Attenuation of Pulmonary Fibrosis by the MyD88 Inhibitor TJ-M2010-5 Through Autophagy Induction in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animals and Animal Treatment
2.3. Histology and Immunohistochemistry
2.4. Bronchoalveolar Lavage Fluid Collection and the Number of Leukocytes in Bronchoalveolar Lavage Fluid
2.5. Measurements of Contents of Hydroxyproline and Activity of Superoxide Dismutase in Lung Tissues
2.6. ELISA Assay
2.7. Real-Time Polymerase Chain Reaction Analysis
2.8. Cell Culture and Drug Interventions
2.9. Cell Immunofluorescence
2.10. Proliferation, Apoptosis, and Cytotoxicity Assays
2.11. Western Blotting
2.12. Transmission Electron Microscopy
2.13. Statistical Analysis
3. Results
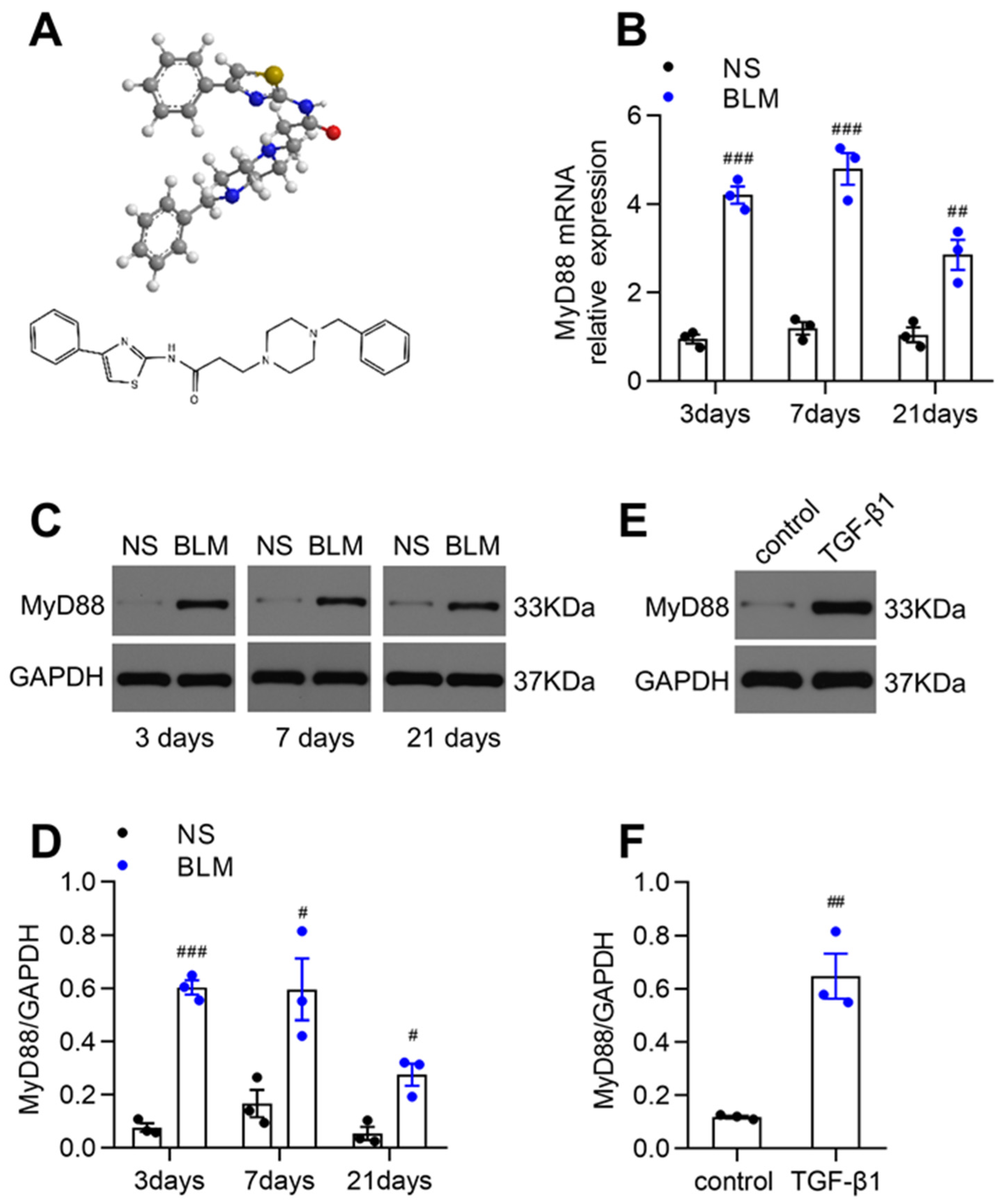
3.1. MyD88 Expression Was Markedly Upregulated in Fibrotic Lungs of BLM-Induced Mice and TGF-β1-Stimulated Fibroblasts
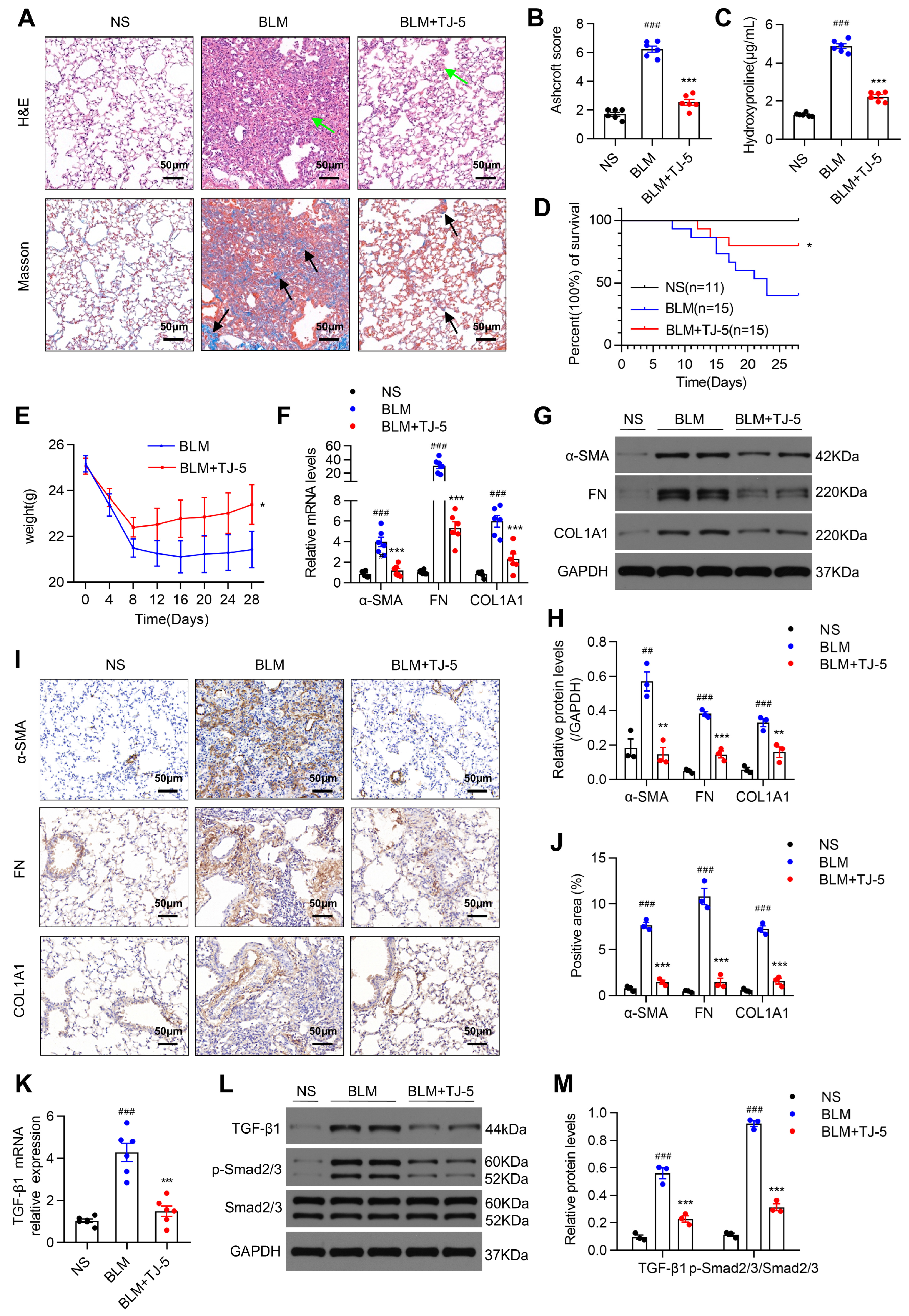
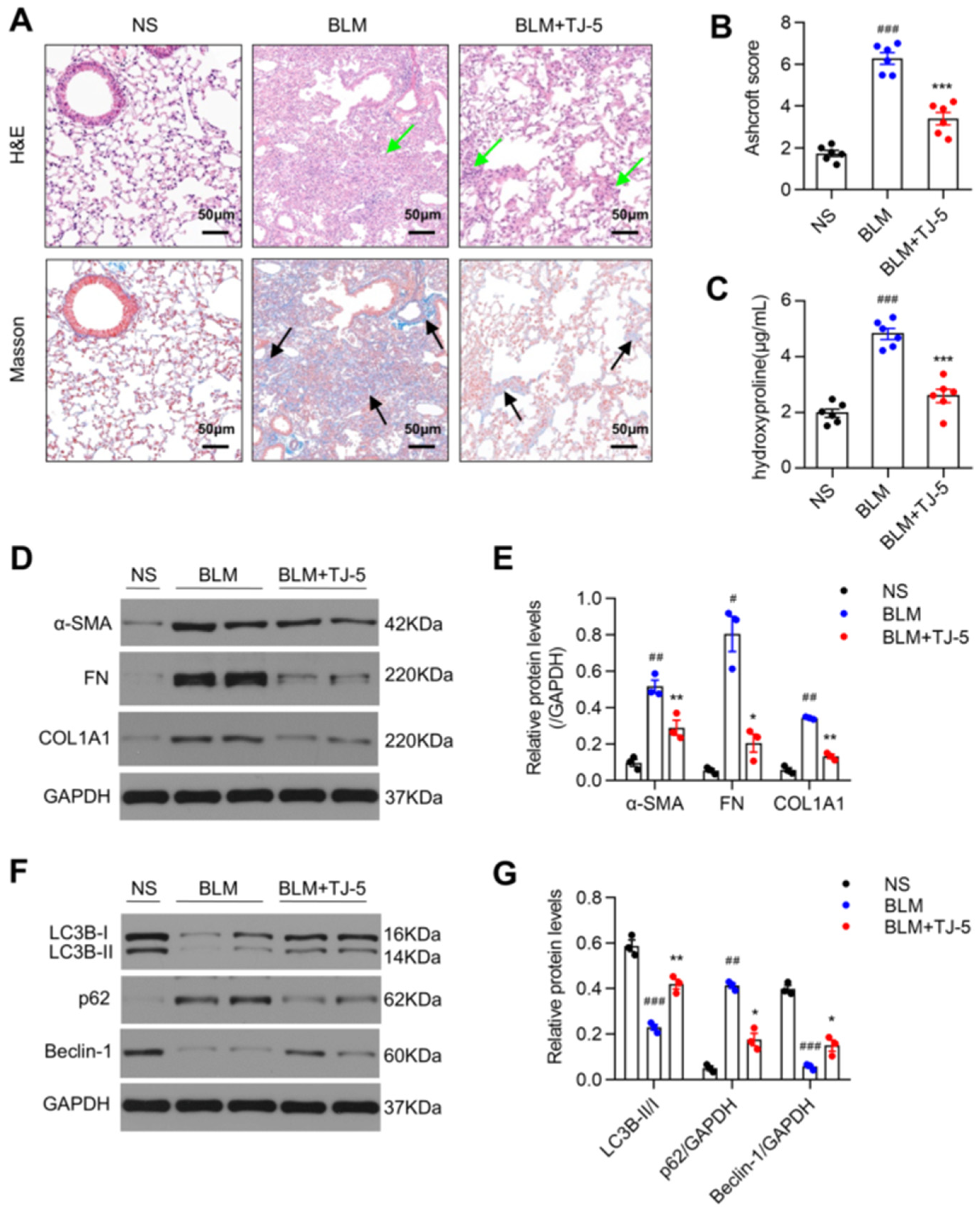
3.2. TJ-5 Ameliorated BLM-Induced Pulmonary Fibrosis and Reduced the Expression of Fibrosis Markers
3.3. TJ-5 Attenuated BLM-Induced Pulmonary Inflammation
3.4. TJ-5 Suppressed TGF-β1-Induced ECM Deposition in Lung Fibroblasts and Inhibited Their Proliferation
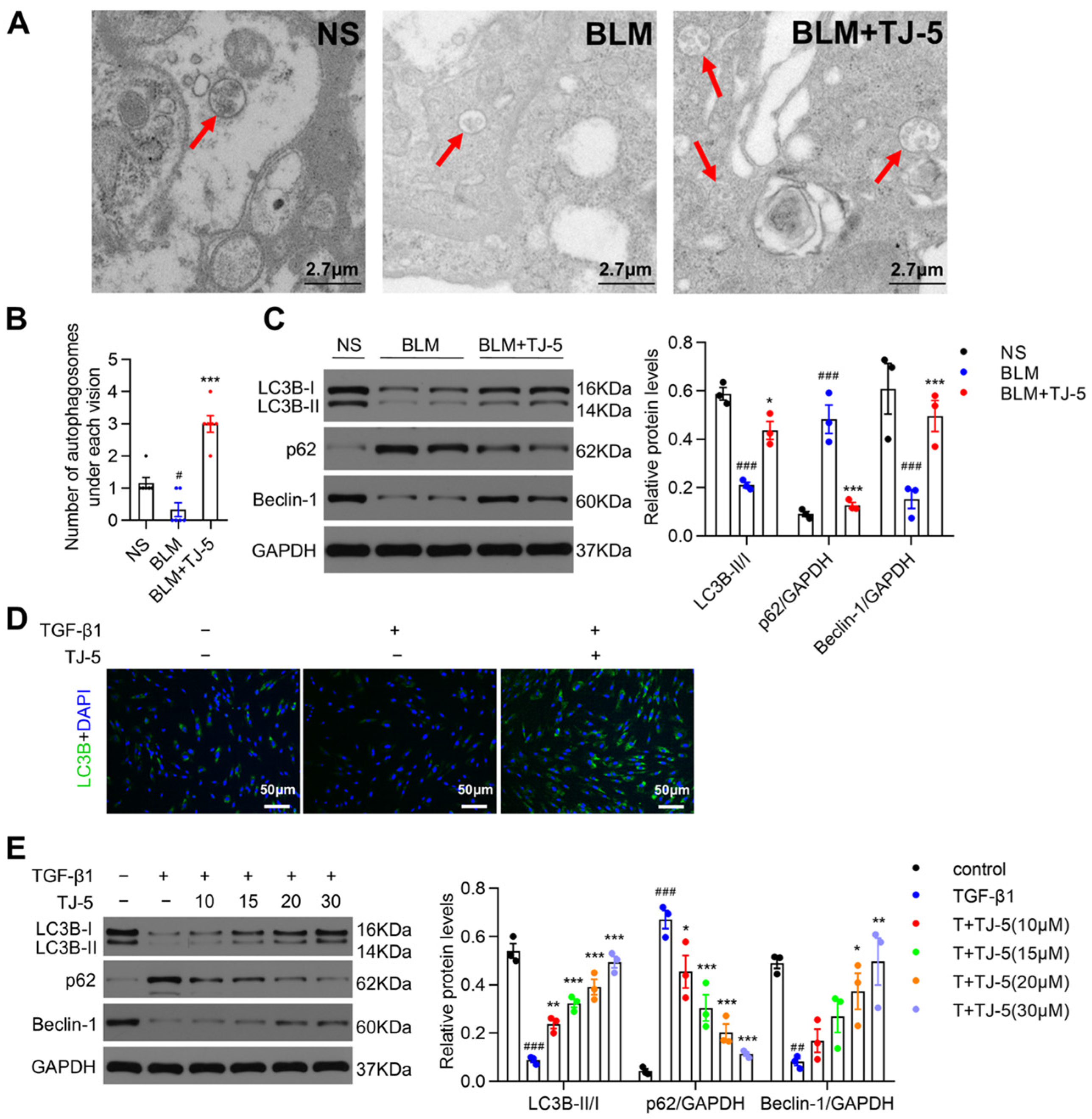
3.5. TJ-5 Induced Autophagy in Fibrotic Lungs of BLM-Stimulated Mice and TGF-β1-Induced Fibroblasts
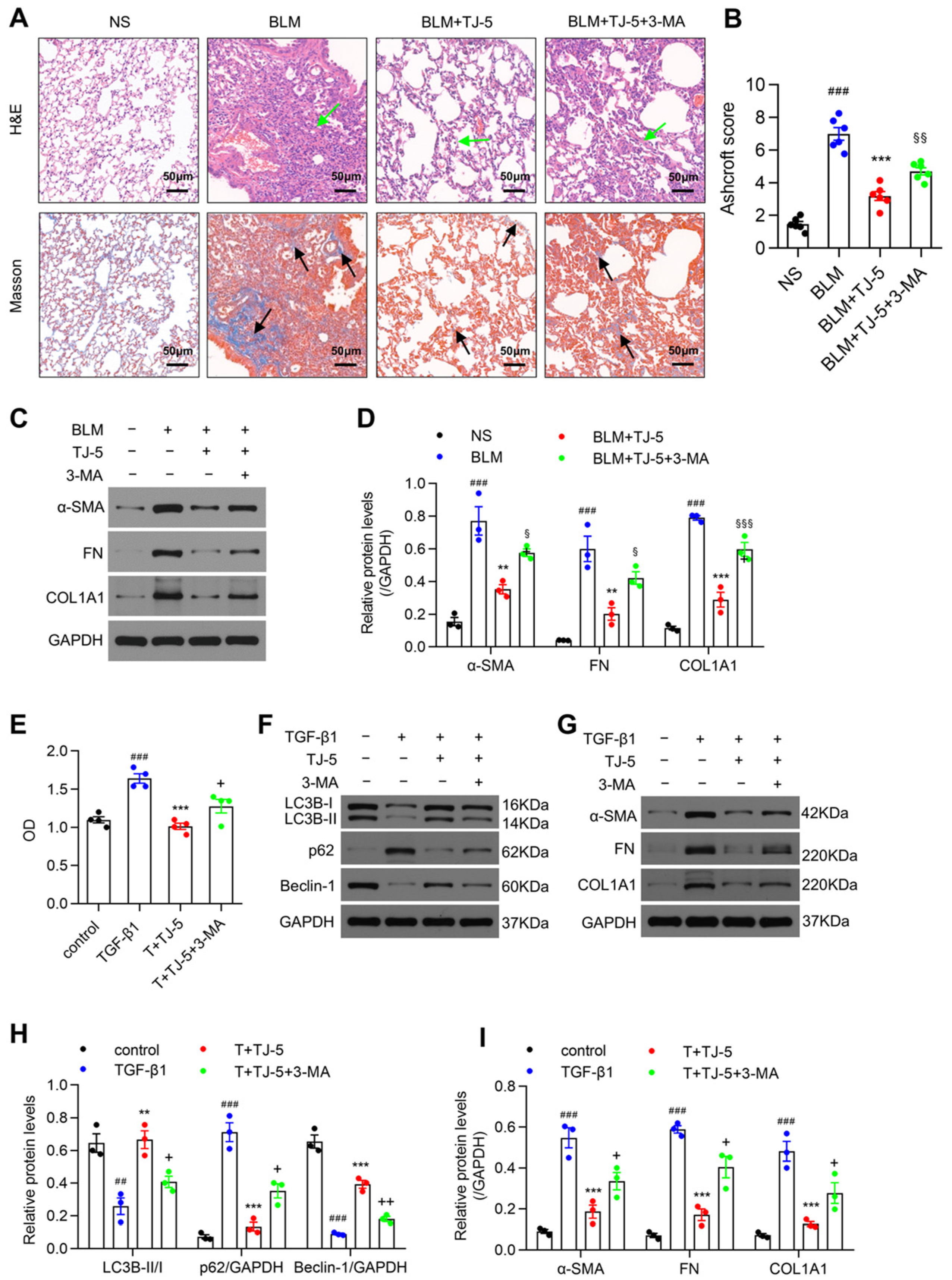
3.6. Inhibition of Autophagy Reversed the Protective Effects of TJ-5
3.7. Therapeutic Intervention with TJ-5 Ameliorated BLM-Induced Pulmonary Fibrosis and Inhibited Lung Fibroblast Activation
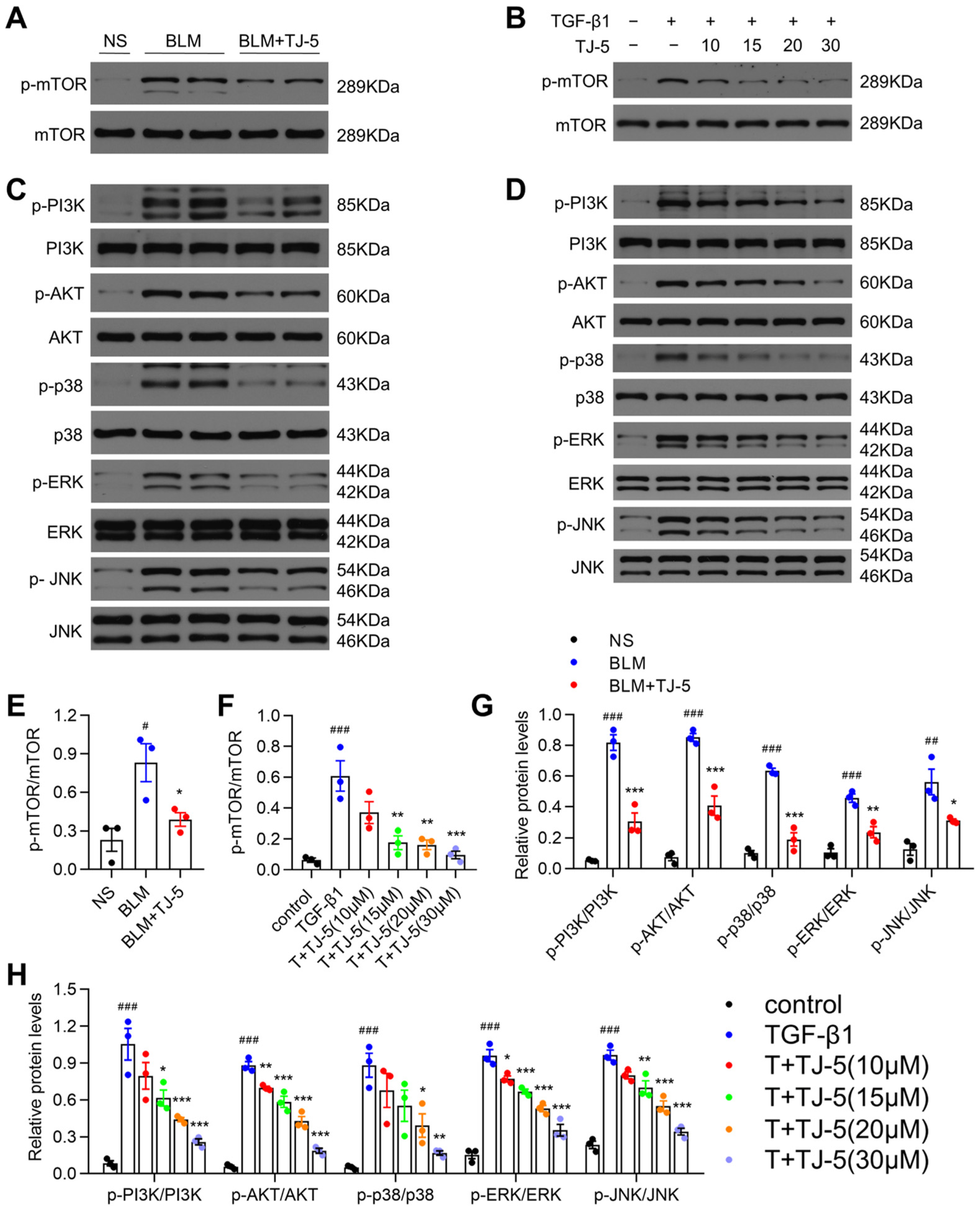
3.8. TJ-5 Triggered Autophagy Possibly by Suppressing PI3K/AKT/mTOR and MAPK/mTOR Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IPF | idiopathic pulmonary fibrosis |
| MyD88 | myeloid differentiation factor 88 |
| BLM | bleomycin |
| ECM | extracellular matrix |
| TLRs | Toll-like receptors |
| BALF | bronchoalveolar lavage fluid |
References
- Moss, B.J.; Ryter, S.W.; Rosas, I.O. Pathogenic Mechanisms Underlying Idiopathic Pulmonary Fibrosis. Annu. Rev. Pathol. Mech. Dis. 2022, 17, 515–546. [Google Scholar] [CrossRef] [PubMed]
- Richeldi, L.; Collard, H.R.; Jones, M.G. Idiopathic pulmonary fibrosis. Lancet 2017, 389, 1941–1952. [Google Scholar] [CrossRef]
- Maher, T.M.; Bendstrup, E.; Dron, L.; Langley, J.; Smith, G.; Khalid, J.M.; Patel, H.; Kreuter, M. Global incidence and prevalence of idiopathic pulmonary fibrosis. Respir. Res. 2021, 22, 197. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Li, T.; Yang, Z.; Yi, W.; Di, S.; Sun, Y.; Wang, D.; Yang, Y. AMPK orchestrates an elaborate cascade protecting tissue from fibrosis and aging. Ageing Res. Rev. 2017, 38, 18–27. [Google Scholar] [CrossRef]
- Spagnolo, P.; Kropski, J.A.; Jones, M.G.; Lee, J.S.; Rossi, G.; Karampitsakos, T.; Maher, T.M.; Tzouvelekis, A.; Ryerson, C.J. Idiopathic pulmonary fibrosis: Disease mechanisms and drug development. Pharmacol. Ther. 2021, 222, 107798. [Google Scholar] [CrossRef]
- Glass, D.S.; Grossfeld, D.; Renna, H.A.; Agarwala, P.; Spiegler, P.; DeLeon, J.; Reiss, A.B. Idiopathic pulmonary fibrosis: Current and future treatment. Clin. Respir. J. 2022, 16, 84–96. [Google Scholar] [CrossRef]
- Mei, Q.; Liu, Z.; Zuo, H.; Yang, Z.; Qu, J. Idiopathic Pulmonary Fibrosis: An Update on Pathogenesis. Front. Pharmacol. 2022, 12, 797292. [Google Scholar] [CrossRef]
- Koudstaal, T.; Wijsenbeek, M.S. Idiopathic pulmonary fibrosis. La Presse Médicale 2023, 52, 104166. [Google Scholar] [CrossRef]
- Hansen, M.; Rubinsztein, D.C.; Walker, D.W. Autophagy as a promoter of longevity: Insights from model organisms. Nat. Rev. Mol. Cell Biol. 2018, 19, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.S.; Lin, L.; Geyer, A.; Haspel, J.A.; An, C.H.; Cao, J.; Rosas, I.O.; Morse, D.; Eickelberg, O. Autophagy in Idiopathic Pulmonary Fibrosis. PLoS ONE 2012, 7, e41394. [Google Scholar] [CrossRef]
- Liao, S.-X.; Sun, P.-P.; Gu, Y.-H.; Rao, X.-M.; Zhang, L.-Y.; Ou-Yang, Y. Autophagy and pulmonary disease. Ther. Adv. Respir. Dis. 2019, 13, 1–13. [Google Scholar] [CrossRef]
- El-Horany, H.E.-S.; Atef, M.M.; Ghafar, M.T.A.; Fouda, M.H.; Nasef, N.A.; Hegab, I.I.; Helal, D.S.; Elseady, W.; Hafez, Y.M.; Hagag, R.Y.; et al. Empagliflozin Ameliorates Bleomycin-Induced Pulmonary Fibrosis in Rats by Modulating Sesn2/AMPK/Nrf2 Signaling and Targeting Ferroptosis and Autophagy. Int. J. Mol. Sci. 2023, 24, 9481. [Google Scholar] [CrossRef]
- Racanelli, A.C.; Kikkers, S.A.; Choi, A.M.K.; Cloonan, S.M. Autophagy and Inflammation in Chronic Respiratory Disease. Autophagy 2018, 14, 221–232. [Google Scholar] [CrossRef]
- Nho, R.S.; Hergert, P.; Gullberg, D. IPF fibroblasts are desensitized to type i collagen matrix-induced cell death by suppressing low autophagy via aberrant Akt/mTOR kinases. PLoS ONE 2014, 9, e94616. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Liu, J.; Wu, Z.; Li, C.; Zhang, Y. Development and validation of the prognostic model based on autophagy-associated genes in idiopathic pulmonary fibrosis. Front. Immunol. 2022, 13, 1049361. [Google Scholar] [CrossRef]
- Ricci, A.; Cherubini, E.; Scozzi, D.; Pietrangeli, V.; Tabbì, L.; Raffa, S.; Leone, L.; Visco, V.; Torrisi, M.R.; Bruno, P.; et al. Decreased expression of autophagic beclin 1 protein in idiopathic pulmonary fibrosis fibroblasts. J. Cell. Physiol. 2012, 228, 1516–1524. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, G.; Wang, Z.; Qin, H.; Mo, B.; Wang, C. Elongation factor-2 kinase acts downstream of p38 MAPK to regulate proliferation, apoptosis and autophagy in human lung fibroblasts. Exp. Cell Res. 2018, 363, 291–298. [Google Scholar] [CrossRef]
- Huebener, P.; Schwabe, R.F. Regulation of wound healing and organ fibrosis by toll-like receptors. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2013, 1832, 1005–1017. [Google Scholar] [CrossRef]
- Li, C.; Yu, Y.; Li, W.; Liu, B.; Jiao, X.; Song, X.; Lv, C.; Qin, S. Phycocyanin attenuates pulmonary fibrosis via the TLR2-MyD88-NF-κB signaling pathway. Sci. Rep. 2017, 7, 5843. [Google Scholar] [CrossRef]
- Yang, H.-Z.; Wang, J.-P.; Mi, S.; Liu, H.-Z.; Cui, B.; Yan, H.-M.; Yan, J.; Li, Z.; Liu, H.; Hua, F.; et al. TLR4 activity is required in the resolution of pulmonary inflammation and fibrosis after acute and chronic lung injury. Am. J. Pathol. 2012, 180, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Meneghin, A.; Choi, E.S.; Evanoff, H.L.; Kunkel, S.L.; Martinez, F.J.; Flaherty, K.R.; Toews, G.B.; Hogaboam, C.M. TLR9 is expressed in idiopathic interstitial pneumonia and its activation promotes in vitro myofibroblast differentiation. Histochem. Cell Biol. 2008, 130, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Gasse, P.; Mary, C.; Guenon, I.; Noulin, N.; Charron, S.; Schnyder-Candrian, S.; Schnyder, B.; Akira, S.; Quesniaux, V.F.; Lagente, V.; et al. IL-1R1/MyD88 signaling and the inflammasome are essential in pulmonary inflammation and fibrosis in mice. J. Clin. Investig. 2007, 117, 3786–3799. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Xu, W.; Wang, J.; Yan, J.; Shi, Y.; Zhang, C.; Ge, W.; Wu, J.; Du, P.; Chen, Y. Boosting mTOR-dependent autophagy via upstream TLR4-MyD88-MAPK signalling and downstream NF-κB pathway quenches intestinal inflammation and oxidative stress injury. EBioMedicine 2018, 35, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.O.; Park, E.K.; Park, D.H.; Song, G.Y.; Bae, J.-S. Therapeutic Effects of Cornuside on Particulate Matter–Induced Lung Injury. Int. J. Mol. Sci. 2023, 24, 4979. [Google Scholar] [CrossRef]
- Yu, T.; Guo, F.; Yu, Y.; Sun, T.; Ma, D.; Han, J.; Qian, Y.; Kryczek, I.; Sun, D.; Nagarsheth, N.; et al. Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell 2017, 170, 548–563.e16. [Google Scholar] [CrossRef]
- Kader, M.; Alaoui-El-Azher, M.; Vorhauer, J.; Kode, B.B.; Wells, J.Z.; Stolz, D.; Michalopoulos, G.; Wells, A.; Scott, M.; Ismail, N.; et al. MyD88-dependent inflammasome activation and autophagy inhibition contributes to Ehrlichia-induced liver injury and toxic shock. PLoS Pathog. 2017, 13, e1006644. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Y.; Qiu, T.; Liu, W.; Yao, P. Autophagy, an important therapeutic target for pulmonary fibrosis diseases. Clin. Chim. Acta 2020, 502, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Guan, K.-L. mTOR: A pharmacologic target for autophagy regulation. J. Clin. Investig. 2015, 125, 25–32. [Google Scholar] [CrossRef]
- Wan, H.; Xie, T.; Xu, Q.; Hu, X.; Xing, S.; Yang, H.; Gao, Y.; He, Z. Thy-1 depletion and integrin β3 upregulation-mediated PI3K-Akt-mTOR pathway activation inhibits lung fibroblast autophagy in lipopolysaccharide-induced pulmonary fibrosis. Mod. Pathol. 2019, 99, 1636–1649. [Google Scholar] [CrossRef] [PubMed]
- Ba, L.; Gao, J.; Chen, Y.; Qi, H.; Dong, C.; Pan, H.; Zhang, Q.; Shi, P.; Song, C.; Guan, X.; et al. Allicin attenuates pathological cardiac hypertrophy by inhibiting autophagy via activation of PI3K/Akt/mTOR and MAPK/ERK/mTOR signaling pathways. Phytomedicine 2019, 58, 152765. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Li, C.; Shang, Y.; Li, D.; Zhuo, Y.; Yang, L.; Cui, N.; Li, Y.; Zhang, S. Chaihu Guizhi Ganjiang Decoction Ameliorates Pancreatic Fibrosis via JNK/mTOR Signaling Pathway. Front. Pharmacol. 2021, 12, 679557. [Google Scholar] [CrossRef]
- Livingston, M.J.; Zhang, M.; Kwon, S.-H.; Chen, J.-K.; Li, H.; Manicassamy, S.; Dong, Z. Autophagy activates EGR1 via MAPK/ERK to induce FGF2 in renal tubular cells for fibroblast activation and fibrosis during maladaptive kidney repair. Autophagy 2023, 20, 1032–1053. [Google Scholar] [CrossRef]
- Li, X.; Jiang, S.; Tapping, R.I. Toll-like receptor signaling in cell proliferation and survival. Cytokine 2010, 49, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Deguine, J.; Barton, G.M. MyD88: A central player in innate immune signaling. F1000Prime Rep. 2014, 6, 97. [Google Scholar] [CrossRef]
- Xie, L.; Jiang, F.-C.; Zhang, L.-M.; He, W.-T.; Liu, J.-H.; Li, M.-Q.; Zhang, X.; Xing, S.; Guo, H.; Zhou, P. Targeting of MyD88 Homodimerization by Novel Synthetic Inhibitor TJ-M2010-5 in Preventing Colitis-Associated Colorectal Cancer. J. Natl. Cancer Inst. 2015, 108, djv364. [Google Scholar] [CrossRef]
- Parsey, M.V.; Tuder, R.M.; Abraham, E. Neutrophils are major contributors to intraparenchymal lung IL-1 beta expression after hemorrhage and endotoxemia. J. Immunol. 1998, 160, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Hübner, R.-H.; Gitter, W.; El Mokhtari, N.E.; Mathiak, M.; Both, M.; Bolte, H.; Freitag-Wolf, S.; Bewig, B. Standardized quantification of pulmonary fibrosis in histological samples. BioTechniques 2008, 44, 507–517. [Google Scholar] [CrossRef]
- Xue, C.; Liu, Y.; Li, C.; Li, Y.; Yang, T.; Xie, L.; Zhou, P. Powerful protection against renal ischemia reperfusion injury by T cell-specific NF-κB inhibition. Transplantation 2014, 97, 391–396. [Google Scholar] [CrossRef]
- Herrera, J.; Henke, C.A.; Bitterman, P.B. Extracellular matrix as a driver of progressive fibrosis. J. Clin. Investig. 2018, 128, 45–53. [Google Scholar] [CrossRef]
- Aono, Y.; Ledford, J.G.; Mukherjee, S.; Ogawa, H.; Nishioka, Y.; Sone, S.; Beers, M.F.; Noble, P.W.; Wright, J.R. Surfactant protein-D regulates effector cell function and fibrotic lung remodeling in response to bleomycin injury. Am. J. Respir. Crit. Care Med. 2012, 185, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qin, W.; Liang, Q.; Zeng, J.; Yang, Q.; Chen, Y.; Wang, J.; Lu, W. Bufei Huoxue Capsule Alleviates Bleomycin-Induced Pulmonary Fibrosis in Mice via TGF-Β1/Smad2/3 Signaling. J. Ethnopharmacol. 2023, 316, 116733. [Google Scholar] [CrossRef]
- Liu, D.; Xu, L.; Zhang, X.; Shi, C.; Qiao, S.; Ma, Z.; Yuan, J. Snapshot: Implications for mTOR in Aging-related Ischemia/Reperfusion Injury. Aging Dis. 2019, 10, 116–133. [Google Scholar] [CrossRef]
- Romero, Y.; Bueno, M.; Ramirez, R.; Álvarez, D.; Sembrat, J.C.; Goncharova, E.A.; Rojas, M.; Selman, M.; Mora, A.L.; Pardo, A. mTORC 1 activation decreases autophagy in aging and idiopathic pulmonary fibrosis and contributes to apoptosis resistance in IPF fibroblasts. Aging Cell 2016, 15, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, X.; Zheng, H.; Liu, Q.; Zhang, H.; Wang, X.; Shen, T.; Wang, S.; Ren, D. Morusin induces apoptosis and autophagy via JNK, ERK and PI3K/Akt signaling in human lung carcinoma cells. Chem. Interact. 2020, 331, 109279. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.-L.; Zhang, M.-Y.; Liu, J.-Y.; Fang, L.-J.; Qu, Y.-Q. The role of autophagy in idiopathic pulmonary fibrosis: From mechanisms to therapies. Ther. Adv. Respir. Dis. 2022, 16, 1–21. [Google Scholar] [CrossRef]
- Huang, G.; Yang, X.; Yu, Q.; Luo, Q.; Ju, C.; Zhang, B.; Chen, Y.; Liang, Z.; Xia, S.; Wang, X.; et al. Overexpression of STX11 alleviates pulmonary fibrosis by inhibiting fibroblast activation via the PI3K/AKT/mTOR pathway. Signal Transduct. Target. Ther. 2024, 9, 306. [Google Scholar] [CrossRef]
- Huang, G.; Xu, X.; Ju, C.; Zhong, N.; He, J.; Tang, X.X. Identification and validation of autophagy-related gene expression for predicting prognosis in patients with idiopathic pulmonary fibrosis. Front. Immunol. 2022, 13, 997138. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.S.; Zeglinski, M.R.; Rattan, S.G.; Landry, N.M.; Ghavami, S.; Wigle, J.T.; Klonisch, T.; Halayko, A.J.; Dixon, I.M.C. Inhibition of Autophagy Inhibits the Conversion of Cardiac Fibroblasts to Cardiac Myofibroblasts. Oncotarget 2016, 7, 78516–78531. [Google Scholar] [CrossRef]
- Wang, Y.; Ping, Z.; Gao, H.; Liu, Z.; Xv, Q.; Jiang, X.; Yu, W. LYC inhibits the AKT signaling pathway to activate autophagy and ameliorate TGFB-induced renal fibrosis. Autophagy 2023, 20, 1114–1133. [Google Scholar] [CrossRef]
- Li, X.; Yang, K.-B.; Chen, W.; Mai, J.; Wu, X.-Q.; Sun, T.; Wu, R.-Y.; Jiao, L.; Li, D.-D.; Ji, J.; et al. CUL3 (cullin 3)-mediated ubiquitination and degradation of BECN1 (beclin 1) inhibit autophagy and promote tumor progression. Autophagy 2021, 17, 4323–4340. [Google Scholar] [CrossRef]
- Hohmann, M.S.; Habiel, D.M.; Coelho, A.L.; Verri, W.A.; Hogaboam, C.M. Quercetin Enhances Ligand-induced Apoptosis in Senescent Idiopathic Pulmonary Fibrosis Fibroblasts and Reduces Lung Fibrosis In Vivo. Am. J. Respir. Cell Mol. Biol. 2019, 60, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, X.; Cao, S.; Sun, Y.; He, X.; Jiang, B.; Yu, Y.; Duan, J.; Qiu, F.; Kang, N. Berberine represses human gastric cancer cell growth in vitro and in vivo by inducing cytostatic autophagy via inhibition of MAPK/mTOR/p70S6K and Akt signaling pathways. Biomed. Pharmacother. 2020, 128, 110245. [Google Scholar] [CrossRef]
- Yu, Z.; Jv, Y.; Cai, L.; Tian, X.; Huo, X.; Wang, C.; Zhang, B.; Sun, C.; Ning, J.; Feng, L.; et al. Gambogic acid attenuates liver fibrosis by inhibiting the PI3K/AKT and MAPK signaling pathways via inhibiting HSP90. Toxicol. Appl. Pharmacol. 2019, 371, 63–73. [Google Scholar] [CrossRef]
- Ma, J.; Ding, L.; Zang, X.; Yang, Y.; Zhang, W.; Li, X.; Zhao, D.; Zhang, Z.; Wang, Z.; Zhao, L.; et al. Qimai Feiluoping Decoction Inhibits EndMT to Alleviate Pulmonary Fibrosis by Reducing PI3K/AKT/mTOR Pathway-Mediated the Restoration of Autophagy. J. Inflamm. Res. 2025, ume 18, 8447–8475. [Google Scholar] [CrossRef]
- Gong, H.; Lyu, X.; Liu, Y.; Peng, N.; Tan, S.; Dong, L.; Zhang, X. Eupatilin inhibits pulmonary fibrosis by activating Sestrin2/PI3K/Akt/mTOR dependent autophagy pathway. Life Sci. 2023, 334, 122218. [Google Scholar] [CrossRef]
- Yu, J.-Z.; Ying, Y.; Liu, Y.; Sun, C.-B.; Dai, C.; Zhao, S.; Tian, S.-Z.; Peng, J.; Han, N.-P.; Yuan, J.-L.; et al. Antifibrotic action of Yifei Sanjie formula enhanced autophagy via PI3K-AKT-mTOR signaling pathway in mouse model of pulmonary fibrosis. Biomed. Pharmacother. 2019, 118, 109293. [Google Scholar] [CrossRef]
- Chen, H.; Chen, H.; Liang, J.; Gu, X.; Zhou, J.; Xie, C.; Lv, X.; Wang, R.; Li, Q.; Mao, Z.; et al. TGF-β1/IL-11/MEK/ERK signaling mediates senescence-associated pulmonary fibrosis in a stress-induced premature senescence model of Bmi-1 deficiency. Exp. Mol. Med. 2020, 52, 130–151. [Google Scholar] [CrossRef] [PubMed]
- van der Velden, J.L.J.; Ye, Y.; Nolin, J.D.; Hoffman, S.M.; Chapman, D.G.; Lahue, K.G.; Abdalla, S.; Chen, P.; Liu, Y.; Bennett, B.; et al. JNK inhibition reduces lung remodeling and pulmonary fibrotic systemic markers. Clin. Transl. Med. 2016, 5, 36. [Google Scholar] [CrossRef]
- von Bernuth, H.; Picard, C.; Puel, A.; Casanova, J. Experimental and natural infections in MyD88- and IRAK-4-deficient mice and humans. Eur. J. Immunol. 2012, 42, 3126–3135. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.F.; Woolley, A.; Sharma, N.S. Impact of MyD88 Deficiency on Innate Immune Function in COVID-19 Infection and Allotransplantation. Transplantation 2023, 107, 2084–2086. [Google Scholar] [CrossRef]
- Picard, C.; von Bernuth, H.; Ghandil, P.; Chrabieh, M.; Levy, O.; Arkwright, P.D.; McDonald, D.; Geha, R.S.; Takada, H.; Krause, J.C.; et al. Clinical Features and Outcome of Patients With IRAK-4 and MyD88 Deficiency. Medicine 2010, 89, 403–425. [Google Scholar] [CrossRef] [PubMed]
- von Bernuth, H.; Picard, C.; Jin, Z.; Pankla, R.; Xiao, H.; Ku, C.-L.; Chrabieh, M.; Ben Mustapha, I.; Ghandil, P.; Camcioglu, Y.; et al. Pyogenic Bacterial Infections in Humans with MyD88 Deficiency. Science 2008, 321, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-H.; He, L.; Zou, Z.-M.; Ding, Z.-C.; Zhang, X.; Wang, H.; Zhou, P.; Xie, L.; Xing, S.; Yi, C.-Z. A novel inhibitor of homodimerization targeting MyD88 ameliorates renal interstitial fibrosis by counteracting TGF-β1-Induced EMT in Vivo and in Vitro. Kidney Blood Press. Res. 2018, 43, 1677–1687. [Google Scholar] [CrossRef]
- Miao, Y.; Ding, Z.; Zou, Z.; Yang, Y.; Yang, M.; Zhang, X.; Li, Z.; Zhou, L.; Zhang, L.; Zhang, X.; et al. Inhibition of MyD88 by a novel inhibitor reverses two-thirds of the infarct area in myocardial ischemia and reperfusion injury. Am. J. Transl. Res. 2020, 12, 5151–5169. [Google Scholar]
- Li, Z.; Zhao, M.; Zhang, X.; Lu, Y.; Yang, Y.; Xie, Y.; Zou, Z.; Zhou, L.; Shang, R.; Zhang, L.; et al. TJ-M2010-5, a novel CNS drug candidate, attenuates acute cerebral ischemia-reperfusion injury through the MyD88/NF-κB and ERK pathway. Front. Pharmacol. 2022, 13, 1080438. [Google Scholar] [CrossRef]
- Franzén, L.; Lindvall, M.O.; Hühn, M.; Ptasinski, V.; Setyo, L.; Keith, B.P.; Collin, A.; Oag, S.; Volckaert, T.; Borde, A.; et al. Mapping spatially resolved transcriptomes in human and mouse pulmonary fibrosis. Nat. Genet. 2024, 56, 1725–1736. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Li, Z.; Zhao, M.; Zhao, Y.; Zou, Z.; Xie, Y.; Zhang, L.; Du, D.; Zhou, P. Attenuation of Pulmonary Fibrosis by the MyD88 Inhibitor TJ-M2010-5 Through Autophagy Induction in Mice. Biomedicines 2025, 13, 2214. https://doi.org/10.3390/biomedicines13092214
Yang Y, Li Z, Zhao M, Zhao Y, Zou Z, Xie Y, Zhang L, Du D, Zhou P. Attenuation of Pulmonary Fibrosis by the MyD88 Inhibitor TJ-M2010-5 Through Autophagy Induction in Mice. Biomedicines. 2025; 13(9):2214. https://doi.org/10.3390/biomedicines13092214
Chicago/Turabian StyleYang, Yang, Zeyang Li, Minghui Zhao, Yuanyuan Zhao, Zhimiao Zou, Yalong Xie, Limin Zhang, Dunfeng Du, and Ping Zhou. 2025. "Attenuation of Pulmonary Fibrosis by the MyD88 Inhibitor TJ-M2010-5 Through Autophagy Induction in Mice" Biomedicines 13, no. 9: 2214. https://doi.org/10.3390/biomedicines13092214
APA StyleYang, Y., Li, Z., Zhao, M., Zhao, Y., Zou, Z., Xie, Y., Zhang, L., Du, D., & Zhou, P. (2025). Attenuation of Pulmonary Fibrosis by the MyD88 Inhibitor TJ-M2010-5 Through Autophagy Induction in Mice. Biomedicines, 13(9), 2214. https://doi.org/10.3390/biomedicines13092214





