What Is New in Spinal Cord Injury Management: A Narrative Review on the Emerging Role of Nanotechnology
Abstract
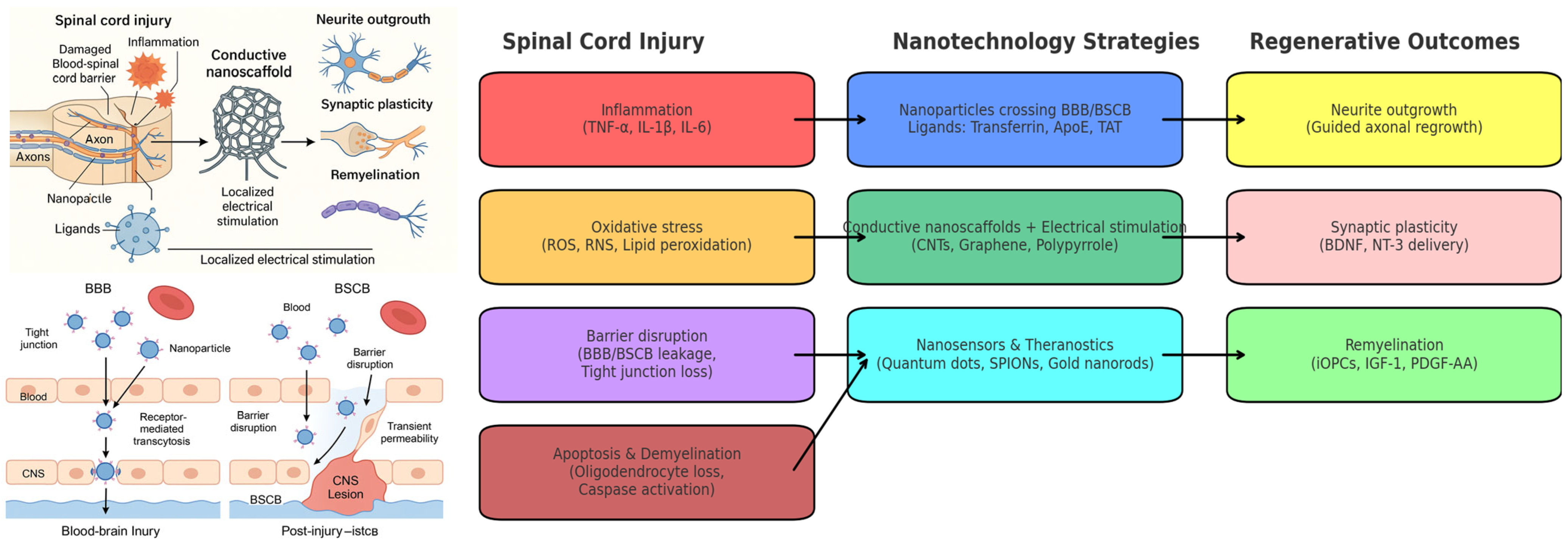
1. Introduction
2. Molecular and Cellular Mechanisms of Injury: Inflammation and Oxidative Stress
3. Nanotechnology for Ultra-Precise Diagnosis
4. Targeted Multi-Modal Therapeutic Strategies
4.1. Nanoparticle-Based Targeted Delivery for the Treatment of Spinal Cord Injury
4.2. Electro-Nanohybrid Stimulation
| Nanomaterial | Application | Reported Outcome vs. Sham | Limitations |
|---|---|---|---|
| Carbon Nanotubes (Cnts) | Scaffolds, hydrogels for axonal guidance and electrical conduction | 20–30% improvement in locomotor recovery and neurite outgrowth [62,63] | Risk of oxidative stress, inflammatory activation, long-term accumulation |
| Graphene Oxide (Go) | Coatings, scaffolds to enhance synaptic maturation and conduction | Higher compound action potentials, faster healing vs. sham [64] | Potential cytotoxicity, ROS generation, persistence in tissue |
| Gold Nanostructures (Nanorods, Nps) | Embedded in hydrogels for conductive and bioactive scaffolds | Improved axonal regrowth and functional recovery in rodents [65] | Non-degradable, long-term persistence in tissue |
| Conductive Polymers (Polypyrrole, Pedot:Pss) | Electroactive scaffolds, drug-releasing conductive systems | 15–40% improvement in locomotor scores, increased axonal density and remyelination [66,67] | Stability issues, possible delamination, toxic degradation byproducts |
5. Nanotechnological Strategies for CNS Drug Delivery
| Parameter | BBB (Blood–Brain Barrier) | BSCB (Blood–Spinal Cord Barrier) | Representative Nanoparticle Strategy | Entry Mechanism | References |
|---|---|---|---|---|---|
| Structure | Continuous endothelium with tight junctions; highly selective | Similar to BBB, slightly more permeable under physiological conditions | Ligand-functionalized nanoparticles (e.g., transferrin, ApoE, RVG) | Receptor-mediated transcytosis | [2,70] |
| Therapeutic challenge | Blocks over 98% of systemically administered drugs | Less restrictive but still limits large or hydrophilic molecules | Lipid or polymeric NPs designed to exploit specific transport pathways | Ligand–receptor binding across endothelial cells | [1] |
| Nanoparticle strategy | Functionalization with ligands for receptor-mediated transcytosis (e.g., transferrin) | Exploitation of increased permeability after injury | Iron oxide or gold NPs for theranostic delivery | Passive diffusion during barrier disruption | [1,70] |
| Optimal timing | Constant, but difficult without targeting ligands | Subacute phase: hours to days post injury, during inflammation | Time-controlled delivery with responsive carriers | Exploitation of transient barrier permeability | [2] |
| Clinical applications | Alzheimer’s, brain tumors, encephalitis | Spinal cord injury, multiple sclerosis, spinal inflammation | SPIONs, liposomes, polymeric nanocarriers | Depends on disease context | [1] |
| Composition/Material | Example of Functionalization | Target Cell/Pathology | Rationale and Reported Outcomes |
|---|---|---|---|
| Lipid nanoparticles (LNPs) | RVG peptide, ApoE | Neurons, endothelial cells | Ionizable lipids enable endosomal escape; RVG improves neuronal uptake. RVG–LNP–siRNA improved silencing efficiency and motor recovery in SCI models [78]. |
| Polymeric nanoparticles (PLGA, PEG-PLA) | PEGylation, BDNF loading | Broad (neurons, glia) | Protect fragile proteins, enable sustained release. PEG–PLGA–BDNF prolonged delivery and improved motor recovery vs. free BDNF [77]. |
| Inorganic nanoparticles (Gold, Iron oxide) | Surface thiol/PEG, Tf | Lesion site, imaging-guided therapy | Provide intrinsic imaging properties (MRI, optical) + therapeutic cargo. Gold NPs in hydrogels enhanced axonal regrowth [65]. |
| Hybrid nanocarriers (Lipid–polymer, polymer–inorganic) | Dual ligands (e.g., mannose + Tf) | Macrophages, neurons | Combine stability, targeting, and multifunctionality. Mannose–PLGA NPs promoted M2 polarization, reducing lesion size [79]. |
| Nanofibrous scaffolds (Collagen, PCL + CNT/Graphene) | Laminin, RGD motifs | Axons, synaptic connections | Provide topographic guidance and electrical stimulation; RGD enhances adhesion/angiogenesis. Graphene scaffolds supported axon elongation and functional recovery [64]. |
6. AI-Guided Personalized Nanomedicine
7. Integration with Conventional Therapies and Personalized Nanomedicine
Physical Rehabilitation
8. Discussion
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tran, A.P.; Warren, P.M.; Silver, J. The Biology of Regeneration Failure and Success after Spinal Cord Injury. Physiol. Rev. 2018, 98, 881–917. [Google Scholar] [CrossRef]
- Silva, G.A. Nanotechnology Approaches to Crossing the Blood–Brain Barrier and Drug Delivery to the CNS. BMC Neurosci. 2008, 9 (Suppl. S3), S4. [Google Scholar] [CrossRef]
- Patel, T.; Zhou, J.; Piepmeier, J.M.; Saltzman, W.M. Polymeric Nanoparticles for Drug Delivery to the Central Nervous System. Adv. Drug Deliv. Rev. 2012, 64, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; John, J.V.; McCarthy, A.; Xie, J. New Forms of Electrospun Nanofiber Materials for Biomedical Applications. J. Mater. Chem. B 2020, 8, 3733–3746. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hou, Y.; Martinez, D.; Kurniawan, D.; Chiang, W.-H.; Bartolo, P. Carbon Nanomaterials for Electro-Active Structures: A Review. Polymers 2020, 12, 2946. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and Surface Engineering of Iron Oxide Nanoparticles for Biomedical Applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef]
- Papa, S.; Rossi, F.; Ferrari, R.; Mariani, A.; De Paola, M.; Caron, I.; Fiordaliso, F.; Bisighini, C.; Sammali, E.; Colombo, C.; et al. Selective nanovector mediated treatment of activated proinflammatory microglia/macrophages in spinal cord injury. ACS Nano 2013, 7, 9881–9895. [Google Scholar] [CrossRef] [PubMed]
- Liaw, K.; Zhang, Z.; Kannan, S. Neuronanotechnology for brain regeneration. Adv. Drug Deliv. Rev. 2019, 148, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Papastefanaki, F.; Matsas, R. From Demyelination to Remyelination: The Road toward Therapies for Spinal Cord Injury. Glia 2015, 63, 1101–1125. [Google Scholar] [CrossRef] [PubMed]
- Jokerst, J.V.; Gambhir, S.S. Molecular Imaging with Theranostic Nanoparticles. Acc. Chem. Res. 2011, 44, 1050–1060. [Google Scholar] [CrossRef]
- Gong, W.; Zhang, T.; Che, M.; Wang, Y.; He, C.; Liu, L.; Lv, Z.; Xiao, C.; Wang, H.; Zhang, S. Recent Advances in Nanomaterials for the Treatment of Spinal Cord Injury. Mater. Today Bio. 2022, 18, 100524. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Blanco-Andujar, C.; Walter, A.; Cotin, G.; Bordeianu, C.; Mertz, D.; Felder-Flesch, D.; Begin-Colin, S. Design of Iron Oxide-Based Nanoparticles for MRI and Magnetic Hyperthermia. Nanomedicine 2016, 11, 1889–1910. [Google Scholar] [CrossRef] [PubMed]
- Dalamagkas, K.; Tsintou, M.; Seifalian, A. Translational Regenerative Therapies for Chronic Spinal Cord Injury. Int. J. Mol. Sci. 2018, 19, 1776. [Google Scholar] [CrossRef]
- Burnouf, T.; Agrahari, V.; Agrahari, V. Extracellular Vesicles as Nanomedicine: Hopes and Hurdles in Clinical Translation. Int. J. Nanomed. 2019, 14, 8847–8859. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.N.; Bosse-Joseph, C.C.; Kumari, R.; Bailey, W.M.; Park, K.A.; Slone, V.K.; Gensel, J.C. Nonresolving Neuroinflammation Regulates Axon Regeneration in Chronic Spinal Cord Injury. J. Neurosci. 2025, 45, e1017242024. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The Devil Is in the Details. J. Neurochem. 2016, 137, 296–309. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- David, S.; Kroner, A. Repertoire of Microglial and Macrophage Responses after Spinal Cord Injury. Brain Res. 2011, 1381, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yin, Y.; Xu, S.-J.; Wu, Y.-P.; Chen, W.-S. Inflammation & Apoptosis in Spinal Cord Injury. Indian J. Med. Res. 2012, 135, 287–296. [Google Scholar] [PubMed]
- Kigerl, K.A.; Gensel, J.C.; Ankeny, D.P.; Alexander, J.K.; Donnelly, D.J.; Popovich, P.G. Identification of Two Distinct Macrophage Subsets with Divergent Effects Causing Either Neurotoxicity or Regeneration after Spinal Cord Injury. J. Neurosci. 2009, 29, 13435–13444. [Google Scholar] [CrossRef]
- Goldmann, T.; Wieghofer, P.; Jordão, M.J.; Prutek, F.; Hagemeyer, N.; Frenzel, K.; Amann, L.; Staszewski, O.; Kierdorf, K.; Krueger, M.; et al. Origin, fate and ynamics of macrophages at central nervous system interfaces. Nat. Immunol. 2016, 17, 797–805. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miron, V.E.; Boyd, A.; Zhao, J.-W.; Yuen, T.J.; Ruckh, J.M.; Shadrach, J.L.; van Wijngaarden, P.; Wagers, A.J.; Williams, A.; Franklin, R.J.M.; et al. M2 Microglia and Macrophages Drive Oligodendrocyte Differentiation during CNS Remyelination. Nat. Neurosci. 2013, 16, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.-M.; Chen, S.-Y.; Xu, Q.-J.; Fu, S.-P.; Yang, Y.-C.; Zou, W.-H.; Zhang, M.; Liu, J.; Wan, W.-H.; Peng, J.-C.; et al. Neuroinflammation and Scarring after Spinal Cord Injury: Therapeutic Roles of MSCs on Inflammation and Glial Scar. Front. Immunol. 2021, 12, 751021. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, Z.-L.; Xie, H.; Tian, X.-B.; Xu, H.-L.; Li, W.; Yao, S.; Zhang, H. Microglial Depletion Impairs Glial Scar Formation and Aggravates Inflammation partly by inhibiting STAT3 phosphorylation in astrocytes after spinal cord injury. Neural Regen. Res. 2023, 18, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Leal-Filho, M.B. Spinal Cord Injury: From Inflammation to Glial Scar. Surg. Neurol. Int. 2011, 2, 112. [Google Scholar] [CrossRef]
- Qian, Z.; Chang, J.; Jiang, F.; Ge, D.; Yang, L.; Li, Y.; Chen, H.; Cao, X. Excess Administration of miR-340-5p Ameliorates Spinal Cord Injury-Induced Neuroinflammation and Apoptosis by Modulating the P38-MAPK Signaling Pathway. Brain Behav. Immun. 2020, 87, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Joshi, H.P.; Kim, K.-T.; Kim, Y.Y.; Yeo, K.; Choi, H.; Kim, Y.W.; Choi, U.Y.; Kumar, H.; Sohn, S.; et al. Combined Treatment with Fasudil and Menthol Improves Functional Recovery in Rat SCI Model. Biomedicines 2020, 8, 258. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gorman, P.H.; Forrest, G.F.; Asselin, P.K.; Scott, W.; Kornfeld, S.; Hong, E.; Spungen, A.M. The Effect of Exoskeletal-Assisted Walking on Spinal Cord Injury Bowel Function: Results from a Randomized Trial and Comparison to Other Physical Interventions. J. Clin. Med. 2021, 10, 964. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jure, I.; Labombarda, F. Spinal Cord Injury Drives Chronic Brain Changes. Neural Regen. Res. 2017, 12, 1044–1047. [Google Scholar] [CrossRef]
- Ankeny, D.P.; Guan, Z.; Popovich, P.G. B Cells Produce Pathogenic Antibodies after Traumatic Spinal Cord. J. Clin. Investig. 2009, 119, 2990–2999. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sandrow-Feinberg, H.R.; Houlé, J.D. Exercise after Spinal Cord Injury as an Agent for Neuroprotection, Regeneration and Rehabilitation. Brain Res. 2015, 1619, 12–21. [Google Scholar] [CrossRef]
- Abe, Y.; Yamamoto, T.; Sugiyama, Y.; Watanabe, T.; Saito, N.; Kayama, H.; Kumagai, T. Apoptotic Cells Associated with Wallerian Degeneration after Experimental SCI. J. Neurotrauma 1999, 16, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Nakamura, M.; Katoh, H.; Miyao, T.; Shimazaki, T.; Ishii, K.; Yamane, J.; Yoshimura, A.; Iwamoto, Y.; Toyama, Y.; et al. Conditional Ablation of Stat3 Prevents Astrocyte Scar Formation after SCI. Nat. Med. 2006, 12, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.-J.; Kim, G.M.; Lee, J.M.; He, Y.Y.; Xu, J.; Hsu, C.Y. JNK Activation Contributes to DP5 Induction and Apoptosis following SCI. Neurobiol. Dis. 2005, 20, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.-J.; Zhang, L.; Samad, O.A.; Suter, M.R.; Yasuhiko, K.; Xu, Z.Z.; Park, J.Y.; Lind, A.L.; Ma, Q.; Ji, R.R. JNK-Induced MCP-1 Production in Spinal Cord Astrocytes Contributes to Neuropathic Pain. J. Neurosci. 2009, 29, 4096–4108. [Google Scholar] [CrossRef] [PubMed]
- Repici, M.; Chen, X.; Morel, M.-P.; Doulazmi, M.; Sclip, A.; Cannaya, V.; Veglianese, P.; Kraftsik, R.; Mariani, J.; Borsello, T.; et al. Specific Inhibition of the JNK Pathway Promotes Locomotor Recovery and Neuroprotection after Mouse Spinal Cord Injury. Neurobiol. Dis. 2012, 46, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.M.; Tep, C.; Yune, T.Y.; Zhou, X.Z.; Uchida, T.; Lu, K.P.; Yoon, S.O. Opposite Regulation of Oligodendrocyte Apoptosis by JNK3 and Pin1 after Spinal Cord Injury. J. Neurosci. 2007, 27, 8395–8404. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Z.; Xu, X.M.; Hu, R.; Du, C.; Zhang, S.X.; McDonald, J.W.; Dong, H.X.; Wu, Y.J.; Fan, G.S.; Jacquin, M.F.; et al. Neuronal and Glial Apoptosis after Traumatic Spinal Cord Injury. J. Neurosci. 1997, 17, 5395–5406. [Google Scholar] [CrossRef]
- Yamauchi, K.; Osuka, K.; Takayasu, M.; Usuda, N.; Nakazawa, A.; Nakahara, N.; Yoshida, M.; Aoshima, C.; Hara, M.; Yoshida, J. Activation of JAK/STAT Signalling in Neurons following Spinal Cord Injury in Mice. J. Neurochem. 2006, 96, 1060–1070. [Google Scholar] [CrossRef]
- Yao, X.; Sun, C.; Fan, B.; Zhao, C.; Zhang, Y.; Duan, H.; Pang, Y.; Shen, W.; Li, B.; Wang, X.; et al. Neurotropin Exerts Neuroprotective Effects after Spinal Cord Injury by Inhibiting Apoptosis and Modulating Cytokines. J. Orthop. Transl. 2021, 27, 97–106. [Google Scholar] [CrossRef]
- Toader, C.; Dumitru, A.V.; Eva, L.; Serban, M.; Covache-Busuioc, R.A.; Ciurea, A.V. Nanoparticle Strategies for Treating CNS Disorders: A Comprehensive Review of Drug Delivery and Theranostic Applications. Int. J. Mol. Sci. 2024, 25, 569. [Google Scholar] [CrossRef]
- Lee, D.-E.; Koo, H.; Sun, I.-C.; Ryu, J.H.; Kim, K.; Kwon, I.C. Multifunctional Nanoparticles for Multimodal Imaging and Theragnosis. Chem. Soc. Rev. 2012, 41, 2656–2672. [Google Scholar] [CrossRef]
- Jokerst, J.V.; Lobovkina, T.; Zare, R.N.; Gambhir, S.S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 2011, 6, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Antaris, A.L.; Dai, H. Near-Infrared Fluorophores for Biomedical Imaging. Nat. Biomed. Eng. 2017, 1, 0010. [Google Scholar] [CrossRef]
- Haraden, C.A.; Huebner, J.L.; Hsueh, M.F.; Li, Y.J.; Kraus, V.B. Synovial fluid biomarkers associated with osteoarthritis severity reflect macrophage and neutrophil related inflammation. Arthritis Res. Ther. 2019, 21, 146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lateef, A.; Petri, M. Unmet medical needs in systemic lupus erythematosus. Arthritis Res. Ther. 2012, 14, S4. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid Nanoparticles for mRNA Delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Burkhart, A.; Melander, F.; Andresen, T.L.; Moos, T. Targeting Transferrin Receptors at the Blood–Brain Barrier: A Systematic Review. Prog. Neurobiol. 2019, 181, 101665. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, R.; Han, L.; Ke, W.; Shao, K.; Ye, L.; Lou, J.; Jiang, C. Brain-Targeting Gene Delivery and Cellular Internalization Mechanisms for Modified Rabies Virus Glycoprotein RVG29 Nanoparticles. Biomaterials 2009, 30, 4195–4202. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yong, J.; Marciano, P.; Doig, H.; Mao, G.; Clark, J. The Translation of Nanomedicines in the Contexts of Spinal Cord Injury and Repair. Cells 2024, 13, 569. [Google Scholar] [CrossRef]
- Wang, L.; Wilhelm, S. Exploiting endothelial transcytosis to reach into the brain. Nat. Mater. 2023, 22, 282–283. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Xu, W.; Ren, Y.; Wang, Z.; He, X.; Huang, R.; Ma, B.; Zhao, J.; Zhu, R.; Cheng, L. Spinal Cord Injury: Molecular Mechanisms and Therapeutic Interventions. Signal Transduct. Target. Ther. 2023, 8, 245. [Google Scholar] [CrossRef]
- Champion, J.A.; Katare, Y.K.; Mitragotri, S. Particle Shape: A New Design Parameter for Micro- and Nanoscale Drug Delivery Carriers. J. Control. Release. 2007, 121, 3–9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ishida, T.; Kiwada, H. Accelerated Blood Clearance (ABC) Phenomenon upon Repeated Injection of PEGylated Liposomes. Int. J. Pharm. 2008, 354, 56–62. [Google Scholar] [CrossRef]
- Lila, A.S.A.; Ishida, T. Liposomal Delivery Systems: Design Optimization and Current Applications. J. Biol. Pharm. Bull. 2017, 40, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Khlebtsov, N.; Dykman, L. Biomedical Applications of Multifunctional Gold-Based Nanocomposites. Chem. Soc. Rev. 2011, 40, 1647–1671. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of siRNA to the Mouse Brain by Systemic Injection of Targeted Exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef]
- Lener, T.; Gimona, M.; Aigner, L.; Börger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Del Portillo, H.A.; et al. Applying Extracellular Vesicles Based Therapeutics in Clinical Trials–An ISEV Position Paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, S.; Wang, B.; Cui, S.; Yan, J. Toxicity of Cationic Lipids and Cationic Polymers in Gene Delivery. J. Control. Release 2006, 114, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Walthers, C.M.; Seidlits, S.K. Gene Delivery Strategies to Promote Spinal Cord Repair. Biomark. Insights. 2015, 10, 11–29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Q.; Zheng, J.; Li, L.; Yeh, J.M.; Xie, X.; Zhao, Y.; Li, C.; Hou, G.; Yan, H. Bioinspired Conductive Oriented Nanofiber Felt with Efficient ROS Clearance and Anti-Inflammation for Inducing M2 Macrophage Polarization and Accelerating Spinal Cord Injury Repair. Bioact. Mater. 2025, 46, 173–194. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cellot, G.; Toma, F.M.; Varley, Z.K.; Laishram, J.; Villari, A.; Quintana, M.; Cipollone, S.; Prato, M.; Ballerini, L. Carbon Nanotube Scaffolds Tune Synaptic Strength in Cultured Neural Circuits: Novel Frontiers in Nanomaterial-Tissue Interactions. J. Neurosci. 2011, 31, 12945–12953. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fabbro, A.; Scaini, D.; León, V.; Vázquez, E.; Cellot, G.; Privitera, G.; Lombardi, L.; Torrisi, F.; Tomarchio, F.; Bonaccorso, F.; et al. Graphene-Based Interfaces Do Not Alter Target Nerve Cells. ACS Nano. 2012, 10, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Park, J.; Sim, S.H.; Sung, M.G.; Kim, K.S.; Hong, B.H.; Hong, S. Enhanced Differentiation of Human Neural Stem Cells into Neurons on Graphene. Adv. Mater. 2011, 23, H263–H267. [Google Scholar] [CrossRef] [PubMed]
- Gui, C.; Cui, D.X. Functionalized Gold Nanorods for tumor imaging and targeted therapy. Cancer Biol. Med. 2012, 9, 221–233. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Emori, Y.; Kawasaki, H.; Imajoh, S.; Imahori, K.; Suzuki, K. Endogenous inhibitor for calcium-dependent cysteine protease contains four internal repeats that could be responsible for its multiple reactive sites. Proc. Natl. Acad. Sci. USA 1987, 84, 3590–3594. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive Polymers: Towards a Smart Biomaterial for Tissue Engineering. Adv. Drug Deliv. Rev. 2014, 82–83, 18–35. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The Blood–Brain Barrier: Structure, Regulation, and Drug Delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, S.; Chen, X.; Yang, T.; Cheng, J.; Liu, E.; Jiang, L.; Song, M.; Shu, H.; Ma, Y. Revealing the Mechanisms of Blood–Brain Barrier in Chronic Neurodegenerative Disease: An Opportunity for Therapeutic Intervention. Rev. Neurosci. 2024, 35, 895–916. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.M.; Patel, B.M. Crossing the Blood–Brain Barrier: Recent Advances in Drug Delivery to the Brain. Adv. Drug Deliv. Rev. 2012, 64, 7–23. [Google Scholar] [CrossRef]
- Bartanusz, V.; Jezova, D.; Alajajian, B.; Digicaylioglu, M. The Blood–Spinal Cord Barrier: Morphology and Clinical Implications. Brain Res. Rev. 2011, 64, 328–363. [Google Scholar] [CrossRef] [PubMed]
- Whetstone, W.D.; Hsu, J.Y.; Eisenberg, M.; Werb, Z.; Noble-Haeusslein, L.J. Blood-spinal cord barrier after spinal cord injury: Relation to revascularization and wound healing. J. Neurosci. 2003, 23, 7820–7828. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Popovich, P.G.; Horner, P.J.; Mullin, B.B.; Stokes, B.T. A quantitative spatial analysis of the blood-spinal cord barrier. I. Perme-ability changes after experimental spinal contusion injury. Exp. Neurol. 1996, 142, 258–275. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood–Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Shi, Y.; Jiang, W.; Han, J.; Huang, S.; Jiang, X. Lactoferrin-Conjugated PEG-PLGA Nanoparticles for Brain Delivery: Preparation, Characterization and Efficacy in Parkinson’s Disease. Int. J. Pharm. 2011, 415, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. Integrins: Bidirectional, Allosteric Signaling Machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, Z.; Guo, M.; Wang, Y.; Liu, J.; Liu, Y.; Li, M.; Wei, T.; Li, P.; Zhao, Y.; et al. TET3-Facilitated Differentiation of Human Umbilical Cord Mesenchymal Stem Cells into Oligodendrocyte Precursor Cells for Spinal Cord Injury Recovery. J. Transl. Med. 2024, 22, 1118. [Google Scholar] [CrossRef]
- Kumar, P.; Wu, H.; McBride, J.L.; Jung, K.-E.; Kim, M.H.; Davidson, B.L.; Lee, S.K.; Shankar, P. Transvascular Delivery of Small Interfering RNA to the Central Nervous System. Nature 2007, 448, 39–43. [Google Scholar] [CrossRef]
- Papa, S.; Ferrari, R.; De Paola, M.; Rossi, F.; Mariani, A.; Caron, I.; Sammali, E.; Peviani, M.; Dell’Oro, V.; Colombo, C.; et al. Polymeric Nanoparticle System to Target Activated Microglia/Macrophages in Spinal Cord Injury. J. Control. Release 2014, 174, 15–26. [Google Scholar] [CrossRef]
- Fortun, J.; Puzis, R.; Pearse, D.D.; Gage, F.H.; Bunge, M.B. Muscle Injection of AAV-NT3 Promotes Anatomical Reorganization of CST Axons and Improves Behavioral Outcome following SCI. J. Neurotrauma 2009, 26, 941–953. [Google Scholar] [CrossRef]
- Li, Q.; Li, C.; Zhang, X. Research Progress on the Effects of Different Exercise Modes on the Secretion of Exerkines after SCI. Cell. Mol. Neurobiol. 2024, 44, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Van de Winckel, A.; Carpentier, S.T.; Deng, W.; Bottale, S.; Zhang, L.; Hendrickson, T.; Linnman, C.; Lim, K.O.; Mueller, B.A.; Philippus, A.; et al. Identifying Body Awareness-Related Brain Network Changes after Cognitive Multisensory Rehabilitation for Neuropathic Pain Relief in Adults with Spinal Cord Injury: Delayed Treatment Arm Phase I Randomized Controlled Trial. medRxiv 2023. [Google Scholar] [CrossRef]
| Nanomarker Type | Functionalization/Target | Imaging Modality | Key Advantages | Potential Applications | Examples/ References |
|---|---|---|---|---|---|
| SPIONs (Superparamagnetic Iron Oxide Nanoparticles) | Antibodies, peptides, small molecules targeting inflammation or apoptosis | MRI | High contrast, biocompatibility, long circulation, tissue-specific accumulation | Non-invasive monitoring of neuroinflammation; lesion tracking | [11,41] |
| Gold nanoparticles/nanorods | Surface conjugation with peptides or antibodies | Photoacoustic imaging | Strong optical absorption, deep tissue penetration, vascular/tumor structure visualization | Vascular imaging; monitoring of oxidative stress and tissue hypoxia | [42,43] |
| Quantum dots (QDs) | Functionalized with ligands for neuronal or glial markers | NIR fluorescence imaging | Bright emission, tunable wavelength, minimal background noise | Intraoperative surgical guidance; neuronal apoptosis detection | [43] |
| Carbon nanotubes | Functionalization with injury-specific ligands | Photoacoustic imaging | High optical absorption, acoustic signal conversion, hybrid optical ultrasound | Mapping vascular and structural alterations; oxidative stress detection | [42] |
| Radiolabeled nanoparticles (e.g., 18F, 68Ga conjugates) | PEGylation, peptides for receptor targeting | PET | Real-time metabolic tracking, high sensitivity, complements anatomical MRI | Functional neuroimaging; early detection of metabolic dysfunction after SCI | [41] |
| Multimodal nanoparticles (SPIONs + QDs, gold + NIR dyes) | Dual/multiple surface ligands | MRI + NIRF/ PET + PA | Combined structural + molecular information, intraoperative guidance | Pre-operative lesion mapping with MRI; real-time intraoperative NIRF guidance | [40] |
| Theranostic nanoparticles | Drug-loaded plus targeting ligands | MRI/ NIRF/ PET | Simultaneous therapy + diagnosis, dynamic monitoring of response | Monitoring treatment efficacy; personalized medicine approaches | [40,41] |
| Cargo Type | Optimal NP Design | Size/Shape Rationale | Targeting Strategies | Advantages | Limitations/Adverse Effects |
|---|---|---|---|---|---|
| Growth factors (BDNF, IGF) | Liposomes, PLGA NPs | >150 nm sustain release, protein stability | PEGylation, antibody conjugation | Protect proteins, controlled release | Burst release, acidic byproducts (PLGA irritation) |
| siRNA/mRNA | Lipid nanoparticles, exosomes, cationic polymers | <100 nm efficient endocytosis and escape | RVG peptide (neurons), mannose (microglia), transferrin (BBB) | Efficient transfection, systemic delivery | Immune activation (TLR), PEG immune reactions |
| CRISPR/Cas components | Hybrid lipid–polymer NPs, gold NPs | <120 nm facilitate nuclear delivery | Nuclear localization peptides, antibody targeting | Genome editing, long-term correction | Off-target effects, immune sensing of Cas proteins |
| Small molecules/ antioxidants | Inorganic NPs (Au, SPIONs), polymeric micelles | Variable; spheres more stable | Often passive or minimal | ROS scavenging, magnetic/optical guidance | Accumulation, oxidative stress, organ retention |
| Nanoparticle Type | Therapeutic Application | Clinical Status | Representative Outcomes | Clinical Trial ID (NCT#) |
|---|---|---|---|---|
| Liposomes | Delivery of corticosteroids, neuroprotective agents | Evaluated in neurological disorders | Improved drug stability and bioavailability | |
| Iron oxide nanoparticles (Ferumoxytol) | Imaging and theranostics | Approved for anemia; tested in CNS imaging | Enhanced MRI contrast at lesion sites | |
| Exosomes/extracellular vesicles | Delivery of RNA, proteins; regenerative therapies | Phase I/II in oncology and neurodegenerative diseases | Good safety profile; immune compatibility | NCT03608631 |
| Polymeric nanoparticles (PLGA, PEG-PLA) | Growth factors, nucleic acids (preclinical SCI, oncology) | Some formulations in early phase clinical trials | Sustained release; neuroprotection | NCT04314895 |
| Application Area | Description | Rehabilitative Benefit | Development Status |
|---|---|---|---|
| Neurotrophic Factor Delivery | Use of nanoparticles to deliver agents like BDNF, NGF, or IGF-1 to enhance plasticity during motor rehabilitation. | Amplifies the effect of activity-based therapies by promoting synaptic and axonal plasticity. | Preclinical |
| Bioelectronic Interfaces | Integration of conductive nanomaterials into scaffolds or implants to restore electrical signaling and support neuronal reactivation. | Enables the functional reactivation of spinal circuits and synergy with FES or robotic training. | Preclinical to early prototyping |
| Nanosensors for Monitoring | Implantable or wearable nanosensors to monitor inflammation, neural activity, or metabolic markers during therapy. | Personalizes rehabilitation intensity and timing based on real-time physiological data. | Emerging technology |
| Gene Modulation via Nanocarriers | Nanoparticles carrying siRNA or miRNA to modulate genes involved in inhibitory signaling or regeneration during rehabilitation phases. | Maximizes the molecular environment’s responsiveness to training by modulating key signaling pathways. | Preclinical studies in animal models |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raciti, L.; Raciti, G.; Calabrò, R.S. What Is New in Spinal Cord Injury Management: A Narrative Review on the Emerging Role of Nanotechnology. Biomedicines 2025, 13, 2176. https://doi.org/10.3390/biomedicines13092176
Raciti L, Raciti G, Calabrò RS. What Is New in Spinal Cord Injury Management: A Narrative Review on the Emerging Role of Nanotechnology. Biomedicines. 2025; 13(9):2176. https://doi.org/10.3390/biomedicines13092176
Chicago/Turabian StyleRaciti, Loredana, Gianfranco Raciti, and Rocco Salvatore Calabrò. 2025. "What Is New in Spinal Cord Injury Management: A Narrative Review on the Emerging Role of Nanotechnology" Biomedicines 13, no. 9: 2176. https://doi.org/10.3390/biomedicines13092176
APA StyleRaciti, L., Raciti, G., & Calabrò, R. S. (2025). What Is New in Spinal Cord Injury Management: A Narrative Review on the Emerging Role of Nanotechnology. Biomedicines, 13(9), 2176. https://doi.org/10.3390/biomedicines13092176







