IL-1β Controls Proliferation, Apoptosis, and Necroptosis Through the PI3K/AKT/Src/NF-κB Pathway in Leukaemic Lymphoblasts
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Protein EXPRESSION via Flow Cytometry
2.3. Proliferation and Apoptosis
2.4. Statistical Analysis
3. Results
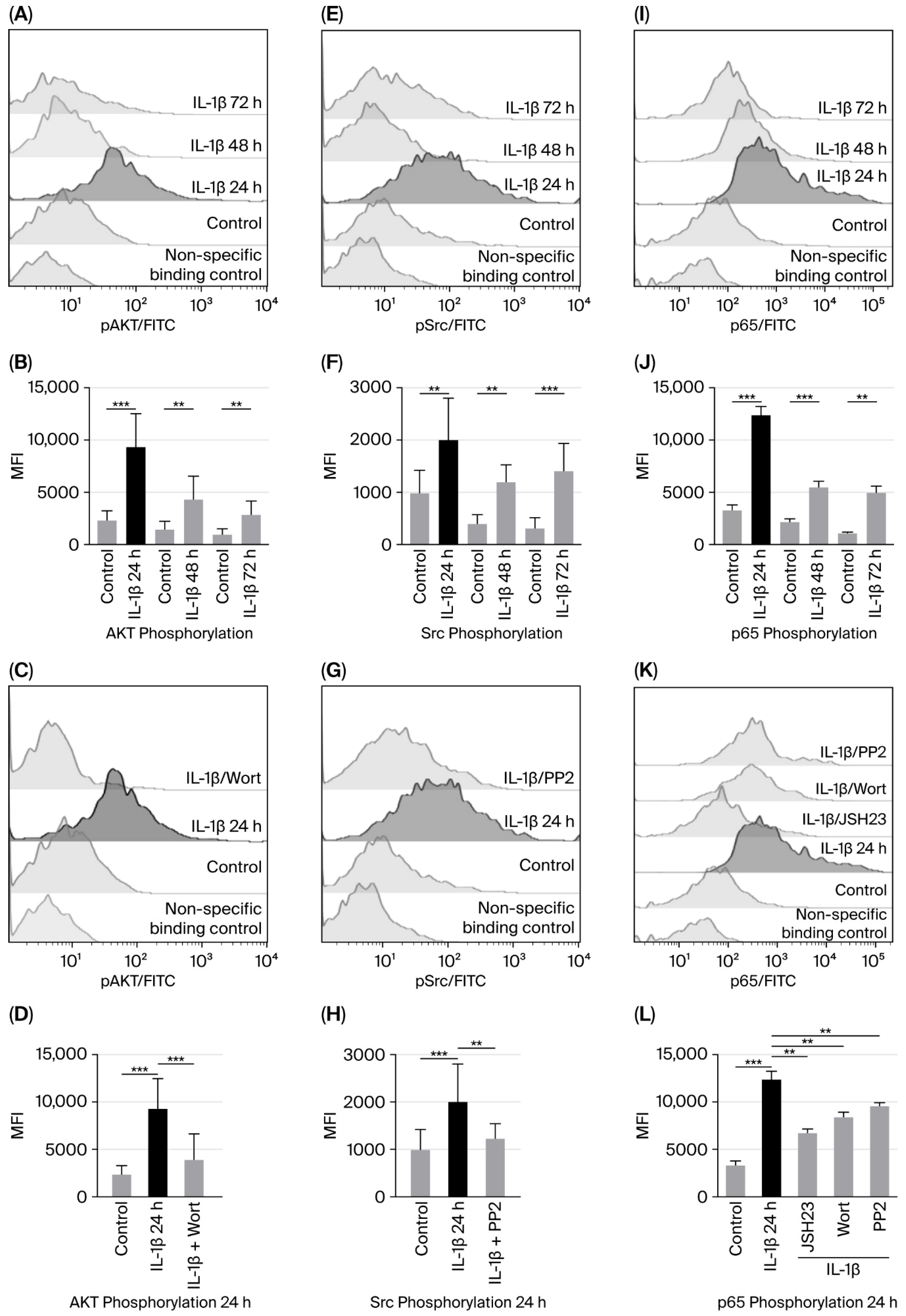
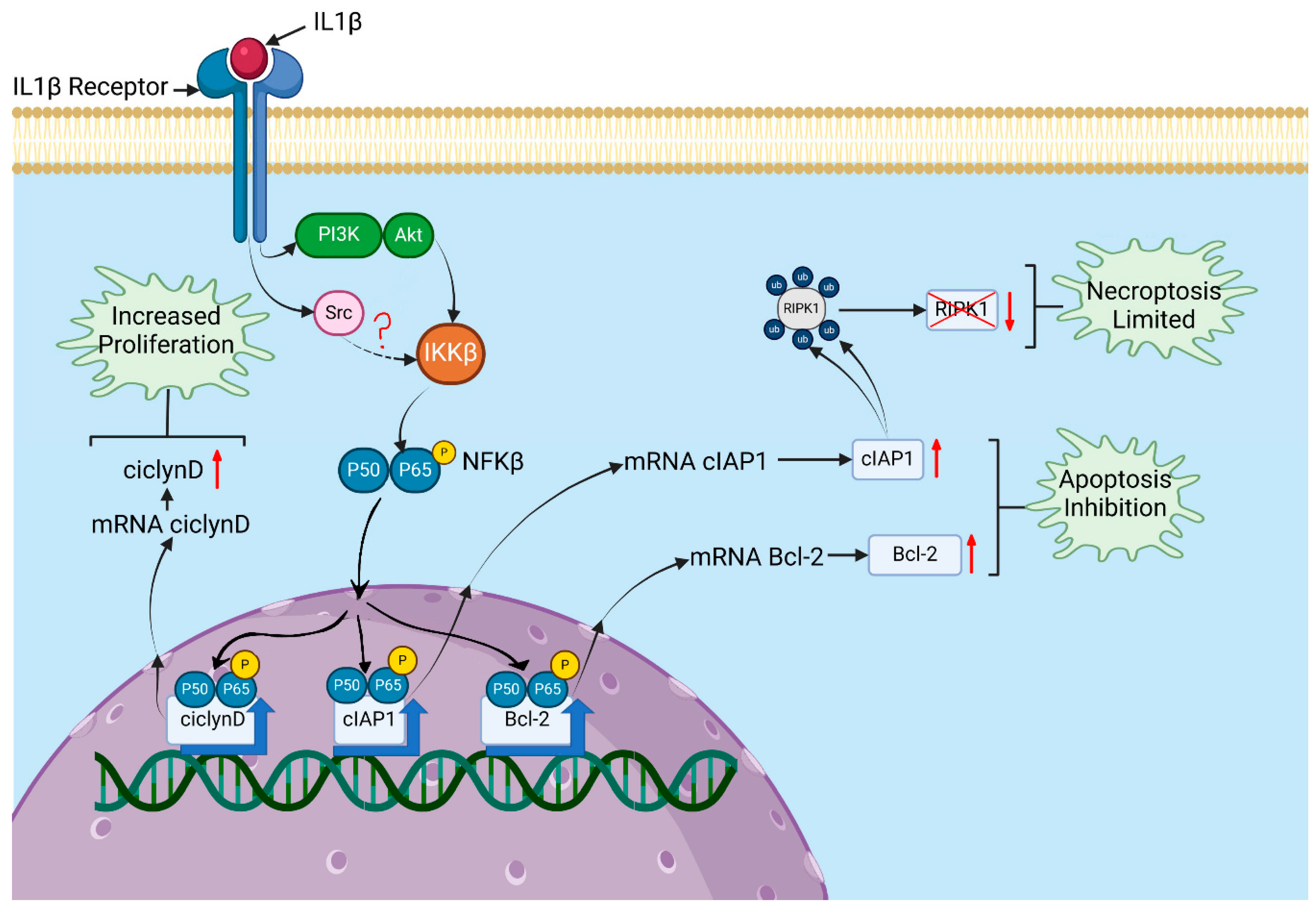
3.1. IL-1β Induces AKT, Src, and NF-κB Activation in RS4:11 Leukaemic Lymphoblasts
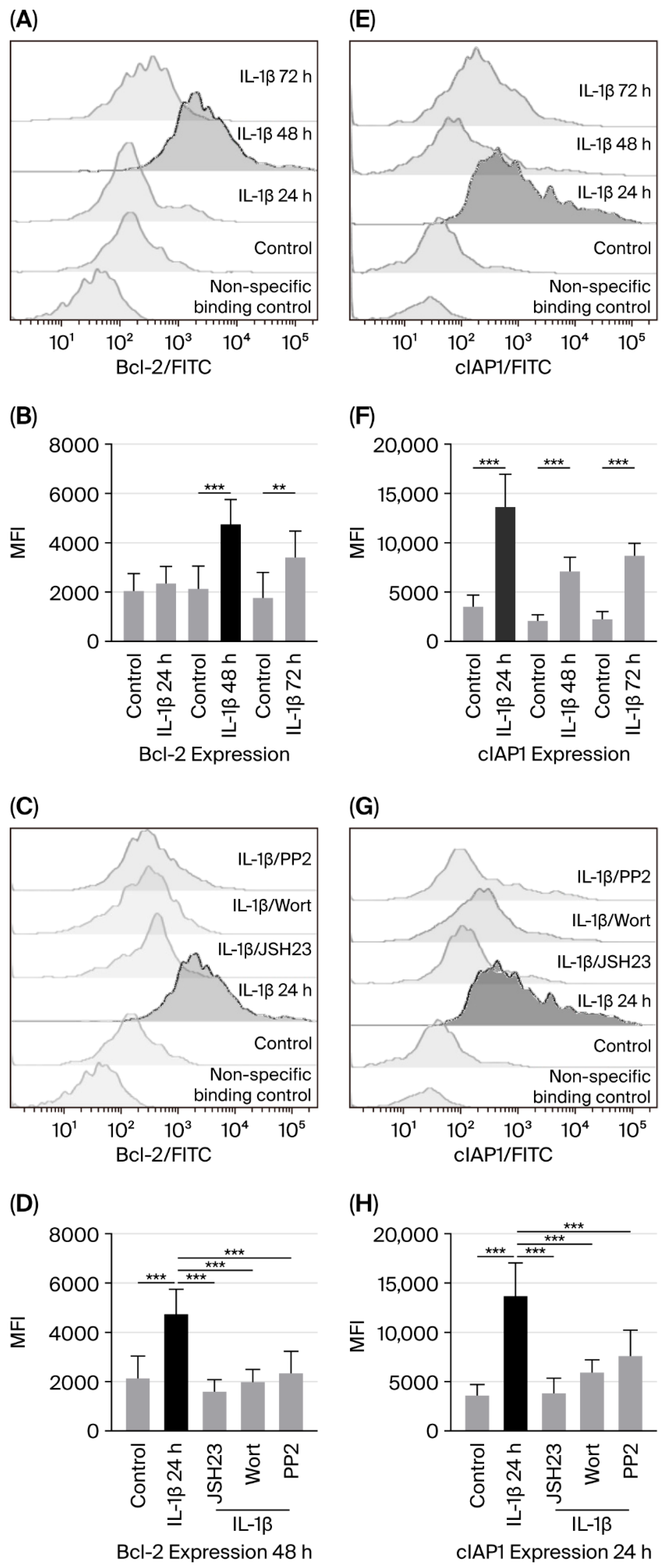
3.2. IL-1β Influences Expression of Bcl-2 and cIAP1 via PI3K/AKT/NF-κB Pathway in RS4:11 Leukaemic Lymphoblasts
3.3. IL-1β Decreases Apoptosis and Limits Necroptosis via the PI3K/AKT/Src/NF-κB Pathway in RS4:11 Leukaemic Lymphoblasts
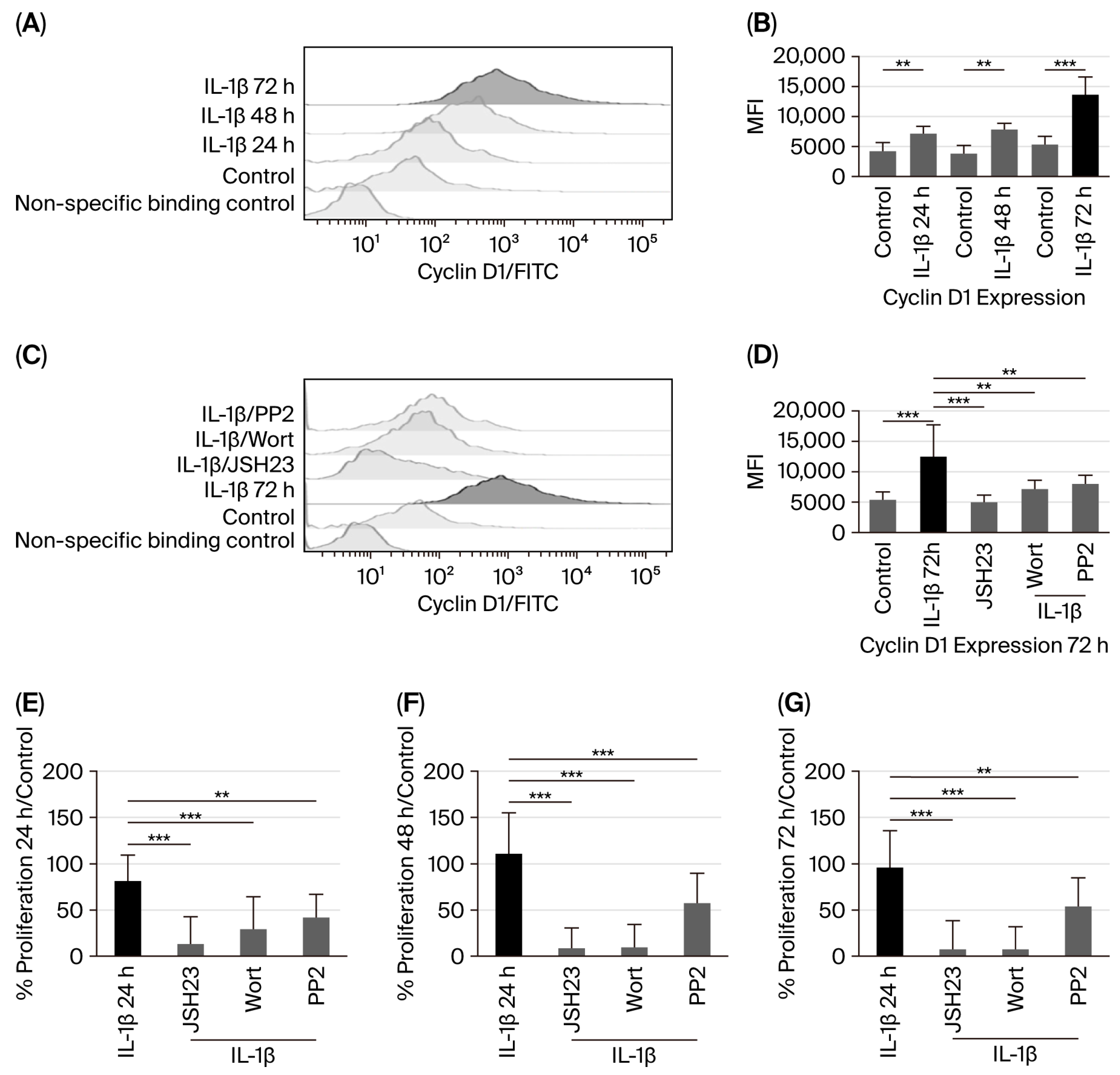
3.4. IL-1β via PI3K/AKT/Src/NF-κB Influences Cyclin D1 Expression and Proliferation of RS4:11 Leukaemic Lymphoblasts
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Braun, T.; Carvalho, G.; Fabre, C.; Grosjean, J.; Fenaux, P.; Kroemer, G. Targeting NF-kB in hematologic malignancies. Cell Death Differ. 2006, 13, 748–758. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, F.; Wu, Y.; Zhu, Y.; Jiang, Y.; Wu, Q.; Dong, Z.; Liu, K. Inflammation in cancer: Therapeutic opportunities from new insights. Mol. Cancer 2025, 24, 2–96. [Google Scholar] [CrossRef]
- Kureshi, C.T.; Dougan, S.K. Cytokines in cancer. Cancer Cell 2025, 43, 15–35. [Google Scholar] [CrossRef] [PubMed]
- Bent, R.; Moll, L.; Grabbe, S.; Bros, M. Interleukin-1 Beta—A Friend or Foe in Malignancies. Int. J. Mol. Sci. 2018, 8, 2155. [Google Scholar] [CrossRef]
- Krzystek-Korpacka, M.; Diakowska, D.; Kapturkiewicz, B.; Bębenek, M.; Gamian, A. Profiles of circulating inflammatory cytokines in colorectal cancer (CRC), high cancer risk conditions, and health are distinct. Possible implications for CRC screening and surveillance. Cancer Lett. 2013, 337, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Matti, B.F.; Saleem, M.A.; Sabir, S.F. Assessment of interleukin 1β serum level in different responder groups stages of chronic myeloid leukemia patients on imatinb mesylate therapy. Indian J. Hematol. Blood Transfus. 2014, 30, 247–252. [Google Scholar] [CrossRef]
- Mitchell, S.; Vargas, J.; Hoffmann, A. Signaling via the NFκB system. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.W. Acute lymphoblastic leukemia. Curr. Probl. Pediatr. Adolesc. Health Care 2002, 32, 40–49. [Google Scholar] [CrossRef]
- Sizemore, N.; Leung, S.; Stark, G.R. Activation of phosphatidylinositol 3-kinase in response to interleukin-1 leads to phosphorylation and activation of the NF-kappaB p65/RelA subunit. Mol. Cell. Biol. 1999, 19, 4798–4805. [Google Scholar] [CrossRef]
- Iliopoulos, D.; Hirsch, H.A.; Struhl, K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell 2009, 139, 693–706. [Google Scholar] [CrossRef]
- American Type Culture Collection, R.S.4.; 11 Cell Line (CRL-1873) Manassas, VA, USA. 2025. Available online: https://www.atcc.org/products/crl-1873 (accessed on 30 October 2025).
- Cyton. RS4:11 Cells. 2025. Available online: https://www.cytion.com/es/RS4-11-Celulas/305360 (accessed on 30 October 2025).
- Nakamura, I.; Takahashi, N.; Sasaki, T.; Tanaka, S.; Udagawa, N.; Murakami, H.; Kimura, K.; Kabuyama, Y.; Kurokawa, T.; Suda, T.; et al. Wortmannin, a specific inhibitor of phosphatidylinositol-3 kinase, blocks osteoclastic bone resorption. FEBS Lett. 1995, 361, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Wang, L.J.; Wu, J.; Shi, Y.X.; Li, G.P.; Yang, X.Q. Src kinase inhibitor PP2 regulates the biological characteristics of A549 cells via the PI3K/AKT signaling pathway. Oncol Lett. 2018, 16, 5059–5065. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, G.; Yuan, Y.; Wu, G.; Wang, S.; Yuan, L. NEK7 interacts with NLRP3 to modulate the pyroptosis in inflammatory bowel disease via NF-κB signaling. Cell Death Dis. 2019, 10, 906. [Google Scholar] [CrossRef]
- Wang, C.Y.; Guttridge, D.C.; Mayo, M.W.; Baldwin, A.S., Jr. NF-kappaB induces expression of the Bcl-2 homologue A1/Bfl-1 to preferentially suppress chemotherapy-induced apoptosis. Mol. Cell. Biol. 1999, 19, 5923–5929. [Google Scholar] [CrossRef]
- Ahmad, I.; Irfan, S.; Ali Beg, M.M.; Kamli, H.; Ali, S.P.; Begum, N.; Alshahrani, M.Y.; Rajagopalan, P. The SMACmimetic AT-101 exhibits anti-tumor in lung adenocarcinoma cells by the IAPs/caspase-dependent apoptosis p65-NFƙBcross-talk. Iran. J. Basic Med. Sci. 2021, 24, 969–977. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, J.; Li, Y.; Shi, Q.; Jin, L.; Li, S.; Zhu, M.; Wang, Q.; Wong, L.L.; Yang, W.; et al. Overexpressed Cyclin D1 and CDK4 proteins are responsible for the resistance to CDK4/6 inhibitor in breast cancer that can be reversed by PI3K/mTOR inhibitors. Sci. China Life Sci. 2023, 66, 94–109. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Turzanski, J.; Grundy, M.; Russell, N.H.; Pallis, M. Interleukin-1beta maintains an apoptosis-resistant phenotype in the blast cells of acute myeloid leukaemia via multiple pathways. Leukemia 2004, 18, 1662–1670. [Google Scholar] [CrossRef]
- Beaupre, D.M.; Talpaz, M.; Marini, F.C., 3rd; Cristiano, R.J.; Roth, J.A.; Estrov, Z.; Albitar, M.; Freedman, M.H.; Kurzrock, R. Autocrine interleukin-1beta production in leukemia: Evidence for the involvement of mutated RAS. Cancer Res. 1999, 15, 2971–2980. [Google Scholar] [PubMed]
- Reddy, S.A.; Huang, J.H.; Liao, W.S. Phosphatidylinositol 3-kinase in interleukin 1 signaling. Physical interaction with the interleukin 1 receptor and requirement in NFkappaB and AP-1 activation. J. Biol. Chem. 1997, 272, 29167–29173. [Google Scholar] [CrossRef]
- Lin, M.T.; Lin, B.R.; Chang, C.C.; Chu, C.Y.; Su, H.J.; Chen, S.T.; Jeng, Y.M.; Kuo, M.L. IL-6 induces AGS gastric cancer cell invasion via activation of the c-Src/RhoA/ROCK signaling pathway. Int. J. Cancer 2007, 120, 2600–2608. [Google Scholar] [CrossRef] [PubMed]
- Diep, S.; Maddukuri, M.; Yamauchi, S.; Geshow, G.; Delk, N.A. Interleukin-1 and Nuclear Factor Kappa B Signaling Promote Breast Cancer Progression and Treatment Resistance. Cells 2022, 11, 1673. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ding, W.; Chen, Y.; Feng, M.; Ouyang, Y.; Yu, Y.; He, Z. Up-regulation of breast cancer resistance protein plays a role in HER2-mediated chemoresistance through PI3K/AKT and nuclear factor-kappa B signaling pathways in MCF7 breast cancer cells. Acta Biochim. Biophys. Sin. 2011, 43, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Ghoneum, A.; Said, N. PI3K-AKT -mTOR and NFκB Pathways in Ovarian Cancer: Implications for Targeted Therapeutics. Cancers 2019, 11, 949. [Google Scholar] [CrossRef]
- Wheeler, D.L.; Iida, M.; Dunn, E.F. The role of Src in solid tumors. Oncologist 2009, 14, 667–678. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Cory, S.; Huang, D.C.; Adams, J.M. The Bcl-2 family: Roles in cell survival and oncogenesis. Oncogene 2003, 22, 8590–8607. [Google Scholar] [CrossRef]
- Rahmani, M.; Aust, M.M.; Attkisson, E.; Williams, D.C., Jr.; Ferreira-Gonzalez, A.; Grant, S. Dual inhibition of Bcl-2 and Bcl-xL strikingly enhances PI3K inhibition-induced apoptosis in human myeloid leukemia cells through a GSK3- and Bim-dependent mechanism. Cancer Res. 2013, 73, 1340–1351. [Google Scholar] [CrossRef]
- Nachmias, B.; Ashhab, Y.; Ben-Yehuda, D. The inhibitor of apoptosis protein family (IAPs): An emerging therapeutic target in cancer. Semin. Cancer Biol. 2004, 14, 231–243. [Google Scholar] [CrossRef]
- Spets, H.; Strömberg, T.; Georgii-Hemming, P.; Siljason, J.; Nilsson, K.; Jernberg-Wiklund, H. Expression of the bcl-2 family of pro- and anti-apoptotic genes in multiple myeloma and normal plasma cells: Regulation during interleukin-6(IL-6)-induced growth and survival. Eur. J. Haematol. 2002, 69, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.J.; Reutelingsperger, C.P.; McGahon, A.J.; Rader, J.A.; van Schie, R.C.; LaFace, D.M.; Green, D.R. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: Inhibition by overexpression of Bcl-2 and Abl. J. Exp. Med. 1995, 82, 1545–1556. [Google Scholar] [CrossRef]
- Earnshaw, W.C.; Martins, L.M.; Kaufmann, S.H. Mammalian caspases: Structure, activation, substrates, and functions during apoptosis. Annu. Rev. Biochem. 1999, 68, 383–424. [Google Scholar] [CrossRef]
- Kayagaki, N.; Webster, J.D.; Newton, K. Control of Cell Death in Health and Disease. Annu. Rev. Pathol. 2024, 19, 157–180. [Google Scholar] [CrossRef]
- Kocab, A.J.; Duckett, C.S. Inhibitor of apoptosis proteins as intracellular signaling intermediates. FEBS J. 2016, 283, 221–231. [Google Scholar] [CrossRef] [PubMed]
- McComb, S.; Cheung, H.H.; Korneluk, R.G.; Wang, S.; Krishnan, L.; Sad, S. cIAP1 and cIAP2 limit macrophage necroptosis by inhibiting Rip1 and Rip3 activation. Cell Death Differ. 2012, 19, 1791–1801. [Google Scholar] [CrossRef] [PubMed]
- Gerges, S.; Rohde, K.; Fulda, S. Cotreatment with Smac mimetics and demethylating agents induces both apoptotic and necroptotic cell death pathways in acute lymphoblastic leukemia cells. Cancer Lett. 2016, 375, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, F.; Schenk, B.; Martens, S.; Vandenabeele, P.; Fulda, S. Sorafenib inhibits therapeutic induction of necroptosis in acute leukemia cells. Oncotarget 2017, 8, 68208–68220. [Google Scholar] [CrossRef]
- Bousserouel, S.; Raymondjean, M.; Brouillet, A.; Béréziat, G.; Andréani, M. Modulation of cyclin D1 and early growth response factor-1 gene expression in interleukin-1beta-treated rat smooth muscle cells by n-6 and n-3 polyunsaturated fatty acids. Eur. J. Biochem. 2004, 271, 4462–4473. [Google Scholar] [CrossRef]
- Ming, J.; Jiang, G.; Zhang, Q.; Qiu, X.; Wang, E. Interleukin-7 up-regulates cyclin D1 via activator protein-1 to promote proliferation of cell in lung cancer. Cancer Immunol. Immunother. 2012, 61, 79–88. [Google Scholar] [CrossRef]
- Baumann, P.; Schneider, L.; Mandl-Weber, S.; Oduncu, F.; Schmidmaier, R. Simultaneous targeting of PI3K and mTOR with NVP-BGT226 is highly effective in multiple myeloma. Anticancer Drugs 2012, 23, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Shishodia, S.; Aggarwal, B.B. Nuclear factor-kappaB: A friend or a foe in cancer? Biochem. Pharmacol. 2004, 68, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wu, K.; Li, C.; Zhang, Z.; Zhai, P.; Guo, H.; Zhang, J. SPP1+ macrophages promote head and neck squamous cell carcinoma progression by secreting TNF-α and IL-1β. J. Exp. Clin. Cancer Res. 2024, 43, 332. [Google Scholar] [CrossRef]
- Teixeira, L.F.S.; Peron, J.P.S.; Bellini, M.H. Silencing of nuclear factor kappa b 1 gene expression inhibits colony formation, cell migration and invasion via the downregulation of interleukin 1 beta and matrix metallopeptidase 9 in renal cell carcinoma. Mol. Biol. Rep. 2020, 47, 1143–1151. [Google Scholar] [CrossRef]
- Viatour, P.; Merville, M.P.; Bours, V.; Chariot, A. Phosphorylation of NF-kappa B and Ikappa B proteins: Implications in cancer and inflammation. Trends Biochem. Sci. 2005, 30, 43–52. [Google Scholar] [CrossRef]
- Gilmore, T.D. Introduction to NF-kappaB: Players, pathways, perspectives. Oncogene 2006, 25, 6680–6684. [Google Scholar] [CrossRef] [PubMed]
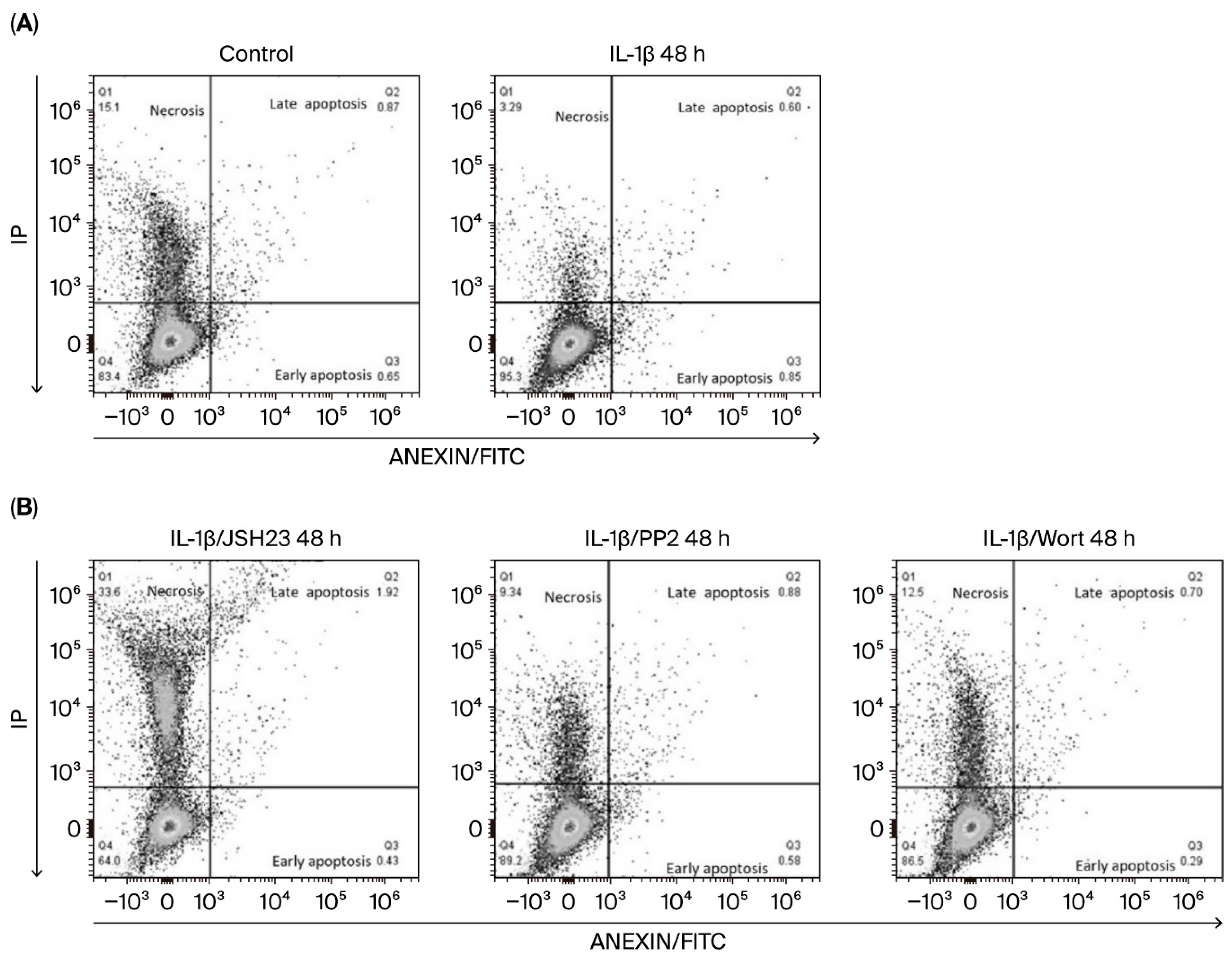
| Treatment | Viable Cells (%) | Early Apoptosis (%) | Late Apoptosis (%) | Necrosis (%) |
|---|---|---|---|---|
| Control | 66.11 ± 7.0 | 5.0 ± 0.08 | 0.84 ± 0.10 | 13.54 ± 2.7 |
| IL-1β | 89.76 ± 5.0 | 0.90 ± 0.06 | 0.91 ± 0.33 | 5.0 ± 1.9 |
| IL-1β + JSH23 | 66.47 ± 7.3 | 0.53 ± 0.12 | 1.9 ± 6.16 | 30.5 ± 3.8 |
| IL-1β + PP2 | 84.90 ± 6.4 | 0.62 ± 0.10 | 0.83 ± 0.09 | 10.53 ± 2.3 |
| IL-1β + Wort | 85.50 ± 5.4 | 0.24 ± 0.05 | 0.75 ± 0.07 | 12.7 ± 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Victoria-Avila, Z.-L.; Reyes-Maldonado, E.; Domínguez-López, M.L.; Vela-Ojeda, J.; Lozada-Ruiz, A.; Alemán, O.R.; Lezama, R.A. IL-1β Controls Proliferation, Apoptosis, and Necroptosis Through the PI3K/AKT/Src/NF-κB Pathway in Leukaemic Lymphoblasts. Biomedicines 2026, 14, 41. https://doi.org/10.3390/biomedicines14010041
Victoria-Avila Z-L, Reyes-Maldonado E, Domínguez-López ML, Vela-Ojeda J, Lozada-Ruiz A, Alemán OR, Lezama RA. IL-1β Controls Proliferation, Apoptosis, and Necroptosis Through the PI3K/AKT/Src/NF-κB Pathway in Leukaemic Lymphoblasts. Biomedicines. 2026; 14(1):41. https://doi.org/10.3390/biomedicines14010041
Chicago/Turabian StyleVictoria-Avila, Zitlal-Lin, Elba Reyes-Maldonado, María Lilia Domínguez-López, Jorge Vela-Ojeda, Aranza Lozada-Ruiz, Omar Rafael Alemán, and Ruth Angélica Lezama. 2026. "IL-1β Controls Proliferation, Apoptosis, and Necroptosis Through the PI3K/AKT/Src/NF-κB Pathway in Leukaemic Lymphoblasts" Biomedicines 14, no. 1: 41. https://doi.org/10.3390/biomedicines14010041
APA StyleVictoria-Avila, Z.-L., Reyes-Maldonado, E., Domínguez-López, M. L., Vela-Ojeda, J., Lozada-Ruiz, A., Alemán, O. R., & Lezama, R. A. (2026). IL-1β Controls Proliferation, Apoptosis, and Necroptosis Through the PI3K/AKT/Src/NF-κB Pathway in Leukaemic Lymphoblasts. Biomedicines, 14(1), 41. https://doi.org/10.3390/biomedicines14010041







