Sex Differences in the Initiation and Progression of Necroptosis Following Kidney Ischemia–Reperfusion Injury
Abstract
1. Introduction
2. Methods
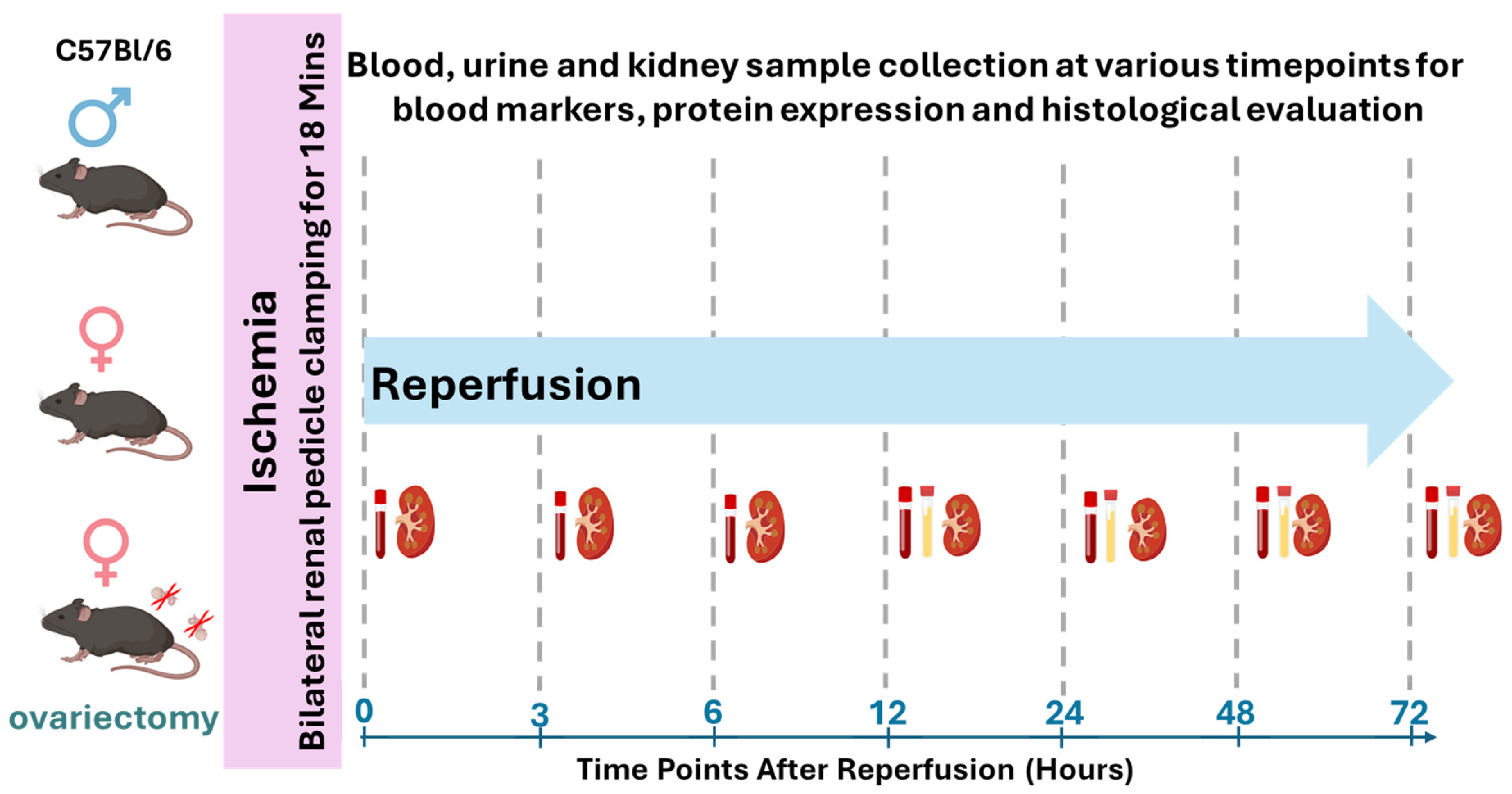
2.1. Study Design and Animals
2.2. IR-AKI Induction
2.3. Ovariectomy
2.4. Assessment of Kidney Injury and Function
2.5. Immunoblotting
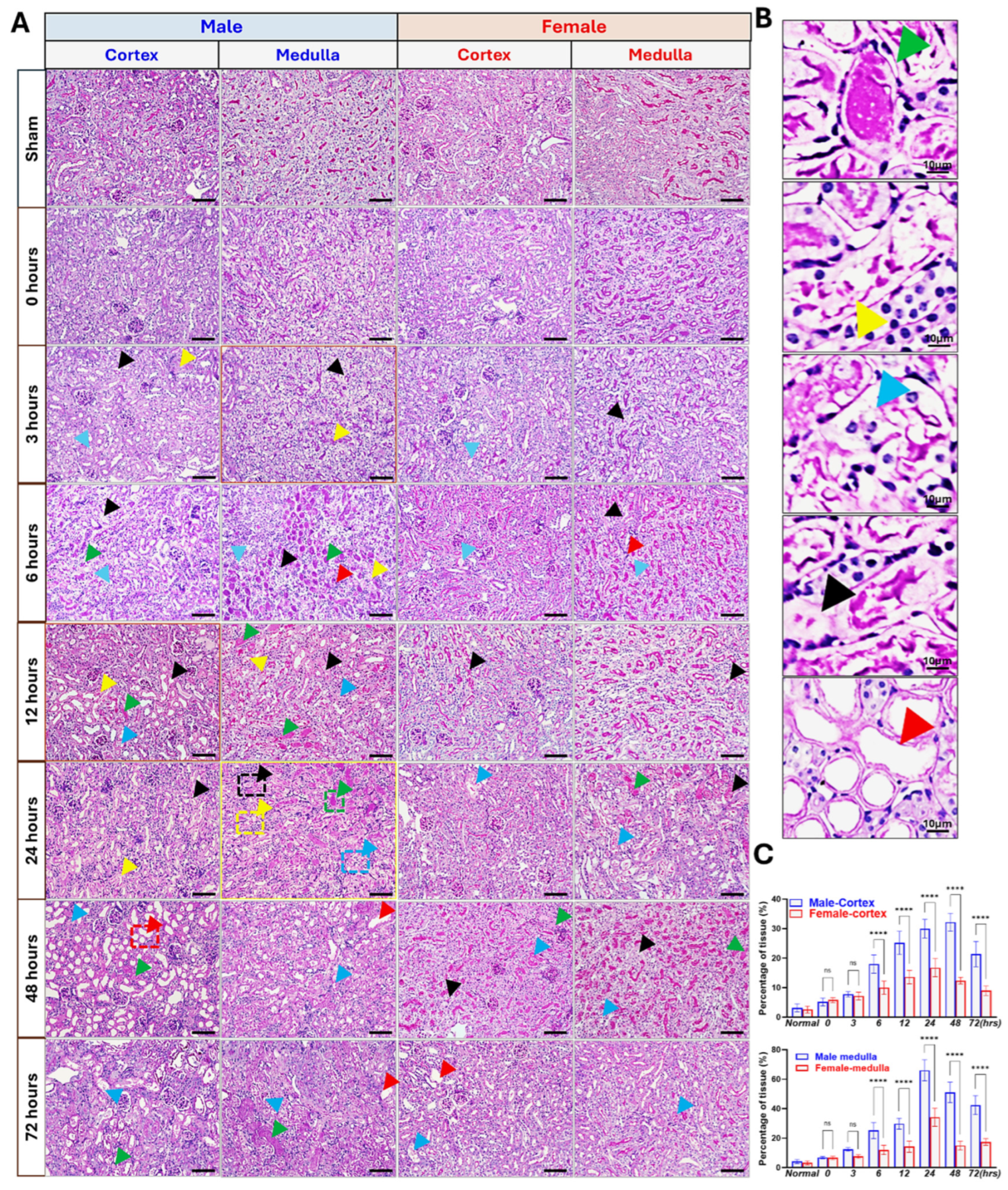
2.6. Histological Analysis
2.7. Real-Time PCR
2.8. Statistical Analyses
3. Results
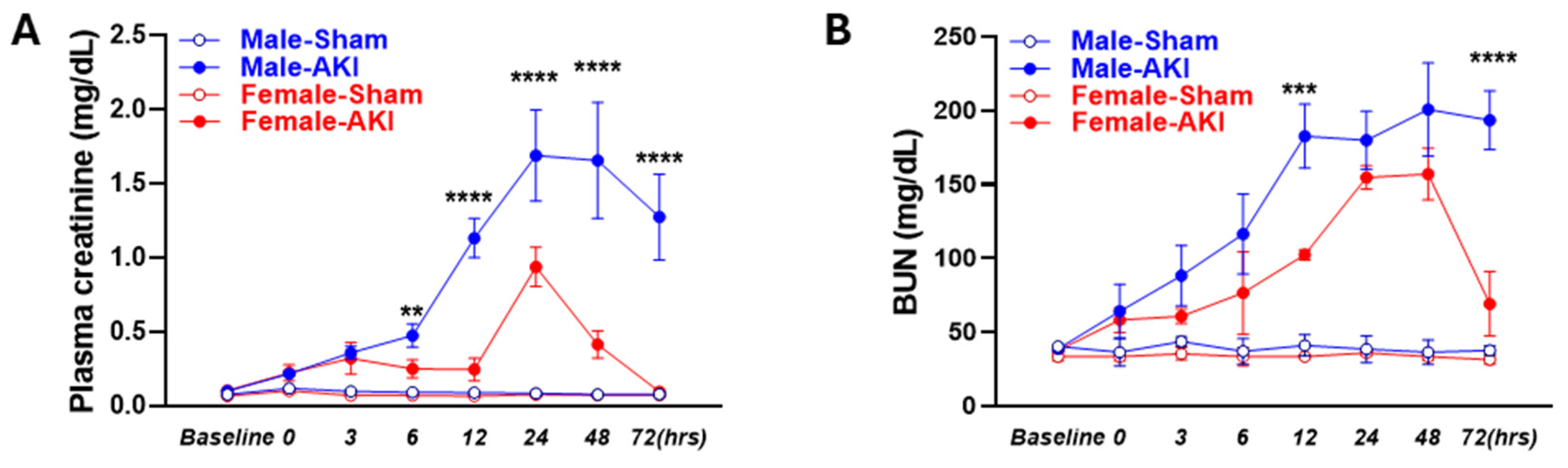
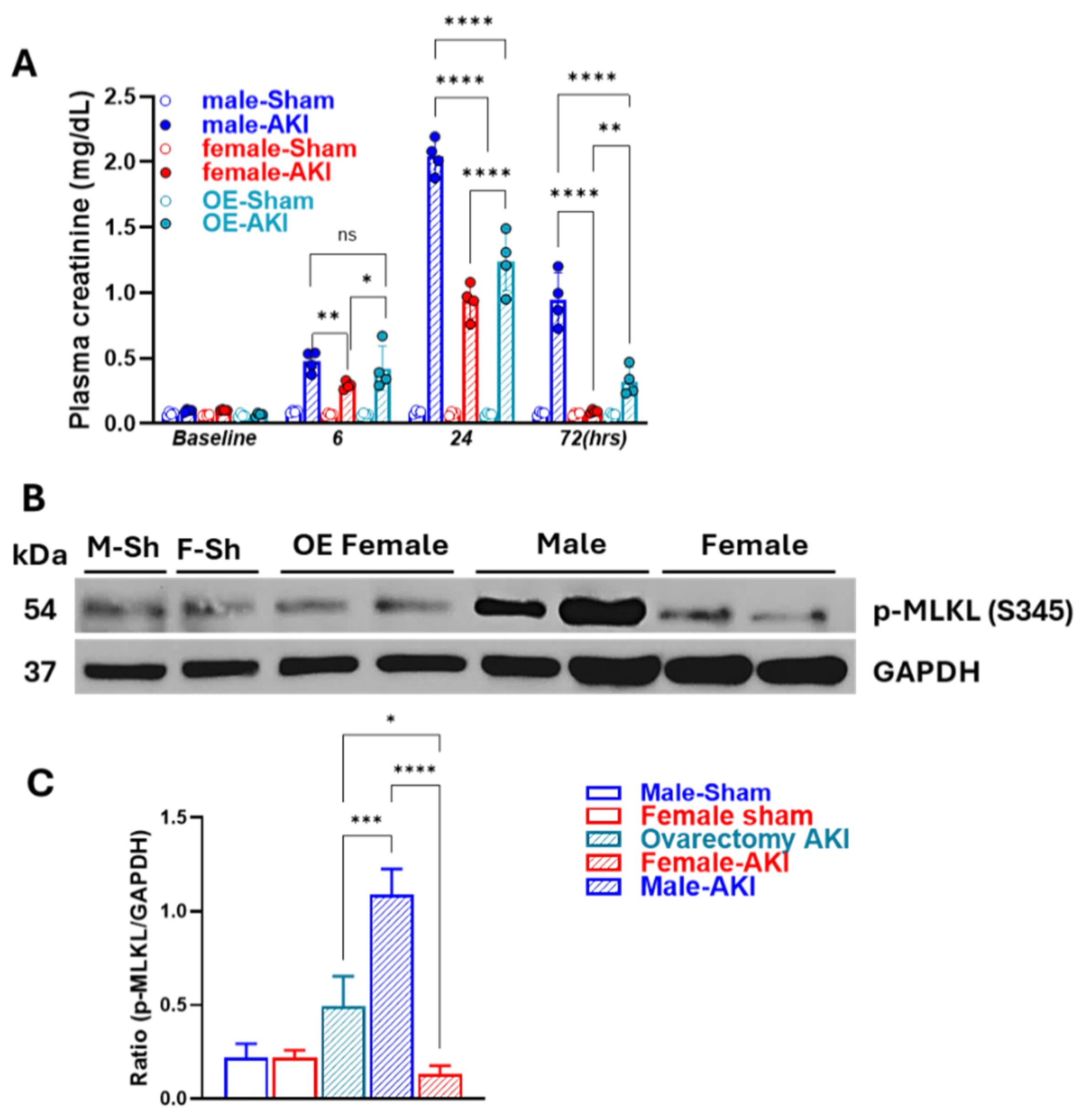
3.1. Sex Differences in IR-Induced Injury and Morphological Changes
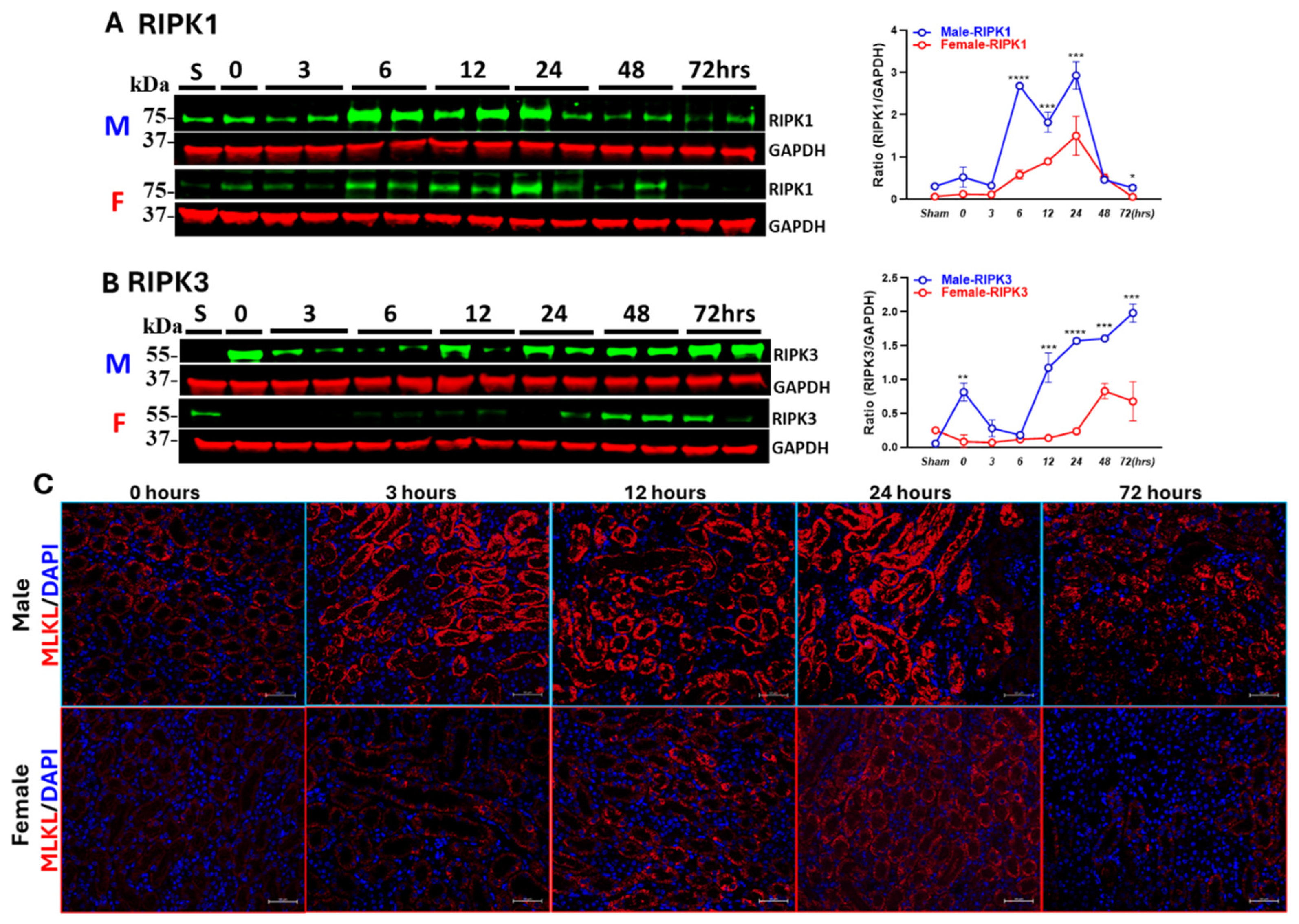
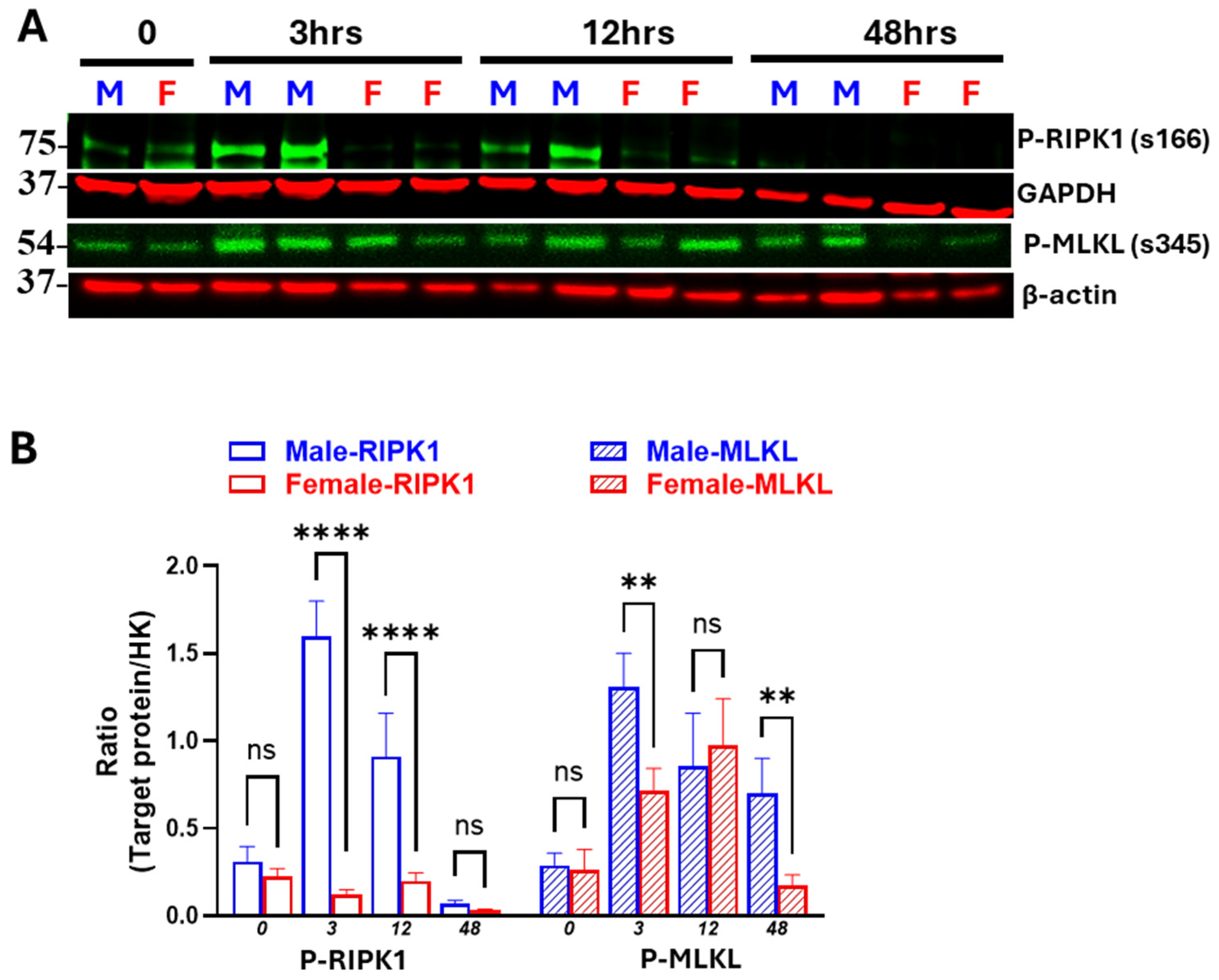
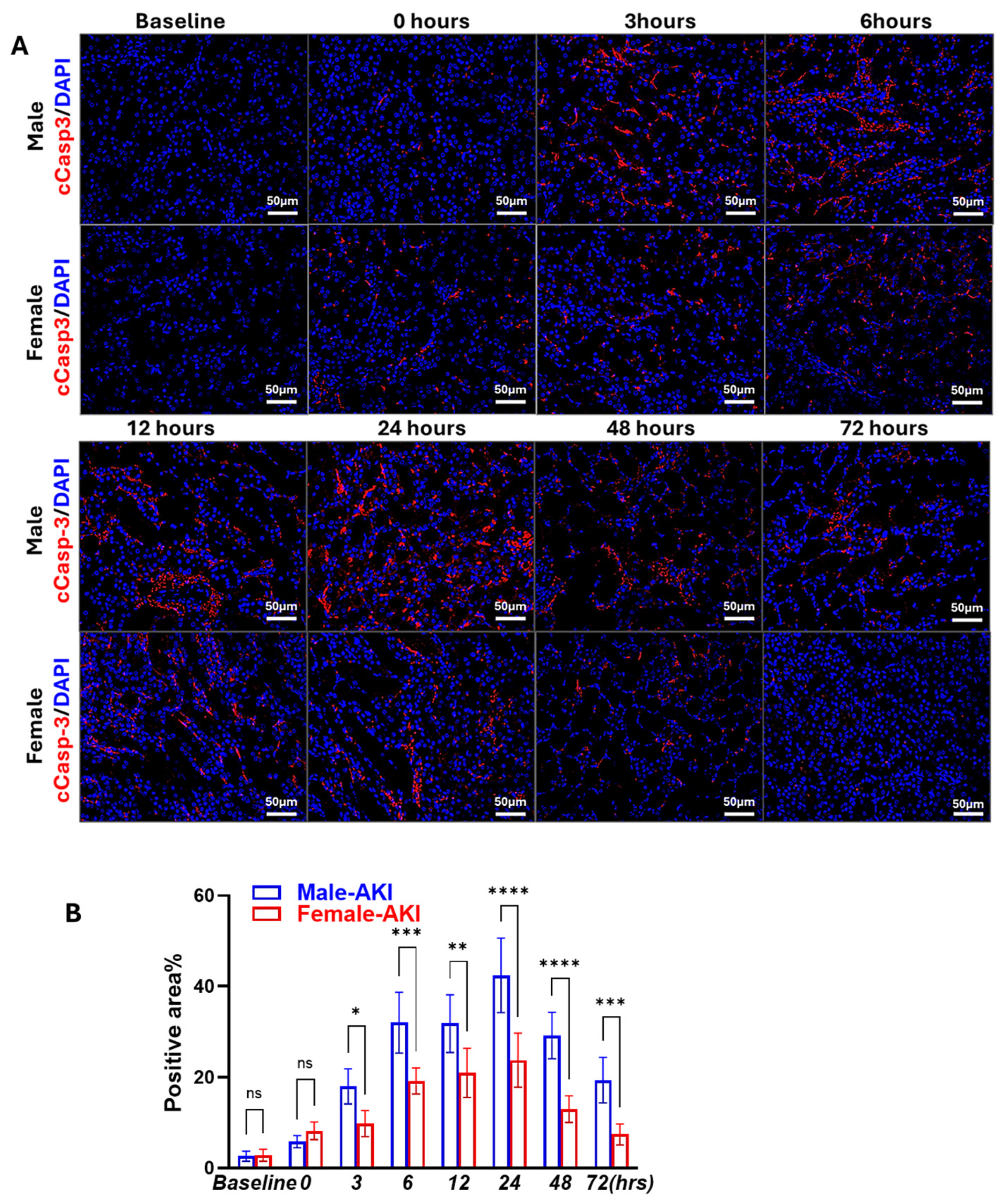
3.2. Sex Differences in IR-Induced RIPK1-Dependent Necroptotic and Apoptotic Markers
3.3. Males Exhibit Earlier and More Persistent Necroptosis Activation than Females
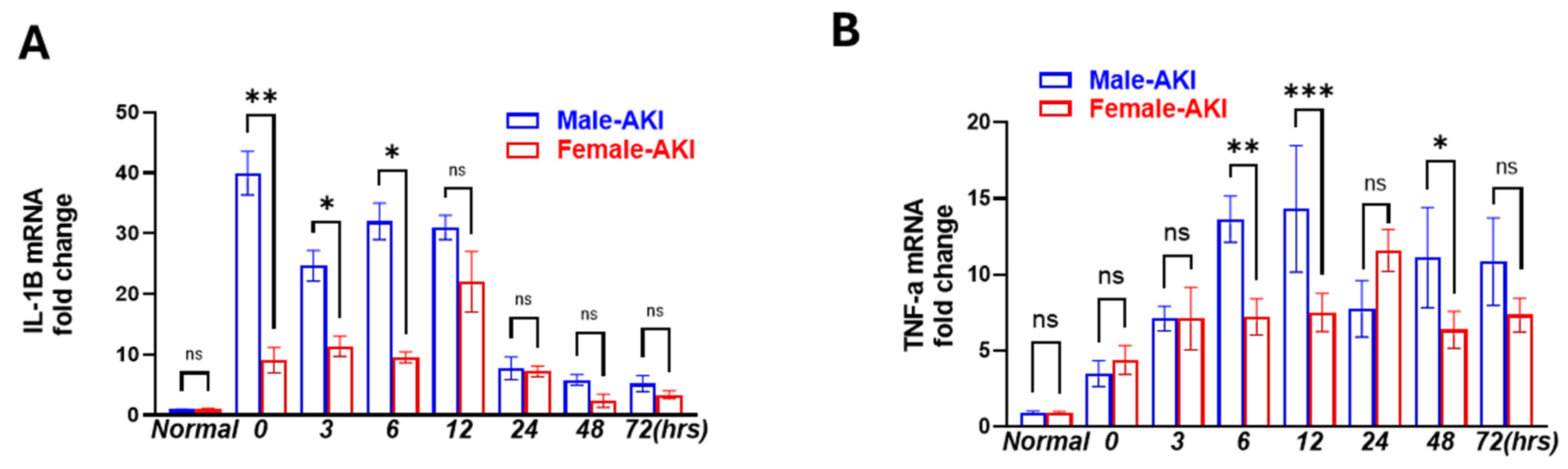
3.4. Male Mice Showed Earlier and More Persisted Inflammatory Response than Females
3.5. Removal of Ovarian Hormones Blunts Sex-Specific Differences in Necroptosis Timing and Intensity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Pefanis, A.; Ierino, F.L.; Murphy, J.M.; Cowan, P.J. Regulated necrosis in kidney ischemia-reperfusion injury. Kidney Int. 2019, 96, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Al-Jaghbeer, M.; Dealmeida, D.; Bilderback, A.; Ambrosino, R.; Kellum, J.A. Clinical Decision Support for In-Hospital AKI. J. Am. Soc. Nephrol. 2018, 29, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Zarjou, A.; Agarwal, A. Sepsis and acute kidney injury. J. Am. Soc. Nephrol. 2011, 22, 999–1006. [Google Scholar] [CrossRef]
- Bonventre, J.V.; Yang, L. Cellular pathophysiology of ischemic acute kidney injury. J. Clin. Investig. 2011, 121, 4210–4221. [Google Scholar] [CrossRef]
- Li, C.; Yu, Y.; Zhu, S.; Hu, Y.; Ling, X.; Xu, L.; Zhang, H.; Guo, K. The emerging role of regulated cell death in ischemia and reperfusion-induced acute kidney injury: Current evidence and future perspectives. Cell Death Discov. 2024, 10, 1–10. [Google Scholar] [CrossRef]
- Sun, L.; Wang, H.; Wang, Z.; He, S.; Chen, S.; Liao, D.; Wang, L.; Yan, J.; Liu, W.; Lei, X.; et al. Mixed Lineage Kinase Domain-like Protein Mediates Necrosis Signaling Downstream of RIP3 Kinase. Cell 2012, 148, 213–227. [Google Scholar] [CrossRef]
- Liu, S.; Liu, H.; Johnston, A.; Hanna-Addams, S.; Reynoso, E.; Xiang, Y.; Wang, Z. MLKL forms disulfide bond-dependent amyloid-like polymers to induce necroptosis. Proc. Natl. Acad. Sci. USA 2017, 114, E7450–E7459. [Google Scholar] [CrossRef]
- He, S.; Wang, X. RIP kinases as modulators of inflammation and immunity. Nat. Immunol. 2018, 19, 912–922. [Google Scholar] [CrossRef]
- He, S.; Wang, L.; Miao, L.; Wang, T.; Du, F.; Zhao, L.; Wang, X. Receptor Interacting Protein Kinase-3 Determines Cellular Necrotic Response to TNF-α. Cell 2009, 137, 1100–1111. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Challa, S.; Moquin, D.; Genga, R.; Ray, T.D.; Guildford, M.; Chan, F.K.-M. Phosphorylation-Driven Assembly of the RIP1-RIP3 Complex Regulates Programmed Necrosis and Virus-Induced Inflammation. Cell 2009, 137, 1112–1123. [Google Scholar] [CrossRef]
- Kearney, C.J.; Martin, S.J. An Inflammatory Perspective on Necroptosis. Mol. Cell 2017, 65, 965–973. [Google Scholar] [CrossRef]
- Pefanis, A.; Bongoni, A.K.; McRae, J.L.; Salvaris, E.J.; Fisicaro, N.; Murphy, J.M.; Ierino, F.L.; Cowan, P.J. Dynamics of necroptosis in kidney ischemia-reperfusion injury. Front. Immunol. 2023, 14, 1251452. [Google Scholar] [CrossRef]
- Linkermann, A.; Bräsen, J.H.; Himmerkus, N.; Liu, S.; Huber, T.B.; Kunzendorf, U.; Krautwald, S. Rip1 (Receptor-interacting protein kinase 1) mediates necroptosis and contributes to renal ischemia/reperfusion injury. Kidney Int. 2012, 81, 751–761. [Google Scholar] [CrossRef]
- Chen, H.; Fang, Y.; Wu, J.; Chen, H.; Zou, Z.; Zhang, X.; Shao, J.; Xu, Y. RIPK3-MLKL-mediated necroinflammation contributes to AKI progression to CKD. Cell Death Dis. 2018, 9, 878. [Google Scholar] [CrossRef] [PubMed]
- Neugarten, J.; Golestaneh, L. Sex Differences in Acute Kidney Injury. Semin. Nephrol. 2022, 42, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Hosszu, A.; Fekete, A.; Szabo, A.J. Sex differences in renal ischemia-reperfusion injury. Am. J. Physiol. Physiol. 2020, 319, F149–F154. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wang, Y.; Zhang, J.; Wang, L.; Fu, L.; Cha, B.J.; Buggs, J.; Liu, R. A mouse model of renal ischemia-reperfusion injury solely induced by cold ischemia. Am. J. Physiol. Physiol. 2019, 317, F616–F622. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, J.; Wang, L.; Jiang, S.; Fu, L.; Buggs, J.; Liu, R. New mouse model of chronic kidney disease transitioned from ischemic acute kidney injury. Am. J. Physiol. Physiol. 2019, 317, F286–F295. [Google Scholar] [CrossRef]
- Luengo-Mateos, M.; González-Vila, A.; Caldas, A.M.T.; Alasaoufi, A.M.; González-Domínguez, M.; López, M.; González-García, I.; Barca-Mayo, O. Protocol for ovariectomy and estradiol replacement in mice. STAR Protoc. 2024, 5, 102910. [Google Scholar] [CrossRef]
- Rowe, A.A.; Issioui, Y.; Johnny, B.; Wert, K.J. Murine Orchiectomy and Ovariectomy to Reduce Sex Hormone Production. J. Vis. Exp. 2023, e64379. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Jiang, S.; Wei, J.; Buggs, J.; Fu, L.; Zhang, J.; Liu, R. Graft function assessment in mouse models of single- and dual-kidney transplantation. Am. J. Physiol. Physiol. 2018, 315, F628–F636. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Song, J.; Wang, S.; Buggs, J.; Chen, R.; Zhang, J.; Wang, L.; Rong, S.; Li, W.; Wei, J.; et al. Cross-sex transplantation alters gene expression and enhances inflammatory response in the transplanted kidneys. Am. J. Physiol. Ren. Physiol. 2017, 313, F326–F338. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, L.; Liang, P.; Mast, J.; Mathis, C.; Liu, C.Y.; Wei, J.; Zhang, J.; Fu, L.; Juncos, L.A.; et al. Reducing ischemic kidney injury through application of a synchronization modulation electric field to maintain Na+/K+ -ATPase functions. Sci. Transl. Med. 2022, 14, eabj4906. [Google Scholar] [CrossRef] [PubMed]
- Mulay, S.R.; Linkermann, A.; Anders, H.J. Necroinflammation in Kidney Disease. J. Am. Soc. Nephrol. 2016, 27, 27–39. [Google Scholar] [CrossRef]
- Linkermann, A.; Stockwell, B.R.; Krautwald, S.; Anders, H.-J. Regulated cell death and inflammation: An auto-amplification loop causes organ failure. Nat. Rev. Immunol. 2014, 14, 759–767. [Google Scholar] [CrossRef]
- Neugarten, J.; Golestaneh, L. Influence of Sex on the Progression of Chronic Kidney Disease. Mayo Clin. Proc. 2019, 94, 1339–1356. [Google Scholar] [CrossRef]
- Müller, V.; Losonczy, G.; Heemann, U.; Vannay, A.; Fekete, A.; Reusz, G.; Tulassay, T.; Szabó, A.J. Sexual dimorphism in renal ischemia-reperfusion injury in rats: Possible role of endothelin. Kidney Int. 2002, 62, 1364–1371. [Google Scholar] [CrossRef]
- Cai, Z.; Jitkaew, S.; Zhao, J.; Chiang, H.-C.; Choksi, S.; Liu, J.; Ward, Y.; Wu, L.-G.; Liu, Z.-G. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat. Cell Biol. 2013, 16, 55–65. [Google Scholar] [CrossRef]
- Zhao, J.; Jitkaew, S.; Cai, Z.; Choksi, S.; Li, Q.; Luo, J.; Liu, Z.-G. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc. Natl. Acad. Sci. USA 2012, 109, 5322–5327. [Google Scholar] [CrossRef]
- Hanna-Addams, S.; Liu, S.; Liu, H.; Chen, S.; Wang, Z. CK1alpha, CK1delta, and CK1epsilon are necrosome components which phosphorylate serine 227 of human RIPK3 to activate necroptosis. Proc. Natl. Acad. Sci. USA 2020, 117, 1962–1970. [Google Scholar] [CrossRef]
- Wang, H.; Sun, L.; Su, L.; Rizo, J.; Liu, L.; Wang, L.-F.; Wang, F.-S.; Wang, X. Mixed Lineage Kinase Domain-like Protein MLKL Causes Necrotic Membrane Disruption upon Phosphorylation by RIP3. Mol. Cell 2014, 54, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Du, F.; Wang, X. TNF-α Induces Two Distinct Caspase-8 Activation Pathways. Cell 2008, 133, 693–703. [Google Scholar] [CrossRef]
- Satake, A.; Takaoka, M.; Nishikawa, M.; Yuba, M.; Shibata, Y.; Okumura, K.; Kitano, K.; Tsutsui, H.; Fujii, K.; Kobuchi, S.; et al. Protective effect of 17β-estradiol on ischemic acute renal failure through the PI3K/Akt/eNOS pathway. Kidney Int. 2008, 73, 308–317. [Google Scholar] [CrossRef]
- Kang, K.P.; Lee, J.E.; Lee, A.S.; Jung, Y.J.; Kim, D.; Lee, S.; Hwang, H.P.; Kim, W.; Park, S.K. Effect of gender differences on the regulation of renal ischemia-reperfusion-induced inflammation in mice. Mol. Med. Rep. 2014, 9, 2061–2068. [Google Scholar] [CrossRef]
- Hutchens, M.P.; Fujiyoshi, T.; Komers, R.; Herson, P.S.; Anderson, S. Estrogen protects renal endothelial barrier function from ischemia-reperfusion in vitro and in vivo. Am. J. Physiol. Physiol. 2012, 303, F377–F385. [Google Scholar] [CrossRef]
- Sharma, S.; Eghbali, M. Influence of sex differences on microRNA gene regulation in disease. Biol. Sex Differ. 2014, 5, 3. [Google Scholar] [CrossRef]
- McDonough, A.A.; Foley, T.S.; Ralph, D.L.; Schwindt, S.; Soong, J.; Gaytan, R.C.; Lasaad, S.; Nelson, J.W.; Edwards, A.; Kleyman, T.R.; et al. Effect of sex chromosome complement versus gonadal hormones on abundance of renal transporters. Am. J. Physiol. Physiol. 2025, 328, F638–F646. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Holliday, M.; Sheikh-Hamad, D.; Li, Q.; Tong, Q.; Hamad, C.D.; Pan, J.S. Sirtuin-3 mediates sex differences in kidney ischemia-reperfusion injury. Transl. Res. 2021, 235, 15–31. [Google Scholar] [CrossRef]
- Rodríguez, F.; Nieto-Cerón, S.; Fenoy, F.J.; López, B.; Hernández, I.; Martinez, R.R.; Soriano, M.J.G.; Salom, M.G. Sex differences in nitrosative stress during renal ischemia. Am. J. Physiol. Integr. Comp. Physiol. 2010, 299, R1387–R1395. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, M.H.; Parris, C.L.; Liu, C.; Oropeza, A.; Esquivel, C.; Rani, A.; Fan, Y.; Fu, L.; Buggs, J.; Wang, L. Sex Differences in the Initiation and Progression of Necroptosis Following Kidney Ischemia–Reperfusion Injury. Biomedicines 2025, 13, 2085. https://doi.org/10.3390/biomedicines13092085
Tran MH, Parris CL, Liu C, Oropeza A, Esquivel C, Rani A, Fan Y, Fu L, Buggs J, Wang L. Sex Differences in the Initiation and Progression of Necroptosis Following Kidney Ischemia–Reperfusion Injury. Biomedicines. 2025; 13(9):2085. https://doi.org/10.3390/biomedicines13092085
Chicago/Turabian StyleTran, Minh H., Colby L. Parris, Catherin Liu, Andrea Oropeza, Carlos Esquivel, Alka Rani, Yingxiang Fan, Liying Fu, Jacentha Buggs, and Lei Wang. 2025. "Sex Differences in the Initiation and Progression of Necroptosis Following Kidney Ischemia–Reperfusion Injury" Biomedicines 13, no. 9: 2085. https://doi.org/10.3390/biomedicines13092085
APA StyleTran, M. H., Parris, C. L., Liu, C., Oropeza, A., Esquivel, C., Rani, A., Fan, Y., Fu, L., Buggs, J., & Wang, L. (2025). Sex Differences in the Initiation and Progression of Necroptosis Following Kidney Ischemia–Reperfusion Injury. Biomedicines, 13(9), 2085. https://doi.org/10.3390/biomedicines13092085





