Clinical Implications of Bacteremia Caused by Non-baumannii Acinetobacter Compared with Those of Acinetobacter baumannii Bacteremia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Endpoints
2.3. Definitions
2.4. Statistical Analysis
3. Results
3.1. Study Population Characteristics
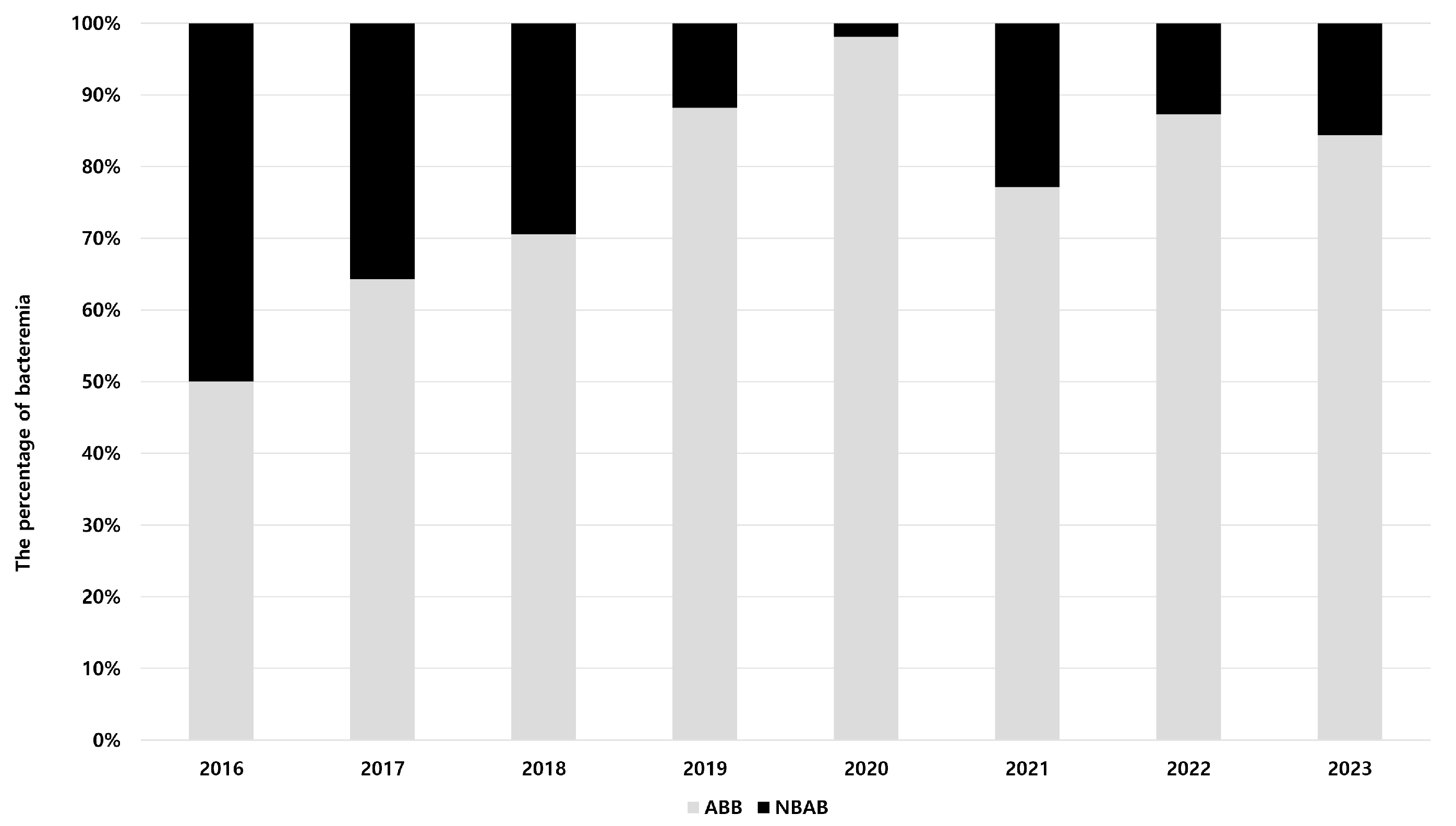
3.2. Epidemiology of Acinetobacter Species Bacteremia
3.3. Antimicrobial Susceptibilities
3.4. Factors Associated with NBAB
3.5. Risk Factors for the 28-Day Mortality in Patients with Acinetobacter Species Bacteremia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munoz-Price, L.S.; Weinstein, R.A. Acinetobacter infection. N. Engl. J. Med. 2008, 358, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Verma, A.; Venkatesh, V.; Verma, S.; Reddy, D.H.; Agrawal, A. The Clinical Impression of NDM-producing Acinetobacter baumannii in Intensive Care Units of the University Referral Hospital in North India. Indian J. Crit. Care Med. 2024, 28, 1044–1049. [Google Scholar] [CrossRef]
- Gaifer, Z.; Fallatah, R.; Alanazi, A.; Alfagi, R.; Alharbi, L.; Osman, H. Prevalence, risk factors, and outcome of carbapenem-resistant Acinetobacter infections in a community hospital in Madinah, Saudi Arabia. Saudi J. Med. Med. Sci. 2024, 12, 306–313. [Google Scholar] [CrossRef]
- Turton, J.F.; Shah, J.; Ozongwu, C.; Pike, R. Incidence of Acinetobacter species other than A. baumannii among clinical isolates of Acinetobacter: Evidence for emerging species. J. Clin. Microbiol. 2010, 48, 1445–1449. [Google Scholar] [CrossRef]
- Fournier, P.E.; Richet, H. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin. Infect. Dis. 2006, 42, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Kempf, M.; Rolain, J.-M. Emergence of resistance to carbapenems in Acinetobacter baumannii in Europe: Clinical impact and therapeutic options. Int. J. Antimicrob. Agents 2012, 39, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Tewari, R.; Chopra, D.; Wazahat, R.; Dhingra, S.; Dudeja, M. Antimicrobial susceptibility patterns of an emerging multidrug resistant nosocomial pathogen: Acinetobacter baumannii. Malays. J. Med. Sci. 2018, 25, 129–134. [Google Scholar] [CrossRef]
- Cisneros, J.M.; Reyes, M.J.; Pachón, J.; Becerril, B.; Caballero, F.J.; García-Garmendía, J.L.; Ortiz, C.; Cobacho, A.R. Bacteremia due to Acinetobacter baumannii: Epidemiology, clinical findings, and prognostic features. Clin. Infect. Dis. 1996, 22, 1026–1032. [Google Scholar] [CrossRef]
- Jain, A.L.; Harding, C.M.; Assani, K.; Shrestha, C.L.; Haga, M.; Leber, A.; Munson, R.S., Jr.; Kopp, B.T. Characteristics of invasive Acinetobacter species isolates recovered in a pediatric academic center. BMC Infect. Dis. 2016, 16, 346. [Google Scholar] [CrossRef]
- Fitzpatrick, M.A.; Ozer, E.; Bolon, M.K.; Hauser, A.R. Influence of ACB complex genospecies on clinical outcomes in a U.S. hospital with high rates of multidrug resistance. J. Infect. 2015, 70, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, Y.; Jia, X.; Luo, Y.; Song, Q.; Zhao, W.; Wang, Y.; Liu, H.; Zheng, D.; Xia, Y.; et al. Dissemination and characterization of NDM-1-producing Acinetobacter pittii in an intensive care unit in China. Clin. Microbiol. Infect. 2012, 18, E506–E513. [Google Scholar] [CrossRef] [PubMed]
- Karah, N.; Haldorsen, B.; Hegstad, K.; Simonsen, G.S.; Sundsfjord, A.; Samuelsen, Ø.; Norwegian Study Group of Acinetobacter. Species identification and molecular characterization of Acinetobacter spp. blood culture isolates from Norway. J. Antimicrob. Chemother. 2011, 66, 738–744. [Google Scholar] [CrossRef]
- Li, P.; Yang, C.; Xie, J.; Liu, N.; Wang, H.; Zhang, L.; Wang, X.; Wang, Y.; Qiu, S.; Song, H. Acinetobacter calcoaceticus from a fatal case of pneumonia harboring bla(NDM-1) on a widely distributed plasmid. BMC Infect. Dis. 2015, 15, 131. [Google Scholar] [CrossRef]
- Dortet, L.; Legrand, P.; Soussy, C.-J.; Cattoir, V. Bacterial identification, clinical significance, and antimicrobial susceptibilities of Acinetobacter ursingii and Acinetobacter schindleri, two frequently misidentified opportunistic pathogens. J. Clin. Microbiol. 2006, 44, 4471–4478. [Google Scholar] [CrossRef]
- Jeong, S.; Hong, J.S.; Kim, J.O.; Kim, K.H.; Lee, W.; Bae, I.K.; Lee, K.; Jeong, S.H. Identification of Acinetobacter species using matrix-assisted laser desorption ionization-time of flight mass spectrometry. Ann. Lab. Med. 2016, 36, 325–334. [Google Scholar] [CrossRef]
- Li, X.; Tang, Y.; Lu, X. Insight into identification of Acinetobacter species by matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS) in the clinical laboratory. J. Am. Soc. Mass. Spectrom. 2018, 29, 1546–1553. [Google Scholar] [CrossRef]
- Marí-Almirall, M.; Cosgaya, C.; Higgins, P.G.; Van Assche, A.; Telli, M.; Huys, G.; Lievens, B.; Seifert, H.; Dijkshoorn, L.; Roca, I.; et al. MALDI-TOF/MS identification of species from the Acinetobacter baumannii (Ab) group revisited: Inclusion of the novel A. seifertii and A. dijkshoorniae species. Clin. Microbiol. Infect. 2017, 23, 210.e1–210.e9. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.A. Reliability of Acinetobacter baumannii identification with matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Lab. Med. Qual. Assur. 2023, 45, 65–69. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Al-Hasan, M.N.; Baddour, L.M. Resilience of the Pitt bacteremia score: 3 decades and counting. Clin. Infect. Dis. 2020, 70, 1834–1836. [Google Scholar] [CrossRef] [PubMed]
- Joyanes, P.; del Carmen Conejo, M.; Martínez-Martínez, L.; Perea, E.J. Evaluation of the VITEK 2 system for the identification and susceptibility testing of three species of nonfermenting gram-negative rods frequently isolated from clinical samples. J. Clin. Microbiol. 2001, 39, 3247–3253. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, P.-R.; Kuo, L.-C.; Chang, T.-C.; Lee, T.-F.; Teng, S.-H.; Chuang, Y.-C.; Teng, L.J.; Sheng, W.H. Evaluation of the Bruker Biotyper matrix-assisted laser desorption ionization-time of flight mass spectrometry system for identification of blood isolates of Acinetobacter species. J. Clin. Microbiol. 2014, 52, 3095–3100. [Google Scholar] [CrossRef] [PubMed]
- CLSI M100; Performance Standards for Antimicrobial Susceptibility Testing, 34th Edition. Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2024. Available online: https://clsi.org/shop/standards/m100/ (accessed on 2 June 2025).
- Kempf, M.; Bakour, S.; Flaudrops, C.; Berrazeg, M.; Brunel, J.-M.; Drissi, M.; Mesli, E.; Touati, A.; Rolain, J.M. Rapid detection of carbapenem resistance in Acinetobacter baumannii using matrix-assisted laser desorption ionization-time of flight mass spectrometry. PLoS ONE 2012, 7, e31676. [Google Scholar] [CrossRef]
- Horan, T.C.; Andrus, M.; Dudeck, M.A. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect. Control 2008, 36, 309–332. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Harbarth, S.; Garbino, J.; Pugin, J.; Romand, J.A.; Lew, D.; Pittet, D. Inappropriate initial antimicrobial therapy and its effect on survival in a clinical trial of immunomodulating therapy for severe sepsis. Am. J. Med. 2003, 115, 529–535. [Google Scholar] [CrossRef]
- Lee, Y.-T.; Kuo, S.-C.; Yang, S.-P.; Lin, Y.-T.; Tseng, F.-C.; Chen, T.-L.; Fung, C.P. Impact of appropriate antimicrobial therapy on mortality associated with Acinetobacter baumannii bacteremia: Relation to severity of infection. Clin. Infect. Dis. 2012, 55, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, E.M.L.; Kaled, E.S.S. Thrombocytopenia. Crit. Care Nurs. Clin. N. Am. 2013, 25, 427–434. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics 20 Core System User’s Guide; IBM Corp.: Armonk, NY, USA, 2011; Available online: https://eio.upc.edu/ca/qui-som/laboratori-de-calcul/manuals-del-sistema/IBM_SPSS_Statistics_Core_System_Users_Guide.pdf (accessed on 2 June 2025).
- Chusri, S.; Chongsuvivatwong, V.; Rivera, J.I.; Silpapojakul, K.; Singkhamanan, K.; McNeil, E.; Doi, Y. Clinical outcomes of hospital-acquired infection with Acinetobacter nosocomialis and Acinetobacter pittii. Antimicrob. Agents Chemother. 2014, 58, 4172–4179. [Google Scholar] [CrossRef]
- Beig, M.; Parvizi, E.; Navidifar, T.; Bostanghadiri, N.; Mofid, M.; Golab, N.; Sholeh, M. Geographical mapping and temporal trends of Acinetobacter baumannii carbapenem resistance: A comprehensive meta-analysis. PLoS ONE 2024, 19, e0311124. [Google Scholar] [CrossRef]
- Al Atrouni, A.; Joly-Guillou, M.-L.; Hamze, M.; Kempf, M. Reservoirs of non-baumannii Acinetobacter species. Front. Microbiol. 2016, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.; Nielsen, T.B.; Bonomo, R.A.; Pantapalangkoor, P.; Luna, B.; Spellberg, B. Clinical and pathophysiological overview of Acinetobacter infections: A century of challenges. Clin. Microbiol. Rev. 2017, 30, 409–447. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.K.; Postic, B.; Kass, E.H. Infections due to organisms of the genus Herellea. B5W and B. anitratum. Arch. Intern. Med. 1962, 110, 580–591. [Google Scholar] [CrossRef]
- Glew, R.H.; Moellering, R.C., Jr.; Kunz, L.J. Infections with Acinetobacter calcoaceticus (Herellea vaginicola): Clinical and laboratory studies. Medicine 1977, 56, 79–97. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.; Bonomo, R.A. The deadly impact of extreme drug resistance in Acinetobacter baumannii. Crit. Care Med. 2014, 42, 1289–1291. [Google Scholar] [CrossRef]
- Lee, K.; Yong, D.; Jeong, S.H.; Chong, Y. Multidrug-resistant Acinetobacter spp.: Increasingly problematic nosocomial pathogens. Yonsei Med. J. 2011, 52, 879–891. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Huang, Y.-T.; Tan, C.-K.; Kuo, Y.-W.; Liao, C.-H.; Lee, P.-I.; Hsueh, P.R. Acinetobacter baumannii and Acinetobacter genospecies 13TU and 3 bacteraemia: Comparison of clinical features, prognostic factors and outcomes. J. Antimicrob. Chemother. 2011, 66, 1839–1846. [Google Scholar] [CrossRef] [PubMed]
- Barth, P.O.; Roesch, E.W.; Lutz, L.; de Souza, Â.C.; Goldani, L.Z.; Pereira, D.C. Rapid bacterial identification by MALDI-TOF MS directly from blood cultures and rapid susceptibility testing: A simple approach to reduce the turnaround time of blood cultures. Braz. J. Infect. Dis. 2023, 27, 102721. [Google Scholar] [CrossRef]
- Wisplinghoff, H.; Paulus, T.; Lugenheim, M.; Stefanik, D.; Higgins, P.G.; Edmond, M.B.; Wenzel, R.P.; Seifert, H. Nosocomial bloodstream infections due to Acinetobacter baumannii, Acinetobacter pittii and Acinetobacter nosocomialis in the United States. J. Infect. 2012, 64, 282–290. [Google Scholar] [CrossRef]
- Liu, Y.-M.; Lee, Y.-T.; Kuo, S.-C.; Chen, T.-L.; Liu, C.-P.; Liu, C.-E. Comparison between bacteremia caused by Acinetobacter pittii and Acinetobacter nosocomialis. J. Microbiol. Immunol. Infect. 2017, 50, 62–67. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Biswas, I.; Veeraraghavan, B. Accurate identification of clinically important Acinetobacter spp.: An update. Future Sci. OA 2019, 5, FSO395. [Google Scholar] [CrossRef] [PubMed]
- Sheck, E.; Romanov, A.; Shapovalova, V.; Shaidullina, E.; Martinovich, A.; Ivanchik, N.; Mikotina, A.; Skleenova, E.; Oloviannikov, V.; Azizov, I.; et al. Acinetobacter non-baumannii species: Occurrence in infections in hospitalized patients, identification, and antibiotic resistance. Antibiotics 2023, 12, 1301. [Google Scholar] [CrossRef]
- Wang, J.; Ruan, Z.; Feng, Y.; Fu, Y.; Jiang, Y.; Wang, H.; Yu, Y. Species distribution of clinical Acinetobacter isolates revealed by different identification techniques. PLoS ONE 2014, 9, e104882. [Google Scholar] [CrossRef]
- Kang, S.; Lee, S.Y.; Seo, S.H.; Lee, J.; Moon, D.C.; Yoo, J.S.; Choi, Y.H. Antimicrobial Resistance of Major Clinical Pathogens Isolated from General Hospitals in Korea: Results from the 2nd phase (2020-2022) Kor-GLASS. Public Health Wkly. Rep. 2024, 17, 1034–1054. [Google Scholar] [CrossRef]
- Su, C.H.; Wang, J.T.; Hsiung, C.A.; Chien, L.J.; Chi, C.L.; Yu, H.T.; Chang, F.Y.; Chang, S.C. Increase of carbapenem-resistant Acinetobacter baumannii infection in acute care hospitals in Taiwan: Association with hospital antimicrobial usage. PLoS ONE 2012, 7, e37788. [Google Scholar] [CrossRef]
- Da Silva, G.J.; Domingues, S. Insights on the horizontal gene transfer of carbapenemase determinants in the opportunistic pathogen Acinetobacter baumannii. Microorganisms 2016, 4, 29. [Google Scholar] [CrossRef]
- Sotomayor, N.; Villacis, J.E.; Burneo, N.; Reyes, J.; Zapata, S.; Bayas-Rea, R.d.L.Á. Carbapenemase genes in clinical and environmental isolates of Acinetobacter spp. from Quito, Ecuador. Peer J. 2024, 12, e17199. [Google Scholar] [CrossRef]
- Hubeny, J.; Korzeniewska, E.; Buta-Hubeny, M.; Zieliński, W.; Rolbiecki, D.; Harnisz, M. Characterization of carbapenem resistance in environmental samples and Acinetobacter spp. isolates from wastewater and river water in Poland. Sci. Total Environ. 2022, 822, 153437. [Google Scholar] [CrossRef] [PubMed]
- Acolatse, J.E.E.; Portal, E.A.R.; Boostrom, I.; Akafity, G.; Dakroah, M.P.; Chalker, V.J.; Sands, K.; Spiller, O.B. Environmental surveillance of ESBL and carbapenemase-producing gram-negative bacteria in a Ghanaian Tertiary Hospital. Antimicrob. Resist. Infect. Control 2022, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- Azimzadeh, M.; Bahador, A.; Shiralizadeh, S.; Mahshouri, P.; Akbari, L.; Makari, S.; Rezaei, A.; Alikhani, M.S.; Alikhani, M.Y. A single-center analysis of clonal transmission of carbapenem-resistant Acinetobacter baumannii among intensive care unit patients during the COVID-19 pandemic. Sci. Rep. 2024, 14, 25897. [Google Scholar] [CrossRef]
- Tobin, L.A.; Jarocki, V.M.; Kenyon, J.; Drigo, B.; Donner, E.; Djordjevic, S.P.; Hamidian, M. Genomic analysis of diverse environmental Acinetobacter isolates identifies plasmids, antibiotic resistance genes, and capsular polysaccharides shared with clinical strains. Appl. Environ. Microbiol. 2024, 90, e0165423. [Google Scholar] [CrossRef]
- Park, Y.K.; Jung, S.-I.; Park, K.-H.; Kim, D.H.; Choi, J.Y.; Kim, S.H.; Ko, K.S. Changes in antimicrobial susceptibility and major clones of Acinetobacter calcoaceticus-baumannii complex isolates from a single hospital in Korea over 7 years. J. Med. Microbiol. 2012, 61, 71–79. [Google Scholar] [CrossRef]
- Hong, Y.-K.; Ko, K.S. Development of colistin dependence in non-baumannii Acinetobacter species. Int. J. Antimicrob. Agents 2018, 52, 742–743. [Google Scholar] [CrossRef]
- Adams, M.D.; Wright, M.S.; Karichu, J.K.; Venepally, P.; Fouts, D.E.; Chan, A.P.; Richter, S.S.; Jacobs, M.R.; Bonomo, R.A. Rapid replacement of Acinetobacter baumannii strains accompanied by changes in lipooligosaccharide loci and resistance gene repertoire. mBio 2019, 10, e00356-19. [Google Scholar] [CrossRef]
- Novović, K.; Jovčić, B. Colistin Resistance in Acinetobacter baumannii: Molecular Mechanisms and Epidemiology. Antibiotics 2023, 12, 516. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-H.; Shin, J.-H.; Lee, S.Y.; Kim, S.H.; Jang, M.O.; Kang, S.-J.; Jung, S.I.; Chung, E.K.; Ko, K.S.; Jang, H.C. The clinical characteristics, carbapenem resistance, and outcome of Acinetobacter bacteremia according to genospecies. PLoS ONE 2014, 8, e65026. [Google Scholar] [CrossRef]
- Lee, Y.-T.; Kuo, S.-C.; Chiang, M.-C.; Yang, S.-P.; Chen, C.-P.; Chen, T.-L.; Fung, C.P. Emergence of carbapenem-resistant non-baumannii species of Acinetobacter harboring a blaOXA-51-like gene that is intrinsic to A. baumannii. Antimicrob. Agents Chemother. 2012, 56, 1124–1127. [Google Scholar] [CrossRef]
- Mehta, A.A.; Kumar, V.A.; Kumari, I.K.; Nair, S.G.; Dinesh, K.R.; Singh, S.K. Risk factors for mortality in Acinetobacter calcoaceticus–baumannii bacteraemia. Asian Pac. J. Trop. Biomed. 2012, 2, S1852–S1857. [Google Scholar] [CrossRef]
- Suh, J.W.; Park, S.M.; Ju, Y.K.; Yang, K.S.; Kim, J.Y.; Kim, S.B.; Sohn, J.W.; Yoon, Y.K. Clinical and molecular predictors of mortality in patients with carbapenem-resistant Acinetobacter baumannii bacteremia: A retrospective cohort study. J. Microbiol. Immunol. Infect. 2024, 57, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Kim, S.I.; Hong, K.-W.; Kim, Y.R.; Park, Y.J.; Kang, M.-W. Risk factors for mortality in patients with carbapenem-resistant Acinetobacter baumannii bacteremia: Impact of appropriate antimicrobial therapy. J. Korean Med. Sci. 2012, 27, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Gao, Y.; Lin, H.; Fan, Q.; Chen, L.; Feng, Y. Prognostic factors that affect mortality patients with Acinetobacter baumannii bloodstream infection. Infect. Drug Resist. 2024, 17, 3825–3837. [Google Scholar] [CrossRef] [PubMed]
- Nithichanon, A.; Kewcharoenwong, C.; Da-Oh, H.; Surajinda, S.; Khongmee, A.; Koosakunwat, S.; Wren, B.W.; Stabler, R.A.; Brown, J.S.; Lertmemongkolchai, G. Acinetobacter nosocomialis Causes as Severe Disease as Acinetobacter baumannii in Northeast Thailand: Underestimated Role of A. nosocomialis in Infection. Microbiol. Spectr. 2022, 10, e0283622. [Google Scholar] [CrossRef] [PubMed]
- Ayenew, Z.; Tigabu, E.; Syoum, E.; Ebrahim, S.; Assefa, D.; Tsige, E. Multidrug resistance pattern of Acinetobacter species isolated from clinical specimens referred to the Ethiopian Public Health Institute: 2014 to 2018 trend anaylsis. PLoS ONE 2021, 16, e0250896. [Google Scholar] [CrossRef] [PubMed]
| Variables | Total (n = 273) | ABB (n = 224) | NBAB (n = 49) | p-Value |
|---|---|---|---|---|
| Median age, years (IQR) | 69 (60–78) | 69 (60–79) | 69 (61–76) | 0.419 |
| Male n (%) | 167 (61.2) | 139 (62.1) | 28 (57.1) | 0.523 |
| Comorbidities | ||||
| Diabetes mellitus, n (%) | 116 (42.5) | 97 (43.3) | 19 (38.8) | 0.561 |
| Hypertension, n (%) | 142 (52.0) | 119 (53.1) | 23 (46.9) | 0.432 |
| Cardiovascular disease, n (%) | 60 (22.0) | 55 (24.6) | 5 (10.2) | 0.028 |
| Cerebrovascular disease, n (%) | 61 (22.3) | 48 (21.4) | 13 (26.5) | 0.437 |
| Chronic kidney disease, n (%) | 52 (19.0) | 47 (21.0) | 5 (10.2) | 0.082 |
| Chronic pulmonary disease, n (%) | 22 (8.1) | 20 (8.9) | 2 (4.1) | 0.259 |
| Chronic liver disease, n (%) | 25 (9.2) | 20 (8.9) | 5 (10.2) | 0.779 |
| Malignancy, n (%) | ||||
| Solid organ | 103 (37.7) | 81 (36.2) | 22 (44.9) | 0.253 |
| Hematology | 38 (13.9) | 30 (13.4) | 8 (16.3) | 0.591 |
| Organ transplantation, n (%) | 10 (3.7) | 9 (4.0) | 1 (2.0) | 0.505 |
| Charlson comorbidity index (IQR) | 3 (2–5) | 3 (1–4) | 3 (2–6) | 0.127 |
| Clinical severity | ||||
| Pitt bacteremia score (IQR) | 3 (0–8) | 4 (2–8) | 0 (0–1) | <0.001 |
| Sepsis, n (%) | 138 (50.5) | 96 (42.9) | 42 (30.4) | <0.001 |
| Septic shock, n (%) | 135 (49.5) | 128 (57.1) | 7 (14.3) | <0.001 |
| Mechanical ventilator, n (%) | 155 (56.8) | 148 (66.1) | 7 (14.3) | <0.001 |
| ECMO, n (%) | 12 (4.4) | 12 (5.4) | 0 (0) | 0.098 |
| CRRT, n (%) | 70 (25.6) | 66 (29.5) | 4 (8.2) | 0.002 |
| Risk factors | ||||
| ICU stay at onset of bacteremia, n (%) | 162 (59.3) | 151 (67.4) | 11 (22.4) | <0.001 |
| Steroid use in 90 days, n (%) | 45 (46.5) | 38 (17.0) | 7 (14.3) | 0.647 |
| Prior antibiotic use in 30 days, n (%) | 183 (67.0) | 171 (76.3) | 12 (24.5) | <0.001 |
| Inappropriate empirical antibiotic therapy, n (%) | 153 (56.0) | 146 (65.2) | 7 (14.3) | <0.001 |
| Carbapenem-resistance, n (%) | 224 (82.1) | 216 (96.4) | 8 (16.3) | <0.001 |
| Primary infectious origin | ||||
| Pneumonia | 123 (45.1) | 115 (51.3) | 8 (16.3) | <0.001 |
| Intra-abdominal infection, n (%) | 38 (13.9) | 26 (11.6) | 12 (24.5) | 0.018 |
| Urinary tract infection, n (%) | 15 (5.5) | 6 (2.7) | 9 (18.4) | <0.001 |
| Catheter related infection, n (%) | 71 (26.0) | 61 (27.2) | 10 (20.4) | 0.324 |
| Skin and soft tissue infection, n (%) | 12 (4.4) | 7 (3.1) | 5 (10.2) | 0.029 |
| Without primary infection origin, n (%) | 23 (8.4) | 18 (8.0) | 5 (10.2) | 0.621 |
| All-cause mortality | 171 (62.6) | 155 (69.2) | 16 (32.7) | <0.001 |
| 7-day | 102 (37.4) | 91 (40.6) | 11 (22.4) | 0.017 |
| 14-day | 120 (44.0) | 107 (47.8) | 13 (26.5) | 0.007 |
| 28-day | 138 (50.5) | 125 (55.8) | 13 (26.5) | <0.001 |
| 60-day | 152 (55.7) | 136 (60.7) | 16 (32.7) | <0.001 |
| Laboratory parameters | ||||
| WBC count (×103/µL) | 10.1 (6.2–16.8) | 10.7 (6.2–17.6) | 7.6 (6.4–11.6) | 0.039 |
| PLT count (×103/µL) | 108 (48–188) | 103.5 (46.0–179.0) | 145 (62–196.5) | 0.332 |
| CRP (nmol/L) | 99.2 (46.0–161.6) | 101.6 (51.4–162.4) | 89.7 (27.7–161.6) | 0.607 |
| PCT (ng/mL) | 1.26 (0.34–5.69) | 1.34 (0.367–6.38) | 0.60 (0.13–1.87) | 0.607 |
| Albumin (mg/dL) | 2.7 (2.4–3.0) | 2.7 (2.4–2.9) | 3.1 (2.6–3.6) | <0.001 |
| Resistance, n (%) | Total (n = 273) | ABB (n = 224) | NBAB (n = 49) | p-Value |
|---|---|---|---|---|
| Ampicillin-sulbactam, (n = 273) | 220 (80.6) | 212 (94.6) | 8 (16.3) | <0.001 |
| Piperacillin-tazobactam, (n = 273) | 224 (82.1) | 215 (96.0) | 9 (18.4) | <0.001 |
| Cefotaxime, (n = 273) | 234 (85.7) | 219 (97.8) | 15 (30.6) | <0.001 |
| Ceftazidime, (n = 273) | 232 (85.0) | 215 (96.0) | 17 (34.7) | <0.001 |
| Cefepime, (n = 273) | 232 (85.0) | 215 (96.0) | 17 (34.7) | <0.001 |
| Aztreonam, (n = 191) | 179 (93.7) | 152 (99.3) | 27 (71.1) | <0.001 |
| Imipenem, (n = 265) | 221 (83.4) | 215 (96.4) | 6 (14.3) | <0.001 |
| Meropenem, (n = 273) | 221 (81.0) | 215 (96.0) | 6 (12.2) | <0.001 |
| Gentamicin, (n = 218) | 166 (76.1) | 147 (86.5) | 19 (39.6) | <0.001 |
| Ciprofloxacin, (n = 273) | 233 (85.3) | 215 (96.0) | 18 (36.7) | <0.001 |
| Minocycline, (n = 268) | 13 (4.9) | 12 (5.4) | 1 (2.2) | 0.353 |
| Tetracycline, (n = 54) | 28 (51.9) | 27 (84.4) | 1 (4.5) | <0.001 |
| Tigecycline, (n = 220) | 55 (25.0) | 55 (28.8) | 0 (0) | 0.001 |
| Trimethoprim-sulfamethoxazole, (n = 218) | 159 (72.9) | 153 (90.0) | 6 (12.5) | <0.001 |
| Colistin, (n = 133) | 28 (21.1) | 28 (27.5) | 0 (0) | 0.001 |
| Tobramycin, (n = 41) | 28 (68.3) | 20 (83.3) | 8 (47.1) | 0.014 |
| Amikacin, (n = 48) | 24 (50.0) | 18 (72.0) | 6 (26.1) | 0.001 |
| Variables | Odds Ratio | 95% CI | p-Value |
|---|---|---|---|
| Carbapenem-resistance | 0.007 | 0.003–0.020 | <0.001 |
| Variables | Odds Ratio | 95% CI | p-Value |
|---|---|---|---|
| Septic shock | 4.179 | 1.917–9.106 | <0.001 |
| Pitt bacteremic score | 1.148 | 1.031–1.278 | 0.012 |
| CRRT | 2.525 | 1.148–5.555 | 0.021 |
| Inappropriate empirical antibiotic therapy | 2.470 | 1.262–4.834 | 0.008 |
| Thrombocytopenia | 2.882 | 1.405–5.913 | 0.004 |
| Age | 1.024 | 0.998–1.050 | 0.068 |
| Primary infection origin (urinary tract infection) | 0.209 | 0.024–1.822 | 0.156 |
| Primary infection origin (skin soft tissue infection) | 0.180 | 0.018–1.802 | 0.144 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suh, J.W.; Hong, J.Y.; Kim, K.J.; Hong, D.J.; Kim, S.B. Clinical Implications of Bacteremia Caused by Non-baumannii Acinetobacter Compared with Those of Acinetobacter baumannii Bacteremia. Biomedicines 2025, 13, 2304. https://doi.org/10.3390/biomedicines13092304
Suh JW, Hong JY, Kim KJ, Hong DJ, Kim SB. Clinical Implications of Bacteremia Caused by Non-baumannii Acinetobacter Compared with Those of Acinetobacter baumannii Bacteremia. Biomedicines. 2025; 13(9):2304. https://doi.org/10.3390/biomedicines13092304
Chicago/Turabian StyleSuh, Jin Woong, Ji Young Hong, Keun Ju Kim, Duck Jin Hong, and Sun Bean Kim. 2025. "Clinical Implications of Bacteremia Caused by Non-baumannii Acinetobacter Compared with Those of Acinetobacter baumannii Bacteremia" Biomedicines 13, no. 9: 2304. https://doi.org/10.3390/biomedicines13092304
APA StyleSuh, J. W., Hong, J. Y., Kim, K. J., Hong, D. J., & Kim, S. B. (2025). Clinical Implications of Bacteremia Caused by Non-baumannii Acinetobacter Compared with Those of Acinetobacter baumannii Bacteremia. Biomedicines, 13(9), 2304. https://doi.org/10.3390/biomedicines13092304





