Oncolytic Vesicular Stomatitis Virus as a Viro-Immunotherapy: Defeating Cancer with a “Hammer” and “Anvil”
Abstract
:1. Introduction
2. The Immune-Suppressive Tumor Microenvironment
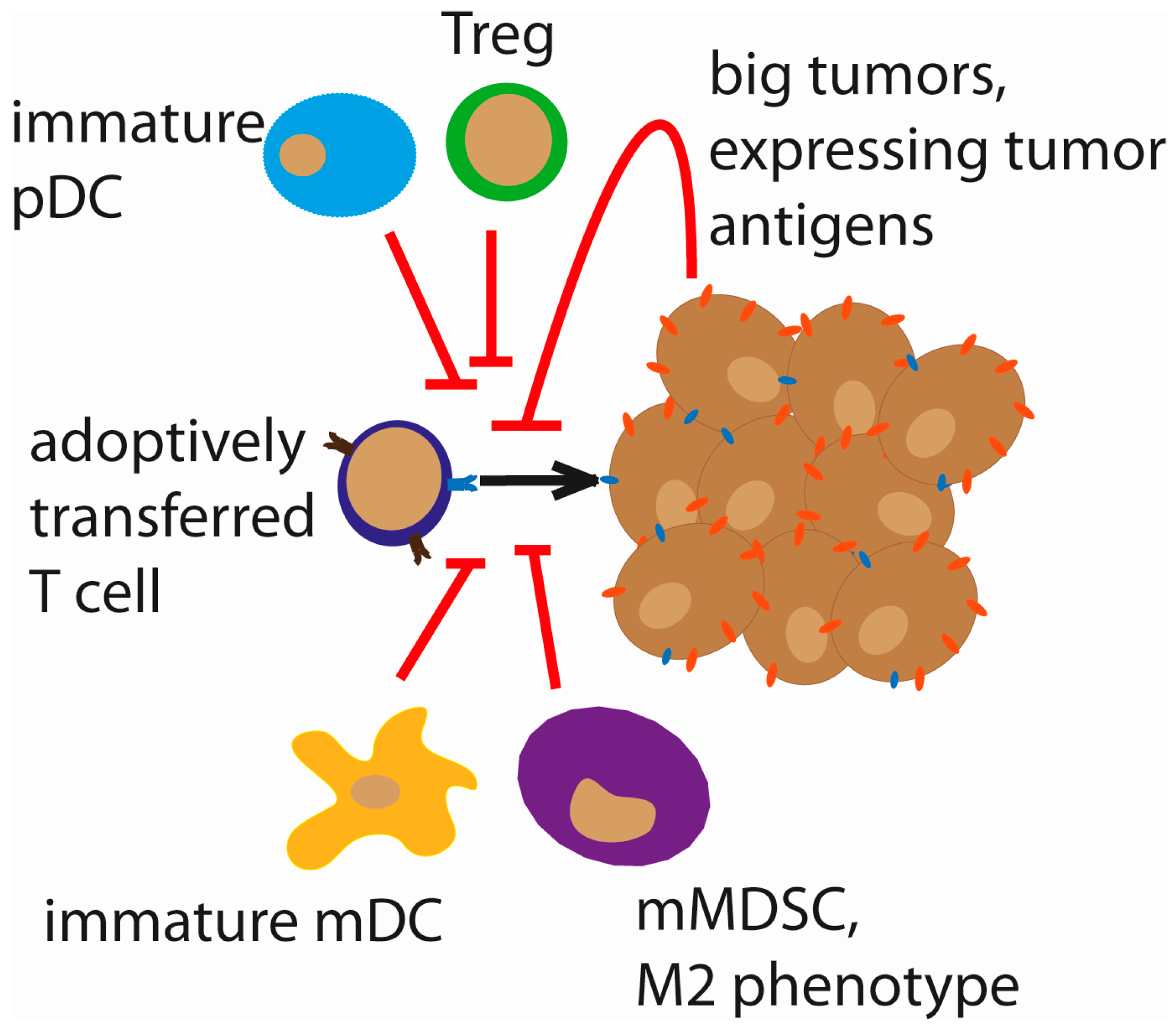
2.1. Immune Suppressor Cells
2.2. Immune Checkpoints
2.3. Dendritic Cell (DC) Maturation and Function
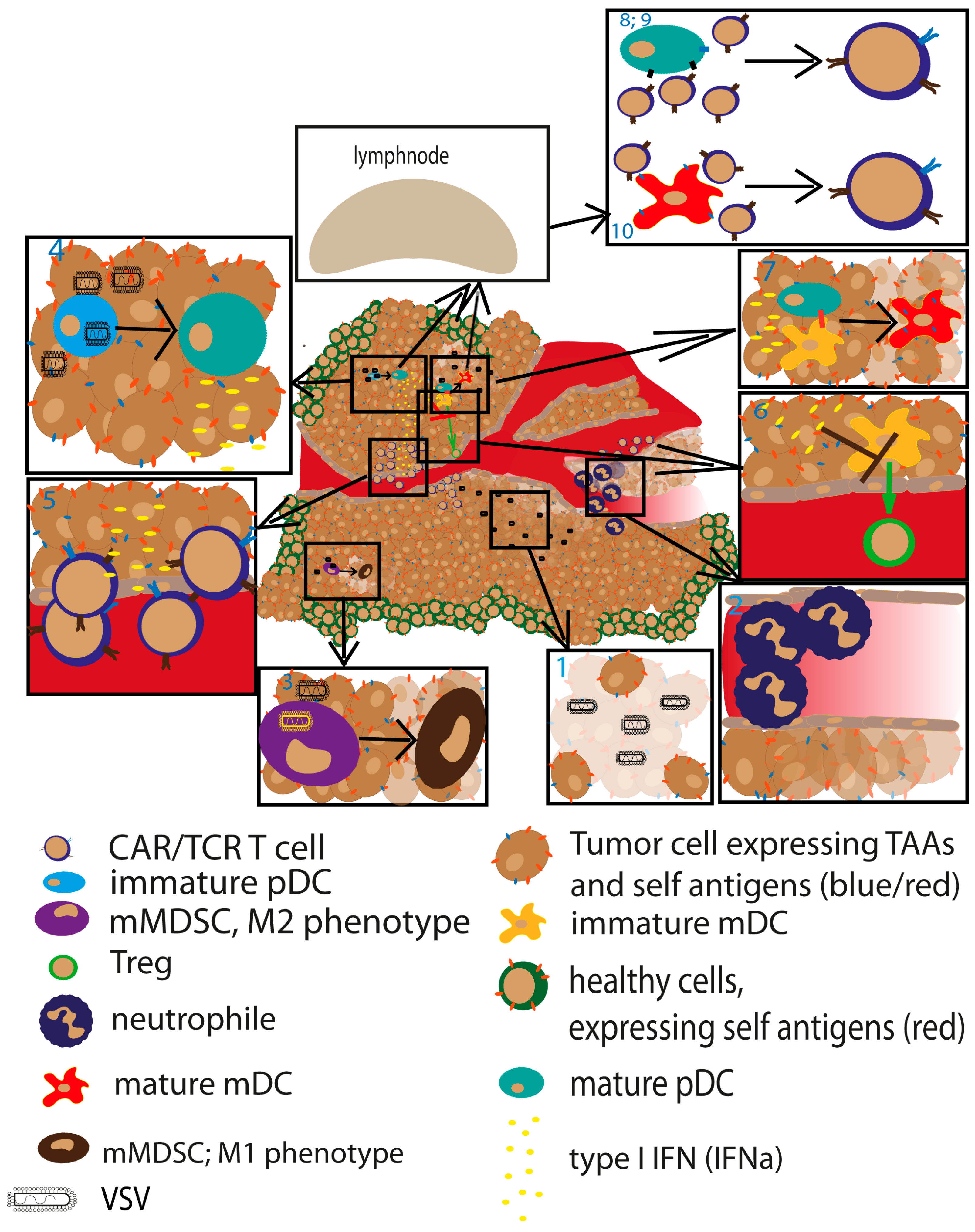
3. Vesicular Stomatitis Virus (VSV) as an Oncolytic Virus
4. Vesicular Stomatitis Virus as an Immune-Stimulating Agent
5. Strategies to Boost the Immune-Stimulating Potential of VSV
5.1. Modification of Endogenous VSV Genes
5.2. VSV-Mediated Cytokine or Immune-Stimulatory Molecule Expression
5.3. VSV as a Vaccination Platform
5.4. VSV in Combination with Adoptive Cell Therapy
6. Summary and Outlook
Acknowledgments
Conflicts of Interest
References
- Henao-Restrepo, A.M.; Longini, I.M.; Egger, M.; Dean, N.E.; Edmunds, W.J.; Camacho, A.; Carroll, M.W.; Doumbia, M.; Draguez, B.; Duraffour, S.; et al. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: Interim results from the guinea ring vaccination cluster-randomised trial. Lancet 2015, 386, 857–866. [Google Scholar] [CrossRef]
- Jenks, N.; Myers, R.; Greiner, S.M.; Thompson, J.; Mader, E.K.; Greenslade, A.; Griesmann, G.E.; Federspiel, M.J.; Rakela, J.; Borad, M.J.; et al. Safety studies on intrahepatic or intratumoral injection of oncolytic vesicular stomatitis virus expressing interferon-β in rodents and nonhuman primates. Hum. Gene Ther. 2010, 21, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.E.; Kuo, P.C. The tumor microenvironment. Surg. Oncol. 2012, 21, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The three Es of cancer immunoediting. Annu. Rev. Immunol. 2004, 22, 329–360. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, G.A.; Gabrilovich, D.; Sotomayor, E.M. Immunosuppressive strategies that are mediated by tumor cells. Annu. Rev. Immunol. 2007, 25, 267–296. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, J.E.; Stephenson, K.B.; Wang, F.; Ashkar, A.A.; Mossman, K.L.; Lenz, L.L.; Rosenthal, K.L.; Bramson, J.L.; Lichty, B.D.; Wan, Y. IL-15 and type I interferon are required for activation of tumoricidal NK cells by virus-infected dendritic cells. Cancer Res. 2011, 71, 2497–2506. [Google Scholar] [CrossRef] [PubMed]
- Zou, W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat. Rev. Cancer 2005, 5, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, R.A.; von Andrian, U.H. How tolerogenic dendritic cells induce regulatory T cells. Adv. Immunol. 2010, 108, 111–165. [Google Scholar] [PubMed]
- Vicari, A.P.; Caux, C.; Trinchieri, G. Tumour escape from immune surveillance through dendritic cell inactivation. Semin. Cancer Biol. 2002, 12, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Oberle, N.; Krammer, P.H. Molecular mechanisms of Treg-mediated T cell suppression. Front. Immunol. 2012, 3, 51. [Google Scholar] [CrossRef] [PubMed]
- Khaled, Y.S.; Ammori, B.J.; Elkord, E. Myeloid-derived suppressor cells in cancer: Recent progress and prospects. Immunol. Cell. Biol. 2013, 91, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Chanmee, T.; Ontong, P.; Konno, K.; Itano, N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel) 2014, 6, 1670–1690. [Google Scholar] [CrossRef] [PubMed]
- Kalathil, S.G.; Thanavala, Y. High immunosuppressive burden in cancer patients: A major hurdle for cancer immunotherapy. Cancer Immunol. Immunother. CII 2016, 65, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Yanofsky, V.R.; Mitsui, H.; Felsen, D.; Carucci, J.A. Understanding dendritic cells and their role in cutaneous carcinoma and cancer immunotherapy. Clin. Dev. Immunol. 2013, 2013, 624123. [Google Scholar] [CrossRef] [PubMed]
- Chevolet, I.; Speeckaert, R.; Schreuer, M.; Neyns, B.; Krysko, O.; Bachert, C.; van Gele, M.; van Geel, N.; Brochez, L. Clinical significance of plasmacytoid dendritic cells and myeloid-derived suppressor cells in melanoma. J. Transl. Med. 2015, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Santegoets, S.J.; Stam, A.G.; Lougheed, S.M.; Gall, H.; Jooss, K.; Sacks, N.; Hege, K.; Lowy, I.; Scheper, R.J.; Gerritsen, W.R.; et al. Myeloid derived suppressor and dendritic cell subsets are related to clinical outcome in prostate cancer patients treated with prostate GVAX and ipilimumab. J. Immunother. Cancer 2014, 2, 31. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, M.; Shim, K.G.; Grams, M.P.; Rajani, K.; Diaz, R.M.; Furutani, K.M.; Thompson, J.; Olivier, K.R.; Park, S.S.; Markovic, S.N.; et al. Definitive management of oligometastatic melanoma in a murine model using combined ablative radiation therapy and viral immunotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Hayman, E.; Pegram, H.J.; Santos, E.; Heller, G.; Sadelain, M.; Brentjens, R. In vivo inhibition of human CD19-targeted effector T cells by natural T regulatory cells in a xenotransplant murine model of B cell malignancy. Cancer Res. 2011, 71, 2871–2881. [Google Scholar] [CrossRef] [PubMed]
- Eisenstein, S.; Coakley, B.A.; Briley-Saebo, K.; Ma, G.; Chen, H.M.; Meseck, M.; Ward, S.; Divino, C.; Woo, S.; Chen, S.H.; et al. Myeloid-derived suppressor cells as a vehicle for tumor-specific oncolytic viral therapy. Cancer Res. 2013, 73, 5003–5015. [Google Scholar] [CrossRef] [PubMed]
- Shime, H.; Matsumoto, M.; Oshiumi, H.; Tanaka, S.; Nakane, A.; Iwakura, Y.; Tahara, H.; Inoue, N.; Seya, T. Toll-like receptor 3 signaling converts tumor-supporting myeloid cells to tumoricidal effectors. Proc. Natl. Acad. Sci. USA 2012, 109, 2066–2071. [Google Scholar] [CrossRef] [PubMed]
- Zamarin, D.; Postow, M.A. Immune checkpoint modulation: Rational design of combination strategies. Pharmacol. Ther. 2015, 150, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Riley, J.L. PD-1 signaling in primary T cells. Immunol. Rev. 2009, 229, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Blank, C.; Brown, I.; Peterson, A.C.; Spiotto, M.; Iwai, Y.; Honjo, T.; Gajewski, T.F. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 2004, 64, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Konishi, J.; Yamazaki, K.; Azuma, M.; Kinoshita, I.; Dosaka-Akita, H.; Nishimura, M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin. Cancer Res. 2004, 10, 5094–5100. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Chen, L. Inhibitory B7-family molecules in the tumour microenvironment. Nat. Rev. Immunol. 2008, 8, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Curiel, T.J.; Wei, S.; Dong, H.; Alvarez, X.; Cheng, P.; Mottram, P.; Krzysiek, R.; Knutson, K.L.; Daniel, B.; Zimmermann, M.C.; et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat. Med. 2003, 9, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Chevolet, I.; Speeckaert, R.; Schreuer, M.; Neyns, B.; Krysko, O.; Bachert, C.; Hennart, B.; Allorge, D.; van Geel, N.; van Gele, M.; et al. Characterization of the in vivo immune network of IDO, tryptophan metabolism, PD-l1, and CTLA-4 in circulating immune cells in melanoma. Oncoimmunology 2015, 4, e982382. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.H.; Gillett, M.D.; Cheville, J.C.; Lohse, C.M.; Dong, H.; Webster, W.S.; Krejci, K.G.; Lobo, J.R.; Sengupta, S.; Chen, L.; et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc. Natl. Acad. Sci. USA 2004, 101, 17174–17179. [Google Scholar] [CrossRef] [PubMed]
- Hamanishi, J.; Mandai, M.; Iwasaki, M.; Okazaki, T.; Tanaka, Y.; Yamaguchi, K.; Higuchi, T.; Yagi, H.; Takakura, K.; Minato, N.; et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc. Natl. Acad. Sci. USA 2007, 104, 3360–3365. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Homet Moreno, B.; Ribas, A. Anti-programmed cell death protein-1/ligand-1 therapy in different cancers. Br. J. Cancer 2015, 112, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, J.S.; Long, G.V.; Scolyer, R.A.; Teng, M.W.; Smyth, M.J. Resistance to PD1/PDL1 checkpoint inhibition. Cancer Treat. Rev. 2017, 52, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Woller, N.; Gurlevik, E.; Fleischmann-Mundt, B.; Schumacher, A.; Knocke, S.; Kloos, A.M.; Saborowski, M.; Geffers, R.; Manns, M.P.; Wirth, T.C.; et al. Viral infection of tumors overcomes resistance to PD-1-immunotherapy by broadening neoantigenome-directed T-cell responses. Mol. Ther. 2015, 23, 1630–1640. [Google Scholar] [CrossRef] [PubMed]
- Zamarin, D.; Holmgaard, R.B.; Subudhi, S.K.; Park, J.S.; Mansour, M.; Palese, P.; Merghoub, T.; Wolchok, J.D.; Allison, J.P. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci. Transl. Med. 2014, 6, 226ra232. [Google Scholar] [CrossRef] [PubMed]
- Demoulin, S.; Herfs, M.; Somja, J.; Roncarati, P.; Delvenne, P.; Hubert, P. HMGB1 secretion during cervical carcinogenesis promotes the acquisition of a tolerogenic functionality by plasmacytoid dendritic cells. Int. J. Cancer 2015, 137, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, E.; Wollenberg, B.; Rothenfusser, S.; Wagner, M.; Wellisch, D.; Mack, B.; Giese, T.; Gires, O.; Endres, S.; Hartmann, G. Identification and functional analysis of tumor-infiltrating plasmacytoid dendritic cells in head and neck cancer. Cancer Res. 2003, 63, 6478–6487. [Google Scholar] [PubMed]
- Saulep-Easton, D.; Vincent, F.B.; Le Page, M.; Wei, A.; Ting, S.B.; Croce, C.M.; Tam, C.; Mackay, F. Cytokine-driven loss of plasmacytoid dendritic cell function in chronic lymphocytic leukemia. Leukemia 2014, 28, 2005–2015. [Google Scholar] [CrossRef] [PubMed]
- Sisirak, V.; Faget, J.; Gobert, M.; Goutagny, N.; Vey, N.; Treilleux, I.; Renaudineau, S.; Poyet, G.; Labidi-Galy, S.I.; Goddard-Leon, S.; et al. Impaired IFN-α production by plasmacytoid dendritic cells favors regulatory T-cell expansion that may contribute to breast cancer progression. Cancer Res. 2012, 72, 5188–5197. [Google Scholar] [CrossRef] [PubMed]
- Sisirak, V.; Vey, N.; Goutagny, N.; Renaudineau, S.; Malfroy, M.; Thys, S.; Treilleux, I.; Labidi-Galy, S.I.; Bachelot, T.; Dezutter-Dambuyant, C.; et al. Breast cancer-derived transforming growth factor-β and tumor necrosis factor-α compromise interferon-α production by tumor-associated plasmacytoid dendritic cells. Int. J. Cancer 2013, 133, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Perrot, I.; Blanchard, D.; Freymond, N.; Isaac, S.; Guibert, B.; Pacheco, Y.; Lebecque, S. Dendritic cells infiltrating human non-small cell lung cancer are blocked at immature stage. J. Immunol. 2007, 178, 2763–2769. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.; Chomarat, P.; Broyles, D.; Netto, G.; Harb, G.M.; Lebecque, S.; Valladeau, J.; Davoust, J.; Palucka, K.A.; Banchereau, J. In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. J. Exp. Med. 1999, 190, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
- Scarpino, S.; Stoppacciaro, A.; Ballerini, F.; Marchesi, M.; Prat, M.; Stella, M.C.; Sozzani, S.; Allavena, P.; Mantovani, A.; Ruco, L.P. Papillary carcinoma of the thyroid: Hepatocyte growth factor (HGF) stimulates tumor cells to release chemokines active in recruiting dendritic cells. Am. J. Pathol. 2000, 156, 831–837. [Google Scholar] [CrossRef]
- Bekeredjian-Ding, I.; Schafer, M.; Hartmann, E.; Pries, R.; Parcina, M.; Schneider, P.; Giese, T.; Endres, S.; Wollenberg, B.; Hartmann, G. Tumour-derived prostaglandin e and transforming growth factor-beta synergize to inhibit plasmacytoid dendritic cell-derived interferon-alpha. Immunology 2009, 128, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Brawand, P.; Fitzpatrick, D.R.; Greenfield, B.W.; Brasel, K.; Maliszewski, C.R.; De Smedt, T. Murine plasmacytoid pre-dendritic cells generated from FLT3 ligand-supplemented bone marrow cultures are immature apcs. J. Immunol. 2002, 169, 6711–6719. [Google Scholar] [CrossRef] [PubMed]
- Vicari, A.P.; Chiodoni, C.; Vaure, C.; Ait-Yahia, S.; Dercamp, C.; Matsos, F.; Reynard, O.; Taverne, C.; Merle, P.; Colombo, M.P.; et al. Reversal of tumor-induced dendritic cell paralysis by cpg immunostimulatory oligonucleotide and anti-interleukin 10 receptor antibody. J. Exp. Med. 2002, 196, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Le Mercier, I.; Poujol, D.; Sanlaville, A.; Sisirak, V.; Gobert, M.; Durand, I.; Dubois, B.; Treilleux, I.; Marvel, J.; Vlach, J.; et al. Tumor promotion by intratumoral plasmacytoid dendritic cells is reversed by tlr7 ligand treatment. Cancer Res. 2013, 73, 4629–4640. [Google Scholar] [CrossRef] [PubMed]
- Mittelbrunn, M.; Martinez del Hoyo, G.; Lopez-Bravo, M.; Martin-Cofreces, N.B.; Scholer, A.; Hugues, S.; Fetler, L.; Amigorena, S.; Ardavin, C.; Sanchez-Madrid, F. Imaging of plasmacytoid dendritic cell interactions with T cells. Blood 2009, 113, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Riedel, J.H.; Paust, H.J.; Turner, J.E.; Tittel, A.P.; Krebs, C.; Disteldorf, E.; Wegscheid, C.; Tiegs, G.; Velden, J.; Mittrucker, H.W.; et al. Immature renal dendritic cells recruit regulatory cxcr6(+) invariant natural killer T cells to attenuate crescentic gn. J. Am. Soc. Nephrol. 2012, 23, 1987–2000. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.; Porosnicu, M.; Markovic, D.; Barber, G.N. Genetically engineered vesicular stomatitis virus in gene therapy: Application for treatment of malignant disease. J. Virol. 2002, 76, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, S.; Porosnicu, M.; Barber, G.N. Oncolytic activity of vesicular stomatitis virus is effective against tumors exhibiting aberrant p53, ras, or myc function and involves the induction of apoptosis. J. Virol. 2001, 75, 3474–3479. [Google Scholar] [CrossRef] [PubMed]
- Stojdl, D.F.; Lichty, B.; Knowles, S.; Marius, R.; Atkins, H.; Sonenberg, N.; Bell, J.C. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat. Med. 2000, 6, 821–825. [Google Scholar] [PubMed]
- Ebert, O.; Shinozaki, K.; Huang, T.G.; Savontaus, M.J.; Garcia-Sastre, A.; Woo, S.L.C. Oncolytic vesicular stomatitis virus for treatment of orthotopic hepatocellular carcinoma in immune-competent rats. Cancer Res. 2003, 63, 611–613. [Google Scholar]
- Ebert, O.; Harbaran, S.; Shinozaki, K.; Woo, S.L. Systemic therapy of experimental breast cancer metastases by mutant vesicular stomatitis virus in immune-competent mice. Cancer Gene Ther. 2005, 12, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Altomonte, J.; Braren, R.; Schulz, S.; Marozin, S.; Rummeny, E.J.; Schmid, R.M.; Ebert, O. Synergistic antitumor effects of transarterial viroembolization for multifocal hepatocellular carcinoma in rats. Hepatology 2008, 48, 1864–1873. [Google Scholar] [CrossRef] [PubMed]
- Hastie, E.; Besmer, D.M.; Shah, N.R.; Murphy, A.M.; Moerdyk-Schauwecker, M.; Molestina, C.; Roy, L.D.; Curry, J.M.; Mukherjee, P.; Grdzelishvili, V.Z. Oncolytic vesicular stomatitis virus in an immunocompetent model of muc1-positive or muc1-null pancreatic ductal adenocarcinoma. J. Virol. 2013, 87, 10283–10294. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.G.; Ebert, O.; Shinozaki, K.; Garcia-Sastre, A.; Woo, S.L. Oncolysis of hepatic metastasis of colorectal cancer by recombinant vesicular stomatitis virus in immune-competent mice. Mol. Ther. 2003, 8, 434–440. [Google Scholar] [CrossRef]
- Balachandran, S.; Barber, G.N. Vesicular stomatitis virus (VSV) therapy of tumors. IUBMB Life 2000, 50, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Stojdl, D.F.; Abraham, N.; Knowles, S.; Marius, R.; Brasey, A.; Lichty, B.D.; Brown, E.G.; Sonenberg, N.; Bell, J.C. The murine double-stranded RNA-dependent protein kinase pkr is required for resistance to vesicular stomatitis virus. J. Virol. 2000, 74, 9580–9585. [Google Scholar] [CrossRef] [PubMed]
- Prestwich, R.J.; Errington, F.; Ilett, E.J.; Morgan, R.S.; Scott, K.J.; Kottke, T.; Thompson, J.; Morrison, E.E.; Harrington, K.J.; Pandha, H.S.; et al. Tumor infection by oncolytic reovirus primes adaptive antitumor immunity. Clin. Cancer Res. 2008, 14, 7358–7366. [Google Scholar] [CrossRef] [PubMed]
- Prestwich, R.J.; Harrington, K.J.; Pandha, H.S.; Vile, R.G.; Melcher, A.A.; Errington, F. Oncolytic viruses: A novel form of immunotherapy. Expert Rev. Anticancer Ther. 2008, 8, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Sobol, P.T.; Boudreau, J.E.; Stephenson, K.; Wan, Y.; Lichty, B.D.; Mossman, K.L. Adaptive antiviral immunity is a determinant of the therapeutic success of oncolytic virotherapy. Mol. Ther. 2011, 19, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.S.; Liu, Z.; Bartlett, D.L. Oncolytic immunotherapy: Dying the right way is a key to eliciting potent antitumor immunity. Front. Oncol. 2014, 4, 74. [Google Scholar] [CrossRef] [PubMed]
- Diaz, R.M.; Galivo, F.; Kottke, T.; Wongthida, P.; Qiao, J.; Thompson, J.; Valdes, M.; Barber, G.; Vile, R.G. Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Res. 2007, 67, 2840–2848. [Google Scholar] [CrossRef] [PubMed]
- Altomonte, J.; Wu, L.; Chen, L.; Meseck, M.; Ebert, O.; Garcia-Sastre, A.; Fallon, J.; Woo, S.L. Exponential enhancement of oncolytic vesicular stomatitis virus potency by vector-mediated suppression of inflammatory responses in vivo. Mol. Ther. 2008, 16, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Huang, T.G.; Meseck, M.; Altomonte, J.; Ebert, O.; Shinozaki, K.; Garcia-Sastre, A.; Fallon, J.; Mandeli, J.; Woo, S.L. RVSV(mΔ51)-m3 is an effective and safe oncolytic virus for cancer therapy. Hum. Gene Ther. 2008, 19, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Wongthida, P.; Diaz, R.M.; Galivo, F.; Kottke, T.; Thompson, J.; Pulido, J.; Pavelko, K.; Pease, L.; Melcher, A.; Vile, R. Type III ifn interleukin-28 mediates the antitumor efficacy of oncolytic virus vsv in immune-competent mouse models of cancer. Cancer Res. 2010, 70, 4539–4549. [Google Scholar] [CrossRef] [PubMed]
- Altomonte, J.; Wu, L.; Meseck, M.; Chen, L.; Ebert, O.; Garcia-Sastre, A.; Fallon, J.; Mandeli, J.; Woo, S.L. Enhanced oncolytic potency of vesicular stomatitis virus through vector-mediated inhibition of NK and NKT cells. Cancer Gene Ther. 2009, 16, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Swiecki, M.; Colonna, M. Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunol. Rev. 2010, 234, 142–162. [Google Scholar] [CrossRef] [PubMed]
- Lund, J.M.; Alexopoulou, L.; Sato, A.; Karow, M.; Adams, N.C.; Gale, N.W.; Iwasaki, A.; Flavell, R.A. Recognition of single-stranded rna viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. USA 2004, 101, 5598–5603. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Barchet, W.; Cella, M.; Odermatt, B.; Asselin-Paturel, C.; Colonna, M.; Kalinke, U. Virus-induced interferon α production by a dendritic cell subset in the absence of feedback signaling in vivo. J. Exp. Med. 2002, 195, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Innate immune recognition of viral infection. Nat. Immunol. 2006, 7, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Frenz, T.; Graalmann, L.; Detje, C.N.; Doring, M.; Grabski, E.; Scheu, S.; Kalinke, U. Independent of plasmacytoid dendritic cell (PDC) infection, PDC triggered by virus-infected cells mount enhanced type I IFN responses of different composition as opposed to pdc stimulated with free virus. J. Immunol. 2014, 193, 2496–2503. [Google Scholar] [CrossRef] [PubMed]
- Swiecki, M.; Gilfillan, S.; Vermi, W.; Wang, Y.; Colonna, M. Plasmacytoid dendritic cell ablation impacts early interferon responses and antiviral nk and CD8+ T cell accrual. Immunity 2010, 33, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Le Boeuf, F.; Diallo, J.S.; McCart, J.A.; Thorne, S.; Falls, T.; Stanford, M.; Kanji, F.; Auer, R.; Brown, C.W.; Lichty, B.D.; et al. Synergistic interaction between oncolytic viruses augments tumor killing. Mol. Ther. 2010, 18, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Wongthida, P.; Diaz, R.M.; Galivo, F.; Kottke, T.; Thompson, J.; Melcher, A.; Vile, R. VSV oncolytic virotherapy in the B16 model depends upon intact Myd88 signaling. Mol. Ther. 2011, 19, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Prestwich, R.J.; Errington, F.; Steele, L.P.; Ilett, E.J.; Morgan, R.S.; Harrington, K.J.; Pandha, H.S.; Selby, P.J.; Vile, R.G.; Melcher, A.A. Reciprocal human dendritic cell-natural killer cell interactions induce antitumor activity following tumor cell infection by oncolytic reovirus. J. Immunol. 2009, 183, 4312–4321. [Google Scholar] [CrossRef] [PubMed]
- Schulz, O.; Diebold, S.S.; Chen, M.; Naslund, T.I.; Nolte, M.A.; Alexopoulou, L.; Azuma, Y.T.; Flavell, R.A.; Liljestrom, P.; Reis e Sousa, C. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature 2005, 433, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Galivo, F.; Diaz, R.M.; Thanarajasingam, U.; Jevremovic, D.; Wongthida, P.; Thompson, J.; Kottke, T.; Barber, G.N.; Melcher, A.; Vile, R.G. Interference of CD40L-mediated tumor immunotherapy by oncolytic vesicular stomatitis virus. Hum. Gene Ther. 2010, 21, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Galivo, F.; Diaz, R.M.; Wongthida, P.; Thompson, J.; Kottke, T.; Barber, G.; Melcher, A.; Vile, R. Single-cycle viral gene expression, rather than progressive replication and oncolysis, is required for VSV therapy of B16 melanoma. Gene Ther. 2010, 17, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Altomonte, J.; Ebert, O. Replicating viral vectors for cancer therapy: Strategies to synergize with host immune responses. Microb. Biotechnol. 2012, 5, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Stanford, M.M.; Breitbach, C.J.; Bell, J.C.; McFadden, G. Innate immunity, tumor microenvironment and oncolytic virus therapy: Friends or foes? Curr. Opin. Mol. Ther. 2008, 10, 32–37. [Google Scholar] [PubMed]
- Lyles, D.S. Cytopathogenesis and inhibition of host gene expression by rna viruses. Microbiol. Mol. Biol. Rev. 2000, 64, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Black, B.L.; Lyles, D.S. Vesicular stomatitis virus matrix protein inhibits host cell-directed transcription of target genes in vivo. J. Virol. 1992, 66, 4058–4064. [Google Scholar] [PubMed]
- Petersen, J.M.; Her, L.S.; Varvel, V.; Lund, E.; Dahlberg, J.E. The matrix protein of vesicular stomatitis virus inhibits nucleocytoplasmic transport when it is in the nucleus and associated with nuclear pore complexes. Mol. Cell. Biol. 2000, 20, 8590–8601. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; McKenzie, M.O.; Puckett, S.; Hojnacki, M.; Poliquin, L.; Lyles, D.S. Ability of the matrix protein of vesicular stomatitis virus to suppress β interferon gene expression is genetically correlated with the inhibition of host RNA and protein synthesis. J. Virol. 2003, 77, 4646–4657. [Google Scholar] [CrossRef] [PubMed]
- Desforges, M.; Charron, J.; Berard, S.; Beausoleil, S.; Stojdl, D.F.; Despars, G.; Laverdiere, B.; Bell, J.C.; Talbot, P.J.; Stanners, C.P.; et al. Different host-cell shutoff strategies related to the matrix protein lead to persistence of vesicular stomatitis virus mutants on fibroblast cells. Virus Res. 2001, 76, 87–102. [Google Scholar] [CrossRef]
- Janelle, V.; Langlois, M.-P.; Lapierre, P.; Charpentier, T.; Poliquin, L.; Lamarre, A. The strength of the T cell response against a surrogate tumor antigen induced by oncolytic VSV therapy does not correlate with tumor control. Mol. Ther. 2014, 22, 1198–1210. [Google Scholar] [CrossRef] [PubMed]
- Stojdl, D.F.; Lichty, B.D.; tenOever, B.R.; Paterson, J.M.; Power, A.T.; Knowles, S.; Marius, R.; Reynard, J.; Poliquin, L.; Atkins, H.; et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003, 4, 263–275. [Google Scholar] [CrossRef]
- Fang, X.; Zhang, S.; Sun, X.; Li, J.; Sun, T. Evaluation of attenuated VSVS with mutated m or/and g proteins as vaccine vectors. Vaccine 2012, 30, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Smedberg, J.R.; Westcott, M.M.; Ahmed, M.; Lyles, D.S. Signaling pathways in murine dendritic cells that regulate the response to vesicular stomatitis virus vectors that express flagellin. J. Virol. 2014, 88, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Brzoza, K.L.; Hiltbold, E.M. Matrix protein mutant of vesicular stomatitis virus stimulates maturation of myeloid dendritic cells. J. Virol. 2006, 80, 2194–2205. [Google Scholar] [CrossRef] [PubMed]
- Lemay, C.G.; Rintoul, J.L.; Kus, A.; Paterson, J.M.; Garcia, V.; Falls, T.J.; Ferreira, L.; Bridle, B.W.; Conrad, D.P.; Tang, V.A.; et al. Harnessing oncolytic virus-mediated antitumor immunity in an infected cell vaccine. Mol. Ther. 2012, 20, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yau, V.K.; Briggs, B.J.; Whittaker, G.R. Role of clathrin-mediated endocytosis during vesicular stomatitis virus entry into host cells. Virology 2005, 338, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Janelle, V.; Brassard, F.; Lapierre, P.; Lamarre, A.; Poliquin, L. Mutations in the glycoprotein of vesicular stomatitis virus affect cytopathogenicity: Potential for oncolytic virotherapy. J. Virol. 2011, 85, 6513–6520. [Google Scholar] [CrossRef] [PubMed]
- Obuchi, M.; Fernandez, M.; Barber, G.N. Development of recombinant vesicular stomatitis viruses that exploit defects in host defense to augment specific oncolytic activity. J. Virol. 2003, 77, 8843–8856. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.J.; Wanna, G.B.; Choi, B.; Aguila, D., 3rd; Ebert, O.; Genden, E.M.; Woo, S.L. Interleukin-12 expression enhances vesicular stomatitis virus oncolytic therapy in murine squamous cell carcinoma. Laryngoscope 2007, 117, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, J.E.; Bridle, B.W.; Stephenson, K.B.; Jenkins, K.M.; Brunelliere, J.; Bramson, J.L.; Lichty, B.D.; Wan, Y. Recombinant vesicular stomatitis virus transduction of dendritic cells enhances their ability to prime innate and adaptive antitumor immunity. Mol. Ther. 2009, 17, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.M.; Bidula, S.M.; Jensen, T.M.; Reiss, C.S. Vesicular stomatitis virus modified with single chain IL-23 exhibits oncolytic activity against tumor cells in vitro and in vivo. Int J. Interferon Cytokine Mediat Res. 2010, 2010, 63–72. [Google Scholar] [PubMed]
- Leveille, S.; Goulet, M.L.; Lichty, B.D.; Hiscott, J. Vesicular stomatitis virus oncolytic treatment interferes with tumor-associated dendritic cell functions and abrogates tumor antigen presentation. J. Virol. 2011, 85, 12160–12169. [Google Scholar] [CrossRef] [PubMed]
- Wongthida, P.; Diaz, R.M.; Pulido, C.; Rommelfanger, D.; Galivo, F.; Kaluza, K.; Kottke, T.; Thompson, J.; Melcher, A.; Vile, R. Activating systemic T-cell immunity against self tumor antigens to support oncolytic virotherapy with vesicular stomatitis virus. Hum. Gene Ther. 2011, 22, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, K.B.; Barra, N.G.; Davies, E.; Ashkar, A.A.; Lichty, B.D. Expressing human interleukin-15 from oncolytic vesicular stomatitis virus improves survival in a murine metastatic colon adenocarcinoma model through the enhancement of anti-tumor immunity. Cancer Gene Ther. 2012, 19, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Breton, C.; Barber, G.N.; Russell, S.J.; Peng, K.W. Retargeting vesicular stomatitis virus using measles virus envelope glycoproteins. Hum. Gene Ther. 2012, 23, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Betancourt, D.; Ramos, J.C.; Barber, G.N. Retargeting oncolytic vesicular stomatitis virus to human T-cell lymphotropic virus type 1-associated adult T-cell leukemia. J. Virol. 2015, 89, 11786–11800. [Google Scholar] [CrossRef] [PubMed]
- Willmon, C.L.; Saloura, V.; Fridlender, Z.G.; Wongthida, P.; Diaz, R.M.; Thompson, J.; Kottke, T.; Federspiel, M.; Barber, G.; Albelda, S.M.; et al. Expression of IFN-β enhances both efficacy and safety of oncolytic vesicular stomatitis virus for therapy of mesothelioma. Cancer Res. 2009, 69, 7713–7720. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Jacobson, B.A.; Ji, Y.; Drees, J.; Tang, S.; Xiong, K.; Wang, H.; Prigge, J.E.; Dash, A.S.; Kratzke, A.K.; et al. Vesicular stomatitis virus expressing interferon-β is oncolytic and promotes antitumor immune responses in a syngeneic murine model of non-small cell lung cancer. Oncotarget 2015, 6, 33165–33177. [Google Scholar] [PubMed]
- Bourgeois-Daigneault, M.C.; Roy, D.G.; Falls, T.; Twumasi-Boateng, K.; St-Germain, L.E.; Marguerie, M.; Garcia, V.; Selman, M.; Jennings, V.A.; Pettigrew, J.; et al. Oncolytic vesicular stomatitis virus expressing interferon-gamma has enhanced therapeutic activity. Mol. Ther. Oncolytics 2016, 3, 16001. [Google Scholar] [CrossRef] [PubMed]
- Francisco-Cruz, A.; Aguilar-Santelises, M.; Ramos-Espinosa, O.; Mata-Espinosa, D.; Marquina-Castillo, B.; Barrios-Payan, J.; Hernandez-Pando, R. Granulocyte-macrophage colony-stimulating factor: Not just another haematopoietic growth factor. Med. Oncol. 2014, 31, 774. [Google Scholar] [CrossRef] [PubMed]
- Bergman, I.; Griffin, J.A.; Gao, Y.; Whitaker-Dowling, P. Treatment of implanted mammary tumors with recombinant vesicular stomatitis virus targeted to her2/neu. Int. J. Cancer 2007, 121, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Melief, C.J.; van Hall, T.; Arens, R.; Ossendorp, F.; van der Burg, S.H. Therapeutic cancer vaccines. J. Clin. Investig. 2015, 125, 3401–3412. [Google Scholar] [CrossRef] [PubMed]
- Zinkernagel, R.M.; Adler, B.; Holland, J.J. Cell-mediated immunity to vesicular stomatitis virus infections in mice. Exp. Cell. Biol. 1978, 46, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Geisbert, T.W.; Feldmann, H. Recombinant vesicular stomatitis virus-based vaccines against ebola and marburg virus infections. J. Infect. Dis. 2011, 204, S1075–S1081. [Google Scholar] [CrossRef] [PubMed]
- Rose, N.F.; Marx, P.A.; Luckay, A.; Nixon, D.F.; Moretto, W.J.; Donahoe, S.M.; Montefiori, D.; Roberts, A.; Buonocore, L.; Rose, J.K. An effective aids vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 2001, 106, 539–549. [Google Scholar] [CrossRef]
- Schwartz, J.A.; Buonocore, L.; Suguitan, A.L., Jr.; Silaghi, A.; Kobasa, D.; Kobinger, G.; Feldmann, H.; Subbarao, K.; Rose, J.K. Potent vesicular stomatitis virus-based avian influenza vaccines provide long-term sterilizing immunity against heterologous challenge. J. Virol. 2010, 84, 4611–4618. [Google Scholar] [CrossRef] [PubMed]
- Bridle, B.W.; Stephenson, K.B.; Boudreau, J.E.; Koshy, S.; Kazdhan, N.; Pullenayegum, E.; Brunelliere, J.; Bramson, J.L.; Lichty, B.D.; Wan, Y. Potentiating cancer immunotherapy using an oncolytic virus. Mol. Ther. 2010, 18, 1430–1439. [Google Scholar] [CrossRef] [PubMed]
- Bridle, B.W.; Boudreau, J.E.; Lichty, B.D.; Brunelliere, J.; Stephenson, K.; Koshy, S.; Bramson, J.L.; Wan, Y. Vesicular stomatitis virus as a novel cancer vaccine vector to prime antitumor immunity amenable to rapid boosting with adenovirus. Mol. Ther. 2009, 17, 1814–1821. [Google Scholar] [CrossRef] [PubMed]
- Tober, R.; Banki, Z.; Egerer, L.; Muik, A.; Behmuller, S.; Kreppel, F.; Greczmiel, U.; Oxenius, A.; von Laer, D.; Kimpel, J. Vsv-gp: A potent viral vaccine vector that boosts the immune response upon repeated applications. J. Virol. 2014, 88, 4897–4907. [Google Scholar] [CrossRef] [PubMed]
- Bridle, B.W.; Clouthier, D.; Zhang, L.; Pol, J.; Chen, L.; Lichty, B.D.; Bramson, J.L.; Wan, Y. Oncolytic vesicular stomatitis virus quantitatively and qualitatively improves primary CD8+ T-cell responses to anticancer vaccines. Oncoimmunology 2013, 2, e26013. [Google Scholar] [CrossRef] [PubMed]
- Bridle, B.W.; Chen, L.; Lemay, C.G.; Diallo, J.S.; Pol, J.; Nguyen, A.; Capretta, A.; He, R.; Bramson, J.L.; Bell, J.C.; et al. Hdac inhibition suppresses primary immune responses, enhances secondary immune responses, and abrogates autoimmunity during tumor immunotherapy. Mol. Ther. 2013, 21, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Kottke, T.; Errington, F.; Pulido, J.; Galivo, F.; Thompson, J.; Wongthida, P.; Diaz, R.M.; Chong, H.; Ilett, E.; Chester, J.; et al. Broad antigenic coverage induced by vaccination with virus-based cDNA libraries cures established tumors. Nat. Med. 2011, 17, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Dargel, C.; Bassani-Sternberg, M.; Hasreiter, J.; Zani, F.; Bockmann, J.H.; Thiele, F.; Bohne, F.; Wisskirchen, K.; Wilde, S.; Sprinzl, M.F.; et al. T cells engineered to express a T-cell receptor specific for glypican-3 to recognize and kill hepatoma cells in vitro and in mice. Gastroenterology 2015, 149, 1042–1052. [Google Scholar] [CrossRef] [PubMed]
- Klar, R.; Schober, S.; Rami, M.; Mall, S.; Merl, J.; Hauck, S.M.; Ueffing, M.; Admon, A.; Slotta-Huspenina, J.; Schwaiger, M.; et al. Therapeutic targeting of naturally presented myeloperoxidase-derived hla peptide ligands on myeloid leukemia cells by TCR-transgenic T cells. Leukemia 2014, 28, 2355–2366. [Google Scholar] [CrossRef] [PubMed]
- Davila, M.L.; Sadelain, M. Biology and clinical application of car T cells for B cell malignancies. Int. J. Hematol. 2016, 104, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Maus, M.V.; Porter, D.L. Chimeric antigen receptor T cell therapy: 25 years in the making. Blood Rev. 2016, 30, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H. T-cell adoptive immunotherapy using tumor-infiltrating T cells and genetically engineered TCR-T cells. Int. Immunol. 2016, 28, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Charo, J.; Perez, C.; Buschow, C.; Jukica, A.; Czeh, M.; Blankenstein, T. Visualizing the dynamic of adoptively transferred T cells during the rejection of large established tumors. Eur. J. Immunol. 2011, 41, 3187–3197. [Google Scholar] [CrossRef] [PubMed]
- Perez, C.; Jukica, A.; Listopad, J.J.; Anders, K.; Kuhl, A.A.; Loddenkemper, C.; Blankenstein, T.; Charo, J. Permissive expansion and homing of adoptively transferred T cells in tumor-bearing hosts. Int. J. Cancer 2015, 137, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, B.A.; Ye, Y.; Etto, T.; Chen, J.Q.; Levitsky, H.I.; Overwijk, W.W.; Cooper, L.J.; Gelovani, J.; Hwu, P. Visualizing fewer than 10 mouse T cells with an enhanced firefly luciferase in immunocompetent mouse models of cancer. Proc. Natl. Acad. Sci. USA 2008, 105, 14342–14346. [Google Scholar] [CrossRef] [PubMed]
- De Aquino, M.T.; Malhotra, A.; Mishra, M.K.; Shanker, A. Challenges and future perspectives of T cell immunotherapy in cancer. Immunol. Lett. 2015, 166, 117–133. [Google Scholar] [CrossRef] [PubMed]
- Spranger, S.; Spaapen, R.M.; Zha, Y.; Williams, J.; Meng, Y.; Ha, T.T.; Gajewski, T.F. Up-regulation of PD-l1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8+ T cells. Sci. Transl. Med. 2013, 5, 200ra116. [Google Scholar] [CrossRef] [PubMed]
- Di, S.; Li, Z. Treatment of solid tumors with chimeric antigen receptor-engineered T cells: Current status and future prospects. Sci. China Life Sci. 2016, 59, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Kaluza, K.M.; Thompson, J.M.; Kottke, T.J.; Flynn Gilmer, H.C.; Knutson, D.L.; Vile, R.G. Adoptive T cell therapy promotes the emergence of genomically altered tumor escape variants. Int. J. Cancer 2012, 131, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.F.; Liu, J.; Li, O.; Zheng, P.; Liu, Y. Antigenic drift as a mechanism for tumor evasion of destruction by cytolytic T lymphocytes. J. Clin. Investig. 2003, 111, 1487–1496. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.F.; Liu, J.Q.; Joshi, P.S.; Wang, L.; Yin, L.; Labanowska, J.; Heerema, N.; Zheng, P.; Liu, Y. Different lineages of P1A-expressing cancer cells use divergent modes of immune evasion for T-cell adoptive therapy. Cancer Res. 2006, 66, 8241–8249. [Google Scholar] [CrossRef] [PubMed]
- Dudley, M.E.; Wunderlich, J.R.; Yang, J.C.; Sherry, R.M.; Topalian, S.L.; Restifo, N.P.; Royal, R.E.; Kammula, U.; White, D.E.; Mavroukakis, S.A.; et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J. Clin. Oncol. 2005, 23, 2346–2357. [Google Scholar] [CrossRef] [PubMed]
- Yee, C.; Thompson, J.A.; Byrd, D.; Riddell, S.R.; Roche, P.; Celis, E.; Greenberg, P.D. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: In vivo persistence, migration, and antitumor effect of transferred T cells. Proc. Natl. Acad. Sci. USA 2002, 99, 16168–16173. [Google Scholar] [CrossRef] [PubMed]
- Villadangos, J.A. Antigen-specific impairment of adoptive T-cell therapy against cancer: Players, mechanisms, solutions and a hypothesis. Immunol. Rev. 2016, 272, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Arina, A.; Bronte, V. Myeloid-derived suppressor cell impact on endogenous and adoptively transferred T cells. Curr. Opin. Immunol. 2015, 33, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Shrikant, P.; Khoruts, A.; Mescher, M.F. CTLA-4 blockade reverses CD8+ T cell tolerance to tumor by a CD4+ T cell- and IL-2-dependent mechanism. Immunity 1999, 11, 483–493. [Google Scholar] [CrossRef]
- Dengel, L.T.; Norrod, A.G.; Gregory, B.L.; Clancy-Thompson, E.; Burdick, M.D.; Strieter, R.M.; Slingluff, C.L., Jr.; Mullins, D.W. Interferons induce cxcr3-cognate chemokine production by human metastatic melanoma. J. Immunother. 2010, 33, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Harlin, H.; Meng, Y.; Peterson, A.C.; Zha, Y.; Tretiakova, M.; Slingluff, C.; McKee, M.; Gajewski, T.F. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009, 69, 3077–3085. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lou, Y.; Lizee, G.; Qin, H.; Liu, S.; Rabinovich, B.; Kim, G.J.; Wang, Y.H.; Ye, Y.; Sikora, A.G.; et al. Plasmacytoid dendritic cells induce NK cell-dependent, tumor antigen-specific T cell cross-priming and tumor regression in mice. J. Clin. Investig. 2008, 118, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.; Erreni, M.; van Brakel, M.; Debets, R.; Allavena, P. Enhanced recruitment of genetically modified Cx3cr1-positive human T cells into fractalkine/cx3cl1 expressing tumors: Importance of the chemokine gradient. J. Immunother. Cancer 2016, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Anz, D.; Rapp, M.; Eiber, S.; Koelzer, V.H.; Thaler, R.; Haubner, S.; Knott, M.; Nagel, S.; Golic, M.; Wiedemann, G.M.; et al. Suppression of intratumoral ccl22 by type I interferon inhibits migration of regulatory T cells and blocks cancer progression. Cancer Res. 2015, 75, 4483–4493. [Google Scholar] [CrossRef] [PubMed]
- Piccioli, D.; Sammicheli, C.; Tavarini, S.; Nuti, S.; Frigimelica, E.; Manetti, A.G.; Nuccitelli, A.; Aprea, S.; Valentini, S.; Borgogni, E.; et al. Human plasmacytoid dendritic cells are unresponsive to bacterial stimulation and require a novel type of cooperation with myeloid dendritic cells for maturation. Blood 2009, 113, 4232–4239. [Google Scholar] [CrossRef] [PubMed]
- Cantisani, R.; Sammicheli, C.; Tavarini, S.; D‘Oro, U.; Wack, A.; Piccioli, D. Surface molecules on stimulated plasmacytoid dendritic cells are sufficient to cross-activate resting myeloid dendritic cells. Hum. Immunol. 2011, 72, 1018–1021. [Google Scholar] [CrossRef] [PubMed]
- Salio, M.; Palmowski, M.J.; Atzberger, A.; Hermans, I.F.; Cerundolo, V. CPG-matured murine plasmacytoid dendritic cells are capable of in vivo priming of functional CD8 T cell responses to endogenous but not exogenous antigens. J. Exp. Med. 2004, 199, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Ardavin, C.; Amigorena, S.; Reis e Sousa, C. Dendritic cells: Immunobiology and cancer immunotherapy. Immunity 2004, 20, 17–23. [Google Scholar] [CrossRef]
- Melief, C.J. Cancer immunotherapy by dendritic cells. Immunity 2008, 29, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Rommelfanger, D.M.; Wongthida, P.; Diaz, R.M.; Kaluza, K.M.; Thompson, J.M.; Kottke, T.J.; Vile, R.G. Systemic combination virotherapy for melanoma with tumor antigen-expressing vesicular stomatitis virus and adoptive T-cell transfer. Cancer Res. 2012, 72, 4753–4764. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Whitaker-Dowling, P.; Barmada, M.A.; Basse, P.H.; Bergman, I. Viral infection of implanted meningeal tumors induces antitumor memory T-cells to travel to the brain and eliminate established tumors. Neuro-Oncology 2015, 17, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Wang, H.; Kottke, T.; Diaz, R.M.; Willmon, C.; Hudacek, A.; Thompson, J.; Parato, K.; Bell, J.; Naik, J.; et al. Loading of oncolytic vesicular stomatitis virus onto antigen-specific T cells enhances the efficacy of adoptive T-cell therapy of tumors. Gene Ther. 2008, 15, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Kottke, T.; Diaz, R.M.; Kaluza, K.; Pulido, J.; Galivo, F.; Wongthida, P.; Thompson, J.; Willmon, C.; Barber, G.N.; Chester, J.; et al. Use of biological therapy to enhance both virotherapy and adoptive T-cell therapy for cancer. Mol. Ther. 2008, 16, 1910–1918. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Kottke, T.; Willmon, C.; Galivo, F.; Wongthida, P.; Diaz, R.M.; Thompson, J.; Ryno, P.; Barber, G.N.; Chester, J.; et al. Purging metastases in lymphoid organs using a combination of antigen-nonspecific adoptive T cell therapy, oncolytic virotherapy and immunotherapy. Nat. Med. 2008, 14, 37–44. [Google Scholar] [CrossRef] [PubMed]
- VanSeggelen, H.; Tantalo, D.G.; Afsahi, A.; Hammill, J.A.; Bramson, J.L. Chimeric antigen receptor-engineered T cells as oncolytic virus carriers. Mol. Ther. Oncolytics 2015, 2, 15014. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.J.; Sampath, P.; Hou, W.; Thorne, S.H. Defining effective combinations of immune checkpoint blockade and oncolytic virotherapy. Clin. Cancer Res. 2015, 21, 5543–5551. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Whitaker-Dowling, P.; Griffin, J.A.; Bergman, I. Treatment with targeted vesicular stomatitis virus generates therapeutic multifunctional anti-tumor memory CD4 T cells. Cancer Gene Ther. 2012, 19, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Patnaik, M.M.; Ruiz, A.; Russell, S.J.; Peng, K.W. Immunovirotherapy with vesicular stomatitis virus and PD-l1 blockade enhances therapeutic outcome in murine acute myeloid leukemia. Blood 2016, 127, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
| Virus | Modification | Action | Murine Tumor Model | Reference |
|---|---|---|---|---|
| VSV IL-4 | Expression of IL-4 Expression of TK | Oncolytic Immunogenic | Melanoma Mammary Adenocarcinoma | [50] |
| VSV mIFNβ VSV hIFNβ VSV rIFNβ | Expression of IFNβ gene murine (m), human (h), rat (r) | Oncolytic Immunogenic | Mammary Adenocarcinoma | [97] |
| VSV IL-12 | Expression of IL-12 | Oncolytic Immunogenic | Squamous Cell Carcinoma | [98] |
| VSV ova | VSV expression of chicken ovalbumin | Oncolytic Immunogenic | Melanoma | [64] |
| VSV hDCT | VSVmΔ51 expression of human DCT | Oncolytic Immunogenic | Melanoma | [99] |
| VSV IL-23 | Expression of IL-23 | Oncolytic Immunogenic Attenuation in the CNS | Mammary Adenocarcinoma | [100] |
| VSV IL-28 | Expression of IL-28 | Oncolytic Immunogenic | Melanoma | [67] |
| VSV Flt3L | VSVmΔ51 expression of human Fl3L (growth factor DC’s activator) | Oncolytic Immunogenic | Lymphoma Melanoma | [101] |
| VSV hgp 100 | VSV expression of hgp100 a tumor-associated antigen | Oncolytic Immunogenic | Melanoma | [102] |
| VSV IL-15 | VSVmΔ51 expression of IL-15 | Oncolytic Immunogenic Safer | Colon Adenocarcinoma | [103] |
| VSV H/F, VSV aEGFR VSV aFR VSV aPSMA | VSV Pseudotyped lacking G gene Displaying single chain antibodies (ScFy) | Oncolytic Immunogenic | Myeloma | [104] |
| VSV HIV-1 gp 160 | VSV expression of human immunodeficiency virus 1 Hybrid fusion protein 160 G | Oncolytic Immunogenic | Leukemia | [105] |
| Virus | Modification | Action | Clinical Trial | Reference |
| VSV rIFNβ | Expression of IFNβ gene | Oncolytic Immunogenic | Phase I Hepatocellular carcinoma | NCT01628640 |
| VSV IFNβ-NIS | Expression of the sodium iodine symporter (NIS) and human interferon β (IFNβ) | Oncolytic Immunogenic | Phase I Refractory solid tumors | NCT02923466 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melzer, M.K.; Lopez-Martinez, A.; Altomonte, J. Oncolytic Vesicular Stomatitis Virus as a Viro-Immunotherapy: Defeating Cancer with a “Hammer” and “Anvil”. Biomedicines 2017, 5, 8. https://doi.org/10.3390/biomedicines5010008
Melzer MK, Lopez-Martinez A, Altomonte J. Oncolytic Vesicular Stomatitis Virus as a Viro-Immunotherapy: Defeating Cancer with a “Hammer” and “Anvil”. Biomedicines. 2017; 5(1):8. https://doi.org/10.3390/biomedicines5010008
Chicago/Turabian StyleMelzer, Michael Karl, Arturo Lopez-Martinez, and Jennifer Altomonte. 2017. "Oncolytic Vesicular Stomatitis Virus as a Viro-Immunotherapy: Defeating Cancer with a “Hammer” and “Anvil”" Biomedicines 5, no. 1: 8. https://doi.org/10.3390/biomedicines5010008
APA StyleMelzer, M. K., Lopez-Martinez, A., & Altomonte, J. (2017). Oncolytic Vesicular Stomatitis Virus as a Viro-Immunotherapy: Defeating Cancer with a “Hammer” and “Anvil”. Biomedicines, 5(1), 8. https://doi.org/10.3390/biomedicines5010008







