; Lim, S.H.; Kim, H.S.; Yoo, K.H.; Jung, K.S.; Song, H.N.; Hong, M.; Do, I.G.; Ahn, T.; et al. Mutational profiling of brain metastasis from breast cancer: Matched pair analysis of targeted sequencing between brain metastasis and primary breast cancer. Oncotarget 2015, 6, 43731–43742. [Google Scholar] [CrossRef] [PubMed]
- Taglieri, L.; Nardo, T.; Vicinanza, R.; Ross, J.M.; Scarpa, S.; Coppotelli, G. Thyroid hormone regulates fibronectin expression through the activation of the hypoxia inducible factor 1. Biochem. Biophys. Res. Commun. 2017, 493, 1304–1310. [Google Scholar] [CrossRef] [PubMed]
- Malik, G.; Knowles, L.M.; Dhir, R.; Xu, S.; Yang, S.; Ruoslahti, E.; Pilch, J. Plasma fibronectin promotes lung metastasis by contributions to fibrin clots and tumor cell invasion. Cancer Res. 2010, 70, 4327–4334. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Celia-Terrassa, T.; Kang, Y. Distinctive properties of metastasis-initiating cells. Genes Dev. 2016, 30, 892–908. [Google Scholar] [CrossRef] [PubMed]
- Pulido, C.; Vendrell, I.; Ferreira, A.R.; Casimiro, S.; Mansinho, A.; Alho, I.; Costa, L. Bone metastasis risk factors in breast cancer. Ecancermedicalscience 2017, 11, 715. [Google Scholar] [CrossRef] [PubMed]
- Hercbergs, A.A.; Goyal, L.K.; Suh, J.H.; Lee, S.; Reddy, C.A.; Cohen, B.H.; Stevens, G.H.; Reddy, S.K.; Peereboom, D.M.; Elson, P.J.; et al. Propylthiouracil-induced chemical hypothyroidism with high-dose tamoxifen prolongs survival in recurrent high grade glioma: A phase I/II study. Anticancer Res. 2003, 23, 617–626. [Google Scholar] [PubMed]
- Hercbergs, A.; Johnson, R.E.; Ashur-Fabian, O.; Garfield, D.H.; Davis, P.J. Medically induced euthyroid hypothyroxinemia may extend survival in compassionate need cancer patients: An observational study. Oncologist 2015, 20, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Iglesias, O.; Garcia-Silva, S.; Regadera, J.; Aranda, A. Hypothyroidism enhances tumor invasiveness and metastasis development. PLoS ONE 2009, 4, e6428. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Iglesias, O.; Garcia-Silva, S.; Tenbaum, S.P.; Regadera, J.; Larcher, F.; Paramio, J.M.; Vennstrom, B.; Aranda, A. Thyroid hormone receptor β1 acts as a potent suppressor of tumor invasiveness and metastasis. Cancer Res. 2009, 69, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Llorente, L.; Ardila-Gonzalez, S.; Fanjul, L.F.; Martinez-Iglesias, O.; Aranda, A. microRNAs 424 and 503 are mediators of the anti-proliferative and anti-invasive action of the thyroid hormone receptor beta. Oncotarget 2014, 5, 2918–2933. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.S.; Lin, Y.H.; Chi, H.C.; Chen, P.Y.; Huang, Y.H.; Yeh, C.T.; Wang, C.S.; Lin, K.H. Thyroid hormone inhibits growth of hepatoma cells through induction of miR-214. Sci. Rep. 2017, 7, 14868. [Google Scholar] [CrossRef] [PubMed]
| Gene | Metastasis of Cancer Type | References |
|---|---|---|
| AKT1 | breast cancer; colorectal cancer; osteosarcoma; esophageal squamous cell carcinoma; tongue squamous cell carcinoma | [48,49,50,51,52,53,54,55] |
| AKT2 | breast cancer; colorectal cancer; multiple cancer types | [51,56,57,58] |
| ERBB2 | prostate cancer; breast cancer; gastric cancer | [59,60,61,62] |
| HRAS | breast cancer | [48] |
| IDH2 | intrahepatic cholangiocarcinoma (biliary cancer); osteosarcoma (loss of tumor suppressor function); hepatocellular carcinoma; low grade diffuse glioma | [63,64,65,66] |
| KIT | breast cancer; prostate cancer; colorectal cancer; melanoma; gastrointestinal stromal tumors | [67,68,69,70,71,72,73,74,75] |
| MAP2K7 (MKK7) | lung cancer; colon cancer; pancreatic cancer | [76,77,78] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mousa, S.A.; Glinsky, G.V.; Lin, H.-Y.; Ashur-Fabian, O.; Hercbergs, A.; Keating, K.A.; Davis, P.J. Contributions of Thyroid Hormone to Cancer Metastasis. Biomedicines 2018, 6, 89. https://doi.org/10.3390/biomedicines6030089
Mousa SA, Glinsky GV, Lin H-Y, Ashur-Fabian O, Hercbergs A, Keating KA, Davis PJ. Contributions of Thyroid Hormone to Cancer Metastasis. Biomedicines. 2018; 6(3):89. https://doi.org/10.3390/biomedicines6030089
Chicago/Turabian StyleMousa, Shaker A., Gennadi V. Glinsky, Hung-Yun Lin, Osnat Ashur-Fabian, Aleck Hercbergs, Kelly A. Keating, and Paul J. Davis. 2018. "Contributions of Thyroid Hormone to Cancer Metastasis" Biomedicines 6, no. 3: 89. https://doi.org/10.3390/biomedicines6030089
APA StyleMousa, S. A., Glinsky, G. V., Lin, H.-Y., Ashur-Fabian, O., Hercbergs, A., Keating, K. A., & Davis, P. J. (2018). Contributions of Thyroid Hormone to Cancer Metastasis. Biomedicines, 6(3), 89. https://doi.org/10.3390/biomedicines6030089