L-Citrulline Improves Recovery of Enterocytes While Not Affecting Gut Microbiota in an In Vitro Model of Chemotherapy-Induced Gastrointestinal Mucositis
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Bacterial Cultures
2.3. Chemical Compounds
2.4. xCELLigence Real-Time Cell Analysis
2.5. Biotek Synergy HT Microplate Reading
2.6. Statistical Analysis
3. Results
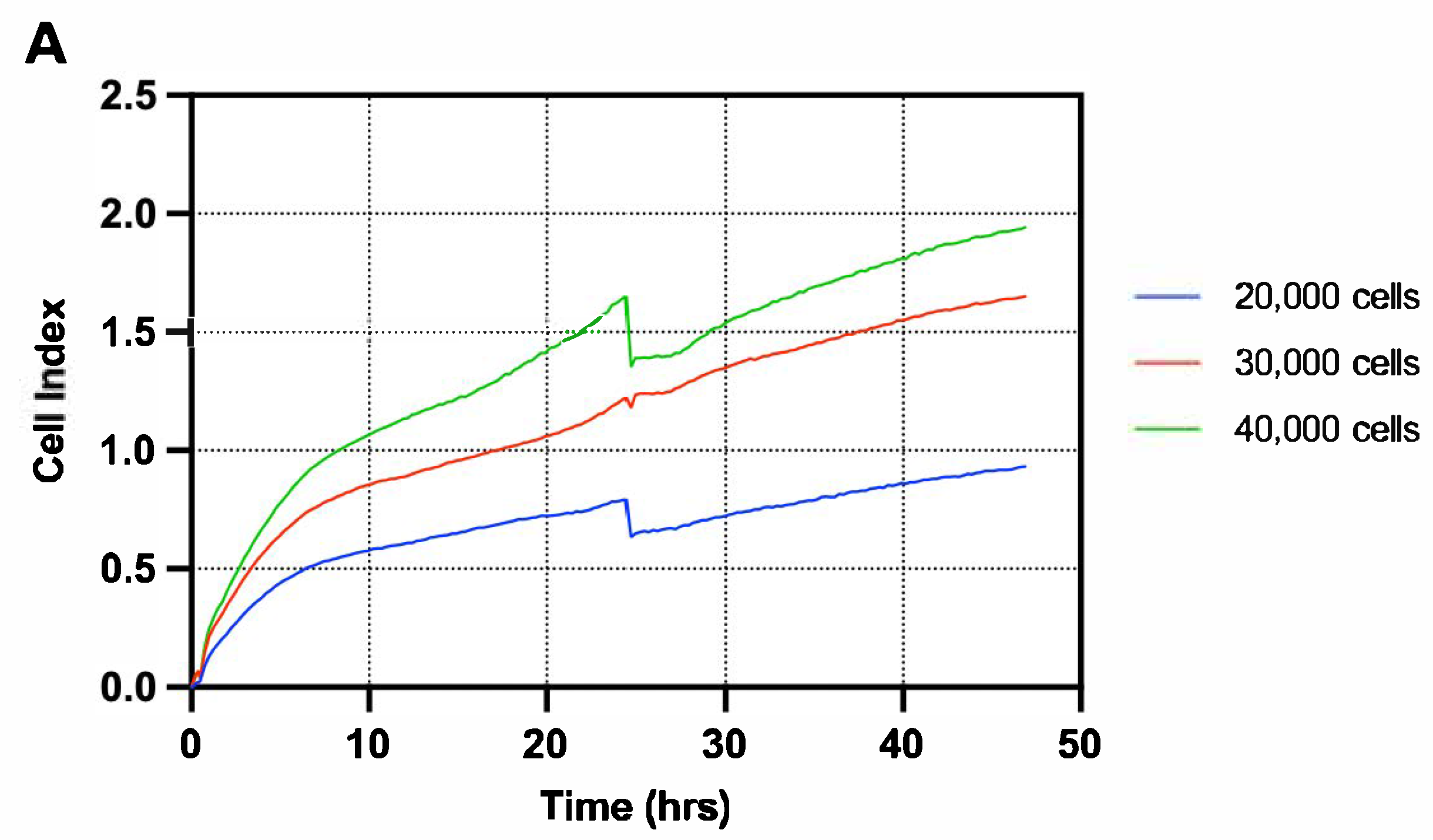
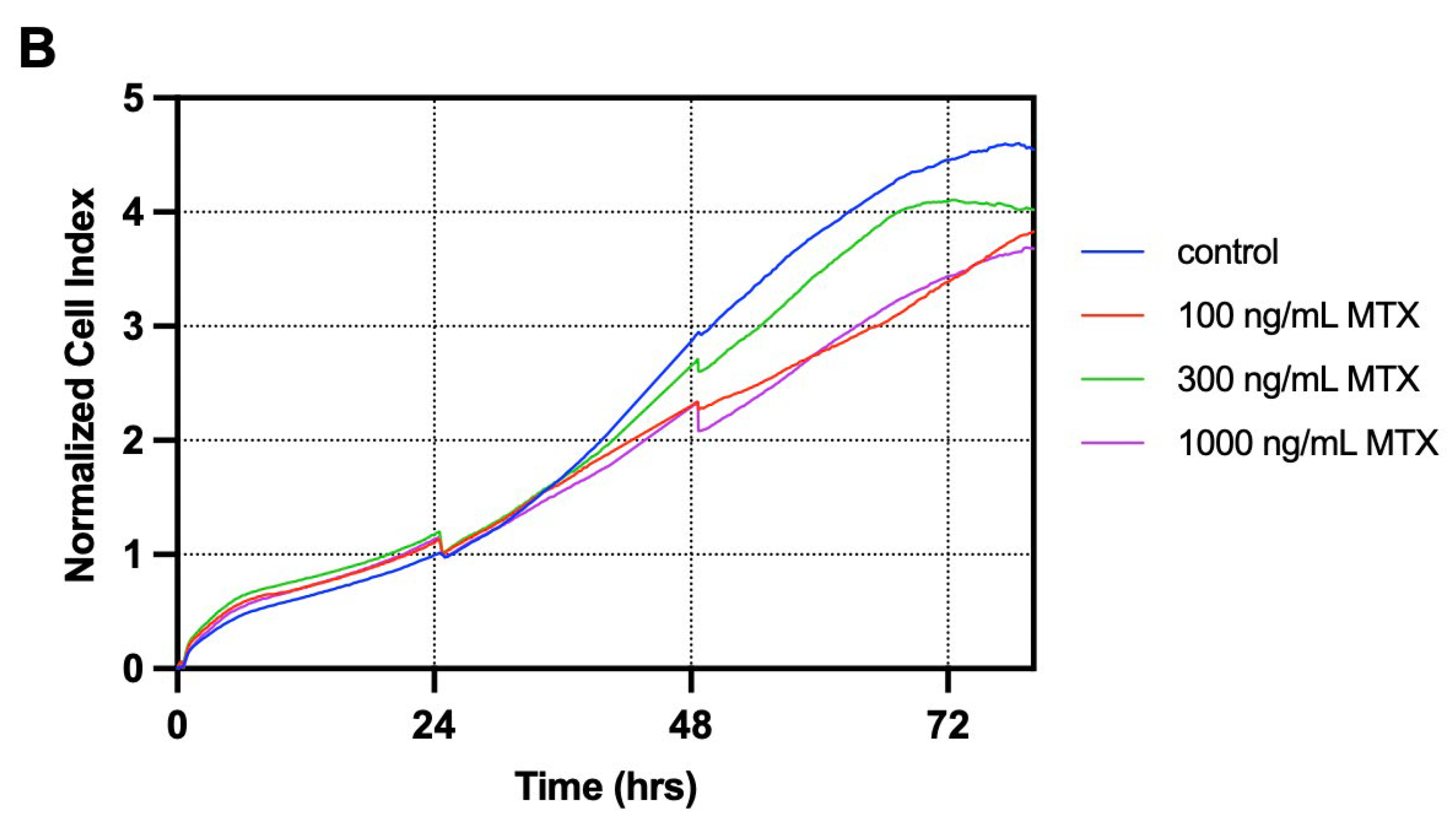
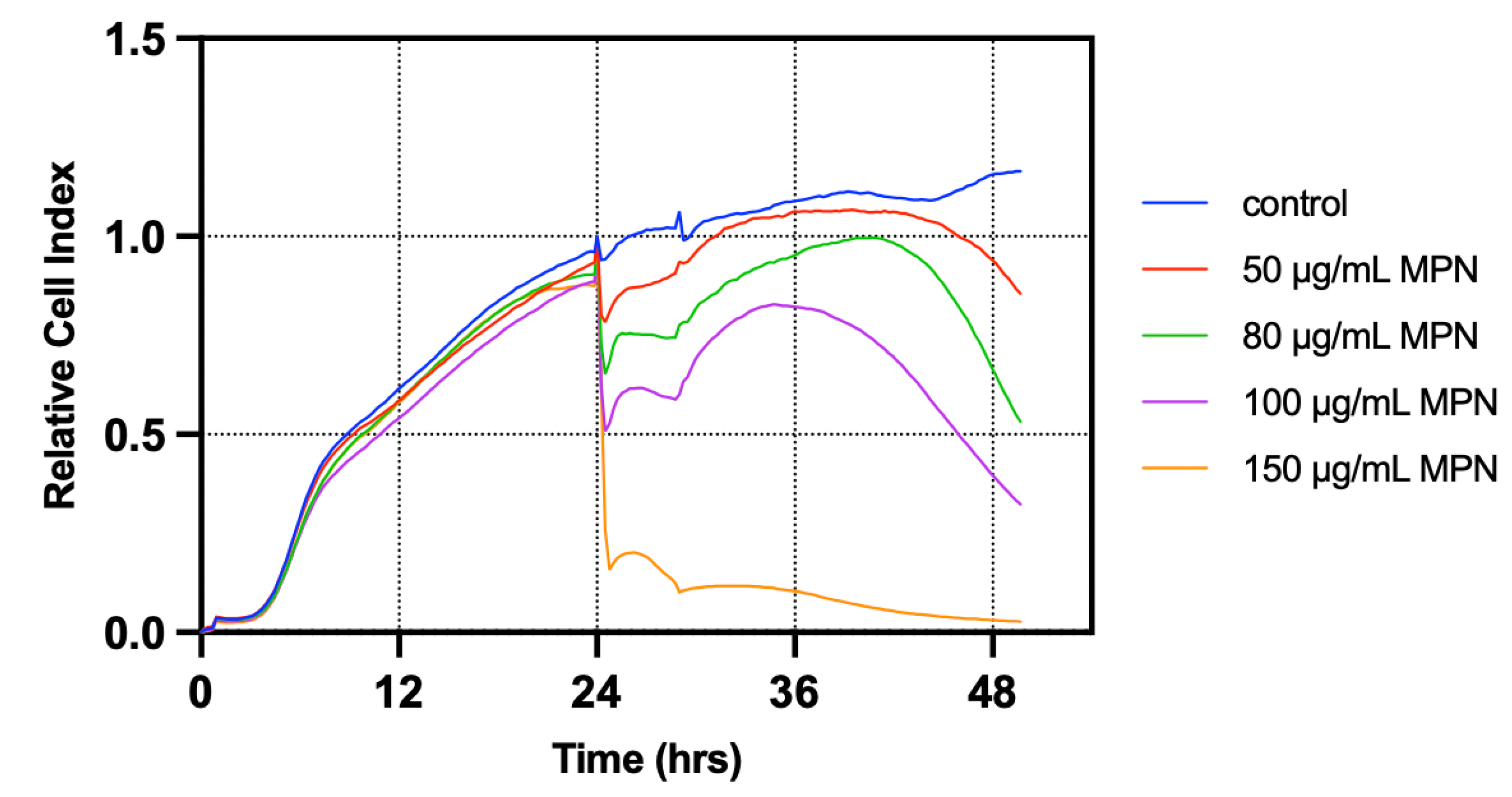
3.1. T84 Cells Treated with Melphalan Constituted the Most Suitable Model for Investigating GIM In Vitro
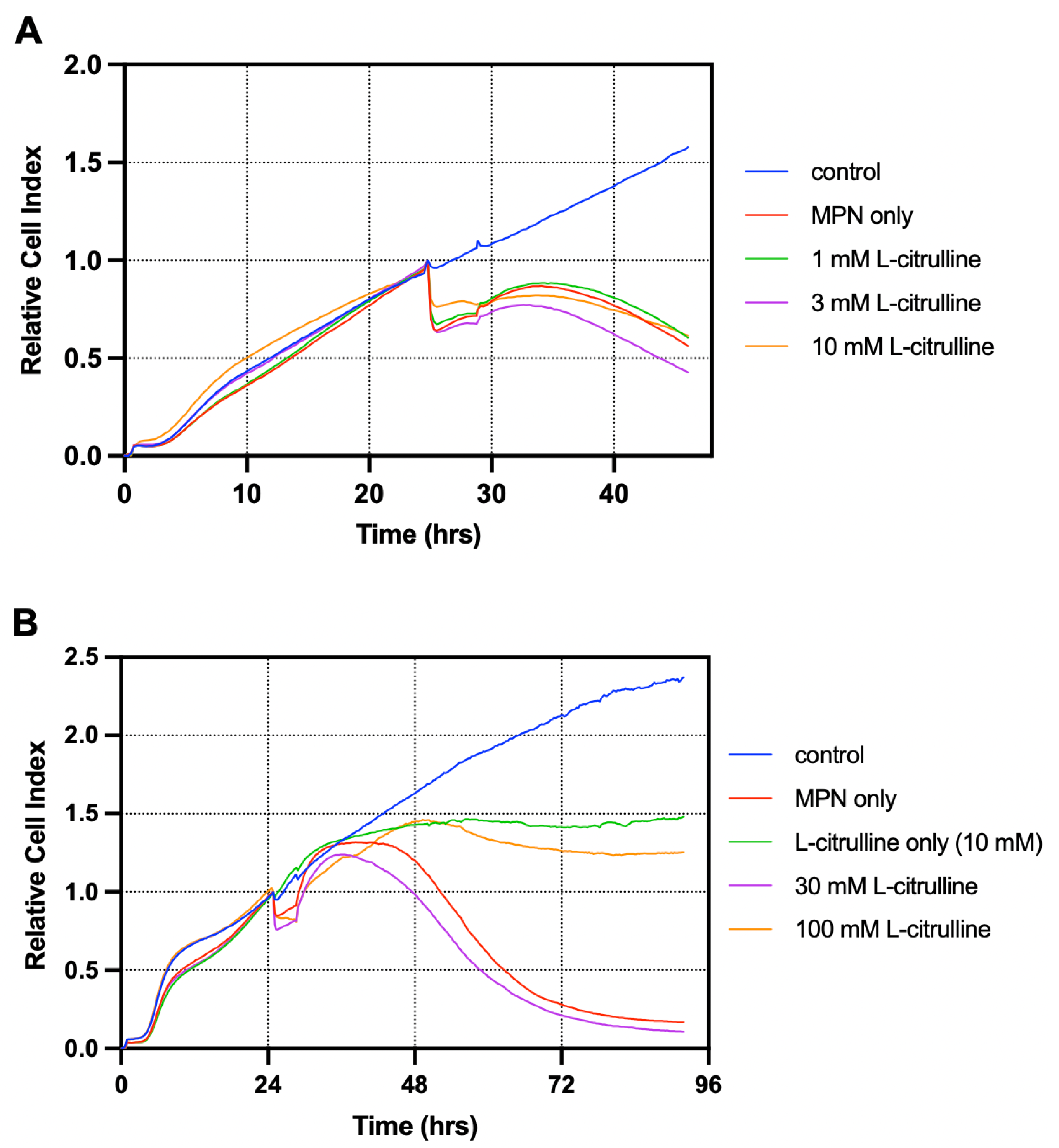
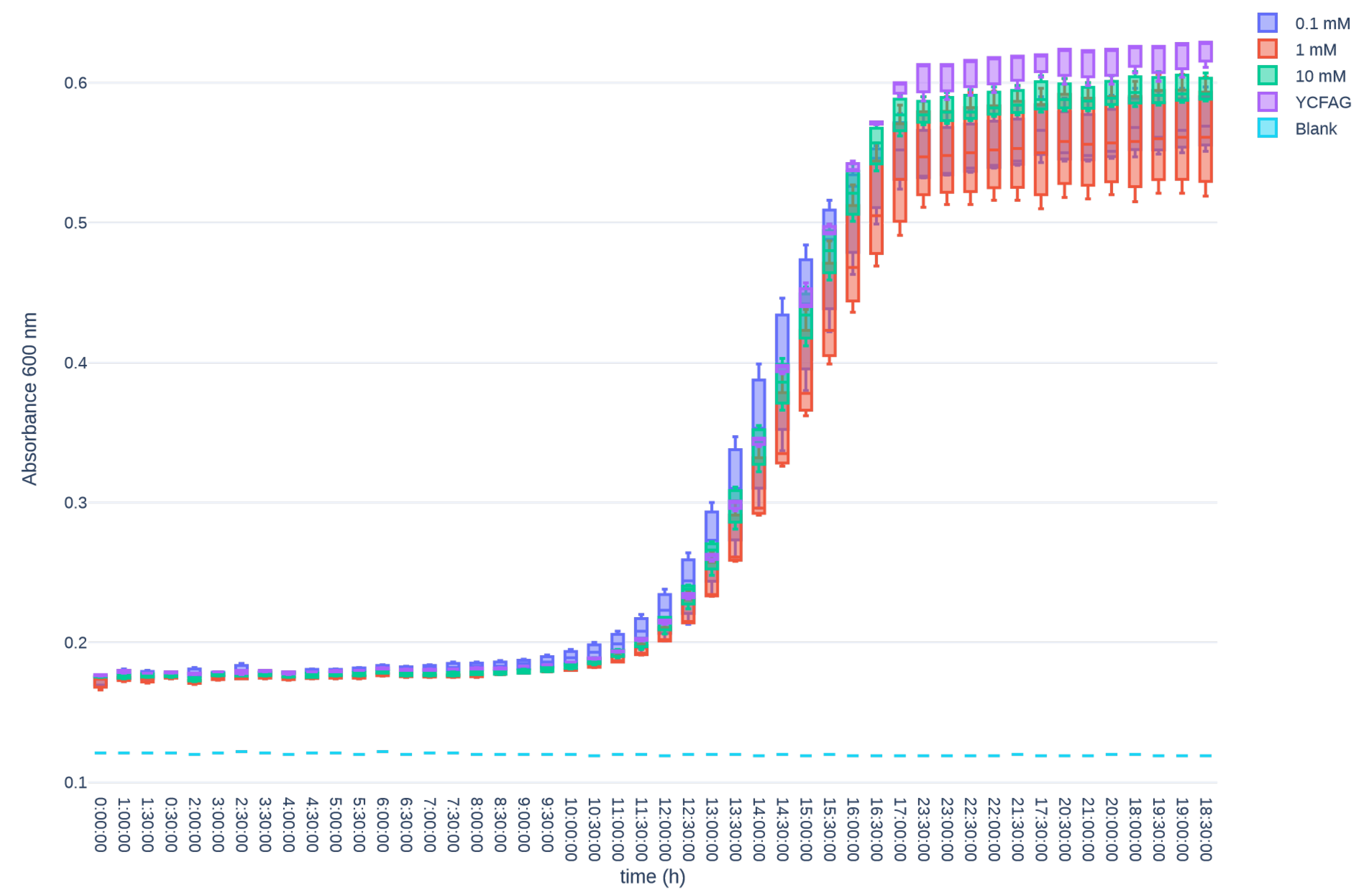
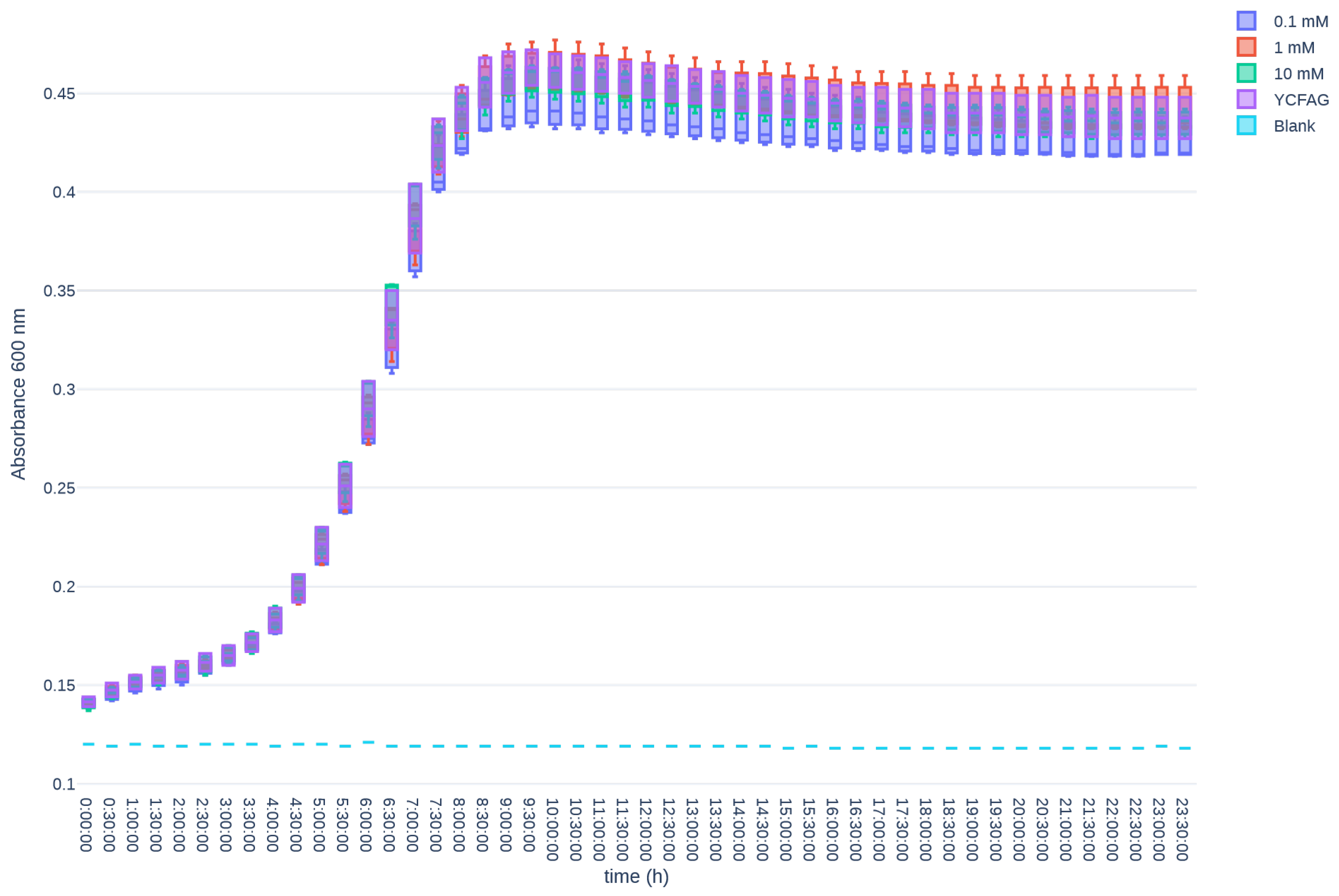
3.2. L-Citrulline Supplementation Improved Cell Recovery in a T84 Cell-Based Model of GIM
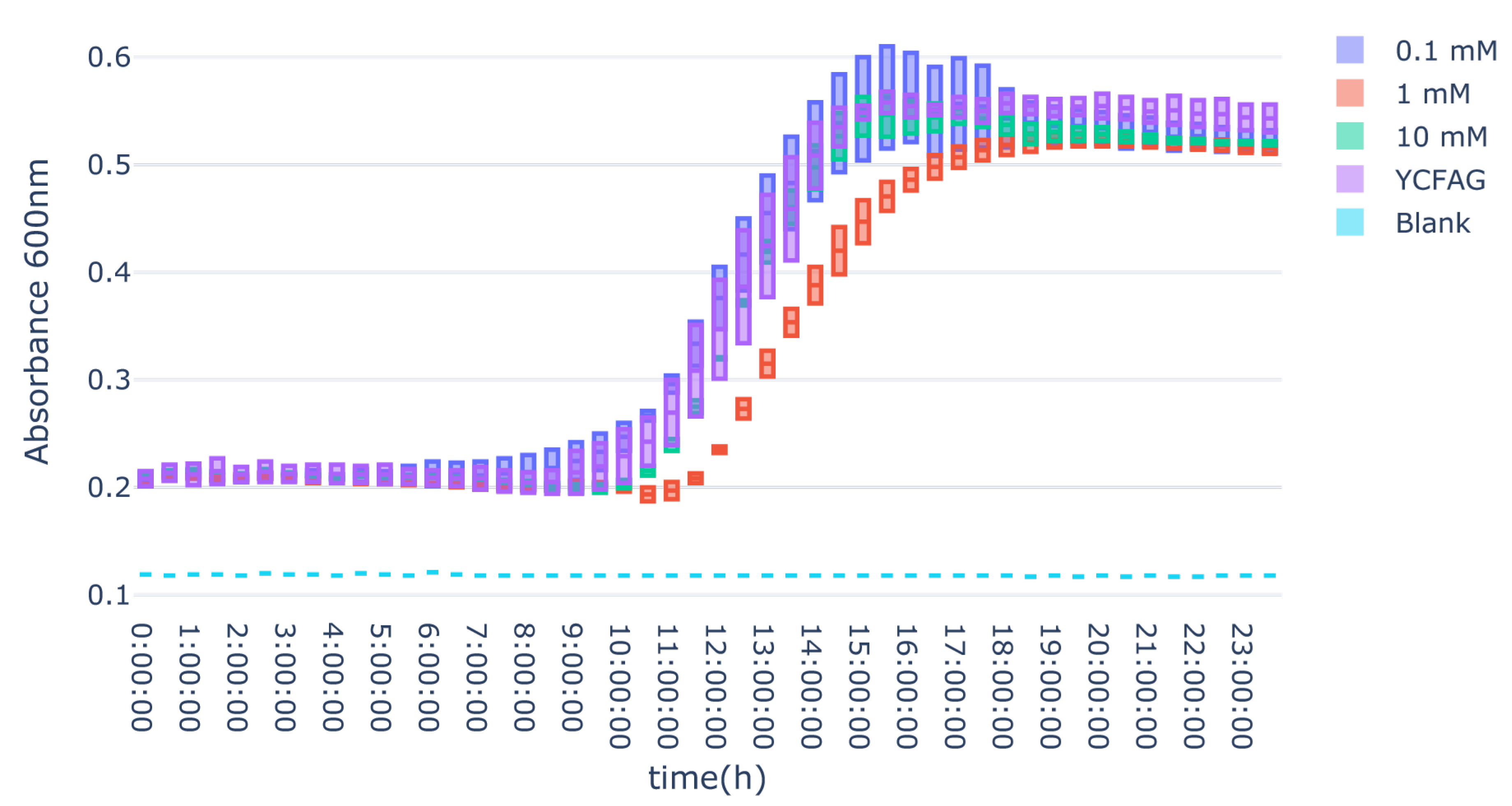
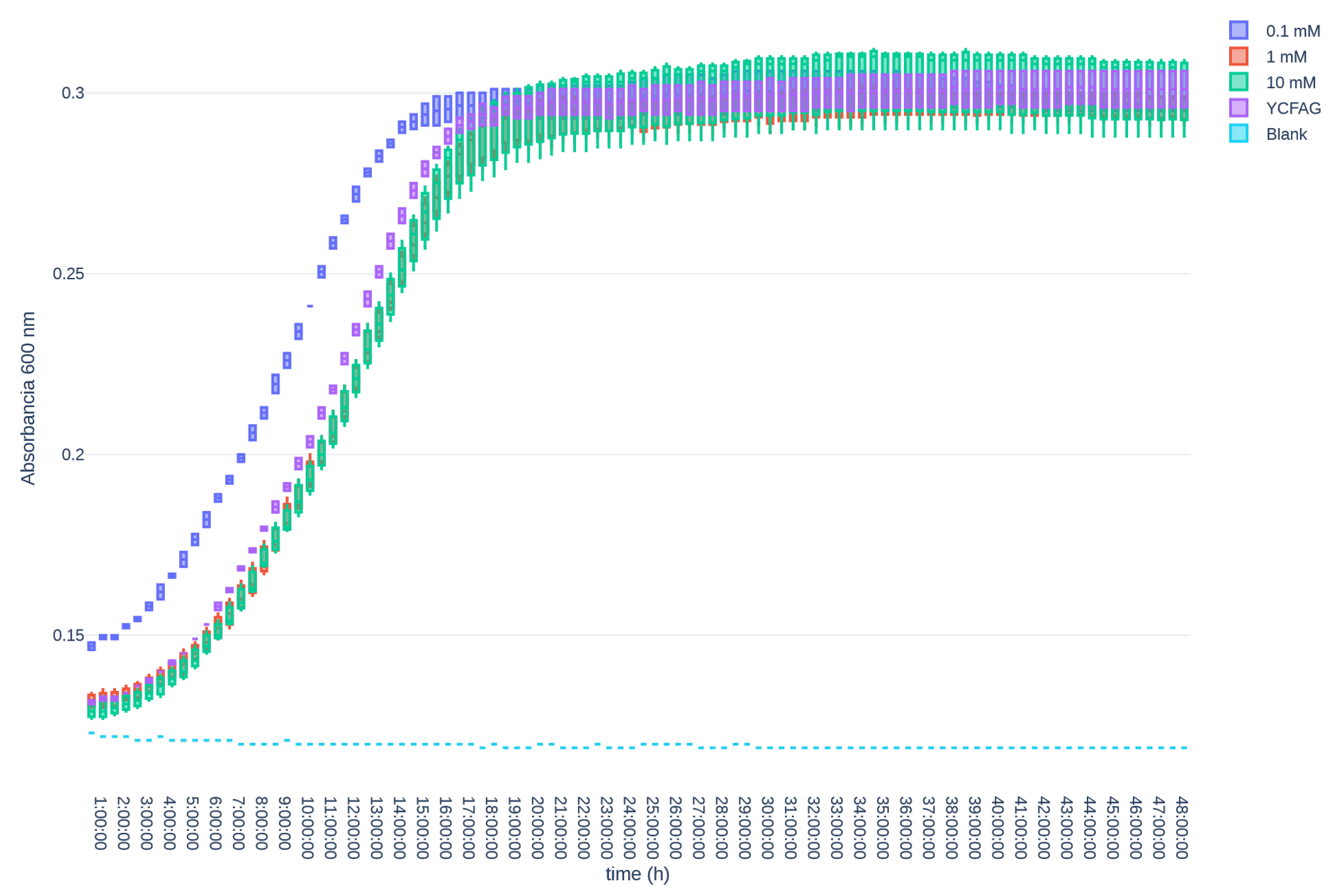
3.3. L-Citrulline Supplementation Did Not Affect the Growth Rate of Selected Gut Bacteria
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Component | Concentration |
|---|---|
| Glucose | 4.5 |
| Casitone | 10 |
| Yeast Extract | 2.5 |
| Sodium Bicarbonate | 4 |
| Dipotassium Hydrogen Phosphate | 0.45 |
| Potassium Dihydrogen Phosphate | 0.45 |
| Sodium Chloride | 0.9 |
| Magnesium (II) Sulfate Heptahydrate | 0.09 |
| Calcium Chloride Dihydrate | 0.12 |
| Sodium Acetate | 2.7 |
| Cysteine | 1 |
| Resazurin (0.02%) | 5 mL |
| Hemin (0.2%) | 5 mL |
| Valerate (≥99% purity) | 100 µL |
| Propionate (≥99% purity) | 600 µL |
| Isobutyrate (≥99% purity) | 100 µL |
| Pink Vitamin Mixture | 1 mL |
| Yellow Vitamin Mixture | 1 mL |
| Pink Vitamin Mixture | Concentration (mg/L) |
| Biotin | 1 |
| Cobalamin | 1 |
| p-Aminobenzoic Acid | 3 |
| Folic Acid | 5 |
| Yellow Vitamin Mixture | Concentration (mg/L) |
| Thiamine | 5 |
| Riboflavin | 5 |
Appendix B



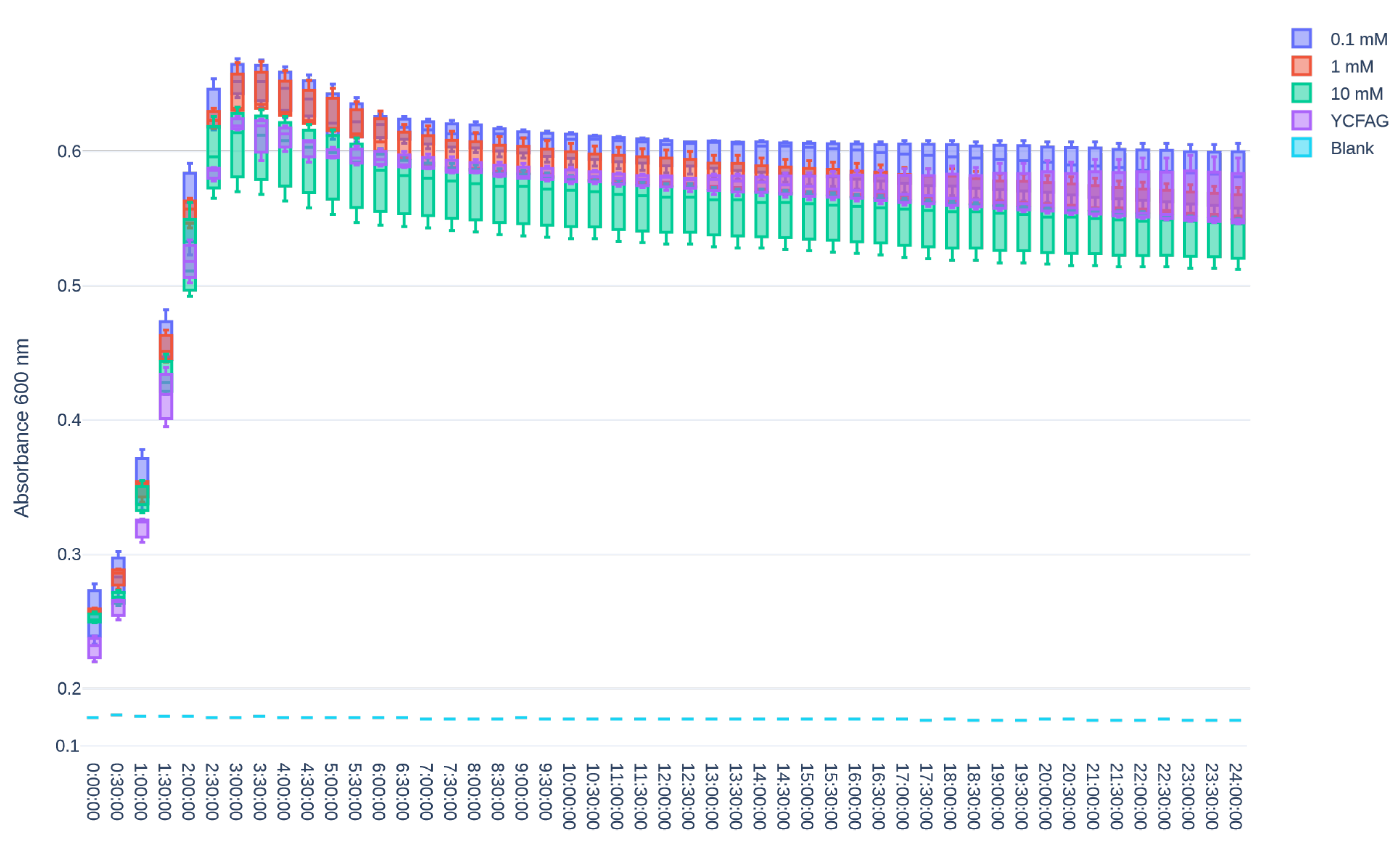
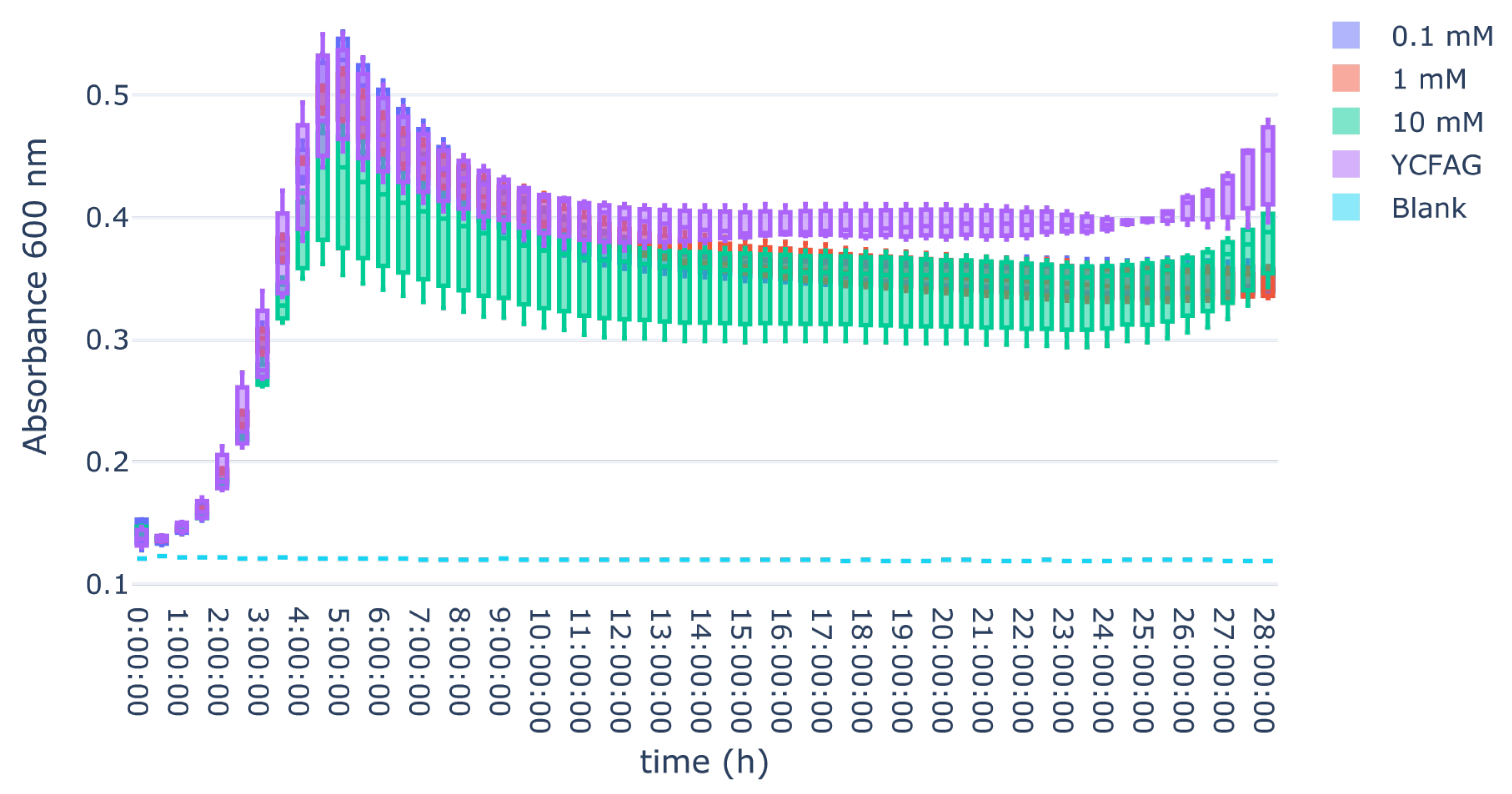
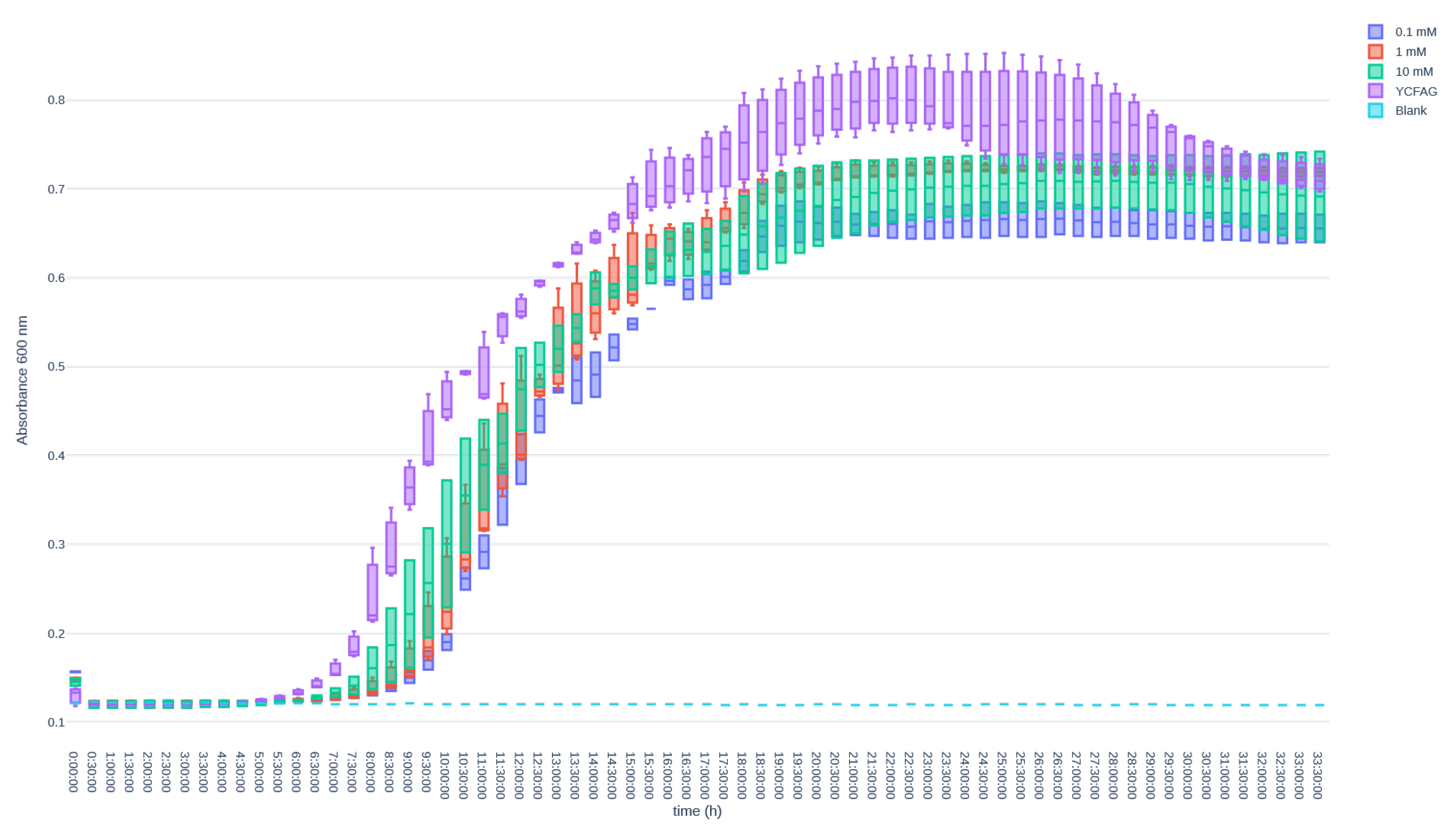
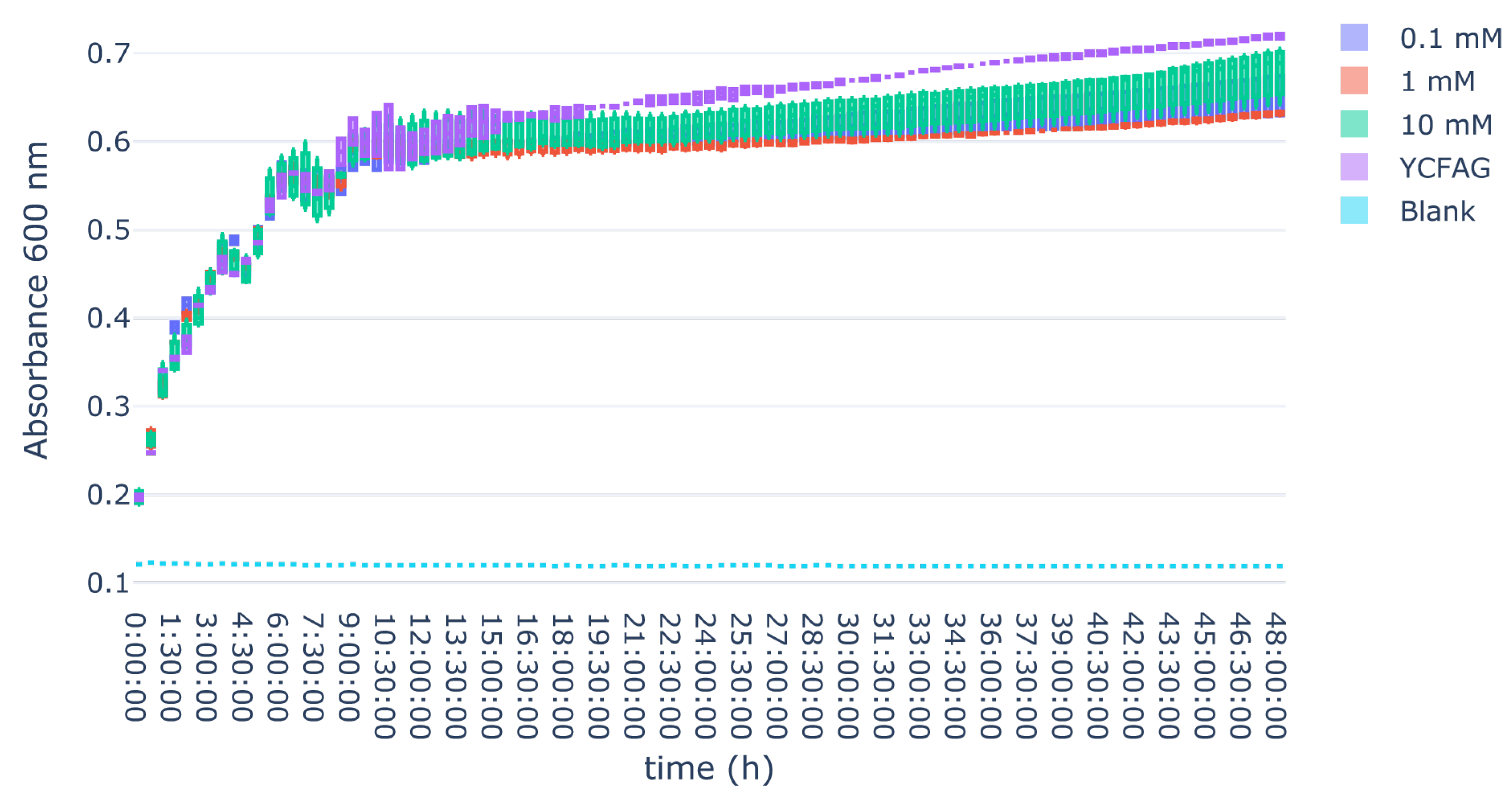
References
- Steliarova-Foucher, E.; Colombet, M.; Ries, L.A.G.; Moreno, F.; Dolya, A.; Bray, F.; Hesseling, P.; Shin, H.Y.; Stiller, C.A.; IICC-3 Contributors; et al. International incidence of childhood cancer, 2001–2010: A population-based registry study. Lancet Oncol. 2017, 18, 719–731. [Google Scholar] [CrossRef]
- Schulpen, M.; Visser, O.; Reedijk, A.M.; Kremer, L.C.; Zwaan, C.M.; Eggermont, A.M.; Coebergh, J.W.; Pieters, R.; Karim-Kos, H.E. Significant improvement in survival of advanced stage childhood and young adolescent cancer in the Netherlands since the 1990s. Eur. J. Cancer 2021, 157, 81–93. [Google Scholar] [CrossRef]
- Bowen, J.M.; Gibson, R.J.; Coller, J.K.; Blijlevens, N.; Bossi, P.; Al-Dasooqi, N.; Bateman, E.H.; Chiang, K.; de Mooij, C.; Mayo, B.; et al. Systematic review of agents for the management of cancer treatment-related gastrointestinal mucositis and clinical practice guidelines. Support. Care Cancer 2019, 27, 4011–4022. [Google Scholar] [CrossRef]
- Weischendorff, S.; de Pietri, S.; Rathe, M.; Schmiegelow, K.; Frandsen, T.L.; Petersen, M.J.; Weimann, A.; Nielsen, C.H.; Enevold, C.; Kocadag, H.B.; et al. Intestinal mucositis, systemic inflammation and bloodstream infections following high-dose methotrexate treatment in childhood acute lymphoblastic leukaemia: Comparison between the NOPHO ALL 2008 protocol and the ALLTogether1 protocol. Int. J. Cancer 2025, 156, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Matherly, L.H.; Taub, J.W. Methotrexate Pharmacology and Resistance in Childhood Acute Lymphoblastic Leukemia. Leuk. Lymphoma 1996, 21, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Maksimovic, V.; Pavlovic-Popovic, Z.; Vukmirovic, S.; Cvejic, J.; Mooranian, A.; Al-Salami, H.; Mikov, M.; Golocorbin-Kon, S. Molecular mechanism of action and pharmacokinetic properties of methotrexate. Mol. Biol. Rep. 2020, 47, 4699–4708. [Google Scholar] [CrossRef] [PubMed]
- Kuczma, M.; Ding, Z.C.; Zhou, G. Immunostimulatory Effects of Melphalan and Usefulness in Adoptive Cell Therapy with Antitumor CD4+ T Cells. Crit. Rev. Immunol. 2016, 36, 179–191. [Google Scholar] [CrossRef]
- da Silva Ferreira, A.R.; Wardill, H.R.; Havinga, R.; Tissing, W.J.E.; Harmsen, H.J.M. Prophylactic Treatment with Vitamins C and B2 for Methotrexate-Induced Gastrointestinal Mucositis. Biomolecules 2020, 11, 34. [Google Scholar] [CrossRef]
- da Silva Ferreira, A.R.; van der Aa, S.A.J.; Wehkamp, T.; Wardill, H.R.; ten Klooster, J.P.; Garssen, J.; Harthoorn, L.F.; Hartog, A.; Harmsen, H.J.M.; Tissing, W.J.E.; et al. Development of a self-limiting model of methotrexate-induced mucositis reinforces butyrate as a potential therapy. Sci. Rep. 2021, 11, 22911. [Google Scholar] [CrossRef]
- Dimitriadou, V.; Minden, M. P3.04: Apraglutide Treatment Reduces Chemotherapy-Induced Gastrointestinal (GI) Damage in Mice and Preserves Cellular Integrity During Chemotherapy. Transplantation 2022, 106, S519–S520. [Google Scholar] [CrossRef]
- Kuiken, N.S.; Rings, E.H.; Tissing, W.J. Risk analysis, diagnosis and management of gastrointestinal mucositis in pediatric cancer patients. Crit. Rev. Oncol. Hematol. 2015, 94, 87–97. [Google Scholar] [CrossRef]
- Kuiken, N.S.; Rings, E.H.; Alffenaar, J.C.; Havinga, R.; Jurdzinski, A.; Groen, A.K.; Tissing, W.J. Tumor Necrosis Factor-Alpha Inhibitor Etanercept Does Not Alter Methotrexate-Induced Gastrointestinal Mucositis in Rats. J. Pediatr. Gastroenterol. Nutr. 2017, 65, e28–e34. [Google Scholar] [CrossRef]
- Kuiken, N.S.; Rings, E.H.; Havinga, R.; van der Aa, S.A.; Groen, A.K.; Tissing, W.J. Effect of Oral Insulin on the Severity and Recovery of Methotrexate-Induced Gastrointestinal Mucositis in the Rat. J. Pediatr. Gastroenterol. Nutr. 2017, 64, e27–e34. [Google Scholar] [CrossRef]
- Pattanakitsakul, P.; Chongviriyaphan, N.; Pakakasama, S.; Apiwattanakul, N. Effect of vitamin A on intestinal mucosal injury in pediatric patients receiving hematopoietic stem cell transplantation and chemotherapy: A quasai-randomized trial. BMC Res. Notes 2020, 13, 464. [Google Scholar] [CrossRef] [PubMed]
- Wardill, H.R.; Bowen, J.M.; Gibson, R.J. New pharmacotherapy options for chemotherapy-induced alimentary mucositis. Expert. Opin. Biol. Ther. 2014, 14, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Wardill, H.R.; da Silva Ferreira, A.R.; Lichtenberg Cloo, S.; Havinga, R.; Harmsen, H.J.M.; Vermeij, W.P.; Tissing, W.J.E. Pre-therapy fasting slows epithelial turnover and modulates the microbiota but fails to mitigate methotrexate-induced gastrointestinal mucositis. Gut Microbes 2020, 12, 1809332. [Google Scholar] [CrossRef] [PubMed]
- Van Sebille, Y.Z.A.; Stansborough, R.; Wardill, H.R.; Bateman, E.; Gibson, R.J.; Keefe, D.M. Management of Mucositis During Chemotherapy: From Pathophysiology to Pragmatic Therapeutics. Curr. Oncol. Rep. 2015, 17, 50. [Google Scholar] [CrossRef]
- Curis, E.; Nicolis, I.; Moinard, C.; Osowska, S.; Zerrouk, N.; Bénazeth, S.; Cynober, L. Almost all about citrulline in mammals. Amino Acids 2005, 29, 177–205. [Google Scholar] [CrossRef]
- Kuiken, N.S.S.; Rings, E.H.H.M.; Blijlevens, N.M.A.; Tissing, W.J.E. Biomarkers and non-invasive tests for gastrointestinal mucositis. Support. Care Cancer 2017, 25, 2933–2941. [Google Scholar] [CrossRef]
- Wardill, H.R.; de Mooij, C.E.M.; da Silva Ferreira, A.R.; van de Peppel, I.P.; Havinga, R.; Harmsen, H.J.M.; Tissing, W.J.E.; Blijlevens, N.M.A. Translational model of melphalan-induced gut toxicity reveals drug-host-microbe interactions that drive tissue injury and fever. Cancer Chemother. Pharmacol. 2021, 88, 173–188. [Google Scholar] [CrossRef]
- Dekker, I.M.; Bruggink, H.; van der Meij, B.S.; Wierdsma, N.J. State of the art: The role of citrulline as biomarker in patients with chemotherapy- or graft-versus-host-disease-induced mucositis. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 416–427. [Google Scholar] [CrossRef]
- Curis, E.; Crenn, P.; Cynober, L. Citrulline and the gut. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 620–626. [Google Scholar] [CrossRef]
- Molendijk, E.B.D.; Blijlevens, N.M.A. NO, way to go: Critical amino acids to replenish nitric oxide production in treating mucositis. Curr. Opin. Support. Palliat. Care 2021, 15, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Sougiannis, A.T.; VanderVeen, B.N.; Davis, J.M.; Fan, D.; Murphy, E.A. Understanding chemotherapy-induced intestinal mucositis and strategies to improve gut resilience. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G712–G719. [Google Scholar] [CrossRef] [PubMed]
- Uyanga, V.A.; Amevor, F.K.; Liu, M.; Cui, Z.; Zhao, X.; Lin, H. Potential Implications of Citrulline and Quercetin on Gut Functioning of Monogastric Animals and Humans: A Comprehensive Review. Nutrients 2021, 13, 3782. [Google Scholar] [CrossRef]
- Fijlstra, M.; Ferdous, M.; Koning, A.M.; Rings, E.H.H.M.; Harmsen, H.J.M.; Tissing, W.J.E. Substantial decreases in the number and diversity of microbiota during chemotherapy-induced gastrointestinal mucositis in a rat model. Support. Care Cancer 2015, 23, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.S.; Li, A.; Wardill, H.R. Role of mucositis in predicting gut microbiota composition in people with cancer. Curr. Opin. Support. Palliat. Care 2024, 18, 73–77. [Google Scholar] [CrossRef]
- van Vliet, M.J.; Harmsen, H.J.M.; de Bont, E.S.J.M.; Tissing, W.J.E. The Role of Intestinal Microbiota in the Development and Severity of Chemotherapy-Induced Mucositis. PLoS Pathog. 2010, 6, e1000879. [Google Scholar] [CrossRef]
- van Vliet, M.J.; Tissing, W.J.E.; Dun, C.A.J.; Meessen, N.E.L.; Kamps, W.A.; de Bont, E.S.J.M.; Harmsen, H.J.M. Chemotherapy Treatment in Pediatric Patients with Acute Myeloid Leukemia Receiving Antimicrobial Prophylaxis Leads to a Relative Increase of Colonization with Potentially Pathogenic Bacteria in the Gut. Clin. Infect. Dis. 2009, 49, 262–270. [Google Scholar] [CrossRef]
- Wardill, H.R.; van der Aa, S.A.; da Silva Ferreira, A.R.; Havinga, R.; Tissing, W.J.; Harmsen, H.J. Antibiotic-induced disruption of the microbiome exacerbates chemotherapy-induced diarrhoea and can be mitigated with autologous faecal microbiota transplantation. Eur. J. Cancer 2021, 153, 27–39. [Google Scholar] [CrossRef]
- Devriese, S.; Van den Bossche, L.; Van Welden, S.; Holvoet, T.; Pinheiro, I.; Hindryckx, P.; De Vos, M.; Laukens, D. T84 monolayers are superior to Caco-2 as a model system of colonocytes. Histochem. Cell Biol. 2017, 148, 85–93. [Google Scholar] [CrossRef]
- Fagundes, R.R.; Bourgonje, A.R.; Saeed, A.; Vich Vila, A.; Plomp, N.; Blokzijl, T.; Sadabad, M.S.; von Martels, J.Z.H.; van Leeuwen, S.S.; Weersma, R.K.; et al. Inulin-grown Faecalibacterium prausnitzii cross-feeds fructose to the human intestinal epithelium. Gut Microbes 2021, 13, 1993582. [Google Scholar] [CrossRef] [PubMed]
- Plomp, N.; Liu, L.; Walters, L.; Bus-Spoor, C.; Khan, M.; Sheridan, P.; Veloo, A.; Walker, A.; Harmsen, H.; Tsompanidou, E. A convenient and versatile culturomics platform to expand the human gut culturome of Lachnospiraceae and Oscillospiraceae. Benef. Microbes 2024, 16, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Sajith, M.; Pawar, A.; Bafna, V.; Bartakke, S.; Subramanian, K.; Vaidya, N. Serum Methotrexate Level and Side Effects of High Dose Methotrexate Infusion in Pediatric Patients with Acute Lymphoblastic Leukaemia (ALL). Indian. J. Hematol. Blood Transfus. 2020, 36, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Pinguet, F.; Martel, P.; Fabbro, M.; Petit, I.; Canal, P.; Culine, S.; Astre, C.; Bressolle, F. Pharmacokinetics of high-dose intravenous melphalan in patients undergoing peripheral blood hematopoietic progenitor-cell transplantation. Anticancer. Res. 1997, 17, 605–611. [Google Scholar]
- Peters, J.H.; Lin, S.C.; Berridge, B.J.; Cummings, J.G.; Chao, W.R. Amino Acids, Including Asparagine and Glutamine, in Plasma and Urine of Normal Human Subjects. Exp. Biol. Med. 1969, 131, 281–288. [Google Scholar] [CrossRef]
- Moinard, C.; Nicolis, I.; Neveux, N.; Darquy, S.; Bénazeth, S.; Cynober, L. Dose-ranging effects of citrulline administration on plasma amino acids and hormonal patterns in healthy subjects: The Citrudose pharmacokinetic study. Br. J. Nutr. 2008, 99, 855–862. [Google Scholar] [CrossRef]
- Martinez-Serra, J.; Gutierrez, A.; Navarro-Palou, M.; Ros, T.; Amat, J.C.; Marcus, T.F.; Fueyo, L.; Suquia, A.G.; Rubio, F.; Besalduch, J.; et al. xCELLigence system for real-time label-free monitoring of growth and viability of cell lines from hematological malignancies. OTT 2014, 12, 985–994. [Google Scholar] [CrossRef]
- Hiers, R.D.; Huebner, P.; Khajotia, S.S.; Florez, F.L.E. Characterization of Experimental Nanoparticulated Dental Adhesive Resins with Long-Term Antibacterial Properties. Nanomaterials 2022, 12, 3732. [Google Scholar] [CrossRef]
- Van Sebille, Y.Z.; Gibson, R.J.; Wardill, H.R.; Ball, I.A.; Keefe, D.M.; Bowen, J.M. Dacomitinib-induced diarrhea: Targeting chloride secretion with crofelemer. Int. J. Cancer 2018, 142, 369–380. [Google Scholar] [CrossRef]
- Tam, J.S.Y.; Coller, J.K.; Prestidge, C.A.; Bowen, J.M. Investigation of TLR4 Antagonists for Prevention of Intestinal Inflammation. Inflammation 2023, 46, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Van Sebille, Y.Z.A.; Gibson, R.J.; Wardill, H.R.; Secombe, K.R.; Ball, I.A.; Keefe, D.M.K.; Finnie, J.W.; Bowen, J.M. Dacomitinib-induced diarrhoea is associated with altered gastrointestinal permeability and disruption in ileal histology in rats. Int. J. Cancer 2017, 140, 2820–2829. [Google Scholar] [CrossRef]
- Wang, H.; Jatmiko, Y.D.; Bastian, S.E.; Mashtoub, S.; Howarth, G.S. Effects of Supernatants from Escherichia coli Nissle 1917 and Faecalibacterium prausnitzii on Intestinal Epithelial Cells and a Rat Model of 5-Fluorouracil-Induced Mucositis. Nutr. Cancer 2017, 69, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Boeing, T.; Speca, S.; de Souza, P.; Mena, A.M.; Bertin, B.; Desreumax, P.; Mota da Silva, L.; Faloni de Andrade, S.; Dubuqoy, L. The PPARγ-dependent effect of flavonoid luteolin against damage induced by the chemotherapeutic irinotecan in human intestinal cells. Chem. Biol. Interact. 2022, 351, 109712. [Google Scholar] [CrossRef]
- Cheah, K.Y.; Howarth, G.S.; Bindon, K.A.; Kennedy, J.A.; Bastian, S.E. Low molecular weight procyanidins from grape seeds enhance the impact of 5-Fluorouracil chemotherapy on Caco-2 human colon cancer cells. PLoS ONE 2014, 9, e98921. [Google Scholar] [CrossRef] [PubMed]
- Guabiraba, R.; Besnard, A.G.; Menezes, G.B.; Secher, T.; Jabir, M.S.; Amaral, S.S.; Braun, H.; Lima-Junior, R.C.; Ribeiro, A.R.; Cunha, F.Q.; et al. IL-33 targeting attenuates intestinal mucositis and enhances effective tumor chemotherapy in mice. Mucosal Immunol. 2014, 7, 1079–1093. [Google Scholar] [CrossRef]
- Justino, P.F.C.; Franco, A.X.; Pontier-Bres, R.; Monteiro, C.E.S.; Barbosa, A.L.R.; Souza, M.H.L.P.; Czerucka, D.; Soares, P.M.G. Modulation of 5-fluorouracil activation of toll-like/MyD88/NF-κB/MAPK pathway by Saccharomyces boulardii CNCM I-745 probiotic. Cytokine 2020, 125, 154791. [Google Scholar] [CrossRef]
- Marsh, D.T.; Smid, S.D. Selected phytocannabinoids inhibit SN-38- and cytokine-evoked increases in epithelial permeability and improve intestinal barrier function in vitro. Toxicol. Vitr. 2024, 99, 105888. [Google Scholar] [CrossRef]
- Yue, X.; Wen, S.; Long-Kun, D.; Man, Y.; Chang, S.; Min, Z.; Shuang-Yu, L.; Xin, Q.; Jie, M.; Liang, W. Three important short-chain fatty acids (SCFAs) attenuate the inflammatory response induced by 5-FU and maintain the integrity of intestinal mucosal tight junction. BMC Immunol. 2022, 23, 19. [Google Scholar] [CrossRef]
- Youmba, S.B.; Belmonte, L.; Galas, L.; Boukhettala, N.; Bôle-Feysot, C.; Déchelotte, P.; Coëffier, M. Methotrexate modulates tight junctions through NF-κB, MEK, and JNK pathways. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 463–470. [Google Scholar] [CrossRef]
- Sukhotnik, I.; Geyer, T.; Pollak, Y.; Mogilner, J.G.; Coran, A.G.; Berkowitz, D. The role of Wnt/β-catenin signaling in enterocyte turnover during methotrexate-induced intestinal mucositis in a rat. PLoS ONE 2014, 9, e110675. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wei, B.; Wang, D.; Wu, J.; Gao, J.; Zhong, H.; Sun, Y.; Xu, Q.; Liu, W.; Gu, Y.; et al. DNA damage repair promotion in colonic epithelial cells by andrographolide downregulated cGAS-STING pathway activation and contributed to the relief of CPT-11-induced intestinal mucositis. Acta Pharm. Sin. B 2022, 12, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Hudita, A.; Radu, I.C.; Galateanu, B.; Ginghina, O.; Herman, H.; Balta, C.; Rosu, M.; Zaharia, C.; Costache, M.; Tanasa, E.; et al. Bioinspired silk fibroin nano-delivery systems protect against 5-FU induced gastrointestinal mucositis in a mouse model and display antitumor effects on HT-29 colorectal cancer cells in vitro. Nanotoxicology 2021, 15, 973–994. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.M.; Leocádio, P.C.; Teixeira, L.G.; Leonel, A.J.; Cara, D.C.; Menezes, G.B.; Generoso, S.d.V.; Cardoso, V.N.; Alvarez-Leite, J.I.; Correia, M.I.T.D. Pretreatment With L-Citrulline Positively Affects the Mucosal Architecture and Permeability of the Small Intestine in a Murine Mucositis Model. JPEN J. Parenter. Enter. Nutr. 2016, 40, 279–286. [Google Scholar] [CrossRef]
- Cai, B.; Zhou, M.H.; Huang, H.L.; Zhou, A.C.; Chu, Z.D.; Huang, X.D.; Li, C.-W.; Chen, L. Protective effects of citrulline supplementation in ulcerative colitis rats. PLoS ONE 2020, 15, e0240883. [Google Scholar] [CrossRef]
- Chen, J.; Xu, R.; Qiu, L. Protective effect of citrulline on the intestinal mucosal barrier of mice during sepsis. Pak. J. Pharm. Sci. 2023, 36, 1079–1084. [Google Scholar] [CrossRef]
- Jegatheesan, P.; Beutheu, S.; Freese, K.; Waligora-Dupriet, A.J.; Nubret, E.; Butel, M.J.; Bergheim, I.; De Bandt, J.-P. Preventive effects of citrulline on Western diet-induced non-alcoholic fatty liver disease in rats. Br. J. Nutr. 2016, 116, 191–203. [Google Scholar] [CrossRef]
- Kvidera, S.K.; Mayorga, E.J.; McCarthy, C.S.; Horst, E.A.; Abeyta, M.A.; Baumgard, L.H. Effects of supplemental citrulline on thermal and intestinal morphology parameters during heat stress and feed restriction in growing pigs. J. Anim. Sci. 2024, 102, skae120. [Google Scholar] [CrossRef]
- Lai, C.H.; Lee, C.H.; Hung, C.Y.; Lo, H.C. Oral Citrulline Mitigates Inflammation and Jejunal Damage via the Inactivation of Neuronal Nitric Oxide Synthase and Nuclear Factor-κB in Intestinal Ischemia and Reperfusion. JPEN J. Parenter. Enter. Nutr. 2017, 41, 422–435. [Google Scholar] [CrossRef]
- Rajcic, D.; Baumann, A.; Hernández-Arriaga, A.; Brandt, A.; Nier, A.; Jin, C.J.; Sánchez, V.; Jung, F.; Camarinha-Silva, A.; Bergheim, I. Citrulline supplementation attenuates the development of non-alcoholic steatohepatitis in female mice through mechanisms involving intestinal arginase. Redox Biol. 2021, 41, 101879. [Google Scholar] [CrossRef]
- Rajcic, D.; Kromm, F.; Hernández-Arriaga, A.; Brandt, A.; Baumann, A.; Staltner, R.; Camarinha-Silva, A.; Bergheim, I. Supplementing L-Citrulline Can Extend Lifespan in C. elegans and Attenuate the Development of Aging-Related Impairments of Glucose Tolerance and Intestinal Barrier in Mice. Biomolecules 2023, 13, 1579. [Google Scholar] [CrossRef]
- Sellmann, C.; Jin, C.J.; Engstler, A.J.; De Bandt, J.P.; Bergheim, I. Oral citrulline supplementation protects female mice from the development of non-alcoholic fatty liver disease (NAFLD). Eur. J. Nutr. 2017, 56, 2519–2527. [Google Scholar] [CrossRef]
- Xie, Y.; Irwin, S.; Nelson, B.; van Daelen, M.; Fontenot, L.; Jacobs, J.P.; Cappelletti, M.; Feng, H.; Li, Y.; Koon, H.W. Citrulline Inhibits Clostridioides difficile Infection with Anti-inflammatory Effects. Cell Mol. Gastroenterol. Hepatol. 2025, 19, 101474. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Gao, Y.; Chen, Y.; Zhang, Y.; Deng, Y.; Niu, S.; Dai, H. L-Citrulline Ameliorates Iron Metabolism and Mitochondrial Quality Control via Activating AMPK Pathway in Intestine and Improves Microbiota in Mice with Iron Overload. Mol. Nutr. Food Res. 2024, 68, e2300723. [Google Scholar] [CrossRef] [PubMed]
- Zoghroban, H.S.; Ibrahim, F.M.; Nasef, N.A.; Saad, A.E. The impact of L-citrulline on murine intestinal cell integrity, immune response, and arginine metabolism in the face of Giardia lamblia infection. Acta Trop. 2023, 237, 106748. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, T.; Chen, S.; Shen, H.; Wang, J. Dietary supplementation with L-citrulline improves amino acid composition and broiler performance, and modulates gut microbiota. Front. Microbiol. 2025, 16, 1551012. [Google Scholar] [CrossRef]
- Du, J.; Gan, M.; Xie, Z.; Zhou, C.; Jing, Y.; Li, M.; Liu, C.; Wang, M.; Dai, H.; Huang, Z.; et al. Effects of dietary L-Citrulline supplementation on growth performance, meat quality, and fecal microbial composition in finishing pigs. Front. Microbiol. 2023, 14, 1209389. [Google Scholar] [CrossRef]
- Hueso, T.; Ekpe, K.; Mayeur, C.; Gatse, A.; Joncquel-Chevallier Curt, M.; Gricourt, G.; Rodriguez, C.; Burdet, C.; Ulmann, G.; Neut, C.; et al. Impact and consequences of intensive chemotherapy on intestinal barrier and microbiota in acute myeloid leukemia: The role of mucosal strengthening. Gut Microbes 2020, 12, 1800897. [Google Scholar] [CrossRef]
- Anderson, C.J.; Boeckaerts, L.; Chin, P.; Cardas, J.B.; Xie, W.; Gonçalves, A.; Blancke, G.; Benson, S.; Rogatti, S.; Simpson, M.S.; et al. Metabolite-based inter-kingdom communication controls intestinal tissue recovery following chemotherapeutic injury. Cell Host Microbe 2024, 32, 1469–1487.e9. [Google Scholar] [CrossRef]
- Duncan, S.H.; Hold, G.L.; Harmsen, H.J.; Stewart, C.S.; Flint, H.J. Growth requirements and fermentation products of Fusobacterium prausnitzii, and a proposal to reclassify it as Faecalibacterium prausnitzii gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 2002, 52, 2141–2146. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van der Laan, W.; Gallardo Molina, P.A.; Koonen, D.P.Y.; Harmsen, H.J.M.; Tissing, W.J.E. L-Citrulline Improves Recovery of Enterocytes While Not Affecting Gut Microbiota in an In Vitro Model of Chemotherapy-Induced Gastrointestinal Mucositis. Biomedicines 2025, 13, 2244. https://doi.org/10.3390/biomedicines13092244
van der Laan W, Gallardo Molina PA, Koonen DPY, Harmsen HJM, Tissing WJE. L-Citrulline Improves Recovery of Enterocytes While Not Affecting Gut Microbiota in an In Vitro Model of Chemotherapy-Induced Gastrointestinal Mucositis. Biomedicines. 2025; 13(9):2244. https://doi.org/10.3390/biomedicines13092244
Chicago/Turabian Stylevan der Laan, Wally, Pablo A. Gallardo Molina, Debby P. Y. Koonen, Hermie J. M. Harmsen, and Wim J. E. Tissing. 2025. "L-Citrulline Improves Recovery of Enterocytes While Not Affecting Gut Microbiota in an In Vitro Model of Chemotherapy-Induced Gastrointestinal Mucositis" Biomedicines 13, no. 9: 2244. https://doi.org/10.3390/biomedicines13092244
APA Stylevan der Laan, W., Gallardo Molina, P. A., Koonen, D. P. Y., Harmsen, H. J. M., & Tissing, W. J. E. (2025). L-Citrulline Improves Recovery of Enterocytes While Not Affecting Gut Microbiota in an In Vitro Model of Chemotherapy-Induced Gastrointestinal Mucositis. Biomedicines, 13(9), 2244. https://doi.org/10.3390/biomedicines13092244






