Dexamethasone Suppression Testing in Patients with Adrenal Incidentalomas with/Without Mild Autonomous Cortisol Secretion: Spectrum of Cortisol Cutoffs and Additional Assays (An Updated Analysis)
Abstract
1. Introduction
Objective
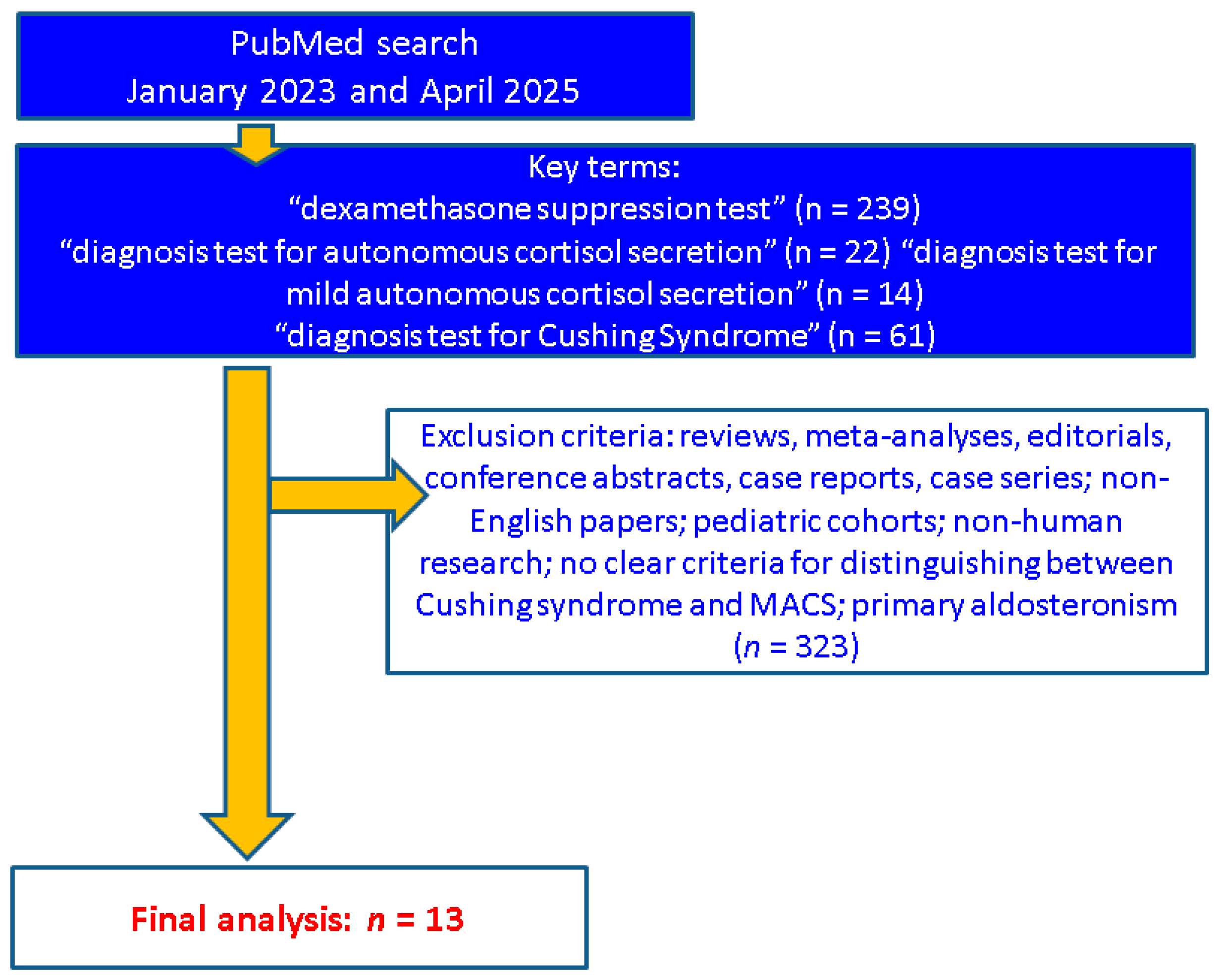
2. Methods
3. Sample-Focused Analysis
3.1. Post-DST Cortisol Cutoffs in Relationship with the Spectrum of Comorbidities
3.2. DST and Additional Assays
3.2.1. Baseline Morning Blood ACTH
3.2.2. Urinary Steroid Profile
3.2.3. Salivary Cortisone
3.2.4. DHEAS
3.3. DST Results: Variations During Long-Term Surveillance
4. Discussion
4.1. The Spectrum of DST: Challenges and Pitfalls
4.2. From DST to MACS: A Modern Pathway, a Traditional Road
4.3. Current Limits and Further Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACTH | adrenocorticotropic hormone |
| ACS | autonomous cortisol secretion |
| AI | adrenal incidentaloma |
| AUC | area under the curve |
| BMI | body mass index |
| CI | confidence interval |
| DHEAS | dehydroepiandrosterone sulfate |
| DST | 1-mg dexamethasone suppression test |
| eGFR | estimated glomerular filtration rate |
| F | female |
| FGF | Fibroblast Growth Factor |
| HPA | hypothalamic-pituitaryadrenal |
| HR | hazard ratio |
| IQR | interquartile range |
| LNSC | late-night salivary cortisol |
| LNSE | late-night salivary cortisone |
| LDDST | low-dose dexamethasone suppression test |
| MACS | mild autonomous cortisol secretion |
| M | male |
| NFA | non-functioning adrenal adenomas |
| n | number of studies |
| N | number of patients |
| OR | odds ratio |
| ROC | Receiver Operating Characteristic |
| SD | standard deviation |
| THF | tetrahydrocortisol |
| THS | tetrahydro-11-deoxycortisol |
| THE | tetrahydrocortisone |
| UFC | urinary free cortisol |
| vs. | versus |
| y | years |
References
- Asirvatham, A.R.; Balachandran, K.; Mahadevan, S.; Balasubramaniam, S.K. Hypothalamic-Pituitary-Adrenal Axis Recovery Following the 1-mg Overnight Dexamethasone Suppression Test in Healthy Volunteers. Int. J. Endocrinol. Metab. 2020, 18, e94908. [Google Scholar] [CrossRef]
- Ferrante, E.; Simeoli, C.; Mantovani, G.; Pivonello, R. Who and how to screen for endogenous hypercortisolism in patients with mood disorders. J. Endocrinol. Investig. 2025, 48 (Suppl. S1), 75–82. [Google Scholar] [CrossRef] [PubMed]
- Carsote, M.; Valea, A.; Dumitru, N.; Terzea, D.; Petrova, E.; Albu, S.; Buruiana, A.; Ghemigian, A. Metastases in daily endocrine practice. Arch. Balk. Med. Union 2016, 51, 476–480. [Google Scholar]
- Petersenn, S. Overnight 1 mg dexamethasone suppression test and 24 h urine free cortisol-accuracy and pitfalls when screening for Cushing’s syndrome. Pituitary 2022, 25, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Galm, B.P.; Qiao, N.; Klibanski, A.; Biller, B.M.K.; Tritos, N.A. Accuracy of Laboratory Tests for the Diagnosis of Cushing Syndrome. J. Clin. Endocrinol. Metab. 2020, 105, dgaa105. [Google Scholar] [CrossRef]
- Fleseriu, M.; Auchus, R.; Bancos, I.; Ben-Shlomo, A.; Bertherat, J.; Biermasz, N.R.; Boguszewski, C.L.; Bronstein, M.D.; Buchfelder, M.; Carmichael, J.D.; et al. Consensus on diagnosis and management of Cushing’s disease: A guideline update. Lancet Diabetes Endocrinol. 2021, 9, 847–875. [Google Scholar] [CrossRef]
- Ueland, G.Å.; Grinde, T.; Methlie, P.; Kelp, O.; Løvås, K.; Husebye, E.S. Diagnostic testing of autonomous cortisol secretion in adrenal incidentalomas. Endocr. Connect. 2020, 9, 963–970. [Google Scholar] [CrossRef]
- Park, S.S.; Kim, J.H. Recent Updates on the Management of Adrenal Incidentalomas. Endocrinol. Metab. 2023, 38, 373–380. [Google Scholar] [CrossRef]
- Janiak, K.; Józwik-Plebanek, K.; Kamiński, G. Recent guidelines for diagnostic and therapeutic management of accidentally detected adrenal tumours (incidentaloma) in adults. Endokrynol. Pol. 2024, 75, 385–394. [Google Scholar] [CrossRef]
- Berndt, V.; Dahlqvist, P.; de Verdier, J.; Ryberg, H.; Ragnarsson, O. The diagnostic value of salivary cortisol and salivary cortisone in patients with suspected hypercortisolism. Front. Endocrinol. 2022, 13, 1028804. [Google Scholar] [CrossRef]
- Carafone, L.E.; Zhang, C.D.; Li, D.; Lazik, N.; Hamidi, O.; Hurtado, M.D.; Young, W.F., Jr.; Thomas, M.A.; Dy, B.M.; Lyden, M.L.; et al. Diagnostic Accuracy of Dehydroepiandrosterone Sulfate and Corticotropin in Autonomous Cortisol Secretion. Biomedicines 2021, 9, 741. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, D.J.; Alam, U.; Davison, A.S.; Belfield, J.; Shore, S.L.; Vinjamuri, S. Investigation and assessment of adrenal incidentalomas. Clin. Med. 2023, 23, 135–140. [Google Scholar] [CrossRef]
- Kebebew, E. Adrenal incidentaloma. N. Engl. J. Med. 2021, 384, 1542–1551. [Google Scholar] [CrossRef]
- Bancos, I.; Prete, A. Approach to the Patient With Adrenal Incidentaloma. J. Clin. Endocrinol. Metab. 2021, 106, 3331–3353. [Google Scholar] [CrossRef]
- Reimondo, G.; Castellano, E.; Grosso, M.; Priotto, R.; Puglisi, S.; Pia, A.; Pellegrino, M.; Borretta, G.; Terzolo, M. Adrenal Incidentalomas are Tied to Increased Risk of Diabetes: Findings from a Prospective Study. J. Clin. Endocrinol. Metab. 2020, 105, dgz284. [Google Scholar] [CrossRef]
- Podbregar, A.; Janez, A.; Goricar, K.; Jensterle, M. The prevalence and characteristics of non-functioning and autonomous cortisol secreting adrenal incidentaloma after patients’ stratification by body mass index and age. BMC Endocr. Disord. 2020, 20, 118. [Google Scholar] [CrossRef]
- Ebbehoj, A.; Li, D.; Kaur, R.J.; Zhang, C.; Singh, S.; Li, T.; Atkinson, E.; Achenbach, S.; Khosla, S.; Arlt, W.; et al. Epidemiology of adrenal tumours in Olmsted County, Minnesota, USA: A population-based cohort study. Lancet Diabetes Endocrinol. 2020, 8, 894–902. [Google Scholar] [CrossRef]
- Jing, Y.; Hu, J.; Luo, R.; Mao, Y.; Luo, Z.; Zhang, M.; Yang, J.; Song, Y.; Feng, Z.; Wang, Z.; et al. Prevalence and Characteristics of Adrenal Tumors in an Unselected Screening Population: A Cross-Sectional Study. Ann. Intern. Med. 2022, 175, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Sconfienza, E.; Tetti, M.; Forestiero, V.; Veglio, F.; Mulatero, P.; Monticone, S. Prevalence of Functioning Adrenal Incidentalomas: A Systematic Review and Meta-analysis. J. Clin. Endocrinol. Metab. 2023, 108, 1813–1823. [Google Scholar] [CrossRef]
- Ichijo, T.; Ueshiba, H.; Nawata, H.; Yanase, T. A nationwide survey of adrenal incidentalomas in Japan: The first report of clinical and epidemiological features. Endocr. J. 2020, 67, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Prete, A.; Subramanian, A.; Bancos, I.; Chortis, V.; Tsagarakis, S.; Lang, K.; Macech, M.; Delivanis, D.A.; Pupovac, I.D.; Reimondo, G.; et al. Cardiometabolic Disease Burden and Steroid Excretion in Benign Adrenal Tumors: A Cross-Sectional Multicenter Study. Ann. Intern. Med. 2022, 175, 325–334. [Google Scholar] [CrossRef]
- Manea, M.M.; Dragos, D.; Ghenu, M.I.; Enache, I.I.; Stoican, I.C.; Ciulavu, C.; Vasiliu, O.; Sirbu, C.A.; Tuta, S. The Neurocardiogenic Impact of Ischemic Stroke: Intricacies of Cardiac Enzymes and the Vegetative System. Rom. J. Mil. Med. 2025, CXXVIII, 36–42. [Google Scholar] [CrossRef]
- Cassinello, J.M.J.; Vega-Beyhart, A.; Iriarte, M.B.; Donato, S.; Herrera-Martínez, A.D.; Marazuela, M.; Araujo-Castro, M. Mild autonomous cortisol secretion: Impact on bone health and quality of life. Rev. Endocr. 2025, 88, 693–700. [Google Scholar] [CrossRef]
- Popa, F.L.; Boicean, L.C.; Iliescu, M.G.; Stanciu, M. The importance of association between sex steroids deficiency, reduction of bone mineral density and falling risk in men with implications in medical rehabilitation. Balneo PRM Res. J. 2021, 12, 318–322. [Google Scholar] [CrossRef]
- Zavatta, G.; Vicennati, V.; Altieri, P.; Tucci, L.; Colombin, G.; Coscia, K.; Mosconi, C.; Balacchi, C.; Fanelli, F.; Malagrinò, M.; et al. Mild autonomous cortisol secretion in adrenal incidentalomas and risk of fragility fractures: A large cross-sectional study. Eur. J. Endocrinol. 2023, 188, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Pipernea, R.; Popa, F.L.; Ciortea, V.M.; Irsay, L.; Ungur, R.A.; Pintea, A.L.; Iliescu, M.G.; Cipăian, R.C.; Stanciu, M. The role of rehabilitation and anabolic treatment in severe osteoporosis associated with significant vitamin D deficiency—Case report. Balneo PRM Res. J. 2023, 14, 539. [Google Scholar] [CrossRef]
- Dumitru, N.; Carsote, M.; Cocolos, A.; Petrova, E.; Olaru, M.; Dumitrache, C.; Ghemigian, A. The Link Between Bone Osteocalcin and Energy Metabolism in a Group of Postmenopausal Women. Curr. Health Sci. J. 2019, 45, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, J.F.D.; Costa, J.M.; Junqueira, F.D.; Fonseca, A.O.; de Almeida, A.B.A.B.S.; Moraes, A.B.; Vieira Neto, L. Adrenal incidentaloma: Do patients with apparently nonfunctioning mass or autonomous cortisol secretion have similar or different clinical and metabolic features? Clin. Endocrinol. 2023, 98, 662–669. [Google Scholar] [CrossRef]
- Dogra, P.; Šambula, L.; Saini, J.; Thangamuthu, K.; Athimulam, S.; Delivanis, D.A.; Baikousi, D.A.; Nathani, R.; Zhang, C.D.; Genere, N.; et al. High prevalence of frailty in patients with adrenal adenomas and adrenocortical hormone excess: A cross-sectional multi-centre study with prospective enrolment. Eur. J. Endocrinol. 2023, 189, 318–326. [Google Scholar] [CrossRef]
- Favero, V.; Parazzoli, C.; Bernasconi, D.P.; Chiodini, I. Cardiometabolic comorbidities and cardiovascular events in “non-functioning” adrenal incidentalomas: A systematic review and meta-analysis. J. Endocrinol. Investig. 2024, 47, 2929–2942. [Google Scholar] [CrossRef]
- Deutschbein, T.; Reimondo, G.; Di Dalmazi, G.; Bancos, I.; Patrova, J.; Vassiliadi, D.A.; Nekić, A.B.; Debono, M.; Lardo, P.; Ceccato, F.; et al. Age-dependent and sex-dependent disparity in mortality in patients with adrenal incidentalomas and autonomous cortisol secretion: An international, retrospective, cohort study. Lancet Diabetes Endocrinol. 2022, 10, 499–508. [Google Scholar] [CrossRef]
- Patrova, J.; Mannheimer, B.; Lindh, J.D.; Falhammar, H. Mortality in Patients With Nonfunctional Adrenal Tumors. JAMA Intern. Med. 2023, 183, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Morelli, V.; Frigerio, S.; Aresta, C.; Passeri, E.; Pugliese, F.; Copetti, M.; Barbieri, A.M.; Fustinoni, S.; Polledri, E.; Corbetta, S.; et al. Adrenalectomy Improves Blood Pressure and Metabolic Control in Patients With Possible Autonomous Cortisol Secretion: Results of a RCT. Front. Endocrinol. 2022, 13, 898084. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, I.; Tomaselli, A.; Angelini, F.; Ferrari, D.; De Alcubierre, D.; Hasenmajer, V.; Sbardella, E.; Cozzolino, A.; Paganini, A.M.; Isidori, A.M.; et al. Predicting postoperative hypocortisolism in patients with non-aldosterone-producing adrenocortical adenoma: A retrospective single-centre study. J. Endocrinol. Investig. 2024, 47, 1751–1762. [Google Scholar] [CrossRef]
- Olsen, H.; Olsen, M. Associations of age, BMI, and renal function to cortisol after dexamethasone suppression in patients with adrenal incidentalomas. Front. Endocrinol. 2023, 13, 1055298. [Google Scholar] [CrossRef]
- Rahimi, L.; Kittithaworn, A.; Gregg Garcia, R.; Saini, J.; Dogra, P.; Atkinson, E.J.; Achenbach, S.J.; Kattah, A.; Bancos, I. Kidney Function in Patients With Adrenal Adenomas: A Single-Center Retrospective Cohort Study. J. Clin. Endocrinol. Metab. 2024, 109, e1750–e1758. [Google Scholar] [CrossRef]
- Favero, V.; Aresta, C.; Parazzoli, C.; Cairoli, E.; Eller-Vainicher, C.; Palmieri, S.; Salcuni, A.S.; Arosio, M.; Persani, L.; Scillitani, A.; et al. The degree of cortisol secretion is associated with diabetes mellitus and hypertension in patients with nonfunctioning adrenal tumors. Cardiovasc. Diabetol. 2023, 22, 102. [Google Scholar] [CrossRef] [PubMed]
- Araujo-Castro, M.; Ramírez, P.P.; Rojas-Marcos, P.M.; Centeno, R.G.; Gimeno, P.G.; Fernández-Ladreda, M.T.; Núñez, M.A.S.; Higueruela, C.; Lázaro, C.R. Nonfunctioning adrenal incidentalomas with cortisol post-dexamethasone suppression test > 0.9 µg/dL have a higher prevalence of cardiovascular disease than those with values ≤ 0.9 µg/dL. Endocrine 2023, 79, 384–391. [Google Scholar] [CrossRef]
- Güneş, E.; Güneş, M. Are nonfunctioning adrenal incidentalomas really nonfunctioning? A retrospective single-center study. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 9895–9901. [Google Scholar] [CrossRef]
- Pelsma, I.C.M.; Fassnacht, M.; Tsagarakis, S.; Terzolo, M.; Tabarin, A.; Sahdev, A.; Newell-Price, J.; Marina, L.; Lorenz, K.; Bancos, I.; et al. Comorbidities in mild autonomous cortisol secretion and the effect of treatment: Systematic review and meta-analysis. Eur. J. Endocrinol. 2023, 189, S88–S101. [Google Scholar] [CrossRef]
- Chen, A.X.; Radhakutty, A.; Drake, S.M.; Kiu, A.; Thompson, C.H.; Burt, M.G. Cardiovascular Risk Markers in Adults With Adrenal Incidentaloma and Mild Autonomous Cortisol Secretion. J. Clin. Endocrinol. Metab. 2024, 109, e1020–e1028. [Google Scholar] [CrossRef]
- Kjellbom, A.; Lindgren, O.; Puvaneswaralingam, S.; Löndahl, M.; Olsen, H. Association Between Mortality and Levels of Autonomous Cortisol Secretion by Adrenal Incidentalomas: A Cohort Study. Ann. Intern. Med. 2021, 174, 1041–1049. [Google Scholar] [CrossRef]
- Morelli, V.; Aresta, C.; Gaudio, A.; Eller-Vainicher, C.; Zhukouskaya, V.V.; Merlotti, D.; Orsi, E.; Maria Barbieri, A.; Fustinoni, S.; Polledri, E.; et al. Prediction of hypertension, diabetes and fractures in eucortisolemic women by measuring parameters of cortisol milieu. Endocrine 2020, 68, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Kufukihara, R.; Takeda, T.; Hakozaki, K.; Yasumizu, Y.; Tanaka, N.; Matsumoto, K.; Morita, S.; Kosaka, T.; Mizuno, R.; Asanuma, H.; et al. Predictors of renal function after adrenalectomy in patients with Cushing or subclinical Cushing syndrome. Int. J. Urol. 2022, 29, 1447–1454. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Girerd, S.; Jaisser, F.; Barrera-Chimal, J. Nonepithelial mineralocorticoid receptor activation as a determinant of kidney disease. Kidney Int. Suppl. 2022, 12, 12–18. [Google Scholar] [CrossRef]
- Eller-Vainicher, C.; Morelli, V.; Aresta, C.; Salcuni, A.S.; Falchetti, A.; Carnevale, V.; Persani, L.; Scillitani, A.; Chiodini, I. Defining Nonfunctioning Adrenal Adenomas on the Basis of the Occurrence of Hypocortisolism after Adrenalectomy. J. Endocr. Soc. 2020, 4, bvaa079. [Google Scholar] [CrossRef]
- Puvaneswaralingam, S.; Kjellbom, A.; Lindgren, O.; Löndahl, M.; Olsen, H. ACTH following overnight dexamethasone suppression can be used in the verification of autonomous cortisol secretion in patients with adrenal incidentalomas. Clin. Endocrinol. 2021, 94, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Nica, S.; Sionel, R.; Maciuca, R.; Csutak, O.; Ciobica, M.L.; Nica, M.I.; Chelu, I.; Radu, I.; Toma, M. Gender-Dependent Associations Between Digit Ratio and Genetic Polymorphisms, BMI, and Reproductive Factors. Rom. J. Mil. Med. 2025, 128, 78–86. [Google Scholar] [CrossRef]
- Vasiliu, O. Therapeutic management of atypical antipsychotic-related metabolic dysfunctions using GLP-1 receptor agonists: A systematic review. Exper. Ther. Med. 2023, 26, 355. [Google Scholar] [CrossRef]
- Turan Erdogan, B.; Evranos Ogmen, B.; Sacikara, M.; Aydin, C.; Topaloglu, O.; Ersoy, R.; Cakir, B. The relationship between mild autonomous cortisol secretion and metabolic diseases in cases with adrenal incidentaloma. Endokrynol. Pol. 2025, 76, 172–181. [Google Scholar] [CrossRef]
- Efthymiadis, A.; Loo, H.; Shine, B.; James, T.; Keevil, B.; Tomlinson, J.W.; Pal, A.; Pofi, R. Development of diagnostic algorithm for Cushing’s syndrome: A tertiary centre experience. J. Endocrinol. Investig. 2024, 47, 2449–2459. [Google Scholar] [CrossRef]
- Al-Waeli, D.; Alidrisi, H.; Mansour, A. Utilizing dehydroepiandrosterone sulfate and its ratio for detecting mild autonomous cortisol excess in patients with adrenal incidentaloma. J. Med. Life 2023, 16, 1456–1461. [Google Scholar] [CrossRef]
- Araujo-Castro, M.; Hanzu, F.A.; Pascual-Corrales, E.; García Cano, A.M.; Marchan, M.; Escobar-Morreale, H.F.; Valderrabano, P.; Casals, G. Is the 1 mg-dexamethasone suppression test a precise marker of glucocorticoid excess and cardiometabolic risk in patients with adrenal incidentalomas? Endocrine 2023, 82, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Issa, B.G.; Hanna, F.W.F.; Fryer, A.A.; Ensah, G.; Ebere, I.; Marshall, D.; Keevil, B. The Utility of Salivary Cortisone in the Overnight Dexamethasone Suppression Test in Adrenal Incidentalomas. J. Clin. Endocrinol. Metab. 2023, 108, e937–e943. [Google Scholar] [CrossRef] [PubMed]
- Wu, V.C.; Chan, C.K.; Wu, W.C.; Peng, K.Y.; Chang, Y.S.; Yeh, F.Y.; Chiang, J.Y.; Lee, Y.J.; Liu, K.L.; Wang, S.M.; et al. New-onset diabetes mellitus risk associated with concurrent autonomous cortisol secretion in patients with primary aldosteronism. Hypertens. Res. 2023, 46, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Bancos, I.; Taylor, A.E.; Chortis, V.; Sitch, A.J.; Jenkinson, C.; Davidge-Pitts, C.J.; Lang, K.; Tsagarakis, S.; Macech, M.; Riester, A.; et al. Urine steroid metabolomics for the differential diagnosis of adrenal incidentalomas in the EURINE-ACT study: A prospective test validation study. Lancet Diabetes Endocrinol. 2020, 8, 773–781. [Google Scholar] [CrossRef]
- Berke, K.; Constantinescu, G.; Masjkur, J.; Kimpel, O.; Dischinger, U.; Peitzsch, M.; Kwapiszewska, A.; Dobrowolski, P.; Nölting, S.; Reincke, M.; et al. Plasma Steroid Profiling in Patients With Adrenal Incidentaloma. J. Clin. Endocrinol. Metab. 2022, 107, e1181–e1192. [Google Scholar] [CrossRef]
- Ku, E.J.; Lee, C.; Shim, J.; Lee, S.; Kim, K.A.; Kim, S.W.; Rhee, Y.; Kim, H.J.; Lim, J.S.; Chung, C.H.; et al. Metabolic Subtyping of Adrenal Tumors: Prospective Multi-Center Cohort Study in Korea. Endocrinol. Metab. 2021, 36, 1131–1141. [Google Scholar] [CrossRef]
- Mitrica, M.; Vasiliu, O.; Plesa, A.; Sirbu, O.M. Multinodular and vacuolating neuronal tumor. Rom. J. Mil. Med. 2025, 128, 10–16. [Google Scholar] [CrossRef]
- Huayllas, M.K.P.; Smith, L.M.; Gallagher, J.C.; Netzel, B.C.; Singh, R.J.; Kater, C.E. Steroidogenesis in patients with adrenal incidentalomas: Extended steroid profile measured by liquid chromatography-mass spectrometry after ACTH stimulation and dexamethasone suppression. Clin. Endocrinol. 2021, 95, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Zenoaga-Barbăroșie, C.; Berca, L.; Vassu-Dimov, T.; Toma, M.; Nica, M.; Alexiu-Toma, O.; Ciornei, C.; Albu, A.; Nica, S.; Nistor, C.; et al. The Predisposition for Type 2 Diabetes Mellitus and Metabolic Syndrome. Balk. J. Med. Genet. 2023, 26, 21–26. [Google Scholar] [CrossRef]
- Nistor, C.-E.; Găvan, C.S.; Ciritel, A.-A.; Nemes, A.F.; Ciuche, A. The Association of Minimally Invasive Surgical Approaches and Mortality in Patients with Malignant Pleuropericarditis—A 10 Year Retrospective Observational Study. Medicina 2022, 58, 718. [Google Scholar] [CrossRef] [PubMed]
- Chortis, V.; Bancos, I.; Nijman, T.; Gilligan, L.C.; Taylor, A.E.; Ronchi, C.L.; O’Reilly, M.W.; Schreiner, J.; Asia, M.; Riester, A.; et al. Urine Steroid Metabolomics as a Novel Tool for Detection of Recurrent Adrenocortical Carcinoma. J. Clin. Endocrinol. Metab. 2020, 105, e307–e318. [Google Scholar] [CrossRef] [PubMed]
- Teuber, J.P.; Nanba, K.; Turcu, A.F.; Chen, X.; Zhao, L.; Else, T.; Auchus, R.J.; Rainey, W.E.; Rege, J. Intratumoral steroid profiling of adrenal cortisol-producing adenomas by liquid chromatography- mass spectrometry. J. Steroid Biochem. Mol. Biol. 2021, 212, 105924. [Google Scholar] [CrossRef]
- Debono, M.; Elder, C.J.; Lewis, J.; Fearnside, J.; Caunt, S.; Dixon, S.; Jacques, R.M.; Newell-Price, J.; Whitaker, P.M.J.; Keevil, B.; et al. Home waking salivary cortisone to screen for adrenal insufficiency. NEJM Evid. 2023, 2, 2. [Google Scholar] [CrossRef]
- Kvam Hellan, K.; Lyngstad, M.; Methlie, P.; Løvås, K.; Husebye, E.S.; Ueland, G.Å. Utility of Salivary Cortisol and Cortisone in the Diagnostics of Adrenal Insufficiency. J. Clin. Endocrinol. Metab. 2025, 110, 1218–1223. [Google Scholar] [CrossRef]
- Savas, M.; Mehta, S.; Agrawal, N.; van Rossum, E.F.C.; Feelders, R.A. Approach to the Patient: Diagnosis of Cushing Syndrome. J. Clin. Endocrinol. Metab. 2022, 107, 3162–3174. [Google Scholar] [CrossRef]
- Anderson, T.; Wideman, L. The association between the cortisol and cortisone awakening responses. Psychoneuroendocrinology 2023, 152, 106075. [Google Scholar] [CrossRef] [PubMed]
- Olsen, H.; Kjellbom, A.; Löndahl, M.; Lindgren, O. Suppressed ACTH Is Frequently Unrelated to Autonomous Cortisol Secretion in Patients With Adrenal Incidentalomas. J. Clin. Endocrinol. Metab. 2019, 104, 506–512. [Google Scholar] [CrossRef]
- Ciftci, S.; Soyluk, O.; Selek, A.; Erol, S.; Hekimsoy, Z.; Esen, A.; Dursun, H.; Sahin, S.; Oruk, G.; Mert, M.; et al. The Importance of DHEA-S Levels in Cushing’s Syndrome; Is There a Cut-off Value in the Differential Diagnosis? Horm. Metab. Res. 2022, 54, 232–237. [Google Scholar] [CrossRef]
- Elhassan, Y.S.; Alahdab, F.; Prete, A.; Delivanis, D.A.; Khanna, A.; Prokop, L.; Murad, M.H.; O’Reilly, M.W.; Arlt, W.; Bancos, I. Natural History of Adrenal Incidentalomas With and Without Mild Autonomous Cortisol Excess: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2019, 171, 107–116. [Google Scholar] [CrossRef]
- Araujo-Castro, M.; Robles Lázaro, C.; Parra Ramírez, P.; García Centeno, R.; Gracia Gimeno, P.; Fernández-Ladreda, M.T.; Sampedro Núñez, M.A.; Marazuela, M.; Escobar-Morreale, H.F.; Valderrabano, P. Maximum adenoma diameter, regardless of uni- or bilaterality, is a risk factor for autonomous cortisol secretion in adrenal incidentalomas. J. Endocrinol. Investig. 2021, 44, 2349–2357. [Google Scholar] [CrossRef] [PubMed]
- Muangnoo, N.; Manosroi, W.; Leelathanapipat, N.; Meejun, T.; Chowchaiyaporn, P.; Teetipsatit, P. Predictive Factors of Functioning Adrenal Incidentaloma: A 15-Year Retrospective Study. Medicina 2022, 58, 597. [Google Scholar] [CrossRef] [PubMed]
- Podbregar, A.; Kocjan, T.; Rakuša, M.; Popović, P.; Garbajs, M.; Goricar, K.; Janez, A.; Jensterle, M. Natural history of nonfunctioning adrenal incidentalomas: A 10-year longitudinal follow-up study. Endocr. Connect. 2021, 10, 637–645. [Google Scholar] [CrossRef]
- Falcetta, P.; Orsolini, F.; Benelli, E.; Agretti, P.; Vitti, P.; Di Cosmo, C.; Tonacchera, M. Clinical features, risk of mass enlargement, and development of endocrine hyperfunction in patients with adrenal incidentalomas: A long-term follow-up study. Endocrine 2021, 71, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Araujo-Castro, M.; Parra Ramírez, P.; Robles Lázaro, C.; García Centeno, R.; Gracia Gimeno, P.; Fernández-Ladreda, M.T.; Sampedro Núñez, M.A.; Marazuela, M.; Escobar-Morreale, H.F.; Valderrabano, P. Predictors of Tumour Growth and Autonomous Cortisol Secretion Development during Follow-Up in Non-Functioning Adrenal Incidentalomas. J. Clin. Med. 2021, 10, 5509. [Google Scholar] [CrossRef]
- Petramala, L.; Circosta, F.; Marino, L.; Palombi, E.; Costanzo, M.L.; Servello, A.; Galardo, G.; Letizia, C. Clinical Evaluation of Adrenal Incidentaloma: The Experience of a Referral Center. Biomedicines 2024, 12, 1910. [Google Scholar] [CrossRef]
- Araujo-Castro, M.; García Cano, A.M.; Escobar-Morreale, H.F.; Valderrabano, P. Predictive model for autonomous cortisol secretion development in non-functioning adrenal incidentalomas. Hormones 2023, 22, 51–59. [Google Scholar] [CrossRef]
- Maas, M.; Nassiri, N.; Bhanvadia, S.; Carmichael, J.D.; Duddalwar, V.; Daneshmand, S. Discrepancies in the Recommended Management of Adrenal Incidentalomas by Various Guidelines. J. Urol. 2021, 205, 52–59. [Google Scholar] [CrossRef]
- Remde, H.; Kranz, S.; Morell, S.M.; Altieri, B.; Kroiss, M.; Detomas, M.; Fassnacht, M.; Deutschbein, T. Clinical course of patients with adrenal incidentalomas and cortisol autonomy: A German retrospective single center cohort study. Front. Endocrinol. 2023, 14, 1123132. [Google Scholar] [CrossRef]
- Lovato, C.M.; Thévenot, T.; Borot, S.; Di Martino, V.; Qualls, C.R.; Urban, F.K.; Dorin, R.I. Decreased maximal cortisol secretion rate in patients with cirrhosis: Relation to disease severity. JHEP Rep. 2021, 3, 100277. [Google Scholar] [CrossRef]
- Vanek, C.; Loriaux, L. The 1 mg overnight dexamethasone suppression test: A danger to the adrenal gland? Curr. Opin. Endocrinol. Diabetes Obes. 2022, 29, 403–405. [Google Scholar] [CrossRef]
- Vogg, N.; Kurlbaum, M.; Deutschbein, T.; Gräsl, B.; Fassnacht, M.; Kroiss, M. Method-Specific Cortisol and Dexamethasone Thresholds Increase Clinical Specificity of the Dexamethasone Suppression Test for Cushing Syndrome. Clin. Chem. 2021, 67, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Roper, S.M. Yield of Serum Dexamethasone Measurement for Reducing False-Positive Results of Low-Dose Dexamethasone Suppression Testing. J. Appl. Lab. Med. 2021, 6, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Genere, N.; Kaur, R.J.; Athimulam, S.; Thomas, M.A.; Nippoldt, T.; Van Norman, M.; Singh, R.; Grebe, S.; Bancos, I. Interpretation of Abnormal Dexamethasone Suppression Test is Enhanced With Use of Synchronous Free Cortisol Assessment. J. Clin. Endocrinol. Metab. 2022, 107, e1221–e1230. [Google Scholar] [CrossRef]
- Berlińska, A.; Świątkowska-Stodulska, R.; Sworczak, K. Factors Affecting Dexamethasone Suppression Test Results. Exp. Clin. Endocrinol. Diabetes 2020, 128, 667–671. [Google Scholar] [CrossRef]
- Ceccato, F.; Artusi, C.; Barbot, M.; Lizzul, L.; Pinelli, S.; Costantini, G.; Niero, S.; Antonelli, G.; Plebani, M.; Scaroni, C. Dexamethasone measurement during low-dose suppression test for suspected hypercortisolism: Threshold development with and validation. J. Endocrinol. Investig. 2020, 43, 1105–1113. [Google Scholar] [CrossRef]
- Valea, A.; Ghervan, C.; Morar, A.; Pop, D.D.; Carsote, M.; Albu, S.E.; Georgescu, C.E.; Chiorean, A. Hashimoto’s thyroiditis and breast cancer: Coincidence or correlation? Arch. Balk. Med. Union 2016, 51, 129–132. [Google Scholar]
- Ceccato, F.; Tizianel, I.; Voltan, G.; Maggetto, G.; Merante Boschin, I.; Quaia, E.; Crimì, F.; Scaroni, C. Attenuation Value in Adrenal Incidentalomas: A Longitudinal Study. Front. Endocrinol. 2021, 12, 794197. [Google Scholar] [CrossRef]
- Farinelli, D.G.; Oliveira, K.C.; Hayashi, L.F.; Kater, C.E. Overnight 1-mg Dexamethasone Suppression Test for Screening Cushing Syndrome and Mild Autonomous Cortisol Secretion (MACS): What Happens when Serum Dexamethasone Is Below Cutoff? How Frequent Is it? Endocr. Pract. 2023, 29, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Atkins, J.S.; Hawley, J.M.; Owen, L.J.; Clayton, J.; Scargill, J.; Keevil, B.G. Serum cortisol assay performance following the 1 mg overnight dexamethasone suppression test. Ann. Clin. Biochem. 2023, 60, 386–395. [Google Scholar] [CrossRef]
- Cappola, A.R.; Auchus, R.J.; El-Hajj Fuleihan, G.; Handelsman, D.J.; Kalyani, R.R.; McClung, M.; Stuenkel, C.A.; Thorner, M.O.; Verbalis, J.G. Hormones and Aging: An Endocrine Society Scientific Statement. J. Clin. Endocrinol. Metab. 2023, 108, 1835–1874. [Google Scholar] [CrossRef]
- Hepsen, S.; Sencar, E.; Sakiz, D.; Akhanli, P.; Ucan, B.; Unsal, I.; Ozbek, M.; Cakal, E. Serum cortisol level after low dose dexamethasone suppression test may be predictive for diabetes mellitus and hypertension presence in obese patients: A retrospective study. Diabetes Res. Clin. Pract. 2020, 161, 108081. [Google Scholar] [CrossRef] [PubMed]
- Popa, F.L.; Diaconu, C.; Canciu, A.; Ciortea, V.M.; Iliescu, M.G.; Stanciu, M. Medical management and rehabilitation in posttraumatic common peroneal nerve palsy. Balneo PRM Res. J. 2022, 13, 496. [Google Scholar] [CrossRef]
- Favero, V.; Cremaschi, A.; Falchetti, A.; Gaudio, A.; Gennari, L.; Scillitani, A.; Vescini, F.; Morelli, V.; Aresta, C.; Chiodini, I. Management and Medical Therapy of Mild Hypercortisolism. Int. J. Mol. Sci. 2021, 22, 11521. [Google Scholar] [CrossRef] [PubMed]
- Araujo-Castro, M.; Valderrábano, P.; Escobar-Morreale, H.F.; Hanzu, F.A.; Casals, G. Urine steroid profile as a new promising tool for the evaluation of adrenal tumors. Lit. Rev. Endocr. 2021, 72, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Araujo-Castro, M. Cardiometabolic profile and urinary metabolomic alterations in non-functioning adrenal incidentalomas: A review. Clin. Endocrinol. 2022, 97, 693–701. [Google Scholar] [CrossRef]
- Liu, M.S.; Lou, Y.; Chen, H.; Wang, Y.J.; Zhang, Z.W.; Li, P.; Zhu, D.L. Performance of DHEAS as a Screening Test for Autonomous Cortisol Secretion in Adrenal Incidentalomas: A Prospective Study. J. Clin. Endocrinol. Metab. 2022, 107, e1789–e1796. [Google Scholar] [CrossRef]
- Yozamp, N.; Vaidya, A. Assessment of mild autonomous cortisol secretion among incidentally discovered adrenal masses. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101491. [Google Scholar] [CrossRef]
- Wilson, M.P.; Randhawa, S.; Bao, B.; Croutze, R.; Murad, M.H.; McInnes, M.D.F.; Low, G. Impact of Size Thresholds on the Diagnosis of Incidental Adrenal Lesions: A Systematic Review and Meta-Analysis. J. Am. Coll. Radiol. 2024, 21, 107–117. [Google Scholar] [CrossRef]
- Qi, S.; Zuo, Y.; Chang, R.; Huang, K.; Liu, J.; Zhang, Z. Using CT radiomic features based on machine learning models to subtype adrenal adenoma. BMC Cancer 2023, 23, 111. [Google Scholar] [CrossRef]
- Toniolo, A.; Agostini, E.; Ceccato, F.; Tizianel, I.; Cabrelle, G.; Lupi, A.; Pepe, A.; Campi, C.; Quaia, E.; Crimì, F. Could CT Radiomic Analysis of Benign Adrenal Incidentalomas Suggest the Need for Further Endocrinological Evaluation? Curr. Oncol. 2024, 31, 4917–4926. [Google Scholar] [CrossRef]
- Araujo-Castro, M.; Paja Fano, M.; Pla Peris, B.; González Boillos, M.; Pascual-Corrales, E.; García-Cano, A.M.; Parra Ramírez, P.; Rojas-Marcos, P.M.; Ruiz-Sanchez, J.G.; Vicente, A.; et al. Autonomous cortisol secretion in patients with primary aldosteronism: Prevalence and implications on cardiometabolic profile and on surgical outcomes. Endocr. Connect. 2023, 12, e230043. [Google Scholar] [CrossRef]
- Peng, K.Y.; Liao, H.W.; Chan, C.K.; Lin, W.C.; Yang, S.Y.; Tsai, Y.C.; Huang, K.H.; Lin, Y.H.; Chueh, J.S.; Wu, V.C. Presence of Subclinical Hypercortisolism in Clinical Aldosterone-Producing Adenomas Predicts Lower Clinical Success. Hypertension 2020, 76, 1537–1544. [Google Scholar] [CrossRef]
- Katabami, T.; Matsuba, R.; Kobayashi, H.; Nakagawa, T.; Kurihara, I.; Ichijo, T.; Tsuiki, M.; Wada, N.; Ogawa, Y.; Sone, M.; et al. Primary aldosteronism with mild autonomous cortisol secretion increases renal complication risk. Eur. J. Endocrinol. 2022, 186, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Cakir, S.D.; Cakir, A.; Ozturk, F.Y.; Basmaz, S.E.; Batman, A.; Saygili, E.S.; Erol, R.S.; Sen, E.C.; Canat, M.M.; Altuntas, Y. Choroidal Thickness in Mild Autonomous Cortisol Secretion. Sisli Etfal Hast. Tip Bul. 2024, 58, 204–209. [Google Scholar] [CrossRef]
- Siemińska, L.; Siemińska, K.; Dittfeld, A.; Kotecka-Blicharz, A. Fibroblast growth factor 21 in patients with mild autonomic cortisol secretion and non-functioning adrenal incidentalomas. Endokrynol. Pol. 2024, 75, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhou, J.; Lyu, X.; Li, Y.; Ma, L.; Liu, Y.; Fan, H.; Zhang, Y. A novel model using leukocytes to differentiating mild autonomous cortisol secretion and non-functioning adrenal adenoma. Sci. Rep. 2024, 14, 23557. [Google Scholar] [CrossRef]
- Athanasouli, F.; Georgiopoulos, G.; Asonitis, N.; Petychaki, F.; Savelli, A.; Panou, E.; Angelousi, A. Nonfunctional adrenal adenomas and impaired glucose metabolism: A systematic review and meta-analysis. Endocrine 2021, 74, 50–60. [Google Scholar] [CrossRef]
- Valea, A.; Carsote, M.; Moldovan, C.; Georgescu, C. Chronic autoimmune thyroiditis and obesity. Arch. Balk. Med. Union 2018, 53, 64–69. [Google Scholar]
- Liu, L.; Li, X.; Ma, W.; Fan, F.; Yang, Y.; Zhang, L.; Yi, T.; Zhang, J.; Guo, J.; Gao, Y. Comparative analysis of cardiac dysfunction in non-functioning adrenal adenomas, primary aldosteronism, and essential hypertension. J. Endocrinol. Investig. 2025, 48, 1811–1817. [Google Scholar] [CrossRef]
- Zhang, X.; Hou, A.; Cao, J.; Liu, Y.; Lou, J.; Li, H.; Ma, Y.; Song, Y.; Mi, W.; Liu, J. Association of Diabetes Mellitus With Postoperative Complications and Mortality After Non-Cardiac Surgery: A Meta-Analysis and Systematic Review. Front. Endocrinol. 2022, 13, 841256. [Google Scholar] [CrossRef] [PubMed]
- Uygur, M.M.; di Filippo, L.; Frara, S.; Menotti, S.; Giustina, A. Pathophysiology and evaluation of bone health in adrenal diseases. Endocrine 2025, 89, 325–337. [Google Scholar] [CrossRef]
- Nistor, C.E.; Bugala, N.M.; Daguci, C.; Daguci, L.; Diaconu, O.A.; Rica, A.M. Multiple endocrine neoplasia type 2 syndrome and osteoporosis. Aging Clin. Exp. Res. 2023, 35, S387. [Google Scholar]
- Zavatta, G.; Di Dalmazi, G. Mild Autonomous Cortisol Secretion (MACS)—Related Osteoporosis. Exp. Clin. Endocrinol. Diabetes 2024, 132, 712–722. [Google Scholar] [CrossRef]
- Nistor, C.E.; Gavan, C.S.; Pantile, D.; Tanase, N.V.; Ciuche, A. Cervico-Thoracic Air Collections in COVID-19 Pneumonia Patients—Our Experience and Brief Review. Chirurgia 2022, 117, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Minnetti, M.; Hasenmajer, V.; Sbardella, E.; Angelini, F.; Simeoli, C.; Di Paola, N.; Cozzolino, A.; Pivonello, C.; De Alcubierre, D.; Chiloiro, S.; et al. Susceptibility and characteristics of infections in patients with glucocorticoid excess or insufficiency: The ICARO tool. Eur. J. Endocrinol. 2022, 187, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Nistor, C.; Ciuche, A.; Constantinescu, I. Emergency surgical tracheal decompression in a huge retrosternal goiter. Acta Endocrinol. 2017, 13, 370–374. [Google Scholar] [CrossRef]
- Kastelan, D.; Dusek, T. Do adrenal incidentalomas have an impact on mental health? A comprehensive review. Eur. J. Endocrinol. 2025, 192, R1–R6. [Google Scholar] [CrossRef]
- Nistor, C.; Ranetti, A.E.; Ciuche, A.; Pantile, D.; Constantin, L.M.; Brincoveanu, R. Betadine in chemical pleurodesis. Farmacia 2014, 62, 897–906. [Google Scholar]
- Farah, S.; Nasr, L.; Eid Fares, J. An Overlooked Disease: Minimal Autonomous Cortisol Secretion (MACS). A Narrative Review. Metab. Immune Disord. Drug Targets 2024, 24, 1518–1524. [Google Scholar] [CrossRef] [PubMed]
- Czapla-Iskrzycka, A.; Świątkowska-Stodulska, R.; Sworczak, K. Comorbidities in Mild Autonomous Cortisol Secretion—A Clinical Review of Literature. Exp. Clin. Endocrinol. Diabetes 2022, 130, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Candemir, B.; Kisip, K.; Akın, Ş.; Sanal, H.T.; Taşar, M.; Candemir, M.; Gülçelik, N.E. Prevalence and Predictive Features of CT-Derived Nonalcoholic Fatty Liver Disease in Metabolically Healthy MACS. Clin. Endocrinol. 2025, 102, 380–388. [Google Scholar] [CrossRef]
- Lamas, C.; Araujo-Castro, M.; Ostermair, L.; Petersenn, E.; Parra Ramírez, P.; Rebollo-Román, Á.; Stuefchen, I.; Bruedgam, D.; Ruiz-Sanchez, J.G.; Michalopoulou, T.; et al. Impact of Cortisol-Cosecretion on Adrenal Venous Sampling Results in Primary Aldosteronism: Study of 225 Cases. Biomedicines 2024, 12, 2430. [Google Scholar] [CrossRef]
- Umakoshi, H. Mild autonomous cortisol secretion in primary aldosteronism: The origin of cortisol. Hypertens. Res. 2025, 48, 2272–2273. [Google Scholar] [CrossRef] [PubMed]


| First Author Year of Publication Reference Number | Study Design | Number of Patients Sex Ratio (F/M) Age (Years) | Outcomes |
|---|---|---|---|
| Bonaventura 2024 [34] | Retrospective | N = 32 AI F/M = 19/13 Age median (IQR) = 61 (51–66) y N = 25 with MACS (1 mg DST > 1.8 µg/dL) F/M = 14/11 Age median (IQR) = 58 (9–66) y N = 7 with NFA (1 mg DST ≤ 1.8 µg/dL) F/M = 5/2 Age median (IQR) = 66 (64–67) | Preoperative cortisol after 1 mg-DST (4.75 µg/dL) as the only significant predictor of 6-week adrenal recovery following adrenalectomy Sensitivity = 89.5% Specificity = 72.7% AUC = 0.87, p < 0.001 Diabetes was associated with a significantly reduced chance of post-surgery recovery (OR = 24.55, p = 0.036). |
| Olsen 2023 [35] | Cross-sectional | N = 631 with AI (1 mg DST <1.8 µg/dL) F/M = 352/279 Age median (IQR) = 63.2 (55.1–69.7) y N = 516 with AI (1 mg DST ≥ 1.8 µg/dL) F/M = 322/194 Age median (IQR) = 67.3 (61.0–74.4) y | Negative correlation between 1 mg DST and eGFR, with 1 mg DST increasing by 9% (95% CI: 6–11%) for each 10 mL/min/1.73 m2 decrease in eGFR |
| Rahimi 2023 [36] | Cohort | N = 972 with AI F/M = 629/343 Age median (IQR) = 60.9 (52.6–68.7) y N = 429 with MACS (1 mg DST ≥ 1.8 µg/dL) F/M = 285/144 Age median (IQR)= 62.8 (54.6–71.1) y N = 543 with NFA (1 mg DST < 1.8 µg/dL) F/M = 344/199 Age median (IQR)= 59.4 (50.5–67.3) y | eGFR MACS vs. NFA: 79.6 vs. 83.8 mL/min/1.73 m2, p < 0.001) Higher 1 mg DST cortisol levels were independently associated with a decline in kidney function, each doubling of cortisol was linked to a decrease of 1.01 mL/min/1.73 m2 in eGFR (p = 0.017), even after adjusting for age (−7.94; p = 0.001) and hypertension (−2.72; p = 0.038), highlighting the role of subtle cortisol excess in renal impairment. |
| Favero 2023 [37] | Retrospective cross-sectional | N = 615 F/M = 289/326 Age (mean ± SD) = 60.15 ± 11.8 y N = 289 NFA (1 mg DST < 1.2 µg/dL) F/M = 182/107 Age (mean ± SD) = 57.5 ± 12.3 y N = 326 NFA (1 mg DST ≥ 1.2 µg/dL) F/M = 195/131 Age (mean ± SD) = 62.5 ± 10.9 y | 1 mg DST 1.2 µg/dL was the cutoff with the highest accuracy in identifying patients with either hypertension or diabetes mellitus: AUC = 0.604 (95% CI: 0.560–0.649) Sensitivity = 60.2% Specificity = 56.0% Hypertension or diabetes mellitus AUC = 0.611 (95% CI: 0.545–0.675) Sensitivity = 60.4% Specificity = 69.6% Compared to patients with 1 mg DST below 1.2 µg/dL (N = 289) vs. 1.2–1.79 µg/dL (N = 326) had: Lower ACTH levels (15.3 ± 10.1 vs. 17.7 ± 11.9 pg/mL, p = 0.008), Older age (62.5 ± 10.9 vs. 57.5 ± 12.3 y, p < 0.001) Higher prevalence of: Hypertension (52.5% vs. 38.1%, p < 0.001) Diabetes mellitus (23.3% vs. 13.1%, p = 0.001) Both hypertension and diabetes mellitus (16.9% vs. 8.3%, p < 0.002) Cardiovascular events (7.3% vs. 3.2%, p = 0.028) After adjusting for confounders (age, gender, obesity, dyslipidemia, and either hypertension or diabetes mellitus), 1 mg DST levels between 1.2–1.79 µg/dL remained significantly associated with: Hypertension (OR = 1.55, 95% CI: 1.08–2.23, p = 0.018) Diabetes mellitus (OR = 1.60, 95% CI: 1.01–2.57, p = 0.045) Both hypertension and diabetes mellitus (OR = 1.96, 95% CI: 1.12–3.41, p = 0.018) |
| Araujo-Castro 2023 [38] | Retrospective | N = 593 NFA F/M = 343/250 Age (mean ± SD) = 62.3 ± 10.83 y N = 442 NFA (1 mg DST ≤ 1.4 µg/dL) F/M = 257/185 Age (mean ± SD) = 61.3 ± 10.42 y N = 151 NFA (1 mg-DST > 1.4 µg/dL) F/M = 86/65 Age (mean ± SD) = 64.9 ± 11.58 y N = 412 NFA (1 mg DST ≤ 0.9 µg/dL) F/M = 241/171 Age (mean ± SD) = 59.6 ± 10.79 y N = 181 NFA (1 mg-DST > 0.9 µg/dL) F/M = 104/77 Age (mean ± SD) = 63.4 ± 10.66 y | 1 mg DST 0.9 µg/dL threshold proves to be useful in identifying patients with NFA at higher cardiovascular risk OR = 2.23 (1.10–4.53) |
| Güneş 2023 [39] | Retrospective | N = 123 with AI F/M = 90/33 Age (mean ± SD) = 53.0 ± 10.9 N = 114 controls F/M = 91/23 Age (mean ± SD) = 52.9 ± 7.4 y | ROC analysis identified the optimal 1 mg DST level for HT, which was 0.87 μg/dL. HT by DST level: <0.87 μg/dL → 42.6% ≥0.87 μg/dL → 66.1%, p = 0.009 Independent predictors of HT (binary logistic regression): Age: β = 0.068, OR = 1.07 (95% CI: 1.02–1.12), p = 0.004 DST level: β = 1.18, OR = 3.24, 95% CI: 1.02–10.34, p = 0.047 |
| First Author Year of Publication Reference Number | Study Design | Number of Patients Sex Ratio (F/M) Age (Years) | Outcomes |
|---|---|---|---|
| Turan Erdogan 2024 [50] | Retrospective study | N = 461 with AI F/M = 309/152 Age (mean ± SD) = 54.8 ± 10.19 y N = 77 with MACS (1 mg DST > 1.8 µg/dL) F/M = 56/21 Age (mean ± SD) = 56.87 ± 10.67 y N = 384 with NFA (1 mg DST ≤ 1.8 µg/dL) F/M = 253/131 Age (mean ± SD) = 54.39 ± 10.05 y | Predicted MACS: DHEAS ≤ 49.31 µg/dL Sensitivity = 61% Specificity = 73% AUC = 0.704 (95% CI: 0.636–0.771, p < 0.001) |
| Efthymiadis 2024 [51] | Retrospective study | N = 53 with CS of these 24 with MACS (1 mg DST > 1.8 µg/dL) and 27 with Cushing disease F/M = 42/11 Age (mean ± SD) = 56 ± 16 y | MACS 1 mg DST (cutoff 1.8 µg/dL) Sensitivity = 100% (95% CI: 82.4–100.0) Specificity = 52.2% (95% CI: 30.6–73.2) AUC = 0.76 (95% CI: 0.66–1.00, p = 0.004) LNSC (cutoff 1.7 nmol/L) Sensitivity = 77.2% (95% CI: 54.6–92.2) Specificity = 64.8% (95% CI: 47.5–79.8) AUC = 0.71 (95% CI: 0.574–0.848, p = 0.007) LDDST (cutoff 1.8 µg/dL) Sensitivity = 93.8% (95% CI: 69.8–99.8) Specificity = 72.7% (95% CI: 39.0–94.0) AUC = 0.83 (95% CI: 0.66–1.00, p = 0.004) UFC (cutoff 135 nmol/L) Sensitivity = 17.7% (95% CI: 3.8–43.4) Specificity = 58.8% (95% CI: 32.9–81.6) AUC = 0.62 (95% CI: 0.43–0.80, p = 0.242) LNSE (cutoff 15.2 nmol/L) Sensitivity = 27.8% (95% CI: 9.7–53.5) Specificity = 96.1% (95% CI: 80.4–99.9) AUC = 0.66 (95% CI: 0.47–0.85, p = 0.102) Combining these with ACTH > 12.6 pmol/L as cutoff distinguishing Cushing disease from MACS Sensitivity = 100% Specificity = 86.7% AUC = 0.98 (95% CI: 0.87–1.00, p < 0.001) |
| Al-Waeli 2023 [52] | Cross-sectional study | N = 38 with AI of these 5 were diagnosed with MACS (1 mg DST > 1.8 µg/dL) F/M = 23/15 Age (mean ± SD) = 47.6 ± 18.3 y | DHEAS ≤ 75 µg/dL Sensitivity = 80% Specificity = 73.3% Negative predictive values = 95.7% Positive predictive values = 33.3% DHEAS ratio ≤ 1.7 Sensitivity = 80% Specificity = 76.6% Negative predictive values = 95.8% Positive predictive values = 36.4% |
| Araujo-Castro, 2023 [53] | Cross-sectional study | N = 49 AI ACS = 25 (1 mg DST > 1.8 µg/dL) F/M = 17/8 Age (mean ± SD) = 67.4 ± 9.68 y NFA = 24 (1 mg DST ≤ 1.8 µg/dL) F/M = 16/8 Age (mean ± SD) = 70.2 ± 7.83 y | ACS-related comorbidities were moderately accurately predicted by post-DST cortisol alone: AUC = 0.767 (95% CI: 0.634–0.882) Post-DST cortisol + urinary cortisone, α-cortol, and THS provided the highest diagnosis accuracy: AUC = 0.813 (95% CI: 0.680–0.912) Post-DST cortisol + glucocorticoid metabolites + DHEAS: AUC = 0.853 (95% CI: 0.712–0.954) |
| Issa 2023 [54] | Retrospective study | N = 173 with AI F/M = 96/77 Age (mean ± SD) = 64.2 ± 11.3 y | Correlation between 1 mg DST salivary cortisone and serum cortisol with an r = 0.95 (p < 0.001) Sensitivity = 83.3% Specificity = 91.4% Accuracy = 88.2% Four predictive parameters: post-dexamethasone salivary cortisone, baseline serum cortisol, the salivary cortisone suppression ratio (pre-/post-dexamethasone), and sex yielded a: Sensitivity = 88.5% Specificity = 91.2% Salivary cortisone alone (cutoff < 2.7 nmol/L) for predicting a 1 mg DST ≤ 1.8 µg/dL: Sensitivity = 85.3% Specificity = 91.7% |
| First Author Year of Publication Reference Number | Study Design | Number of Patients Sex Ratio (F/M) Age (Years) | Outcomes |
|---|---|---|---|
| Petramala 2024 [77] | Retrospective study | N = 132 with AI F/M = 76/56 Age (mean ± SD) = 61.7 ± 10.8 y N = 90 AI (1 mg DST < 1.8 µg/dL) F/M = 27/17 Age (mean ± SD) = 61.6 ± 11.5 y N = 43 AI (1 mg DST > 1.8 µg/dL) F/M = 11/11 Age (mean ± SD) = 61.8 ± 9.4 y | Follow-up (annually for at least 5 years): 29.2% of subjects developed MACS (1 mg DST > 1.8 µg/dL) At the end of follow-up, MACS patients showed higher diastolic blood pressure values: NFA vs. MACS: 81.6 ± 10.5 vs. 83.7 ± 9.7 mmHg, p < 0.05 |
| Araujo-Castro 2023 [78] | Retrospective study | N = 331 with NFA F/M = 197/134 Age (mean ± SD) = 62.0 ± 10.6 y During a median follow-up time of 35.7 months N = 73 (22.1%) develop ACS (1 mg DST > 1.8 µg/dL) | The greatest predictor of ACS development during follow-up was a combination of age, post-DST serum cortisol, and bilaterality at presentation, which demonstrated good diagnosis accuracy AUC = 0.70 (95% CI: 0.64–0.76) DST being the threshold of 1.3 µg/dL for the prediction of ACS development AUC = 0.701 (95% CI: 0.637–0.765) Sensitivity = 70% Specificity = 62% Positive predictive value = 0.37% Negative predictive value = 99% The lowest probability of developing ACS: patients under 50 with cortisol post-DST values < 0.45 µg/dL and unilateral tumors had (2.42%) Baseline post-DST serum cortisol levels at diagnosis were significantly associated with the development of ACS throughout follow-up (hazard ratio 3.56 for each µg/dL, p < 0.001) Follow-up 1 mg DST < 0.9 µg/dL, follow-up is probably unnecessary 1 mg DST 0.9–1.3 µg/dL, repeating the DST every 2–3 years for five 1 mg DST > 1.3 µg/dL requires annual re-evaluation for at least five years |
| Araujo-Castro 2023 [38] | Retrospective study | N = 593 NFA F/M = 343/250 Age (mean ± SD) = 62.3 ± 10.83 y N = 442 NFA (1 mg DST ≤ 1.4 µg/dL) F/M = 257/185 Age (mean ± SD) = 61.3 ± 10.42 y N = 151 NFA (1 mg-DST > 1.4 µg/dL) F/M = 86/65 Age (mean ± SD) = 64.9 ± 11.58 y N = 412 NFA (1 mg DST ≤ 0.9 µg/dL) F/M = 241/171 Age (mean ± SD) = 59.6 ± 10.79 y N = 181 NFA (1 mg-DST > 0.9 µg/dL) F/M = 104/77 Age (mean ± SD) = 63.4 ± 10.66 y | Follow-up of 40.4 ± 51.17 months, 11.8% of the patients developed ACS ACS was increased in patients with higher blood cortisol post-DST levels (HR = 6.45 for each µg/dL, p = 0.001) Increased risk of ACS development when the DST level exceeded 1.4 µg/dL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trandafir, A.-I.; Carsote, M. Dexamethasone Suppression Testing in Patients with Adrenal Incidentalomas with/Without Mild Autonomous Cortisol Secretion: Spectrum of Cortisol Cutoffs and Additional Assays (An Updated Analysis). Biomedicines 2025, 13, 2169. https://doi.org/10.3390/biomedicines13092169
Trandafir A-I, Carsote M. Dexamethasone Suppression Testing in Patients with Adrenal Incidentalomas with/Without Mild Autonomous Cortisol Secretion: Spectrum of Cortisol Cutoffs and Additional Assays (An Updated Analysis). Biomedicines. 2025; 13(9):2169. https://doi.org/10.3390/biomedicines13092169
Chicago/Turabian StyleTrandafir, Alexandra-Ioana, and Mara Carsote. 2025. "Dexamethasone Suppression Testing in Patients with Adrenal Incidentalomas with/Without Mild Autonomous Cortisol Secretion: Spectrum of Cortisol Cutoffs and Additional Assays (An Updated Analysis)" Biomedicines 13, no. 9: 2169. https://doi.org/10.3390/biomedicines13092169
APA StyleTrandafir, A.-I., & Carsote, M. (2025). Dexamethasone Suppression Testing in Patients with Adrenal Incidentalomas with/Without Mild Autonomous Cortisol Secretion: Spectrum of Cortisol Cutoffs and Additional Assays (An Updated Analysis). Biomedicines, 13(9), 2169. https://doi.org/10.3390/biomedicines13092169






