The Extracellular Matrix and the Immune System in Acute Lung Injury: Partners in Damage and Repair
Abstract
1. Introduction
2. Structural and Histopathological Hallmarks of Acute Lung Injury
2.1. The Alveolar Septum and ECM in the Healthy Lung
2.2. Compositional Shift of the ECM in ALI
2.3. Hyaline Membranes as Active Structural and Inflammatory Entities
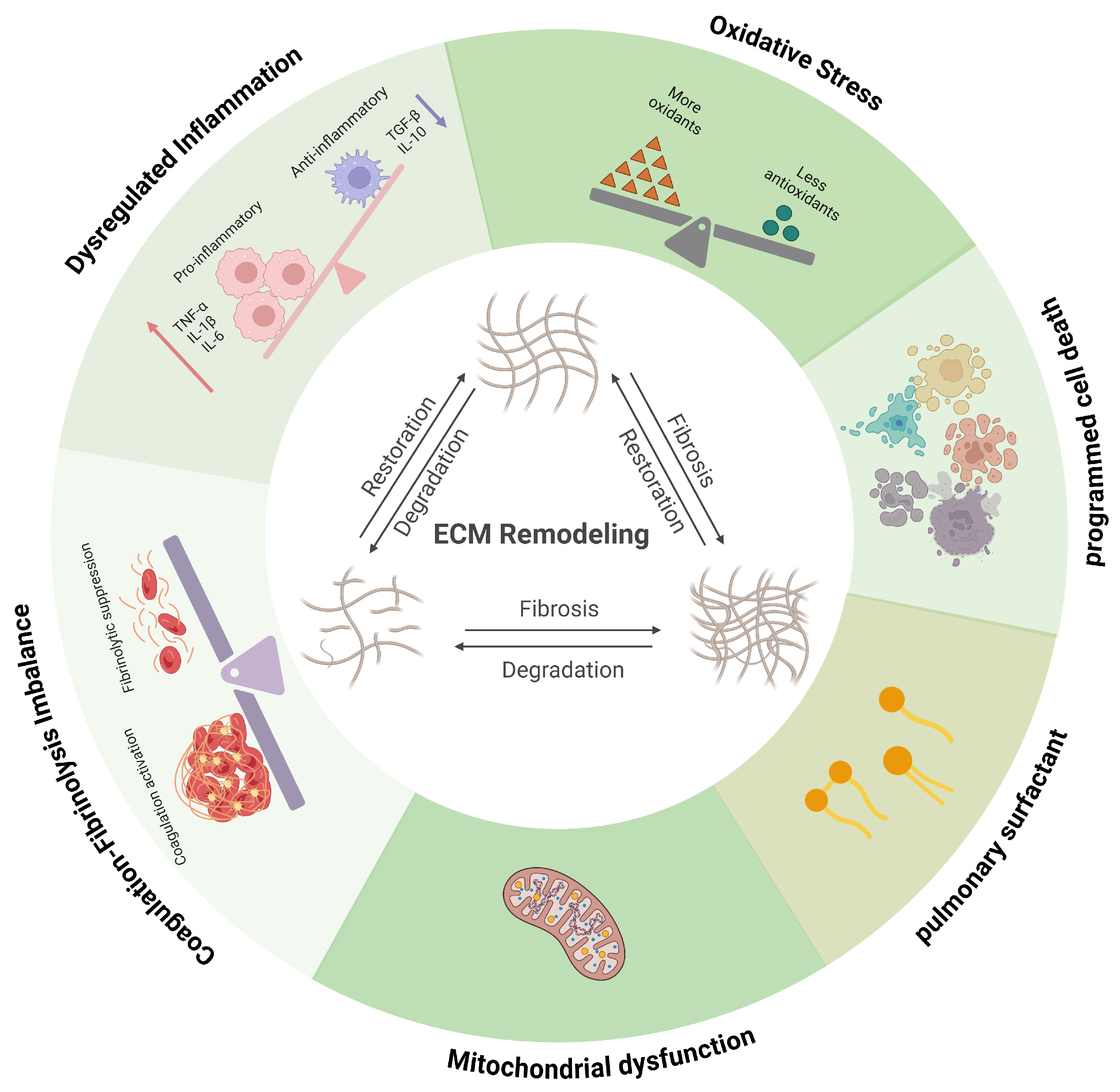
3. Temporal Landscape of ECM Remodeling in ALI
3.1. Classical ALI Staging Revisited in the Context of ECM Remodeling
3.2. Evidence for ECM Synthesis–Degradation Disequilibrium
3.3. ECM as an Active Signaling in Early Lung Injury
4. Immune Dysregulation as a Driver of ECM Remodeling
4.1. Immune Activation and Amplification
4.2. Cytokine Networks as Modulators of ECM Remodeling
4.3. Immune-Driven Protease Release and ECM Degradation
5. Oxidative Stress and ECM Structural Damage
5.1. ROS-Mediated ECM Oxidation and Fragmentation
5.2. Mitochondrial Dysfunction as an Upstream Driver of ECM Injury
5.3. Surfactant Disruption and Secondary ECM Instability
6. Coagulation–Fibrinolysis Imbalance and Matrix–Immune Coupling
6.1. Procoagulant Activation and Provisional Matrix Formation
6.2. Impaired Fibrinolysis and Pro-Fibrotic Signaling
7. Biochemical Remodeling of the ECM and Immune Regulation
7.1. Collagen-Derived Matrikines and Immune Regulation
7.2. Elastin-Derived Matrikines and Immune Regulation
7.3. Hyaluronan-Derived Matrikines and Immune Regulation
7.4. Fibrin/Laminin-Derived Matrikines and Immune Regulation
7.5. Heparan Sulfate Proteoglycans as Cytokine Reservoirs and Immune Modulators
8. Physical Remodeling of the ECM and Mechanoregulation of Immunity
8.1. Mechanical Cues as Immune Modulators
8.2. Mechanotransduction Pathways in Immune Cells
8.3. Immune Cell Sensitivity to ECM Stiffness
8.4. ECM Stiffening and Inflammation as a Positive Feedback Loop
8.5. Altered Basement Membrane Architecture in ALI
9. Novel Intervention Strategies Targeting ECM Remodeling
9.1. Interventions Targeting the Protease System
9.2. Interventions Modulating Cell–Matrix Interactions
9.3. Stem Cell-Based Strategies for ECM Regeneration
9.4. Nanotechnology-Enabled Targeted Delivery Systems
9.5. Multi-Target Modulation by Natural Compounds
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALI | acute lung injury |
| ECM | extracellular matrix |
| ARDS | acute respiratory distress syndrome |
| ICU | intensive care unit |
| AECC | American–European Consensus Conference |
| HA | hyaluronic acid |
| HSPGs | heparan sulfate proteoglycans |
| HFNO | high-flow nasal oxygen |
| BALF | bronchoalveolar lavage fluid |
| PPRs | pattern recognition receptors |
| PaO2/FIO2 | partial pressure of arterial oxygen to fraction of inspired oxygen |
| DAMPs | damage-associated molecular patterns |
| PAMPs | pathogen-associated molecular patterns |
| IL-1β | interleukin-1β |
| NETs | neutrophil extracellular traps |
| TLR2 | Toll-like receptor 2 |
| FPR1 | formyl peptide receptor 1 |
| MMPs | multiple matrix metalloproteinases |
| mtROS | mitochondrial ROS |
| mtDNA | mitochondrial DNA |
| TIMPs | tissue inhibitors of metalloproteinases |
| TGF-β | transforming growth factor-β |
| ROS | reactive oxygen species |
| PAI-1 | plasminogen activator inhibitor-1 |
| uPA | urokinase-type plasminogen activator |
| APC | activated protein C |
| PGP | proline–glycine–proline |
| PE | prolyl endopeptidase |
| CPAP | continuous positive airway pressure |
| PEEP | positive end-expiratory pressure |
| LTA4H | leukotriene A4 hydrolase |
| LFA-1 | lymphocyte function-associated antigen 1 |
| GHK | glycyl–L-histidyl–L-lysine |
| SPARC | Secreted Protein Acidic and Rich in Cysteine |
| COPD | chronic obstructive pulmonary disease |
| VGVAPG | Val-Gly-Val-Ala-Pro-Gly |
| HMW | high molecular weight |
| LER | low expression regions |
| CGS | CGS27023AM |
| RBCM | red blood cell membranes |
| MSCs | Mesenchymal stem cells |
| EVs | extracellular vesicles |
| ECMO | extracorporeal membrane oxygenation |
References
- Briel, M.; Meade, M.; Mercat, A.; Brower, R.G.; Talmor, D.; Walter, S.D.; Slutsky, A.S.; Pullenayegum, E.; Zhou, Q.; Cook, D.; et al. Higher vs. lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: Systematic review and meta-analysis. JAMA 2010, 303, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lin, W.; Hu, R.; Bai, S.; Ruan, Y.; Fan, Y.; Qiao, S.; Wen, X.; Liu, R.; Chen, H.; et al. Crosstalk between lung and extrapulmonary organs in sepsis-related acute lung injury/acute respiratory distress syndrome. Ann. Intensive Care 2025, 15, 97. [Google Scholar] [CrossRef]
- Liu, H.; Dong, J.; Xu, C.; Ni, Y.; Ye, Z.; Sun, Z.; Fan, H.; Chen, Y. Acute lung injury: Pathogenesis and treatment. J. Transl. Med. 2025, 23, 926. [Google Scholar] [CrossRef]
- Gorman, E.A.; O’Kane, C.M.; McAuley, D.F. Acute respiratory distress syndrome in adults: Diagnosis, outcomes, long-term sequelae, and management. Lancet 2022, 400, 1157–1170. [Google Scholar] [CrossRef]
- Xu, H.; Sheng, S.; Luo, W.; Xu, X.; Zhang, Z. Acute respiratory distress syndrome heterogeneity and the septic ARDS subgroup. Front. Immunol. 2023, 14, 1277161. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Hochhegger, B.; Mukhopadhyay, S.; Teixeira, E.S.T.P.P.; Mohammed, T.L. Acute Lung Injury. J. Thorac. Imaging 2025, 40, e0820. [Google Scholar] [CrossRef]
- Mowery, N.T.; Terzian, W.T.H.; Nelson, A.C. Acute lung injury. Curr. Probl. Surg. 2020, 57, 100777. [Google Scholar] [CrossRef]
- Matthay, M.A.; Zemans, R.L. The acute respiratory distress syndrome: Pathogenesis and treatment. Annu. Rev. Pathol. 2011, 6, 147–163. [Google Scholar] [CrossRef]
- Matthay, M.A.; Ware, L.B.; Zimmerman, G.A. The acute respiratory distress syndrome. J. Clin. Investig. 2012, 122, 2731–2740. [Google Scholar] [CrossRef]
- Matuschak, G.M.; Lechner, A.J. Acute lung injury and the acute respiratory distress syndrome: Pathophysiology and treatment. Mo. Med. 2010, 107, 252–258. [Google Scholar] [PubMed]
- Bernard, G.R.; Artigas, A.; Brigham, K.L.; Carlet, J.; Falke, K.; Hudson, L.; Lamy, M.; Legall, J.R.; Morris, A.; Spragg, R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am. J. Respir. Crit. Care Med. 1994, 149, 818–824. [Google Scholar] [CrossRef]
- Fan, E.; Needham, D.M.; Stewart, T.E. Ventilatory management of acute lung injury and acute respiratory distress syndrome. JAMA 2005, 294, 2889–2896. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, S.; Yang, Y.; Yao, J.Q.; Tang, W.F.; Lyon, C.J.; Hu, T.Y.; Wan, M.H. Extracellular vesicles in the pathogenesis and treatment of acute lung injury. Mil. Med. Res. 2022, 9, 61. [Google Scholar] [CrossRef]
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef]
- Matthay, M.A.; Thompson, B.T.; Ware, L.B. The Berlin definition of acute respiratory distress syndrome: Should patients receiving high-flow nasal oxygen be included? Lancet Respir. Med. 2021, 9, 933–936. [Google Scholar] [CrossRef]
- Gattinoni, L.; Marini, J.J.; Collino, F.; Maiolo, G.; Rapetti, F.; Tonetti, T.; Vasques, F.; Quintel, M. The future of mechanical ventilation: Lessons from the present and the past. Crit. Care 2017, 21, 183. [Google Scholar] [CrossRef]
- Marcozzi, C.; Moriondo, A.; Solari, E.; Reguzzoni, M.; Severgnini, P.; Protasoni, M.; Passi, A.; Pelosi, P.; Negrini, D. Regional lung tissue changes with mechanical ventilation and fluid load. Exp. Lung Res. 2015, 41, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Nieman, G.F.; Andrews, P.; Satalin, J.; Wilcox, K.; Kollisch-Singule, M.; Madden, M.; Aiash, H.; Blair, S.J.; Gatto, L.A.; Habashi, N.M. Acute lung injury: How to stabilize a broken lung. Crit. Care 2018, 22, 136. [Google Scholar] [CrossRef]
- Nieman, G.F.; Gatto, L.A.; Habashi, N.M. Impact of mechanical ventilation on the pathophysiology of progressive acute lung injury. J. Appl. Physiol. 2015, 119, 1245–1261, Erratum in J. Appl. Physiol. 2016, 121, 364. https://doi.org/10.1152/japplphysiol.00659.2015.. [Google Scholar] [CrossRef] [PubMed]
- Nieman, G.F.; Satalin, J.; Kollisch-Singule, M.; Andrews, P.; Aiash, H.; Habashi, N.M.; Gatto, L.A. Physiology in Medicine: Understanding dynamic alveolar physiology to minimize ventilator-induced lung injury. J. Appl. Physiol. 2017, 122, 1516–1522. [Google Scholar] [CrossRef] [PubMed]
- Wick, K.D.; Ware, L.B.; Matthay, M.A. Acute respiratory distress syndrome. BMJ 2024, 387, e076612. [Google Scholar] [CrossRef]
- Qian, F.; van den Boom, W.; See, K.C. The new global definition of acute respiratory distress syndrome: Insights from the MIMIC-IV database. Intensive Care Med. 2024, 50, 608–609. [Google Scholar] [CrossRef]
- Matthay, M.A.; Arabi, Y.; Arroliga, A.C.; Bernard, G.; Bersten, A.D.; Brochard, L.J.; Calfee, C.S.; Combes, A.; Daniel, B.M.; Ferguson, N.D.; et al. A New Global Definition of Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2024, 209, 37–47. [Google Scholar] [CrossRef]
- Burgstaller, G.; Oehrle, B.; Gerckens, M.; White, E.S.; Schiller, H.B.; Eickelberg, O. The instructive extracellular matrix of the lung: Basic composition and alterations in chronic lung disease. Eur. Respir. J. 2017, 50, 1601805. [Google Scholar] [CrossRef]
- Massey, V.L.; Beier, J.I.; Ritzenthaler, J.D.; Roman, J.; Arteel, G.E. Potential Role of the Gut/Liver/Lung Axis in Alcohol-Induced Tissue Pathology. Biomolecules 2015, 5, 2477–2503. [Google Scholar] [CrossRef]
- Sorokin, L. The impact of the extracellular matrix on inflammation. Nat. Rev. Immunol. 2010, 10, 712–723. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Fan, D.; Mo, X.N. Prohibitin and the extracellular matrix are upregulated in murine alveolar epithelial cells with LPS-induced acute injury. Mol. Med. Rep. 2018, 17, 7769–7773. [Google Scholar] [CrossRef] [PubMed]
- Ayala, P.; Torres, J.; Vivar, R.; Olmos, P.R.; Meneses, M.; Borzone, G.R. Changes in the pattern of fibrosis in the rat lung with repetitive orotracheal instillations of gastric contents: Evidence of persistent collagen accumulation. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 315, L390–L403. [Google Scholar] [CrossRef]
- Karsdal, M.A.; Nielsen, S.H.; Leeming, D.J.; Langholm, L.L.; Nielsen, M.J.; Manon-Jensen, T.; Siebuhr, A.; Gudmann, N.S.; Rønnow, S.; Sand, J.M.; et al. The good and the bad collagens of fibrosis—Their role in signaling and organ function. Adv. Drug Deliv. Rev. 2017, 121, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Kharitidi, D.; Apaja, P.M.; Manteghi, S.; Suzuki, K.; Malitskaya, E.; Roldan, A.; Gingras, M.C.; Takagi, J.; Lukacs, G.L.; Pause, A. Interplay of Endosomal pH and Ligand Occupancy in Integrin α5β1 Ubiquitination, Endocytic Sorting, and Cell Migration. Cell Rep. 2015, 13, 599–609. [Google Scholar] [CrossRef]
- Hughes, K.T.; Beasley, M.B. Pulmonary Manifestations of Acute Lung Injury: More Than Just Diffuse Alveolar Damage. Arch. Pathol. Lab. Med. 2017, 141, 916–922. [Google Scholar] [CrossRef]
- Knudsen, L.; Ochs, M. The micromechanics of lung alveoli: Structure and function of surfactant and tissue components. Histochem. Cell Biol. 2018, 150, 661–676. [Google Scholar] [CrossRef]
- Mayorca-Guiliani, A.E.; Leeming, D.J.; Henriksen, K.; Mortensen, J.H.; Nielsen, S.H.; Anstee, Q.M.; Sanyal, A.J.; Karsdal, M.A.; Schuppan, D. ECM formation and degradation during fibrosis, repair, and regeneration. npj Metab. Health Dis. 2025, 3, 25. [Google Scholar] [CrossRef]
- Leclech, C.; Natale, C.F.; Barakat, A.I. The basement membrane as a structured surface-role in vascular health and disease. J. Cell Sci. 2020, 133, jcs239889. [Google Scholar] [CrossRef]
- Jandl, K.; Marsh, L.M.; Hoffmann, J.; Mutgan, A.C.; Baum, O.; Bloch, W.; Thekkekara-Puthenparampil, H.; Kolb, D.; Sinn, K.; Klepetko, W.; et al. Basement Membrane Remodeling Controls Endothelial Function in Idiopathic Pulmonary Arterial Hypertension. Am. J. Respir. Cell Mol. Biol. 2020, 63, 104–117. [Google Scholar] [CrossRef]
- Thuringer, M.; Zent, R.; Lennon, R.; Plosa, E.J. Basement membranes in lung development, disease, and repair. Matrix Biol. 2025, 140, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, E.T.; Uhl, F.E.; Asarian, L.; Deng, B.; Becker, C.; Uriarte, J.J.; Downs, I.; Young, B.; Weiss, D.J. Regional and disease specific human lung extracellular matrix composition. Biomaterials 2023, 293, 121960. [Google Scholar] [CrossRef] [PubMed]
- Kligerman, S. Imaging of the Spectrum of Acute Lung Injury. Clin. Chest Med. 2024, 45, 357–371. [Google Scholar] [CrossRef]
- Liu, X.; Fang, Y.; Noble, P.W.; Que, J.; Jiang, D. Disruption of respiratory epithelial basement membrane in COVID-19 patients. Mol. Biomed. 2021, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Marquis, K.M.; Hammer, M.M.; Steinbrecher, K.; Henry, T.S.; Lin, C.Y.; Shifren, A.; Raptis, C.A. CT Approach to Lung Injury. Radiographics 2023, 43, e220176. [Google Scholar] [CrossRef]
- Fan, Y.; Moser, J.; Jongman, R.M.; Borghuis, T.; Vonk, J.M.; Timens, W.; van Meurs, M.; Pillay, J.; Burgess, J.K. Compositional changes of the lung extracellular matrix in acute respiratory distress syndrome. Am. J. Physiol. Cell Physiol. 2025, 328, C1279–C1292. [Google Scholar] [CrossRef]
- de Souza Xavier Costa, N.; Ribeiro Júnior, G.; do Nascimento, E.C.T.; de Brito, J.M.; Antonangelo, L.; Faria, C.S.; Monteiro, J.S.; Setubal, J.C.; Pinho, J.R.R.; Pereira, R.V.; et al. COVID-19 induces more pronounced extracellular matrix deposition than other causes of ARDS. Respir. Res. 2023, 24, 281. [Google Scholar] [CrossRef]
- Narasaraju, T.; Neeli, I.; Criswell, S.L.; Krishnappa, A.; Meng, W.; Silva, V.; Bila, G.; Vovk, V.; Serhiy, Z.; Bowlin, G.L.; et al. Neutrophil Activity and Extracellular Matrix Degradation: Drivers of Lung Tissue Destruction in Fatal COVID-19 Cases and Implications for Long COVID. Biomolecules 2024, 14, 236. [Google Scholar] [CrossRef] [PubMed]
- Cantor, J. The Role of the Extracellular Matrix in the Pathogenesis and Treatment of Pulmonary Emphysema. Int. J. Mol. Sci. 2024, 25, 10613. [Google Scholar] [CrossRef] [PubMed]
- Barker, T.H.; Engler, A.J. The provisional matrix: Setting the stage for tissue repair outcomes. Matrix Biol. 2017, 60–61, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422, Erratum in Lancet Respir. Med. 2020, 8, e26. https://doi.org/10.1016/s2213-2600(20)30076-x.. [Google Scholar] [CrossRef]
- Bradley, B.T.; Maioli, H.; Johnston, R.; Chaudhry, I.; Fink, S.L.; Xu, H.; Najafian, B.; Deutsch, G.; Lacy, J.M.; Williams, T.; et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: A case series. Lancet 2020, 396, 320–332, Erratum in Lancet. 2020, 396, 312. https://doi.org/10.1016/s0140-6736(20)31305-2.. [Google Scholar] [CrossRef]
- Majumdar, S.; Murphy, P.M. Chemokine Regulation During Epidemic Coronavirus Infection. Front. Pharmacol. 2020, 11, 600369. [Google Scholar] [CrossRef]
- Bisharyan, M.S.; Arsenyan, K.A.; Khachatryan, P.S.; Muradyan, M.Z.; Tonoyan, A.A. Histopathological autopsy findings in lungs of pregnant and postpartum women who died of COVID-19 infections. Rechtsmedizin 2023, 33, 403–409. [Google Scholar] [CrossRef]
- Lindstedt, S.; Wang, Q.; Niroomand, A.; Stenlo, M.; Hyllen, S.; Pierre, L.; Olm, F.; Bechet, N.B. High resolution fluorescence imaging of the alveolar scaffold as a novel tool to assess lung injury. Sci. Rep. 2024, 14, 6662. [Google Scholar] [CrossRef] [PubMed]
- Wight, T.N. Provisional matrix: A role for versican and hyaluronan. Matrix Biology 2017, 60–61, 38–56. [Google Scholar] [CrossRef]
- Marshall, R.P.; Bellingan, G.; Webb, S.; Puddicombe, A.; Goldsack, N.; McAnulty, R.J.; Laurent, G.J. Fibroproliferation occurs early in the acute respiratory distress syndrome and impacts on outcome. Am. J. Respir. Crit. Care Med. 2000, 162, 1783–1788. [Google Scholar] [CrossRef]
- Meduri, G.U.; Tolley, E.A.; Chinn, A.; Stentz, F.; Postlethwaite, A. Procollagen types I and III aminoterminal propeptide levels during acute respiratory distress syndrome and in response to methylprednisolone treatment. Am. J. Respir. Crit. Care Med. 1998, 158, 1432–1441, Erratum in Am. J. Respir. Crit. Care Med. 2013, 188, 1477. https://doi.org/10.1164/ajrccm.158.5.9801107.. [Google Scholar] [CrossRef]
- Entzian, P.; Hückstädt, A.; Kreipe, H.; Barth, J. Determination of serum concentrations of type III procollagen peptide in mechanically ventilated patients. Pronounced augmented concentrations in the adult respiratory distress syndrome. Am. Rev. Respir. Dis. 1990, 142, 1079–1082. [Google Scholar] [CrossRef]
- Fligiel, S.E.; Standiford, T.; Fligiel, H.M.; Tashkin, D.; Strieter, R.M.; Warner, R.L.; Johnson, K.J.; Varani, J. Matrix metalloproteinases and matrix metalloproteinase inhibitors in acute lung injury. Hum. Pathol. 2006, 37, 422–430. [Google Scholar] [CrossRef]
- O’Kane, C.M.; McKeown, S.W.; Perkins, G.D.; Bassford, C.R.; Gao, F.; Thickett, D.R.; McAuley, D.F. Salbutamol up-regulates matrix metalloproteinase-9 in the alveolar space in the acute respiratory distress syndrome. Crit. Care Med. 2009, 37, 2242–2249. [Google Scholar] [CrossRef]
- Lanchou, J.; Corbel, M.; Tanguy, M.; Germain, N.; Boichot, E.; Theret, N.; Clement, B.; Lagente, V.; Malledant, Y. Imbalance between matrix metalloproteinases (MMP-9 and MMP-2) and tissue inhibitors of metalloproteinases (TIMP-1 and TIMP-2) in acute respiratory distress syndrome patients. Crit. Care Med. 2003, 31, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Ayala, P.; Vivar, R.; Montalva, R.; Olmos, P.; Meneses, M.; Borzone, G.R. Elastin degradation products in acute lung injury induced by gastric contents aspiration. Respir. Res. 2018, 19, 165. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.H.; Grote, M.N.; Lane, N.E.; Maunder, R.J. Changes in plasma fibronectin isoform levels predict distinct clinical outcomes in critically ill patients. Biomark. Insights 2011, 6, 59–68. [Google Scholar] [CrossRef]
- Leng, L.; Cao, R.; Ma, J.; Mou, D.; Zhu, Y.; Li, W.; Lv, L.; Gao, D.; Zhang, S.; Gong, F.; et al. Pathological features of COVID-19-associated lung injury: A preliminary proteomics report based on clinical samples. Signal Transduct. Target. Ther. 2020, 5, 240. [Google Scholar] [CrossRef]
- Goligorsky, M.S.; Sun, D. Glycocalyx in Endotoxemia and Sepsis. Am. J. Pathol. 2020, 190, 791–798. [Google Scholar] [CrossRef]
- Greco, N.; Masola, V.; Onisto, M. Heparan Sulfate Proteoglycans (HSPGs) and Their Degradation in Health and Disease. Biomolecules 2025, 15, 1597. [Google Scholar] [CrossRef]
- Xie, M.; Li, J.P. Heparan sulfate proteoglycan—A common receptor for diverse cytokines. Cell Signal 2019, 54, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.P.; Yang, Y.; Janssen, W.J.; Gandjeva, A.; Perez, M.J.; Barthel, L.; Zemans, R.L.; Bowman, J.C.; Koyanagi, D.E.; Yunt, Z.X.; et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat. Med. 2012, 18, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Masola, V.; Bellin, G.; Gambaro, G.; Onisto, M. Heparanase: A Multitasking Protein Involved in Extracellular Matrix (ECM) Remodeling and Intracellular Events. Cells 2018, 7, 236. [Google Scholar] [CrossRef]
- Arokiasamy, S.; King, R.; Boulaghrasse, H.; Poston, R.N.; Nourshargh, S.; Wang, W.; Voisin, M.B. Heparanase-Dependent Remodeling of Initial Lymphatic Glycocalyx Regulates Tissue-Fluid Drainage During Acute Inflammation in vivo. Front. Immunol. 2019, 10, 2316. [Google Scholar] [CrossRef]
- Golden, G.J.; Toledo, A.G.; Marki, A.; Sorrentino, J.T.; Morris, C.; Riley, R.J.; Spliid, C.; Chen, Q.; Cornax, I.; Lewis, N.E.; et al. Endothelial Heparan Sulfate Mediates Hepatic Neutrophil Trafficking and Injury during Staphylococcus aureus Sepsis. mBio J. 2021, 12, e0118121. [Google Scholar] [CrossRef] [PubMed]
- Maquart, F.X.; Siméon, A.; Pasco, S.; Monboisse, J.C. Regulation of cell activity by the extracellular matrix: The concept of matrikines. J. Soc. Biol. 1999, 193, 423–428. [Google Scholar] [CrossRef]
- Maquart, F.X.; Pasco, S.; Ramont, L.; Hornebeck, W.; Monboisse, J.C. An introduction to matrikines: Extracellular matrix-derived peptides which regulate cell activity. Implication in tumor invasion. Crit. Rev. Oncol. Hematol. 2004, 49, 199–202. [Google Scholar] [CrossRef]
- Burgess, J.K.; Weckmann, M. Matrikines and the lungs. Pharmacol. Ther. 2012, 134, 317–337. [Google Scholar] [CrossRef]
- Davis, G.E.; Bayless, K.J.; Davis, M.J.; Meininger, G.A. Regulation of tissue injury responses by the exposure of matricryptic sites within extracellular matrix molecules. Am. J. Pathol. 2000, 156, 1489–1498. [Google Scholar] [CrossRef]
- Romo, M.; López-Vicario, C.; Pérez-Romero, N.; Casulleras, M.; Martínez-Puchol, A.I.; Sánchez, B.; Flores-Costa, R.; Alcaraz-Quiles, J.; Duran-Güell, M.; Ibarzábal, A.; et al. Small fragments of hyaluronan are increased in individuals with obesity and contribute to low-grade inflammation through TLR-mediated activation of innate immune cells. Int. J. Obes. 2022, 46, 1960–1969. [Google Scholar] [CrossRef]
- Hoarau, A.; Polette, M.; Coraux, C. Lung Hyaluronasome: Involvement of Low Molecular Weight Ha (Lmw-Ha) in Innate Immunity. Biomolecules 2022, 12, 658. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Wang, Y.; Sui, H.; Chen, Z.; Ye, T.; Zhong, Y.; Qian, J.; Wu, B.; Huang, J.; Tian, T.; et al. Elastin-derived extracellular matrix fragments drive aging through innate immune activation. Nature Aging 2025, 5, 2380–2398. [Google Scholar] [CrossRef] [PubMed]
- Halsey, G.; Sinha, D.; Dhital, S.; Wang, X.; Vyavahare, N. Role of elastic fiber degradation in disease pathogenesis. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2023, 1869, 166706. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. Pulmonary Innate Immune Response Determines the Outcome of Inflammation During Pneumonia and Sepsis-Associated Acute Lung Injury. Front. Immunol. 2020, 11, 1722. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z. The role of macrophages polarization in sepsis-induced acute lung injury. Front. Immunol. 2023, 14, 1209438. [Google Scholar] [CrossRef]
- Zou, S.; Jie, H.; Han, X.; Wang, J. The role of neutrophil extracellular traps in sepsis and sepsis-related acute lung injury. Int. Immunopharmacol. 2023, 124, 110436. [Google Scholar] [CrossRef]
- Scozzi, D.; Liao, F.; Krupnick, A.S.; Kreisel, D.; Gelman, A.E. The role of neutrophil extracellular traps in acute lung injury. Front. Immunol. 2022, 13, 953195. [Google Scholar] [CrossRef]
- Cho, C.; Kang, L.J.; Jang, D.; Jeon, J.; Lee, H.; Choi, S.; Han, S.J.; Oh, E.; Nam, J.; Kim, C.S.; et al. Cirsium japonicum var. maackii and apigenin block Hif-2α-induced osteoarthritic cartilage destruction. J. Cell Mol. Med. 2019, 23, 5369–5379. [Google Scholar] [CrossRef] [PubMed]
- Huuskonen, L.; Anglenius, H.; Ahonen, I.; Tiihonen, K. Effects of Bacterial Lysates and Metabolites on Collagen Homeostasis in TNF-α-Challenged Human Dermal Fibroblasts. Microorganisms 2023, 11, 1465. [Google Scholar] [CrossRef]
- Lee, C.W.; Lin, C.C.; Lin, W.N.; Liang, K.C.; Luo, S.F.; Wu, C.B.; Wang, S.W.; Yang, C.M. TNF-alpha induces MMP-9 expression via activation of Src/EGFR, PDGFR/PI3K/Akt cascade and promotion of NF-kappaB/p300 binding in human tracheal smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 292, L799–L812. [Google Scholar] [CrossRef] [PubMed]
- Al-Roub, A.; Akhter, N.; Al-Rashed, F.; Wilson, A.; Alzaid, F.; Al-Mulla, F.; Sindhu, S.; Ahmad, R. TNFα induces matrix metalloproteinase-9 expression in monocytic cells through ACSL1/JNK/ERK/NF-kB signaling pathways. Sci. Rep. 2023, 13, 14351. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Shen, J.; Chai, Y.; Chen, H. IL-1β Impaired Diabetic Wound Healing by Regulating MMP-2 and MMP-9 through the p38 Pathway. Mediat. Inflamm. 2021, 2021, 6645766. [Google Scholar] [CrossRef]
- Huang, Q.; Lan, F.; Wang, X.; Yu, Y.; Ouyang, X.; Zheng, F.; Han, J.; Lin, Y.; Xie, Y.; Xie, F.; et al. IL-1β-induced activation of p38 promotes metastasis in gastric adenocarcinoma via upregulation of AP-1/c-fos, MMP2 and MMP9. Mol. Cancer 2014, 13, 18. [Google Scholar] [CrossRef]
- Kim, K.H.; Burkhart, K.; Chen, P.; Frevert, C.W.; Randolph-Habecker, J.; Hackman, R.C.; Soloway, P.D.; Madtes, D.K. Tissue inhibitor of metalloproteinase-1 deficiency amplifies acute lung injury in bleomycin-exposed mice. Am. J. Respir. Cell Mol. Biol. 2005, 33, 271–279. [Google Scholar] [CrossRef]
- Dik, W.A.; De Krijger, R.R.; Bonekamp, L.; Naber, B.A.; Zimmermann, L.J.; Versnel, M.A. Localization and potential role of matrix metalloproteinase-1 and tissue inhibitors of metalloproteinase-1 and -2 in different phases of bronchopulmonary dysplasia. Pediatr. Res. 2001, 50, 761–766. [Google Scholar] [CrossRef]
- Davey, A.; McAuley, D.F.; O’Kane, C.M. Matrix metalloproteinases in acute lung injury: Mediators of injury and drivers of repair. Eur. Respir. J. 2011, 38, 959–970. [Google Scholar] [CrossRef]
- Chen, G.; Ge, D.; Zhu, B.; Shi, H.; Ma, Q. Salvia miltiorrhiza Injection Alleviates LPS-Induced Acute Lung Injury by Adjusting the Balance of MMPs/TIMPs Ratio. Evid. Based Complement. Altern. Med. 2020, 2020, 9617081. [Google Scholar] [CrossRef]
- O’Reilly, S.; Ciechomska, M.; Cant, R.; van Laar, J.M. Interleukin-6 (IL-6) trans signaling drives a STAT3-dependent pathway that leads to hyperactive transforming growth factor-β (TGF-β) signaling promoting SMAD3 activation and fibrosis via Gremlin protein. J. Biol. Chem. 2014, 289, 9952–9960. [Google Scholar] [CrossRef]
- Lorente, L.; Martín, M.M.; Solé-Violán, J.; Blanquer, J.; Labarta, L.; Díaz, C.; Borreguero-León, J.M.; Orbe, J.; Rodríguez, J.A.; Jiménez, A.; et al. Association of sepsis-related mortality with early increase of TIMP-1/MMP-9 ratio. PLoS ONE 2014, 9, e94318. [Google Scholar] [CrossRef]
- Zeng, W.; Song, Y.; Wang, R.; He, R.; Wang, T. Neutrophil elastase: From mechanisms to therapeutic potential. J. Pharm. Anal. 2023, 13, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Christopoulou, M.E.; Papakonstantinou, E.; Stolz, D. Matrix Metalloproteinases in Chronic Obstructive Pulmonary Disease. Int. J. Mol. Sci. 2023, 24, 3786. [Google Scholar] [CrossRef] [PubMed]
- Hussell, T.; Bell, T.J. Alveolar macrophages: Plasticity in a tissue-specific context. Nat. Rev. Immunol. 2014, 14, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Silva de França, F.; Tambourgi, D.V. Hyaluronan breakdown by snake venom hyaluronidases: From toxins delivery to immunopathology. Front. Immunol. 2023, 14, 1125899. [Google Scholar] [CrossRef]
- Masola, V.; Greco, N.; Gambaro, G.; Franchi, M.; Onisto, M. Heparanase as active player in endothelial glycocalyx remodeling. Matrix Biol. Plus 2022, 13, 100097. [Google Scholar] [CrossRef]
- Goldberg, R.; Meirovitz, A.; Hirshoren, N.; Bulvik, R.; Binder, A.; Rubinstein, A.M.; Elkin, M. Versatile role of heparanase in inflammation. Matrix Biol. 2013, 32, 234–240. [Google Scholar] [CrossRef]
- Watson, W.H.; Ritzenthaler, J.D.; Roman, J. Lung extracellular matrix and redox regulation. Redox Biol. 2016, 8, 305–315. [Google Scholar] [CrossRef]
- Hayashi, A.; Ryu, A.; Suzuki, T.; Kawada, A.; Tajima, S. In vitro degradation of tropoelastin by reactive oxygen species. Arch. Dermatol. Res. 1998, 290, 497–500. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Tanaka, H.; Okada, T.; Konishi, H.; Takahashi, M.; Ito, M.; Asai, J. Effect of reactive oxygen species on the elastin mRNA expression in cultured human dermal fibroblasts. Free Radic. Biol. Med. 1997, 23, 162–165. [Google Scholar] [CrossRef]
- Kliment, C.R.; Tobolewski, J.M.; Manni, M.L.; Tan, R.J.; Enghild, J.; Oury, T.D. Extracellular superoxide dismutase protects against matrix degradation of heparan sulfate in the lung. Antioxid. Redox Signal. 2008, 10, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Koenitzer, J.R.; Tobolewski, J.M.; Jiang, D.; Liang, J.; Noble, P.W.; Oury, T.D. Extracellular superoxide dismutase inhibits inflammation by preventing oxidative fragmentation of hyaluronan. J. Biol. Chem. 2008, 283, 6058–6066. [Google Scholar] [CrossRef] [PubMed]
- Berdiaki, A.; Neagu, M.; Spyridaki, I.; Kuskov, A.; Perez, S.; Nikitovic, D. Hyaluronan and Reactive Oxygen Species Signaling-Novel Cues from the Matrix? Antioxidants 2023, 12, 824. [Google Scholar] [CrossRef]
- Yang-Jensen, K.C.; Jørgensen, S.M.; Chuang, C.Y.; Davies, M.J. Modification of extracellular matrix proteins by oxidants and electrophiles. Biochem. Soc. Trans. 2024, 52, 1199–1217. [Google Scholar] [CrossRef]
- Lominadze, D.; Tyagi, N.; Sen, U.; Ovechkin, A.; Tyagi, S.C. Homocysteine alters cerebral microvascular integrity and causes remodeling by antagonizing GABA-A receptor. Mol. Cell Biochem. 2012, 371, 89–96. [Google Scholar] [CrossRef]
- Qipshidze, N.; Tyagi, N.; Metreveli, N.; Lominadze, D.; Tyagi, S.C. Autophagy mechanism of right ventricular remodeling in murine model of pulmonary artery constriction. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H688–H696. [Google Scholar] [CrossRef]
- Poitevin, S.; Garnotel, R.; Antonicelli, F.; Gillery, P.; Nguyen, P. Type I collagen induces tissue factor expression and matrix metalloproteinase 9 production in human primary monocytes through a redox-sensitive pathway. J. Thromb. Haemost. 2008, 6, 1586–1594. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, F.S.; Lanzetti, M.; Nesi, R.T.; Nagato, A.C.; Silva, C.P.E.; Kennedy-Feitosa, E.; Melo, A.C.; Cattani-Cavalieri, I.; Porto, L.C.; Valenca, S.S. Oxidative Stress and Inflammation in Acute and Chronic Lung Injuries. Antioxidants 2023, 12, 548. [Google Scholar] [CrossRef]
- Grazioli, S.; Pugin, J. Mitochondrial Damage-Associated Molecular Patterns: From Inflammatory Signaling to Human Diseases. Front. Immunol. 2018, 9, 832. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; You, G.; Zheng, D.; He, Z.; Guo, W.; Antonina, K.; Shukhrat, Z.; Ding, B.; Zan, J.; et al. Tangeretin attenuates acute lung injury in septic mice by inhibiting ROS-mediated NLRP3 inflammasome activation via regulating PLK1/AMPK/DRP1 signaling axis. Inflamm. Res. 2024, 73, 47–63. [Google Scholar] [CrossRef]
- Yang, L.; Ren, Q.; Wang, Y.; Zheng, Y.; Du, F.; Wang, F.; Zhou, J.; Gui, L.; Chen, S.; Chen, X.; et al. Research progress of mitochondrial dysfunction induced pyroptosis in acute lung injury. Respir. Res. 2024, 25, 398. [Google Scholar] [CrossRef]
- Pokharel, M.D.; Garcia-Flores, A.; Marciano, D.; Franco, M.C.; Fineman, J.R.; Aggarwal, S.; Wang, T.; Black, S.M. Mitochondrial network dynamics in pulmonary disease: Bridging the gap between inflammation, oxidative stress, and bioenergetics. Redox Biol. 2024, 70, 103049. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tsui, C.K.; Garcia, G.; Joe, L.K.; Wu, H.; Maruichi, A.; Fan, W.; Pandovski, S.; Yoon, P.H.; Webster, B.M.; et al. The extracellular matrix integrates mitochondrial homeostasis. Cell 2024, 187, 4289–4304.e4226. [Google Scholar] [CrossRef] [PubMed]
- Romani, P.; Nirchio, N.; Arboit, M.; Barbieri, V.; Tosi, A.; Michielin, F.; Shibuya, S.; Benoist, T.; Wu, D.; Hindmarch, C.C.T.; et al. Mitochondrial fission links ECM mechanotransduction to metabolic redox homeostasis and metastatic chemotherapy resistance. Nat. Cell Biol. 2022, 24, 168–180. [Google Scholar] [CrossRef]
- Son, S.S.; Jeong, H.S.; Lee, S.W.; Lee, E.S.; Lee, J.G.; Lee, J.H.; Yi, J.; Park, M.J.; Choi, M.S.; Lee, D.; et al. EPRS1-mediated fibroblast activation and mitochondrial dysfunction promote kidney fibrosis. Exp. Mol. Med. 2024, 56, 2673–2689. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Zheng, H.; Meng, H.; Liu, C.; Duan, L.; Zhang, J.; Zhang, Z.; Gao, J.; Zhang, Y.; Sun, T. Mitochondrial DNA induces nucleus pulposus cell pyroptosis via the TLR9-NF-κB-NLRP3 axis. J. Transl. Med. 2023, 21, 389. [Google Scholar] [CrossRef]
- Huang, L.S.; Anas, M.; Xu, J.; Zhou, B.; Toth, P.T.; Krishnan, Y.; Di, A.; Malik, A.B. Endosomal trafficking of two-pore K+ efflux channel TWIK2 to plasmalemma mediates NLRP3 inflammasome activation and inflammatory injury. Elife 2023, 12, e83842. [Google Scholar] [CrossRef]
- Hamzeh, O.; Rabiei, F.; Shakeri, M.; Parsian, H.; Saadat, P.; Rostami-Mansoor, S. Mitochondrial dysfunction and inflammasome activation in neurodegenerative diseases: Mechanisms and therapeutic implications. Mitochondrion 2023, 73, 72–83. [Google Scholar] [CrossRef]
- Kim, H.I.; Park, J.; Gallo, D.; Shankar, S.; Konecna, B.; Han, Y.; Banner-Goodspeed, V.; Capers, K.R.; Ko, S.G.; Otterbein, L.E.; et al. DANGER Signals Activate G -Protein Receptor Kinases Suppressing Neutrophil Function and Predisposing to Infection After Tissue Trauma. Ann. Surg. 2023, 278, e1277–e1288. [Google Scholar] [CrossRef]
- Pouwels, S.D.; Hesse, L.; Faiz, A.; Lubbers, J.; Bodha, P.K.; Ten Hacken, N.H.; van Oosterhout, A.J.; Nawijn, M.C.; Heijink, I.H. Susceptibility for cigarette smoke-induced DAMP release and DAMP-induced inflammation in COPD. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 311, L881–L892. [Google Scholar] [CrossRef]
- Liu, S.W.; Chen, Y.J.; Liu, Y.; Zhou, W.; Liu, X. Regulated programmed cell death in acute lung injury: From pathogenesis to therapy. Front. Immunol. 2025, 16, 1630015. [Google Scholar] [CrossRef]
- Sun, L.; Li, X.; Luo, Z.; Li, M.; Liu, H.; Zhu, Z.; Wang, J.; Lu, P.; Wang, L.; Yang, C.; et al. Purinergic receptor P2X7 contributes to abdominal aortic aneurysm development via modulating macrophage pyroptosis and inflammation. Transl. Res. 2023, 258, 72–85. [Google Scholar] [CrossRef]
- Zu, Y.; Mu, Y.; Li, Q.; Zhang, S.T.; Yan, H.J. Icariin alleviates osteoarthritis by inhibiting NLRP3-mediated pyroptosis. J. Orthop. Surg. Res. 2019, 14, 307. [Google Scholar] [CrossRef]
- Ji, Q.; Sun, Z.; Yang, Z.; Zhang, W.; Ren, Y.; Chen, W.; Yao, M.; Nie, S. Protective effect of ginsenoside Rg1 on LPS-induced apoptosis of lung epithelial cells. Mol. Immunol. 2021, 136, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Batah, S.S.; Fabro, A.T. Pulmonary pathology of ARDS in COVID-19: A pathological review for clinicians. Respir. Med. 2021, 176, 106239. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, Y.; Hu, G.; Shang, X.; Ming, J.; Deng, M.; Li, Y.; Ma, Y.; Liu, S.; Zhou, Y. Innate/Inflammatory Bioregulation of Surfactant Protein D Alleviates Rat Osteoarthritis by Inhibiting Toll-Like Receptor 4 Signaling. Front. Immunol. 2022, 13, 913901. [Google Scholar] [CrossRef]
- Saka, R.; Wakimoto, T.; Nishiumi, F.; Sasaki, T.; Nose, S.; Fukuzawa, M.; Oue, T.; Yanagihara, I.; Okuyama, H. Surfactant protein-D attenuates the lipopolysaccharide-induced inflammation in human intestinal cells overexpressing toll-like receptor 4. Pediatr. Surg. Int. 2016, 32, 59–63. [Google Scholar] [CrossRef]
- Ohya, M.; Nishitani, C.; Sano, H.; Yamada, C.; Mitsuzawa, H.; Shimizu, T.; Saito, T.; Smith, K.; Crouch, E.; Kuroki, Y. Human pulmonary surfactant protein D binds the extracellular domains of Toll-like receptors 2 and 4 through the carbohydrate recognition domain by a mechanism different from its binding to phosphatidylinositol and lipopolysaccharide. Biochemistry 2006, 45, 8657–8664. [Google Scholar] [CrossRef]
- Yang, Z.; Jaeckisch, S.M.; Mitchell, C.G. Enhanced binding of Aspergillus fumigatus spores to A549 epithelial cells and extracellular matrix proteins by a component from the spore surface and inhibition by rat lung lavage fluid. Thorax 2000, 55, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, Y.; Qu, M.; Yu, Y.; Chen, Z.; Zhu, S.; Guo, K.; Chen, W.; Miao, C. Tissue Factor-Enriched Neutrophil Extracellular Traps Promote Immunothrombosis and Disease Progression in Sepsis-Induced Lung Injury. Front. Cell Infect. Microbiol. 2021, 11, 677902. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, X.; Qiao, X.; Shi, M. Friend or foe: The role of platelets in acute lung injury. Front. Immunol. 2025, 16, 1556923. [Google Scholar] [CrossRef] [PubMed]
- Flick, M.J.; Du, X.; Witte, D.P.; Jirousková, M.; Soloviev, D.A.; Busuttil, S.J.; Plow, E.F.; Degen, J.L. Leukocyte engagement of fibrin(ogen) via the integrin receptor alphaMbeta2/Mac-1 is critical for host inflammatory response in vivo. J. Clin. Investig. 2004, 113, 1596–1606. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Yang, Z.; Zhao, J.; Han, L.; Yang, J.; Wang, Y.; Zhang, Z.; Zhou, D. Recombinant Human Fibronectin Mediates Macrophage Polarization via NF-κB/TGF-β1 Pathway to Enhance Fibroblast Proliferation. Biol. Cell 2025, 117, e70025. [Google Scholar] [CrossRef]
- Wilhelm, G.; Mertowska, P.; Mertowski, S.; Przysucha, A.; Strużyna, J.; Grywalska, E.; Torres, K. The Crossroads of the Coagulation System and the Immune System: Interactions and Connections. Int. J. Mol. Sci. 2023, 24, 12563. [Google Scholar] [CrossRef] [PubMed]
- Swieringa, F.; Spronk, H.M.H.; Heemskerk, J.W.M.; van der Meijden, P.E.J. Integrating platelet and coagulation activation in fibrin clot formation. Res. Pract. Thromb. Haemost. 2018, 2, 450–460. [Google Scholar] [CrossRef]
- Chambers, R.C. Procoagulant signalling mechanisms in lung inflammation and fibrosis: Novel opportunities for pharmacological intervention? Br. J. Pharmacol. 2008, 153, S367–S378. [Google Scholar] [CrossRef]
- Tolle, L.B.; Standiford, T.J. Danger-associated molecular patterns (DAMPs) in acute lung injury. J. Pathol. 2013, 229, 145–156. [Google Scholar] [CrossRef]
- Lecut, C.; Feijge, M.A.; Cosemans, J.M.; Jandrot-Perrus, M.; Heemskerk, J.W. Fibrillar type I collagens enhance platelet-dependent thrombin generation via glycoprotein VI with direct support of alpha2beta1 but not alphaIIbbeta3 integrin. Thromb. Haemost. 2005, 94, 107–114. [Google Scholar] [CrossRef]
- Yang, L.; Wu, H.; Lu, L.; He, Q.; Xi, B.; Yu, H.; Luo, R.; Wang, Y.; Zhang, X. A tailored extracellular matrix (ECM)—Mimetic coating for cardiovascular stents by stepwise assembly of hyaluronic acid and recombinant human type III collagen. Biomaterials 2021, 276, 121055. [Google Scholar] [CrossRef]
- Luyendyk, J.P.; Flick, M.J.; Wolberg, A.S. Factor XIII: Driving (cross-)links in hemostasis, thrombosis, and disease. Blood 2025, 146, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- D’Agnillo, F.; Walters, K.A.; Xiao, Y.; Sheng, Z.M.; Scherler, K.; Park, J.; Gygli, S.; Rosas, L.A.; Sadtler, K.; Kalish, H.; et al. Lung epithelial and endothelial damage, loss of tissue repair, inhibition of fibrinolysis, and cellular senescence in fatal COVID-19. Sci. Transl. Med. 2021, 13, eabj7790. [Google Scholar] [CrossRef]
- Ware, L.B.; Matthay, M.A.; Parsons, P.E.; Thompson, B.T.; Januzzi, J.L.; Eisner, M.D. Pathogenetic and prognostic significance of altered coagulation and fibrinolysis in acute lung injury/acute respiratory distress syndrome. Crit. Care Med. 2007, 35, 1821–1828. [Google Scholar] [CrossRef]
- Luo, M.; Ji, Y.; Luo, Y.; Li, R.; Fay, W.P.; Wu, J. Plasminogen activator inhibitor-1 regulates the vascular expression of vitronectin. J. Thromb. Haemost. 2017, 15, 2451–2460. [Google Scholar] [CrossRef]
- Li, N.; Lu, X.-y.; Zhang, J.-y.; Dang, L.-h.; Liu, J.-f.; Wu, F.-y.; Cao, X.-m.; Liang, X.-h.; Sun, J.-h. PAI-1 regulates extracellular matrix remodeling and alters fibroblast profibrotic ability in skeletal muscle repair. Exp. Cell Res. 2025, 450, 114677. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Vaughan, D.E. PAI-1 in tissue fibrosis. J. Cell Physiol. 2012, 227, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xie, Z.; Xie, S.; Wu, J.; Khan, M.; Gao, P.; Li, J. Targeting Urokinase-type plasminogen activator receptor (uPAR) in cancer therapy: Insights from the development of small-molecule inhibitors. Bioorganic Chem. 2025, 163, 108773. [Google Scholar] [CrossRef]
- Andres, S.A.; Edwards, A.B.; Wittliff, J.L. Expression of urokinase-type plasminogen activator (uPA), its receptor (uPAR), and inhibitor (PAI-1) in human breast carcinomas and their clinical relevance. J. Clin. Lab. Anal. 2012, 26, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Culej Bošnjak, D.; Balent, T.; Korać, P.; Antica, M.; Matulić, M. Urokinase Plasminogen Activation System Modulation in Transformed Cell Lines. Int. J. Mol. Sci. 2025, 26, 675. [Google Scholar] [CrossRef]
- Gaggar, A.; Jackson, P.L.; Noerager, B.D.; O’Reilly, P.J.; McQuaid, D.B.; Rowe, S.M.; Clancy, J.P.; Blalock, J.E. A novel proteolytic cascade generates an extracellular matrix-derived chemoattractant in chronic neutrophilic inflammation. J. Immunol. 2008, 180, 5662–5669. [Google Scholar] [CrossRef]
- Akthar, S.; Patel, D.F.; Beale, R.C.; Peiró, T.; Xu, X.; Gaggar, A.; Jackson, P.L.; Blalock, J.E.; Lloyd, C.M.; Snelgrove, R.J. Matrikines are key regulators in modulating the amplitude of lung inflammation in acute pulmonary infection. Nat. Commun. 2015, 6, 8423. [Google Scholar] [CrossRef]
- Weathington, N.M.; van Houwelingen, A.H.; Noerager, B.D.; Jackson, P.L.; Kraneveld, A.D.; Galin, F.S.; Folkerts, G.; Nijkamp, F.P.; Blalock, J.E. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat. Med. 2006, 12, 317–323. [Google Scholar] [CrossRef]
- Xu, X.; Jackson, P.L.; Tanner, S.; Hardison, M.T.; Abdul Roda, M.; Blalock, J.E.; Gaggar, A. A self-propagating matrix metalloprotease-9 (MMP-9) dependent cycle of chronic neutrophilic inflammation. PLoS ONE 2011, 6, e15781. [Google Scholar] [CrossRef]
- Robison, S.W.; Li, J.; Viera, L.; Blackburn, J.P.; Patel, R.P.; Blalock, J.E.; Gaggar, A.; Xu, X. A mechanism for matrikine regulation in acute inflammatory lung injury. JCI Insight 2021, 6, e140750. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.S.; Lal, C.V.; Li, J.D.; Lou, X.Y.; Viera, L.; Abdallah, T.; King, R.W.; Sethi, J.; Kanagarajah, P.; Restrepo-Jaramillo, R.; et al. The neutrophil chemoattractant peptide proline-glycine-proline is associated with acute respiratory distress syndrome. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 315, L653–L661. [Google Scholar] [CrossRef] [PubMed]
- Hahn, C.S.; Scott, D.W.; Xu, X.; Roda, M.A.; Payne, G.A.; Wells, J.M.; Viera, L.; Winstead, C.J.; Bratcher, P.; Sparidans, R.W.; et al. The matrikine N-α-PGP couples extracellular matrix fragmentation to endothelial permeability. Sci. Adv. 2015, 1, e1500175. [Google Scholar] [CrossRef]
- Garnotel, R.; Monboisse, J.-C.; Randoux, A.; Haye, B.; Borel, J.P. The Binding of Type I Collagen to Lymphocyte Function-associated Antigen (LFA) 1 Integrin Triggers the Respiratory Burst of Human Polymorphonuclear Neutrophils: Role of calcium signaling and tyrosine phosphorylation of LFA 1. J. Biol. Chem. 1995, 270, 27495–27503. [Google Scholar] [CrossRef] [PubMed]
- Park, J.R.; Lee, H.; Kim, S.I.; Yang, S.R. The tri-peptide GHK-Cu complex ameliorates lipopolysaccharide-induced acute lung injury in mice. Oncotarget 2016, 7, 58405–58417. [Google Scholar] [CrossRef]
- Maquart, F.X.; Pickart, L.; Laurent, M.; Gillery, P.; Monboisse, J.C.; Borel, J.P. Stimulation of collagen synthesis in fibroblast cultures by the tripeptide-copper complex glycyl-L-histidyl-L-lysine-Cu2+. FEBS Lett. 1988, 238, 343–346. [Google Scholar] [CrossRef]
- Sage, E.H.; Reed, M.; Funk, S.E.; Truong, T.; Steadele, M.; Puolakkainen, P.; Maurice, D.H.; Bassuk, J.A. Cleavage of the matricellular protein SPARC by matrix metalloproteinase 3 produces polypeptides that influence angiogenesis. J. Biol. Chem. 2003, 278, 37849–37857. [Google Scholar] [CrossRef]
- Burgess, J.K.; Boustany, S.; Moir, L.M.; Weckmann, M.; Lau, J.Y.; Grafton, K.; Baraket, M.; Hansbro, P.M.; Hansbro, N.G.; Foster, P.S.; et al. Reduction of tumstatin in asthmatic airways contributes to angiogenesis, inflammation, and hyperresponsiveness. Am. J. Respir. Crit. Care Med. 2010, 181, 106–115. [Google Scholar] [CrossRef]
- Harkness, L.M.; Weckmann, M.; Kopp, M.; Becker, T.; Ashton, A.W.; Burgess, J.K. Tumstatin regulates the angiogenic and inflammatory potential of airway smooth muscle extracellular matrix. J. Cell Mol. Med. 2017, 21, 3288–3297. [Google Scholar] [CrossRef]
- Nissen, G.; Hollaender, H.; Tang, F.S.M.; Wegmann, M.; Lunding, L.; Vock, C.; Bachmann, A.; Lemmel, S.; Bartels, R.; Oliver, B.G.; et al. Tumstatin fragment selectively inhibits neutrophil infiltration in experimental asthma exacerbation. Clin. Exp. Allergy 2018, 48, 1483–1493. [Google Scholar] [CrossRef] [PubMed]
- Jandl, K.; Berg, J.L.; Birnhuber, A.; Fliesser, E.; Borek, I.; Seeliger, B.; David, S.; Schmidt, J.J.; Gorkiewicz, G.; Zacharias, M.; et al. Basement membrane product, endostatin, as a link between inflammation, coagulation and vascular permeability in COVID-19 and non-COVID-19 acute respiratory distress syndrome. Front. Immunol. 2023, 14, 1188079. [Google Scholar] [CrossRef]
- Asif, S.; Ruge, T.; Larsson, A.; Anderberg, S.B.; Lipcsey, M.; Frithiof, R.; Hultström, M. Plasma endostatin correlates with hypoxia and mortality in COVID-19-associated acute respiratory failure. Biomark. Med. 2021, 15, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Mecham, R.P. Elastin in lung development and disease pathogenesis. Matrix Biol. 2018, 73, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Hunninghake, G.W.; Davidson, J.M.; Rennard, S.; Szapiel, S.; Gadek, J.E.; Crystal, R.G. Elastin fragments attract macrophage precursors to diseased sites in pulmonary emphysema. Science 1981, 212, 925–927. [Google Scholar] [CrossRef]
- Senior, R.M.; Griffin, G.L.; Mecham, R.P. Chemotactic activity of elastin-derived peptides. J. Clin. Investig. 1980, 66, 859–862. [Google Scholar] [CrossRef]
- Senior, R.M.; Griffin, G.L.; Mecham, R.P.; Wrenn, D.S.; Prasad, K.U.; Urry, D.W. Val-Gly-Val-Ala-Pro-Gly, a repeating peptide in elastin, is chemotactic for fibroblasts and monocytes. J. Cell Biol. 1984, 99, 870–874. [Google Scholar] [CrossRef]
- Hance, K.A.; Tataria, M.; Ziporin, S.J.; Lee, J.K.; Thompson, R.W. Monocyte chemotactic activity in human abdominal aortic aneurysms: Role of elastin degradation peptides and the 67-kD cell surface elastin receptor. J. Vasc. Surg. 2002, 35, 254–261. [Google Scholar] [CrossRef]
- Teder, P.; Vandivier, R.W.; Jiang, D.; Liang, J.; Cohn, L.; Puré, E.; Henson, P.M.; Noble, P.W. Resolution of lung inflammation by CD44. Science 2002, 296, 155–158. [Google Scholar] [CrossRef]
- Jiang, D.; Liang, J.; Noble, P.W. Regulation of non-infectious lung injury, inflammation, and repair by the extracellular matrix glycosaminoglycan hyaluronan. Anat. Rec. 2010, 293, 982–985. [Google Scholar] [CrossRef]
- Termeer, C.; Benedix, F.; Sleeman, J.; Fieber, C.; Voith, U.; Ahrens, T.; Miyake, K.; Freudenberg, M.; Galanos, C.; Simon, J.C. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J. Exp. Med. 2002, 195, 99–111. [Google Scholar] [CrossRef]
- Termeer, C.C.; Hennies, J.; Voith, U.; Ahrens, T.; Weiss, J.M.; Prehm, P.; Simon, J.C. Oligosaccharides of hyaluronan are potent activators of dendritic cells. J. Immunol. 2000, 165, 1863–1870. [Google Scholar] [CrossRef]
- Jiang, D.; Liang, J.; Fan, J.; Yu, S.; Chen, S.; Luo, Y.; Prestwich, G.D.; Mascarenhas, M.M.; Garg, H.G.; Quinn, D.A.; et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat. Med. 2005, 11, 1173–1179. [Google Scholar] [CrossRef]
- Noble, P.W.; McKee, C.M.; Cowman, M.; Shin, H.S. Hyaluronan fragments activate an NF-kappa B/I-kappa B alpha autoregulatory loop in murine macrophages. J. Exp. Med. 1996, 183, 2373–2378. [Google Scholar] [CrossRef]
- Jiang, D.; Liang, J.; Noble, P.W. Hyaluronan as an immune regulator in human diseases. Physiol. Rev. 2011, 91, 221–264. [Google Scholar] [CrossRef] [PubMed]
- Senior, R.M.; Skogen, W.F.; Griffin, G.L.; Wilner, G.D. Effects of fibrinogen derivatives upon the inflammatory response. Studies with human fibrinopeptide B. J. Clin. Investig. 1986, 77, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Mydel, P.; Shipley, J.M.; Adair-Kirk, T.L.; Kelley, D.G.; Broekelmann, T.J.; Mecham, R.P.; Senior, R.M. Neutrophil elastase cleaves laminin-332 (laminin-5) generating peptides that are chemotactic for neutrophils. J. Biol. Chem. 2008, 283, 9513–9522. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Bartleson, J.M.; Butenko, S.; Alonso, V.; Liu, W.F.; Winer, D.A.; Butte, M.J. Tuning immunity through tissue mechanotransduction. Nat. Rev. Immunol. 2023, 23, 174–188. [Google Scholar] [CrossRef]
- Lei, M.; Chen, G. Integration of mechanics and immunology: Perspective for understanding fibrotic disease mechanisms and innovating therapeutic strategies. Acta Biomater. 2025, 199, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Gruber, E.J.; Leifer, C.A. Molecular regulation of TLR signaling in health and disease: Mechano-regulation of macrophages and TLR signaling. Innate Immun. 2020, 26, 15–25. [Google Scholar] [CrossRef]
- Solis, A.G.; Bielecki, P.; Steach, H.R.; Sharma, L.; Harman, C.C.D.; Yun, S.; de Zoete, M.R.; Warnock, J.N.; To, S.D.F.; York, A.G.; et al. Mechanosensation of cyclical force by PIEZO1 is essential for innate immunity. Nature 2019, 573, 69–74, Erratum in Nature. 2019, 575, E7. https://doi.org/10.1038/s41586-019-1485-8.. [Google Scholar] [CrossRef]
- Gentile, F.; Tirinato, L.; Battista, E.; Causa, F.; Liberale, C.; di Fabrizio, E.M.; Decuzzi, P. Cells preferentially grow on rough substrates. Biomaterials 2010, 31, 7205–7212. [Google Scholar] [CrossRef]
- Shan, S.; Fang, B.; Zhang, Y.; Wang, C.; Zhou, J.; Niu, C.; Gao, Y.; Zhao, D.; He, J.; Wang, J.; et al. Mechanical stretch promotes tumoricidal M1 polarization via the FAK/NF-κB signaling pathway. FASEB J. 2019, 33, 13254–13266. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, L.; Huang, T.; Lu, D.; Song, Y.; Wang, L.; Gao, J. Mechanosensitive cation channel Piezo1 contributes to ventilator-induced lung injury by activating RhoA/ROCK1 in rats. Respir. Res. 2021, 22, 250. [Google Scholar] [CrossRef]
- Liang, G.P.; Xu, J.; Cao, L.L.; Zeng, Y.H.; Chen, B.X.; Yang, J.; Zhang, Z.W.; Kang, Y. Piezo1 induced apoptosis of type II pneumocytes during ARDS. Respir. Res. 2019, 20, 118. [Google Scholar] [CrossRef]
- Meli, V.S.; Atcha, H.; Veerasubramanian, P.K.; Nagalla, R.R.; Luu, T.U.; Chen, E.Y.; Guerrero-Juarez, C.F.; Yamaga, K.; Pandori, W.; Hsieh, J.Y.; et al. YAP-mediated mechanotransduction tunes the macrophage inflammatory response. Sci. Adv. 2020, 6, eabb8471. [Google Scholar] [CrossRef]
- Vining, K.H.; Marneth, A.E.; Adu-Berchie, K.; Grolman, J.M.; Tringides, C.M.; Liu, Y.; Wong, W.J.; Pozdnyakova, O.; Severgnini, M.; Stafford, A.; et al. Mechanical checkpoint regulates monocyte differentiation in fibrotic niches. Nat. Mater. 2022, 21, 939–950. [Google Scholar] [CrossRef] [PubMed]
- Kalashnikov, N.; Moraes, C. Substrate viscoelasticity affects human macrophage morphology and phagocytosis. Soft Matter 2023, 19, 2438–2445. [Google Scholar] [CrossRef] [PubMed]
- Scheraga, R.G.; Abraham, S.; Niese, K.A.; Southern, B.D.; Grove, L.M.; Hite, R.D.; McDonald, C.; Hamilton, T.A.; Olman, M.A. TRPV4 Mechanosensitive Ion Channel Regulates Lipopolysaccharide-Stimulated Macrophage Phagocytosis. J. Immunol. 2016, 196, 428–436. [Google Scholar] [CrossRef]
- Orsini, E.M.; Roychowdhury, S.; Gangadhariah, M.; Cross, E.; Abraham, S.; Reinhardt, A.; Grund, M.E.; Zhou, J.Y.; Stuehr, O.; Pant, B.; et al. TRPV4 Regulates the Macrophage Metabolic Response to Limit Sepsis-induced Lung Injury. Am. J. Respir. Cell Mol. Biol. 2024, 70, 457–467. [Google Scholar] [CrossRef]
- Sridharan, R.; Cavanagh, B.; Cameron, A.R.; Kelly, D.J.; O’Brien, F.J. Material stiffness influences the polarization state, function and migration mode of macrophages. Acta Biomater. 2019, 89, 47–59. [Google Scholar] [CrossRef]
- Tsukui, T.; Wolters, P.J.; Sheppard, D. Alveolar fibroblast lineage orchestrates lung inflammation and fibrosis. Nature 2024, 631, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Roychaudhuri, R.; Hergrueter, A.H.; Polverino, F.; Laucho-Contreras, M.E.; Gupta, K.; Borregaard, N.; Owen, C.A. ADAM9 is a novel product of polymorphonuclear neutrophils: Regulation of expression and contributions to extracellular matrix protein degradation during acute lung injury. J. Immunol. 2014, 193, 2469–2482. [Google Scholar] [CrossRef]
- Wang, X.; Rojas-Quintero, J.; Wilder, J.; Tesfaigzi, Y.; Zhang, D.; Owen, C.A. Tissue Inhibitor of Metalloproteinase-1 Promotes Polymorphonuclear Neutrophil (PMN) Pericellular Proteolysis by Anchoring Matrix Metalloproteinase-8 and -9 to PMN Surfaces. J. Immunol. 2019, 202, 3267–3281. [Google Scholar] [CrossRef] [PubMed]
- Bahr, J.C.; Li, X.Y.; Feinberg, T.Y.; Jiang, L.; Weiss, S.J. Divergent regulation of basement membrane trafficking by human macrophages and cancer cells. Nat. Commun. 2022, 13, 6409. [Google Scholar] [CrossRef]
- Rowe, R.G.; Weiss, S.J. Breaching the basement membrane: Who, when and how? Trends Cell Biol. 2008, 18, 560–574. [Google Scholar] [CrossRef]
- Voisin, M.B.; Woodfin, A.; Nourshargh, S. Monocytes and neutrophils exhibit both distinct and common mechanisms in penetrating the vascular basement membrane in vivo. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Voisin, M.B.; Pröbstl, D.; Nourshargh, S. Venular basement membranes ubiquitously express matrix protein low-expression regions: Characterization in multiple tissues and remodeling during inflammation. Am. J. Pathol. 2010, 176, 482–495. [Google Scholar] [CrossRef]
- Voisin, M.B.; Leoni, G.; Woodfin, A.; Loumagne, L.; Patel, N.S.; Di Paola, R.; Cuzzocrea, S.; Thiemermann, C.; Perretti, M.; Nourshargh, S. Neutrophil elastase plays a non-redundant role in remodeling the venular basement membrane and neutrophil diapedesis post-ischemia/reperfusion injury. J. Pathol. 2019, 248, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Soehnlein, O.; Zernecke, A.; Eriksson, E.E.; Rothfuchs, A.G.; Pham, C.T.; Herwald, H.; Bidzhekov, K.; Rottenberg, M.E.; Weber, C.; Lindbom, L. Neutrophil secretion products pave the way for inflammatory monocytes. Blood 2008, 112, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ravikumar, P.; Nguyen, K.T.; Hsia, C.C.; Hong, Y. Lung protection by inhalation of exogenous solubilized extracellular matrix. PLoS ONE 2017, 12, e0171165. [Google Scholar] [CrossRef]
- Olivares-Martínez, E.; Hernández-Ramírez, D.F.; Núñez-Álvarez, C.A.; Meza-Sánchez, D.E.; Chapa, M.; Méndez-Flores, S.; Priego-Ranero, Á.; Azamar-Llamas, D.; Olvera-Prado, H.; Rivas-Redonda, K.I.; et al. Polymerized Type I Collagen Downregulates STAT-1 Phosphorylation Through Engagement with LAIR-1 in Circulating Monocytes, Avoiding Long COVID. Int. J. Mol. Sci. 2025, 26, 1018. [Google Scholar] [CrossRef]
- Mendieta-Zerón, H.; Cruz-Arenas, E.; Díaz-Meza, S.; Cabrera-Wrooman, A.; Mandujano-Tinoco, E.A.; Salgado, R.M.; Tovar, H.; Muñiz-García, D.; Orozco-Castañeda, L.J.; Hernández-Enríquez, S.; et al. Pharmacological Immunomodulation via Collagen-Polyvinylpyrrolidone or Pirfenidone Plays a Role in the Recovery of Patients with Severe COVID-19 Through Similar Mechanisms of Action Involving the JAK/STAT Signalling Pathway: A Pilot Study. Adv. Respir. Med. 2025, 93, 24. [Google Scholar] [CrossRef] [PubMed]
- Ricard-Blum, S.; Vallet, S.D. Fragments generated upon extracellular matrix remodeling: Biological regulators and potential drugs. Matrix Biol. 2019, 75–76, 170–189. [Google Scholar] [CrossRef]
- McMahon, M.; Ye, S.; Pedrina, J.; Dlugolenski, D.; Stambas, J. Extracellular Matrix Enzymes and Immune Cell Biology. Front. Mol. Biosci. 2021, 8, 703868. [Google Scholar] [CrossRef]
- Fields, G.B. Mechanisms of Action of Novel Drugs Targeting Angiogenesis-Promoting Matrix Metalloproteinases. Front. Immunol. 2019, 10, 1278. [Google Scholar] [CrossRef]
- Dufour, A.; Sampson, N.S.; Li, J.; Kuscu, C.; Rizzo, R.C.; Deleon, J.L.; Zhi, J.; Jaber, N.; Liu, E.; Zucker, S.; et al. Small-molecule anticancer compounds selectively target the hemopexin domain of matrix metalloproteinase-9. Cancer Res. 2011, 71, 4977–4988, Erratum in Cancer Res. 2012, 72, 5141–5142. https://doi.org/10.1158/0008-5472.Can-10-4552.. [Google Scholar] [CrossRef]
- Alford, V.M.; Kamath, A.; Ren, X.; Kumar, K.; Gan, Q.; Awwa, M.; Tong, M.; Seeliger, M.A.; Cao, J.; Ojima, I.; et al. Targeting the Hemopexin-like Domain of Latent Matrix Metalloproteinase-9 (proMMP-9) with a Small Molecule Inhibitor Prevents the Formation of Focal Adhesion Junctions. ACS Chem. Biol. 2017, 12, 2788–2803. [Google Scholar] [CrossRef]
- Muhs, B.E.; Patel, S.; Yee, H.; Marcus, S.; Shamamian, P. Inhibition of matrix metalloproteinases reduces local and distant organ injury following experimental acute pancreatitis. J. Surg. Res. 2003, 109, 110–117. [Google Scholar] [CrossRef]
- Foda, H.D.; Rollo, E.E.; Drews, M.; Conner, C.; Appelt, K.; Shalinsky, D.R.; Zucker, S. Ventilator-induced lung injury upregulates and activates gelatinases and EMMPRIN: Attenuation by the synthetic matrix metalloproteinase inhibitor, Prinomastat (AG3340). Am. J. Respir. Cell Mol. Biol. 2001, 25, 717–724. [Google Scholar] [CrossRef]
- Steinberg, J.; Halter, J.; Schiller, H.J.; Dasilva, M.; Landas, S.; Gatto, L.A.; Maisi, P.; Sorsa, T.; Rajamaki, M.; Lee, H.M.; et al. Metalloproteinase inhibition reduces lung injury and improves survival after cecal ligation and puncture in rats. J. Surg. Res. 2003, 111, 185–195. [Google Scholar] [CrossRef]
- Carney, D.E.; Lutz, C.J.; Picone, A.L.; Gatto, L.A.; Ramamurthy, N.S.; Golub, L.M.; Simon, S.R.; Searles, B.; Paskanik, A.; Snyder, K.; et al. Matrix metalloproteinase inhibitor prevents acute lung injury after cardiopulmonary bypass. Circulation 1999, 100, 400–406. [Google Scholar] [CrossRef]
- Sochor, M.; Richter, S.; Schmidt, A.; Hempel, S.; Hopt, U.T.; Keck, T. Inhibition of matrix metalloproteinase-9 with doxycycline reduces pancreatitis-associated lung injury. Digestion 2009, 80, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Gerber, A.; Goldklang, M.; Stearns, K.; Ma, X.; Xiao, R.; Zelonina, T.; D’Armiento, J. Attenuation of pulmonary injury by an inhaled MMP inhibitor in the endotoxin lung injury model. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2020, 319, L1036–L1047. [Google Scholar] [CrossRef]
- Payne, G.A.; Sharma, N.S.; Lal, C.V.; Song, C.; Guo, L.; Margaroli, C.; Viera, L.; Kumar, S.; Li, J.; Xing, D.; et al. Prolyl endopeptidase contributes to early neutrophilic inflammation in acute myocardial transplant rejection. JCI Insight 2021, 6, e139687. [Google Scholar] [CrossRef]
- Amar, S.; Minond, D.; Fields, G.B. Clinical Implications of Compounds Designed to Inhibit ECM-Modifying Metalloproteinases. Proteomics 2017, 17, 1600389. [Google Scholar] [CrossRef]
- Fischer, T.; Riedl, R. Inhibitory Antibodies Designed for Matrix Metalloproteinase Modulation. Molecules 2019, 24, 2265. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, S.; Yamamoto, K.; Botkjaer, K.; Tape, C.; Dyson, M.R.; McCafferty, J.; Murphy, G.; Nagase, H. Antibody-based exosite inhibitors of ADAMTS-5 (aggrecanase-2). Biochem. J. 2015, 471, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, A.; Mochizuki, S.; Miyakoshi, A.; Kojoh, K.; Okada, Y. Development of human neutralizing antibody to ADAMTS4 (aggrecanase-1) and ADAMTS5 (aggrecanase-2). Biochem. Biophys. Res. Commun. 2016, 469, 62–69. [Google Scholar] [CrossRef]
- Gipson, T.S.; Bless, N.M.; Shanley, T.P.; Crouch, L.D.; Bleavins, M.R.; Younkin, E.M.; Sarma, V.; Gibbs, D.F.; Tefera, W.; McConnell, P.C.; et al. Regulatory effects of endogenous protease inhibitors in acute lung inflammatory injury. J. Immunol. 1999, 162, 3653–3662. [Google Scholar] [CrossRef]
- Mulligan, M.S.; Desrochers, P.E.; Chinnaiyan, A.M.; Gibbs, D.F.; Varani, J.; Johnson, K.J.; Weiss, S.J. In vivo suppression of immune complex-induced alveolitis by secretory leukoproteinase inhibitor and tissue inhibitor of metalloproteinases 2. Proc. Natl. Acad. Sci. USA 1993, 90, 11523–11527. [Google Scholar] [CrossRef]
- Shoari, A.; Coban, M.A.; Hockla, A.; Rezhdo, A.; Dimesa, A.M.; Raeeszadeh-Sarmazdeh, M.; Van Deventer, J.A.; Radisky, E.S. Directed evolution of metalloproteinase inhibitor TIMP-1 for selective inhibition of MMP-9 exploits catalytic and fibronectin domain interactions. J. Biol. Chem. 2025, 301, 110258. [Google Scholar] [CrossRef]
- Brew, K.; Nagase, H. The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Biochim. Biophys. Acta 2010, 1803, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Arpino, V.; Brock, M.; Gill, S.E. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol. 2015, 44–46, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.E.; Huizar, I.; Bench, E.M.; Sussman, S.W.; Wang, Y.; Khokha, R.; Parks, W.C. Tissue inhibitor of metalloproteinases 3 regulates resolution of inflammation following acute lung injury. Am. J. Pathol. 2010, 176, 64–73. [Google Scholar] [CrossRef]
- Kalantar, M.; Hilpert, G.A.; Mosca, E.R.; Raeeszadeh-Sarmazdeh, M. Engineering metalloproteinase inhibitors: Tissue inhibitors of metalloproteinases or antibodies, that is the question. Curr. Opin. Biotechnol. 2024, 86, 103094. [Google Scholar] [CrossRef]
- Shoari, A.; Khalili-Tanha, G.; Coban, M.A.; Radisky, E.S. Structure and computation-guided yeast surface display for the evolution of TIMP-based matrix metalloproteinase inhibitors. Front. Mol. Biosci. 2023, 10, 1321956. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, C.; Li, X.; Guo, K.; Xu, S.; Zhou, J.; Wang, S.; Wu, X.; Yan, G.; Cui, H. JTE-013 reduces inflammation and improves pulmonary fibrosis by regulating the TRAF2/NF-κB/IGF-1 signaling pathway and macrophage polarization. Int. Immunopharmacol. 2025, 167, 115727. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, P.; Xu, Y.; Zhang, J.; Xu, L.; Li, J.; Wang, S.; Li, F.; Chen, S.; Shi, J.; et al. Large-Scale Surface Modification of Decellularized Matrix with Erythrocyte Membrane for Promoting In Situ Regeneration of Heart Valve. Engineering 2024, 41, 216–230. [Google Scholar] [CrossRef]
- Cai, W.X.; Shen, K.; Cao, T.; Wang, J.; Zhao, M.; Wang, K.J.; Zhang, Y.; Han, J.T.; Hu, D.H.; Tao, K. Effects of exosomes from human adipose-derived mesenchymal stem cells on pulmonary vascular endothelial cells injury in septic mice and its mechanism. Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi 2022, 38, 266–275. [Google Scholar] [CrossRef]
- Zhu, B.H.; Lai, H.H.; Wei, C.R.; Shen, Z.; Sun, Y.; Zhu, F.; Wu, G.S. Effects and mechanism of annexin A1-overexpressing human adipose-derived mesenchymal stem cells in the treatment of mice with acute respiratory distress syndrome. Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi 2023, 39, 456–464. [Google Scholar] [CrossRef]
- Chen, J.; Ma, S.; Luo, B.; Hao, H.; Li, Y.; Yang, H.; Zhu, F.; Zhang, P.; Niu, R.; Pan, P. Human umbilical cord mesenchymal stromal cell small extracellular vesicle transfer of microRNA-223-3p to lung epithelial cells attenuates inflammation in acute lung injury in mice. J. Nanobiotechnol. 2023, 21, 295. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Kim, Y.E.; Yang, M.; Ahn, S.Y.; Sung, S.I.; Chang, Y.S. Extracellular vesicles from mesenchymal stromal cells: An emerging therapy for intractable neonatal disorders. Stem Cells Transl. Med. 2025, 14, szaf050. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, Q.; Qi, L.; Dai, X.; Liu, H.; Wang, Y. Low levels of TGF-β1 enhance human umbilical cord-derived mesenchymal stem cell fibronectin production and extend survival time in a rat model of lipopolysaccharide-induced acute lung injury. Mol. Med. Rep. 2016, 14, 1681–1692. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Zhou, W.F.; Hou, W.J.; Fan, M.Z.; Wu, G.S.; Liu, X.B.; Ma, Q.M.; Wang, Y.S.; Zhu, F. Effects of non-muscle myosin Ⅱ silenced bone marrow-derived mesenchymal stem cells transplantation on lung extracellular matrix in rats after endotoxin/lipopolysaccharide-induced acute lung injury. Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi 2022, 38, 422–433. [Google Scholar] [CrossRef]
- Lombardo, M.T.; Gabrielli, M.; Julien-Marsollier, F.; Faivre, V.; Le Charpentier, T.; Bokobza, C.; D’Aliberti, D.; Pelizzi, N.; Halimi, C.; Spinelli, S.; et al. Human Umbilical Cord-Mesenchymal Stem Cells Promote Extracellular Matrix Remodeling in Microglia. Cells 2024, 13, 1665. [Google Scholar] [CrossRef]
- Wu, X.; Tang, Y.; Lu, X.; Liu, Y.; Liu, X.; Sun, Q.; Wang, L.; Huang, W.; Liu, A.; Liu, L.; et al. Endothelial cell-derived extracellular vesicles modulate the therapeutic efficacy of mesenchymal stem cells through IDH2/TET pathway in ARDS. Cell Commun. Signal 2024, 22, 293. [Google Scholar] [CrossRef]
- Zhang, J.; Ji, K.; Ning, Y.; Sun, L.; Fan, M.; Shu, C.; Zhang, Z.; Tu, T.; Cao, J.; Gao, F.; et al. Biological Hyperthermia-Inducing Nanoparticles for Specific Remodeling of the Extracellular Matrix Microenvironment Enhance Pro-Apoptotic Therapy in Fibrosis. ACS Nano 2023, 17, 10113–10128. [Google Scholar] [CrossRef]
- Desu, H.R.; Thoma, L.A.; Wood, G.C. Nebulization of Cyclic Arginine-Glycine-(D)-Aspartic Acid-Peptide Grafted and Drug Encapsulated Liposomes for Inhibition of Acute Lung Injury. Pharm. Res. 2018, 35, 94. [Google Scholar] [CrossRef]
- Pang, P.; Liu, W.; Ma, S.; Liu, J.; Wu, S.; Xue, W.; Zhang, S.; Zhang, J.; Ji, X. Self-assembling natural flavonoid nanomedicines for alveolar macrophage reprogramming by restoring mitochondrial function in acute lung injury therapy. Chem. Eng. J. 2025, 506, 160171, Erratum in Chem. Eng. J. 2025, 522, 167936. https://doi.org/10.1016/j.cej.2025.160171.. [Google Scholar] [CrossRef]
- Wang, H.; Yang, J.; Zhang, Y.; Wang, J.; Song, S.; Gao, L.; Liu, M.; Chen, Z.; Li, X. Harpagide Confers Protection Against Acute Lung Injury Through Multi-Omics Dissection of Immune-Microenvironmental Crosstalk and Convergent Therapeutic Mechanisms. Pharmaceuticals 2025, 18, 1494. [Google Scholar] [CrossRef]
- Li, F.; Yan, W.; Chen, Z.; Dong, W.; Chen, Z. PNSC5325 prevents acute respiratory distress syndrome by alleviating inflammation and inhibiting extracellular matrix degradation of alveolar macrophages. Int. Immunopharmacol. 2024, 143, 113579. [Google Scholar] [CrossRef]
- Boesing, C.; Rocco, P.R.M.; Luecke, T.; Krebs, J. Positive end-expiratory pressure management in patients with severe ARDS: Implications of prone positioning and extracorporeal membrane oxygenation. Crit. Care 2024, 28, 277. [Google Scholar] [CrossRef]
- Teijeiro-Paradis, R.; Gannon, W.D.; Fan, E. Complications Associated With Venovenous Extracorporeal Membrane Oxygenation-What Can Go Wrong? Crit. Care Med. 2022, 50, 1809–1818. [Google Scholar] [CrossRef]
- Shimshoni, E.; Adir, I.; Afik, R.; Solomonov, I.; Shenoy, A.; Adler, M.; Puricelli, L.; Sabino, F.; Savickas, S.; Mouhadeb, O.; et al. Distinct extracellular-matrix remodeling events precede symptoms of inflammation. Matrix Biol. 2021, 96, 47–68. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.K.; Bates, J.H.T.; Krishnan, R.; Kim, J.H.; Deng, Y.; Lutchen, K.R.; Suki, B. Elucidating the interaction between stretch and stiffness using an agent-based spring network model of progressive pulmonary fibrosis. Front. Netw. Physiol. 2024, 4, 1396383. [Google Scholar] [CrossRef] [PubMed]

| Parent Matrix | Matrikines | Protease | Cellular Effects | References |
|---|---|---|---|---|
| Collagen | Proline–Glycine–Proline (PGP) | MMP-9 MMP-8 PE | Promotes neutrophil chemotaxis; exacerbates protease imbalance; degraded by LTA4H to mitigate inflammation | [150,151,155] |
| Acetylated PGP (AcPGP) | N-terminal acetylation of PGP | Resistant to LTA4H degradation; recruit neutrophils; ROS release; persistent inflammation; endothelial barrier dysfunction | [150,152,153,154,156] | |
| Collagen I | DGGRYY peptide | - | Activates neutrophils | [157] |
| Collagen I and matricellular protein SPARC | GHK | MMP3 | Enhances pulmonary antioxidant enzyme activity, suppresses IL-6 and TNF-α expression, and limits neutrophil infiltration | [158,159,160] |
| Collagen IV | Tumstatin | MMP9 | Inhibits eosinophil and lymphocyte infiltration | [161,162,163] |
| Collagen XVIII | Endostatin | - | Regulates neutrophil chemotaxis, platelet aggregation, and endothelial barrier function | [164,165] |
| Elastin | 10–50 kDa elastin fragments | Elastolytic enzymes | chemoattract monocytes and recruit monocyte–macrophages | [167,168] |
| Val-Gly-Val-Ala-Pro-Gly | Elastolytic enzymes | Possesses chemotactic activity for monocytes, macrophages, and fibroblasts | [169,170] | |
| HA | HA fragments (low molecular weight) | Hyaluronidases; ROS | Activates macrophages and dendritic cells; | [172,173,174] |
| Fibrin | Fibrin peptide B | Thrombin | Recruit neutrophils and fibroblasts | [178] |
| Laminin | Laminin fragments | Elastase | Recruit neutrophils | [179] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Xie, F.; Sun, Y.; Wang, J.; Luo, W.; Zhang, X.; Cheng, Y.; Chao, J. The Extracellular Matrix and the Immune System in Acute Lung Injury: Partners in Damage and Repair. Biomedicines 2026, 14, 57. https://doi.org/10.3390/biomedicines14010057
Xie F, Sun Y, Wang J, Luo W, Zhang X, Cheng Y, Chao J. The Extracellular Matrix and the Immune System in Acute Lung Injury: Partners in Damage and Repair. Biomedicines. 2026; 14(1):57. https://doi.org/10.3390/biomedicines14010057
Chicago/Turabian StyleXie, Feiyan, Yuheng Sun, Jing Wang, Wei Luo, Xinxin Zhang, Yusi Cheng, and Jie Chao. 2026. "The Extracellular Matrix and the Immune System in Acute Lung Injury: Partners in Damage and Repair" Biomedicines 14, no. 1: 57. https://doi.org/10.3390/biomedicines14010057
APA StyleXie, F., Sun, Y., Wang, J., Luo, W., Zhang, X., Cheng, Y., & Chao, J. (2026). The Extracellular Matrix and the Immune System in Acute Lung Injury: Partners in Damage and Repair. Biomedicines, 14(1), 57. https://doi.org/10.3390/biomedicines14010057







