Iron Deficiency in Heart Failure: From ESC Guidelines to Clinical Practice at a Romanian Hospital
Abstract
1. Introduction
2. Materials and Methods
2.1. Design of the Study
2.2. Data Collection
2.3. Assessment of ID and Anemia in HF
2.4. Statistical Analysis
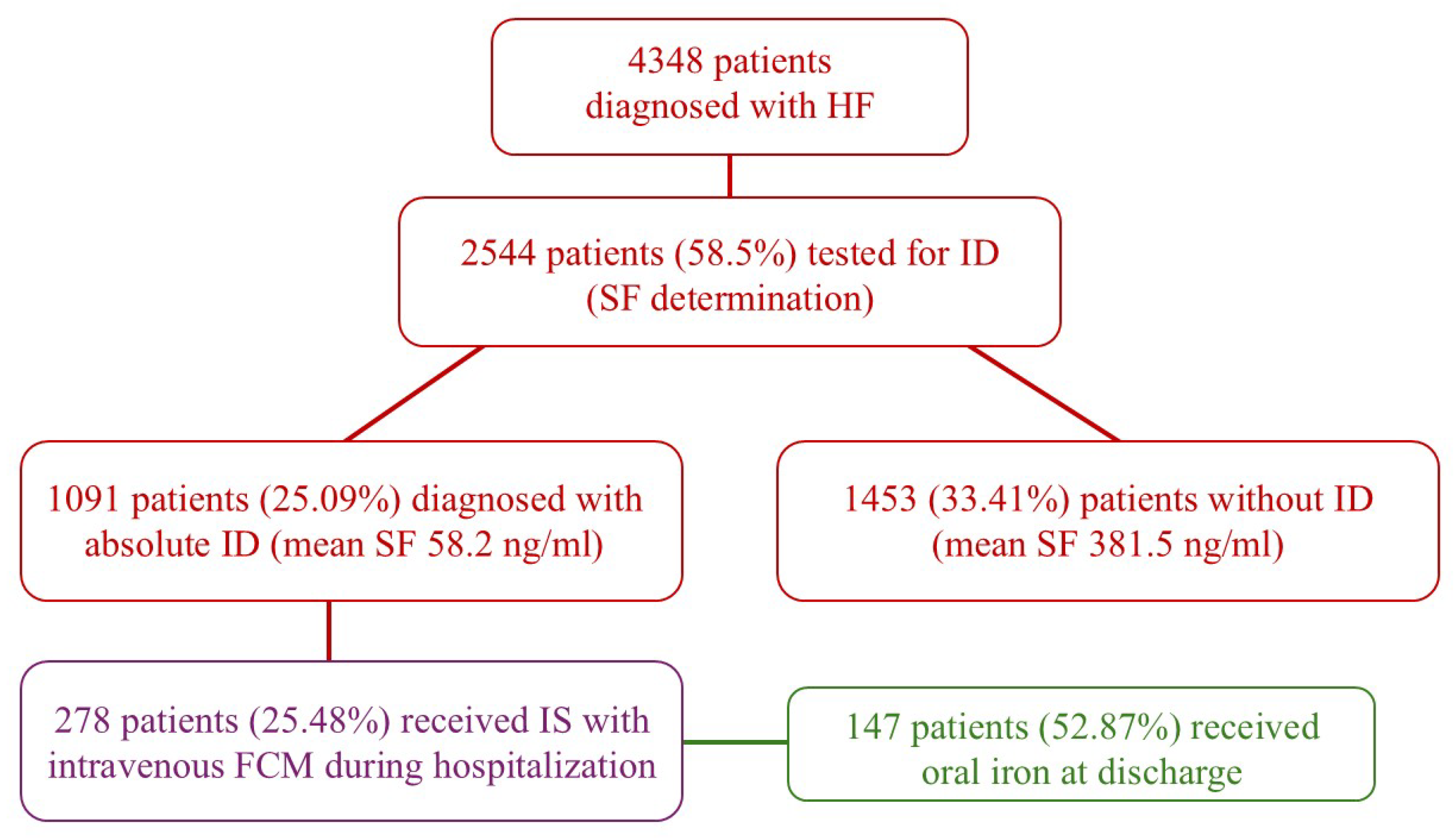
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ID | Iron deficiency |
| HF | Heart failure |
| AHF | Acute heart failure |
| ESC | The European Society of Cardiology |
| SF | Serum ferritin |
| TSAT | Transferrin saturation |
| IS | Iron supplementation |
| FCM | Ferric carboxymaltose |
| LVEF | Left ventricular ejection fraction |
| QoL | Quality of life |
| NYHA | New York Heart Association |
| HFrEF | Reduced ejection fraction |
| HFmrEF | Mildly reduced ejection fraction |
| HFpEF | Preserved ejection fraction |
| IL-6 | Interleukin-6 |
| EF | Ejection fraction |
| LVSD | Left ventricular systolic diameter |
| SVSD | Left ventricular septal thickness |
| RVEDD | Right ventricular end-diastolic diameter |
| LVEDD | Left ventricular end-systolic diameter |
References
- Sindone, A.; Doehner, W.; Manito, N.; McDonagh, T.; Cohen-Solal, A.; Damy, T.; Núñez, J.; Pfister, O.; van der Meer, P.; Comin-Colet, J. Practical Guidance for Diagnosing and Treating Iron Deficiency in Patients with Heart Failure: Why, Who and How? J. Clin. Med. 2022, 11, 2976. [Google Scholar] [CrossRef]
- Del Pinto, R.; Ferri, C. Iron deficiency in heart failure: Diagnosis and clinical implications. Eur. Heart J. Suppl. 2022, 24, I96–I99. [Google Scholar] [CrossRef]
- Sirbu, O. Anemia in heart failure—from guidelines to controversies and challenges. Anatol. J. Cardiol. 2018, 20, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Sirbu, O.; Sorodoc, V.; Jaba, I.M.; Floria, M.; Stoica, A.; Profire, L.; Tuchilus, C.; Rusu, G.; Sorodoc, L. The Influence of Cardiovascular Medications on Iron Metabolism in Patients with Heart Failure. Medicina 2019, 55, 329. [Google Scholar] [CrossRef] [PubMed]
- Beavers, C.J.; Ambrosy, A.P.; Butler, J.; Davidson, B.T.; Gale, S.E.; Piña, I.L.; Mastoris, I.; Reza, N.; Mentz, R.J.; Lewis, G.D. Iron Deficiency in Heart Failure: A Scientific Statement from the Heart Failure Society of America. J. Card. Fail. 2023, 29, 1059–1077. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Nutritional Anaemias. Report of a WHO Scientific Group. World Health Organ. Tech. Rep. Ser. 1968, 405, 5–37.
- von Haehling, S.; Ebner, N.; Evertz, R.; Ponikowski, P.; Anker, S.D. Iron Deficiency in Heart Failure. JACC Heart Fail. 2019, 7, 36–46. [Google Scholar] [CrossRef]
- Loncar, G.; Obradovic, D.; Thiele, H.; von Haehling, S.; Lainscak, M. Iron deficiency in heart failure. ESC Heart Fail. 2021, 8, 2368–2379. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Usman, M.S.; von Haehling, S.; Doehner, W.; Coats, A.J.S. Ferric carboxymaltose for the treatment of iron-deficient heart failure patients: A systematic review and meta-analysis. ESC Heart Fail. 2020, 7, 3392–3400. [Google Scholar] [CrossRef] [PubMed]
- Becher, P.M.; Schrage, B.; Benson, L.; Fudim, M.; Cabrera, C.C.; Dahlström, U.; Rosano, G.M.; Jankowska, E.A.; Anker, S.D.; Lund, L.H.; et al. Phenotyping heart failure patients for iron deficiency and use of intravenous iron therapy: Data from the Swedish Heart Failure Registry. Eur. J. Heart Fail. 2021, 23, 1844–1854. [Google Scholar] [CrossRef]
- Cohen-Solal, A.; Damy, T.; Terbah, M.; Kerebel, S.; Baguet, J.; Hanon, O.; Zannad, F.; Laperche, T.; Leclercq, C.; Concas, V.; et al. High prevalence of iron deficiency in patients with acute decompensated heart failure. Eur. J. Heart Fail. 2014, 16, 984–991. [Google Scholar] [CrossRef]
- Rocha, B.M.; Cunha, G.J.; Falcão, L.F.M. The Burden of Iron Deficiency in Heart Failure. JACC 2018, 71, 782–793. [Google Scholar] [CrossRef]
- Miñana, G.; Lorenzo, M.; Llàcer, P.; Núñez, E.; Palau, P.; Núñez, J. Iron deficiency testing in acute heart failure. Much to do. REC CardioClinics 2022, 57, 296–299. [Google Scholar] [CrossRef]
- Pezel, T.; Audureau, E.; Mansourati, J.; Baudry, G.; Ben Driss, A.; Durup, F.; Fertin, M.; Godreuil, C.; Jeanneteau, J.; Kloeckner, M.; et al. Diagnosis and Treatment of Iron Deficiency in Heart Failure: OFICSel study by the French Heart Failure Working Group. ESC Heart Fail. 2021, 8, 1509–1521. [Google Scholar] [CrossRef]
- Juillière, Y.; Suty-Selton, C.; Riant, E.; Darracq, J.-P.; Dellinger, A.; Labarre, J.-P.; Druelle, J.; Mulak, G.; Danchin, N.; Jourdain, P. Prescription of cardiovascular drugs in the French ODIN cohort of heart failure patients according to age and type of chronic heart failure. Arch. Cardiovasc. Dis. 2013, 107, 21–32. [Google Scholar] [CrossRef][Green Version]
- Cleland, J.; Cohen-Solal, A.; Aguilar, J.C.; Dietz, R.; Eastaugh, J.; Follath, F.; Freemantle, N.; Gavazzi, A.; van Gilst, W.; Hobbs, F.; et al. Management of heart failure in primary care (the IMPROVEMENT of Heart Failure Programme): An international survey. Lancet 2002, 360, 1631–1639. [Google Scholar] [CrossRef]
- Cleland, J.; Daubert, J.; Erdmann, E.; Freemantle, N.; Gras, D.; Kappenberger, L.; Klein, W.; Tavazzi, L. On behalf of the CARE-HF study Steering Committee and Investigators Baseline characteristics of patients recruited into the CARE-HF study. Eur. J. Heart Fail. 2005, 7, 205–214. [Google Scholar] [CrossRef] [PubMed]
- de Groote, P.; Isnard, R.; Assyag, P.; Clerson, P.; Ducardonnet, A.; Galinier, M.; Jondeau, G.; Leurs, I.; Thébaut, J.; Komajda, M. Is the gap between guidelines and clinical practice in heart failure treatment being filled? Insights from the IMPACT RECO survey. Eur. J. Heart Fail. 2007, 9, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- de Groote, P.; Isnard, R.; Clerson, P.; Jondeau, G.; Galinier, M.; Assyag, P.; Demil, N.; Ducardonnet, A.; Thebaut, J.; Komajda, M. Improvement in the management of chronic heart failure since the publication of the updated guidelines of the European Society of Cardiology. Eur. J. Heart Fail. 2009, 11, 85–91. [Google Scholar] [CrossRef]
- Alnuwaysir, R.I.S.; Hoes, M.F.; van Veldhuisen, D.J.; van der Meer, P.; Beverborg, N.G. Iron Deficiency in Heart Failure: Mechanisms and Pathophysiology. J. Clin. Med. 2021, 11, 125. [Google Scholar] [CrossRef] [PubMed]
- Crooks, C.J.; West, J.; Card, T.R. Comorbidities Affect Risk of Nonvariceal Upper Gastrointestinal Bleeding. Gastroenterology 2013, 144, 1384–1393.e2. [Google Scholar] [CrossRef]
- Abraham, N.S. Gastrointestinal bleeding in cardiac patients. Curr. Opin. Gastroenterol. 2014, 30, 609–614. [Google Scholar] [CrossRef]
- Lin, C.-C.; Hu, H.-Y.; Luo, J.-C.; Peng, Y.-L.; Hou, M.-C.; Lin, H.-C.; Lee, F.-Y. Risk factors of gastrointestinal bleeding in clopidogrel users: A nationwide population-based study. Aliment. Pharmacol. Ther. 2013, 38, 1119–1128. [Google Scholar] [CrossRef]
- Martens, P.; Minten, L.; Dupont, M.; Mullens, W. Prevalence of underlying gastrointestinal malignancies in iron-deficient heart failure. ESC Heart Fail. 2018, 6, 37–44. [Google Scholar] [CrossRef]
- Şorodoc, V.; Asaftei, A.; Puha, G.; Ceasovschih, A.; Lionte, C.; Sîrbu, O.; Bologa, C.; Haliga, R.E.; Constantin, M.; Coman, A.E.; et al. Management of Hyponatremia in Heart Failure: Practical Considerations. J. Pers. Med. 2023, 13, 140. [Google Scholar] [CrossRef]
- Sostres, C.; Lanas, A. Gastrointestinal effects of aspirin. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, F.; Lund, L.H.; Benson, L.; Linde, C.; Orsini, N.; Carrero, J.J.; Savarese, G. Iron deficiency in heart failure: Screening, prevalence, incidence and outcome data from the Swedish Heart Failure Registry and the Stockholm CREAtinine Measurements collaborative project. Eur. J. Heart Fail. 2023, 25, 1270–1280. [Google Scholar] [CrossRef]
- Martens, P.; Nijst, P.; Verbrugge, F.H.; Smeets, K.; Dupont, M.; Mullens, W. Impact of iron deficiency on exercise capacity and outcome in heart failure with reduced, mid-range and preserved ejection fraction. Acta Cardiol. 2017, 73, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Bekfani, T.; Pellicori, P.; Morris, D.; Ebner, N.; Valentova, M.; Sandek, A.; Doehner, W.; Cleland, J.G.; Lainscak, M.; Schulze, P.C.; et al. Iron deficiency in patients with heart failure with preserved ejection fraction and its association with reduced exercise capacity, muscle strength and quality of life. Clin. Res. Cardiol. 2019, 108, 203–211. [Google Scholar] [CrossRef]
- Beale, A.L.; Warren, J.L.; Roberts, N.; Meyer, P.; Townsend, N.P.; Kaye, D. Iron deficiency in heart failure with preserved ejection fraction: A systematic review and meta-analysis. Open Heart 2019, 6, e001012. [Google Scholar] [CrossRef]
- Ibrahim, N.E.; Burnett, J.C.; Butler, J.; Camacho, A.; Felker, G.M.; Fiuzat, M.; O’cOnnor, C.; Solomon, S.D.; Vaduganathan, M.; Zile, M.R.; et al. Natriuretic Peptides as Inclusion Criteria in Clinical Trials. JACC Heart Fail. 2020, 8, 347–358. [Google Scholar] [CrossRef]
- Jhund, P.S.; Claggett, B.L.; Voors, A.A.; Zile, M.R.; Packer, M.; Pieske, B.M.; Kraigher-Krainer, E.; Shah, A.M.; Prescott, M.F.; Shi, V.; et al. Elevation in High-Sensitivity Troponin T in Heart Failure and Preserved Ejection Fraction and Influence of Treatment With the Angiotensin Receptor Neprilysin Inhibitor LCZ696. Circ. Heart Fail. 2014, 7, 953–959. [Google Scholar] [CrossRef]
- Thomas, L.; Marwick, T.H.; Popescu, B.A.; Donal, E.; Badano, L.P. Left Atrial Structure and Function, and Left Ventricular Diastolic Dysfunction: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 1961–1977. [Google Scholar] [CrossRef]
- Iyngkaran, P.; Thomas, M.; Horowitz, J.D.; Komesaroff, P.; Jelinek, M.; Hare, D.L. Common Comorbidities that Alter Heart Failure Prognosis—Shaping New Thinking for Practice. Curr. Cardiol. Rev. 2021, 17, 1–13. [Google Scholar] [CrossRef]
- Gigli, L.; Ameri, P.; Secco, G.; De Blasi, G.; Miceli, R.; Lorenzoni, A.; Torre, F.; Chiarella, F.; Brunelli, C.; Canepa, M. Clinical characteristics and prognostic impact of atrial fibrillation in patients with chronic heart failure. World J. Cardiol. 2016, 8, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wang, L.; Kurlansky, P.A.; Schein, J.; Baser, O.; Berger, J.S. Cardiovascular outcomes among elderly patients with heart failure and coronary artery disease and without atrial fibrillation: A retrospective cohort study. BMC Cardiovasc. Disord. 2019, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, E895–E1032. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Hemoglobin Level | p Value for Chi- Square Test/ Fisher’s Exact Test | ||
|---|---|---|---|---|
| ≤12 g/dL (n = 263) | >12 g/dL (n = 15) | Study Group (n = 278) | ||
| Demographic Characteristic | ||||
| Female Sex | 165 (62.7%) | 3 (20.0%) | 168 (60.4%) | 0.001 |
| Age ≥70 years | 189 (71.9%) | 6 (40.0%) | 195 (70.1%) | 0.012 |
| NYHA Class | ||||
| II | 71 (27.0%) | 7 (46.7%) | 78 (28.1%) | 0.045 |
| III | 92 (35.0%) | 7 (46.7%) | 99 (35.6%) | |
| IV | 9 (3.4%) | 0 (0.0%) | 9 (3.2%) | |
| Personal Medical History | ||||
| Aortic Stenosis | 37 (14.1%) | 3 (20.0%) | 40 (14.4%) | 0.369 |
| Myocardial Infarction | 104 (39.5%) | 10 (66.7%) | 114 (41.0%) | 0.036 |
| Chronic Coronary Syndrome | 88 (33.5%) | 4 (28.7%) | 92 (33.1%) | 0.408 |
| Hypertension | 172 (65.4%) | 6 (40.0%) | 178 (64.0%) | 0.045 |
| Atrial Fibrillation | 125 (47.5%) | 6 (40.0%) | 131 (47.1%) | 0.383 |
| Acute Myocardial Infarction | 20 (7.6%) | 2 (13.3%) | 22 (7.9%) | 0.336 |
| Stroke | 30 (11.4%) | 3 (20.0%) | 33 (11.9%) | 0.257 |
| Diabetes Mellitus | 84 (31.9%) | 4 (26.7%) | 88 (31.7%) | 0.457 |
| Valvular Disease | 122 (46.4%) | 11 (73.3%) | 133 (47.8%) | 0.038 |
| Asthma | 6 (2.3%) | 0 (0.0%) | 6 (2.2%) | 0.715 |
| COPD | 7 (2.7%) | 1 (6.7%) | 8 (2.9%) | 0.362 |
| Malignancy | 77 (29.3%) | 4 (9.7%) | 78 (28.1%) | 0.045 |
| Bleeding | 85 (32.3%) | 1 (6.7%) | 86 (30.9%) | 0.027 |
| Treatment | ||||
| Aspirin | 27 (10.3%) | 1 (6.7%) | 28 (10.1%) | 0.541 |
| Clopidogrel | 18 (7.2%) | 1 (6.7%) | 20 (7.2%) | 0.706 |
| Oral Anticoagulant (OAC) | 109 (41.4%) | 6 (40.0%) | 115 (41.4%) | 0.568 |
| Oral Iron at Discharge | 127 (48.3%) | 4 (26.7%) | 131 (47.1%) | 0.084 |
| Beta-Blockers | 130 (49.4%) | 9 (60.0%) | 139 (50.0%) | 0.298 |
| Calcium Channel Blockers (CCB) | 39(14.8%) | 2 (13.3%) | 41 (14.7%) | 0.615 |
| ACEi/ARB | 79 (30.0%) | 3 (20.0%) | 82 (29.5%) | 0.305 |
| Furosemide | 94 (35.7%) | 5 (33.3%) | 99 (35.6%) | 0.544 |
| Spironolactone | 74 (28.1%) | 5 (33.3%) | 79 (28.4%) | 0.430 |
| Parameter | Ejection Fraction | p-Value for Chi- Square Test/ Likelihood Ratio | ||
|---|---|---|---|---|
| ≤40% (n = 46) | >40% (n = 111) | Study Group (n = 157) | ||
| Demographic characteristic | ||||
| Male sex | 24 (52.2%) | 39 (35.1%) | 63 (40.1%) | 0.036 |
| Age ≥70 years | 32 (69.6%) | 86 (77.5%) | 118 (75.2%) | 0.199 |
| NYHA Class | ||||
| II | 9 (19.6%) | 40 (36.0%) | 49 (31.2%) | 0.001 |
| III | 31 (67.4%) | 38 (34.2%) | 69 (43.9%) | |
| IV | 3 (6.5%) | 3 (2.7%) | 6 (3.8%) | |
| Personal Medical History | ||||
| Aortic Stenosis | 11 (23.9%) | 21 (18.9%) | 32 (20.4%) | 0.485 |
| Myocardial Infarction | 36 (78.3%) | 60 (54.1%) | 96 (61.1%) | 0.004 |
| Chronic Coronary Syndrome | 14 (30.4%) | 38 (34.2%) | 52 (33.1%) | 0.395 |
| Hypertension | 26 (56.5%) | 81 (73.0%) | 107 (68.2%) | 0.035 |
| Atrial fibrillation | 27 (58.7%) | 55 (49.5%) | 82 (52.2%) | 0.193 |
| Acute Myocardial Infarction | 7 (15.2%) | 4 (3.6%) | 11 (7.0%) | 0.015 |
| Stroke | 10 (21.7%) | 9 (8.1%) | 19 (12.1%) | 0.020 |
| Diabetes Mellitus | 13 (28.3%) | 29 (26.1%) | 42 (26.8%) | 0.464 |
| Valvular Disease | 38 (82.6%) | 67 (60.4%) | 105 (66.9%) | 0.005 |
| Asthma | 1 (2.2%) | 2 (1.8%) | 3 (1.9%) | 0.649 |
| COPD | 2 (4.3%) | 3 (2.7%) | 5 (3.2%) | 0.458 |
| Malignancy | 9 (19.6%) | 32 (28.8%) | 41 (26.1%) | 0.158 |
| Bleeding | 8 (17.4%) | 31 (27.9%) | 39 (24.8%) | 0.116 |
| Parameter | Ejection Fraction | p Value for t-Student Test | ||
|---|---|---|---|---|
| EF ≤ 40% (n = 46) | EF > 40% (n = 111) | Study Group (n = 157) | ||
| Laboratory Parameter (Mean ± SD) | ||||
| Hemoglobin (g/dL) | 9.97 ± 2.72 | 8.18 ± 2.48 | 8.71 ± 2.72 | 0.001 |
| Hematocrit (%) | 31.07 ± 7.88 | 25.86 ± 8.37 | 27.38 ± 8.54 | 0.001 |
| White blood cells (μL) | 6557 ± 4360 | 7625 ± 3379 | 7898 ± 3703 | 0.152 |
| Iron (μg/dL) | 48.04 ± 7.38 | 35.63 ± 3.67 | 39.27 ± 42.62 | 0.096 |
| Ferritin (ng/mL) | 57.06 ± 3.97 | 31.19 ± 4.47 | 38.77 ± 6.07 | 0.653 |
| Uric acid (mg/dL) | 5.02 ± 4.33 | 4.26 ± 3.51 | 4.48 ± 3.77 | 0.265 |
| Blood glucose (mg/dL) | 117.15 ± 64.81 | 113.66 ± 57.01 | 114.68 ± 59.21 | 0.738 |
| Total cholesterol (mg/dL) | 108.51 ± 72.63 | 96.77 ± 73.46 | 100.15 ± 73.18 | 0.365 |
| HDL-C (mg/dL) | 23.87 ± 16.80 | 27.01 ± 21.80 | 26.09 ± 20.46 | 0.383 |
| LDL-C (mg/dL) | 62.28 ± 60.27 | 55.52 ± 50.04 | 57.50 ± 53.13 | 0.470 |
| VEM (fL) | 80.95 ± 18.71 | 78.38 ± 22.10 | 79.14 ± 21.13 | 0.490 |
| HEM (pg) | 20.51 ± 12.29 | 20.52 ± 12.66 | 20.52 ± 12.51 | 0.995 |
| CHEM (g/dL) | 29.87 ± 7.66 | 29.36 ± 6.46 | 29.51 ± 6.82 | 0.669 |
| GR (×103/μL) | 3617 ± 962 | 3247 ± 981 | 3356 ± 987 | 0.033 |
| RA (mmol/L) | 22.08 ± 7.76 | 20.84 ± 8.68 | 21.20 ± 9.16 | 0.453 |
| Urea (mg/dL) | 60.03 ± 31.04 | 51.94 ± 26.74 | 54.31 ± 28.21 | 0.102 |
| Creatinine (mg/dL) | 1.16 ± 0.44 | 1.02 ± 0.44 | 1.06 ± 0.46 | 0.086 |
| Creatinine clearance (mL/min) | 63.65 ± 25.06 | 67.09 ± 25.36 | 66.08 ± 25.2 | 0.439 |
| CRP (mg/L) | 2.70 ± 4.41 | 4.03 ± 1.22 | 3.64 ± 0.88 | 0.491 |
| NTproBNP (pg/mL) | 4278 ± 1250 | 2034 ± 350 | 2982 ± 455 | 0.001 |
| BNP (pg/mL) | 577.65 ± 144.38 | 464.83 ± 184.48 | 514.45 ± 170.33 | 0.284 |
| Troponin (ng/L) | 163.31 ± 132.80 | 93.22 ± 52.24 | 113.75 ± 53.46 | 0.048 |
| Cardiovascular Parameter | ||||
| LVEDD (mm) | 53.15 ± 23.55 | 40.10 ± 23.20 | 43.92 ± 23.98 | 0.002 |
| LVESD (mm) | 41.09 ± 21.64 | 27.28 ± 20.18 | 31.32 ± 21.49 | 0.001 |
| RVEDD (mm) | 26.67 ± 15.46 | 21.63 ± 14.79 | 23.13 ± 15.12 | 0.050 |
| IVS (mm) | 10.61 ± 4.10 | 9.60 ± 5.31 | 9.89 ± 5.00 | 0.256 |
| LVPW (mm) | 10.20 ± 3.76 | 8.70 ± 5.66 | 9.13 ± 5.22 | 0.105 |
| Parameter | Ejection Fraction | p-Value for Chi2 Test | ||
|---|---|---|---|---|
| ≤40% (n = 46) | >40% (n = 111) | Study Group (n = 157) | ||
| Treatment | ||||
| Aspirin | 6 (13.0%) | 14 (12.6%) | 20 (12.7%) | 0.941 |
| Clopidogrel | 5 (10.9%) | 9 (8.1%) | 14 (8.9%) | 0.587 |
| Oral Anticoagulant (OAC) | 21 (45.7%) | 47 (42.3%) | 68 (43.3%) | 0.704 |
| Oral Iron at Discharge | 21 (45.7%) | 60 (54.1%) | 81(51.6%) | 0.338 |
| Beta-Blockers | 25 (54.3%) | 65 (58.6%) | 90 (57.3%) | 0.378 |
| Calcium Channel Blockers (CCB) | 8 (17.4%) | 17 (15.3%) | 25 (15.9%) | 0.458 |
| ACEi/ARB | 17 (37.0%) | 30 (27.0%) | 47 (29.9%) | 0.148 |
| Furosemide | 23 (50.0%) | 36 (32.4%) | 59 (37.6%) | 0.030 |
| Spironolactone | 17 (37.0%) | 30 (27.0%) | 47 (29.9%) | 0.148 |
| Parameter | Intravenous Ferric Carboxymaltose (FCM) | p Value for Chi- Square Test/ Likelihood Ratio | |
|---|---|---|---|
| 2 Vials (n = 166) | 1 Vials (n = 112) | ||
| NYHA Class | |||
| II | 45 (29.0%) | 31 (27.7%) | 0.207 |
| III | 53 (34.2%) | 43 (38.4%) | |
| IV | 8 (5.2%) | 1 (0.9%) | |
| History of cardiac disease | |||
| Aortic stenosis | 20 (12.9%) | 19 (17.0%) | 0.356 |
| Myocardial Infarction | 61 (39.4%) | 50 (44.6%) | 0.387 |
| Malignacy | 41 (26.5%) | 34 (30.4%) | 0.484 |
| Bleeding | 40 (25.8%) | 42 (37.5%) | 0.042 |
| Treatment | |||
| Aspirin | 21 (13.5%) | 6 (5.4%) | 0.023 |
| Clopidogrel | 13 (8.4%) | 5 (4.5%) | 0.197 |
| Oral Anticoagulant (OAC) | 60 (38.7%) | 47 (42.0%) | 0.593 |
| Laboratory Parameter (Mean ± SD) | |||
| Hemoglobin (g/dL) | 8.63 ± 3.23 | 8.11 ± 2.59 | 0.607 |
| Hematocrit (%) | 27.20 ± 7.49 | 26.53 ± 8.22 | 0.487 |
| VEM (fL) | 79.04 ± 22.53 | 80.41 ± 17.03 | 0.591 |
| HEM (pg) | 21.89 ± 12.40 | 22.62 ± 9.93 | 0.349 |
| CHEM (g/dL) | 29.13 ± 8.03 | 30.47 ± 4.46 | 0.113 |
| Iron (μg/dL) | 40.24 ± 4.15 | 39.60 ± 4.34 | 0.917 |
| Ferritin (ng/mL) | 58.73 ± 11.24 | 62.15 ± 9.87 | 0.124 |
| NTproBNP (pg/mL) | 2947 ± 511 | 1522 ± 282 | 0.028 |
| Troponin (ng/L) | 307.77 ± 187.81 | 8.00 ± 5.51 | 0.001 |
| EF % | 24.77 ± 1.91 | 26.71 ± 2.38 | 0.523 |
| Weight (kg) | 76.96 ± 24.77 | 69.60 ± 16.00 | 0.055 |
| Parameter | Oral Iron at Discharge | p-Value for Chi- Square Test Likelihood Ratio | ||
|---|---|---|---|---|
| Yes (n = 147) | No (n = 131) | Study group (n = 278) | ||
| Demographic Characteristic | ||||
| Female Sex | 85 (57.8%) | 83 (63.4%) | 168 (60.4%) | 0.206 |
| Age ≥70 years | 102 (69.4%) | 93 (71.0%) | 195 (70.1%) | 0.437 |
| NYHA Class | ||||
| II | 35 (23.8%) | 43 (32.8%) | 78 (28.1%) | 0.349 |
| III | 56 (38.1%) | 43 (32.8%) | 99 (35.6%) | |
| IV | 4 (2.7%) | 5 (3.8%) | 9 (3.2%) | |
| Personal Medical History | ||||
| Aortic Stenosis | 23 (15.6%) | 17 (13.0%) | 40 (14.4%) | 0.526 |
| Myocardial Infarction | 58 (39.5%) | 56 (42.7%) | 114 (41.0%) | 0.577 |
| Chronic Coronary Syndrome | 54 (36.7%) | 38 (29.0%) | 92 (33.1%) | 0.108 |
| Hypertension | 86 (58.5%) | 92 (70.2%) | 178 (64.0%) | 0.028 |
| Atrial Fibrillation | 66 (44.9%) | 65 (49.6%) | 131 (47.1%) | 0.252 |
| Acute Myocardial Infarction | 17 (11.6%) | 5 (3.8%) | 22 (7.9%) | 0.014 |
| Stroke | 11 (7.5%) | 22 (16.8%) | 33 (11.9%) | 0.013 |
| Diabetes Mellitus | 46 (68.7%) | 42 (32.1%) | 88 (31.7%) | 0.496 |
| Valvular Disease | 73 (49.7%) | 60 (45.8%) | 133 (47.8%) | 0.301 |
| Asthma | 5 (3.4%) | 1 (0.8%) | 6 (2.2%) | 0.136 |
| COPD | 6 (4.1%) | 2 (1.5%) | 8 (2.9%) | 0.182 |
| Malignancy | 40 (27.2%) | 38 (29.0%) | 78 (28.1%) | 0.739 |
| Bleeding | 34 (23.1%) | 52 (39.7%) | 86 (30.9%) | 0.003 |
| Parameter | Oral Iron at Discharge | p-Value for t-Student Test | ||
|---|---|---|---|---|
| Yes (n = 147) | No (n = 131) | Study Group (n = 278) | ||
| Laboratory Parameters (Mean ± SD) | ||||
| Hemoglobin (g/dL) | 7.88 ± 2.49 | 8.98 ± 3.24 | 8.71 ± 2.67 | 0.002 |
| Hematocrit (%) | 25.45 ± 7.60 | 27.66 ± 8.11 | 27.38 ± 8.54 | 0.020 |
| White blood cells (μL) | 7918 ± 2697 | 8161 ± 3458 | 8047 ± 3667 | 0.582 |
| Iron (μg/dL) | 34.04 ± 3.37 | 43.88 ± 4.57 | 39.24 ± 2.91 | 0.091 |
| Ferritin (ng/mL) | 44.57 ± 9.98 | 51.92 ± 7.67 | 48.03 ± 9.68 | <0.001 |
| Uric acid (mg/dL) | 4.17 ± 3.85 | 3.67 ± 3.65 | 3.91 ± 3.75 | 0.279 |
| Blood glucose (mg/dL) | 118.17 ± 53.81 | 107.61 ± 64.01 | 112.58 ± 59.55 | 0.738 |
| Total cholesterol (mg/dL) | 135.75 ± 49.07 | 139.25 ± 46.92 | 137.45 ± 47.92 | 0.633 |
| HDL-C (mg/dL) | 37.28 ± 13.22 | 39.51 ± 14.20 | 38.35 ± 13.70 | 0.305 |
| LDL-C (mg/dL) | 84.97 ± 42.81 | 87.21 ± 38.29 | 86.04 ± 40.60 | 0.734 |
| Sodium (mEq/L) | 135.77 ± 17.54 | 134.30 ± 23.01 | 134.99 ± 20.59 | 0.553 |
| Potassium (mEq/L) | 4.43 ± 0.89 | 4.27 ± 1.06 | 4.35 ± 0.98 | 0.174 |
| VEM (fL) | 80.80 ± 17.22 | 78.94 ± 22.56 | 79.81 ± 20.22 | 0.445 |
| HEM (pg) | 21.46 ± 11.49 | 22.02 ± 11.35 | 21.75 ± 11.40 | 0.683 |
| CHEM (g/dL) | 29.94 ± 5.31 | 29.39 ± 7.69 | 29.65 ± 6.67 | 0.499 |
| GR (×103/μL) | 3158 ± 897 | 3303 ± 1017 | 3235 ± 964 | 0.212 |
| RA (mmol/L) | 21.26 ± 8.58 | 19.35 ± 10.43 | 20.24 ± 9.64 | 0.104 |
| Urea (mg/dL) | 54.87 ± 32.54 | 57.46 ± 35.95 | 56.24 ± 34.35 | 0.532 |
| Creatinine (mg/dL) | 1.03 ± 0.44 | 1.07 ± 0.55 | 1.06 ± 0.50 | 0.608 |
| Creatinine clearance (mL/min) | 68.35 ± 24.79 | 66.81 ± 32.78 | 67.54 ± 29.25 | 0.663 |
| CRP (mg/L) | 2.81 ± 4.33 | 4.46 ± 0.97 | 3.68 ± 0.54 | 0.029 |
| NTproBNP (pg/mL) | 2345 ± 453 | 2238 ± 426 | 2288 ± 310 | 0.046 |
| Troponin (ng/L) | 60.18 ± 38.38 | 272.81 ± 195.51 | 172.25 ± 104.66 | 0.011 |
| EF% | 47.09 ± 11.67 | 44.41 ± 11.80 | 45.79 ± 11.77 | 0.155 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirbu, O.; Tirnoveanu, A.; Haliga, R.E.; Sorodoc, V.; Sava, M.; Bologa, C.; Petris, O.R.; Morarasu, B.C.; Diaconu, A.D.; Ceasovschih, A.; et al. Iron Deficiency in Heart Failure: From ESC Guidelines to Clinical Practice at a Romanian Hospital. Biomedicines 2025, 13, 2296. https://doi.org/10.3390/biomedicines13092296
Sirbu O, Tirnoveanu A, Haliga RE, Sorodoc V, Sava M, Bologa C, Petris OR, Morarasu BC, Diaconu AD, Ceasovschih A, et al. Iron Deficiency in Heart Failure: From ESC Guidelines to Clinical Practice at a Romanian Hospital. Biomedicines. 2025; 13(9):2296. https://doi.org/10.3390/biomedicines13092296
Chicago/Turabian StyleSirbu, Oana, Andreea Tirnoveanu, Raluca Ecaterina Haliga, Victorita Sorodoc, Miruna Sava, Cristina Bologa, Ovidiu Rusalim Petris, Bianca Codrina Morarasu, Alexandra Diana Diaconu, Alexandr Ceasovschih, and et al. 2025. "Iron Deficiency in Heart Failure: From ESC Guidelines to Clinical Practice at a Romanian Hospital" Biomedicines 13, no. 9: 2296. https://doi.org/10.3390/biomedicines13092296
APA StyleSirbu, O., Tirnoveanu, A., Haliga, R. E., Sorodoc, V., Sava, M., Bologa, C., Petris, O. R., Morarasu, B. C., Diaconu, A. D., Ceasovschih, A., Lionte, C., Morariu, P. C., Morariu, B. A., Statescu, C., Sascau, R. A., Floria, M., & Sorodoc, L. (2025). Iron Deficiency in Heart Failure: From ESC Guidelines to Clinical Practice at a Romanian Hospital. Biomedicines, 13(9), 2296. https://doi.org/10.3390/biomedicines13092296











