Lipin-1 Drives Browning of White Adipocytes via Promotion of Brown Phenotype Markers
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Protein Analysis
2.3. Gene Expression Analysis
2.4. Determination of Lipid Accumulation by Oil Red O Staining
2.5. Statistical Analysis
3. Results
3.1. PPARγ-Driven Regulation of Lipin-1 via the SIRT1–SRSF10 Axis
3.2. Modulation of Lipid Accumulation by SIRT1, SRSF10, and Lipin-1 During Adipocyte Differentiation
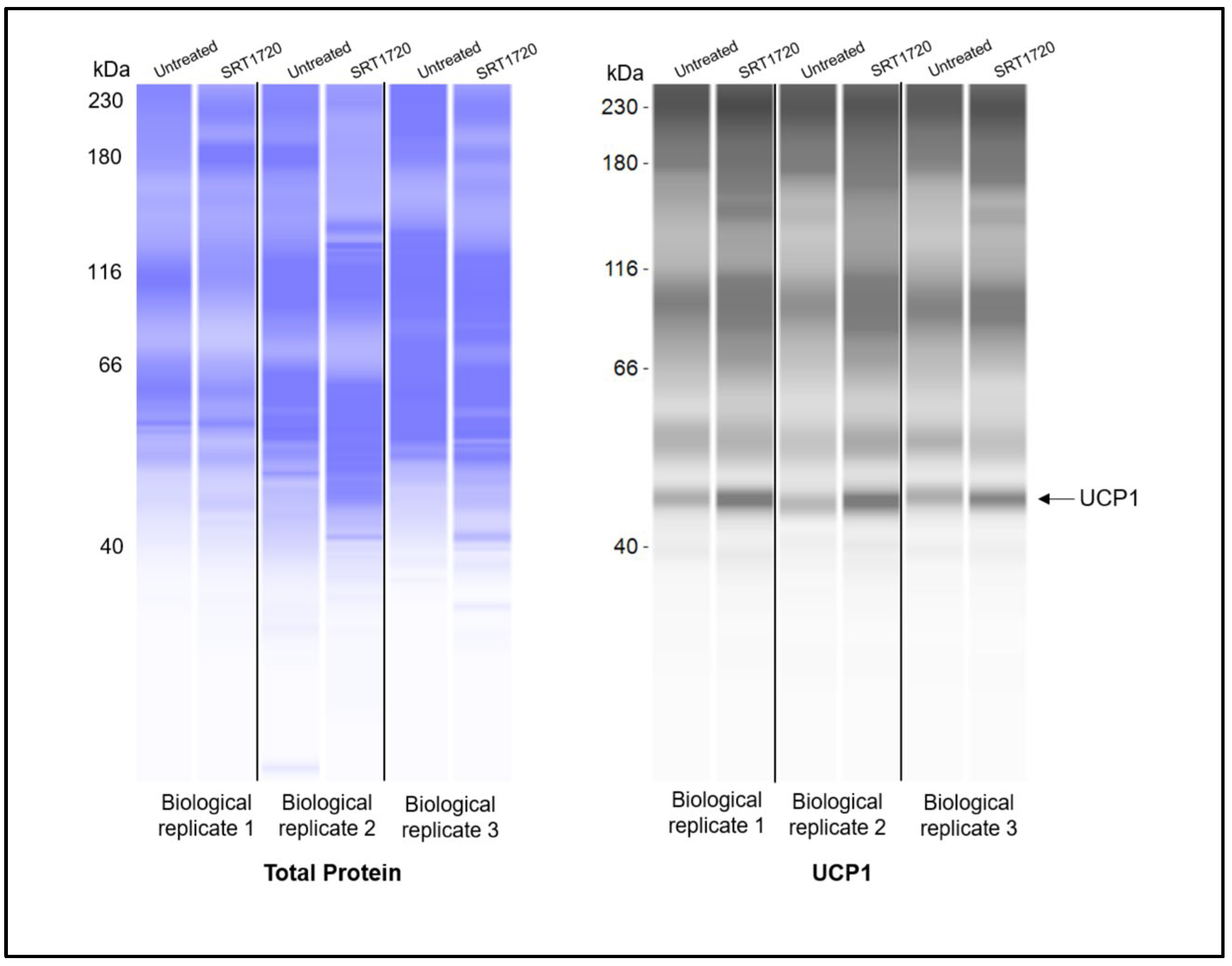
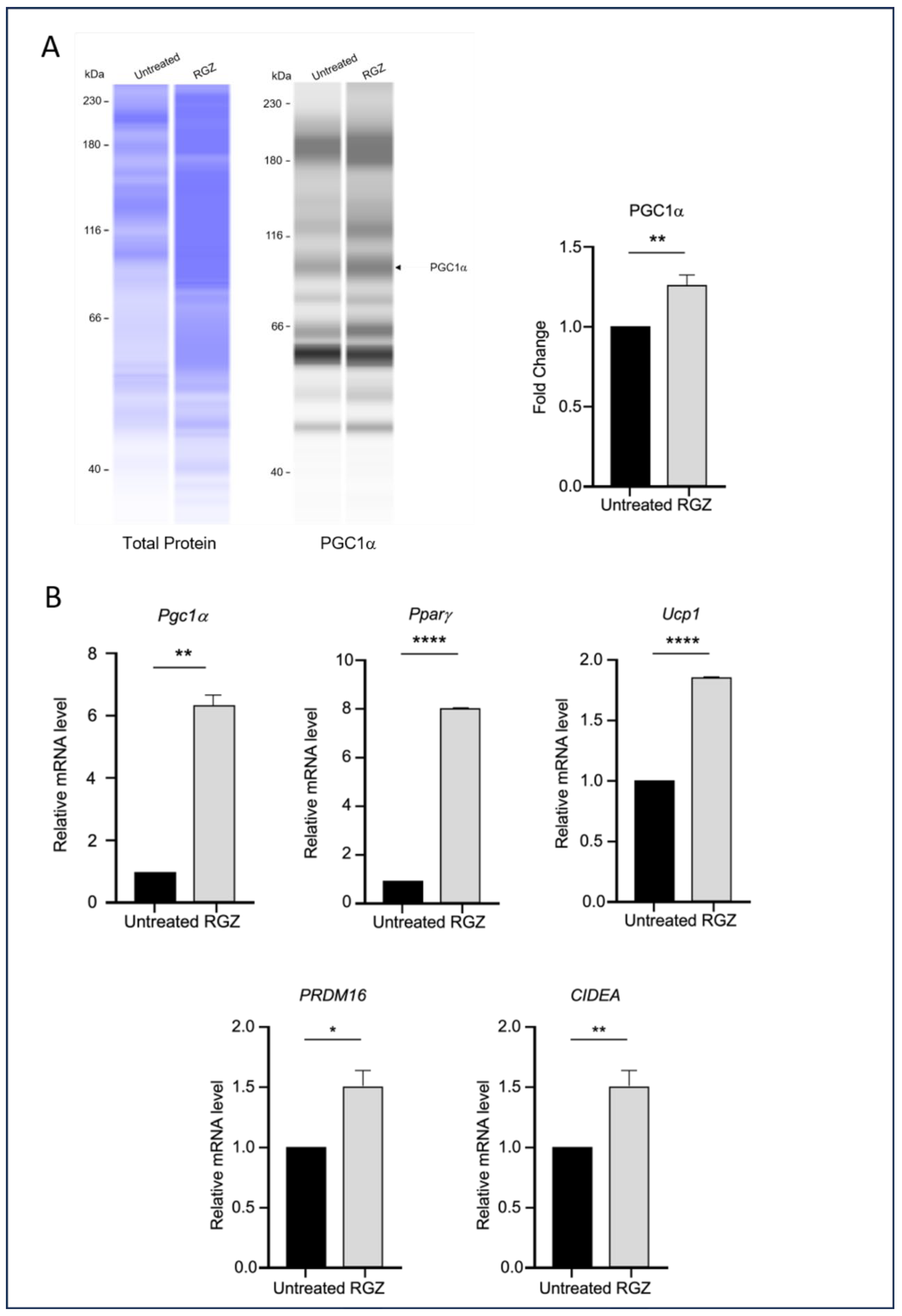
3.3. Elevated Lipin-1 Expression Enhances Browning Marker Expression via Distinct SIRT1-and PPARγ-Dependent Mechanisms
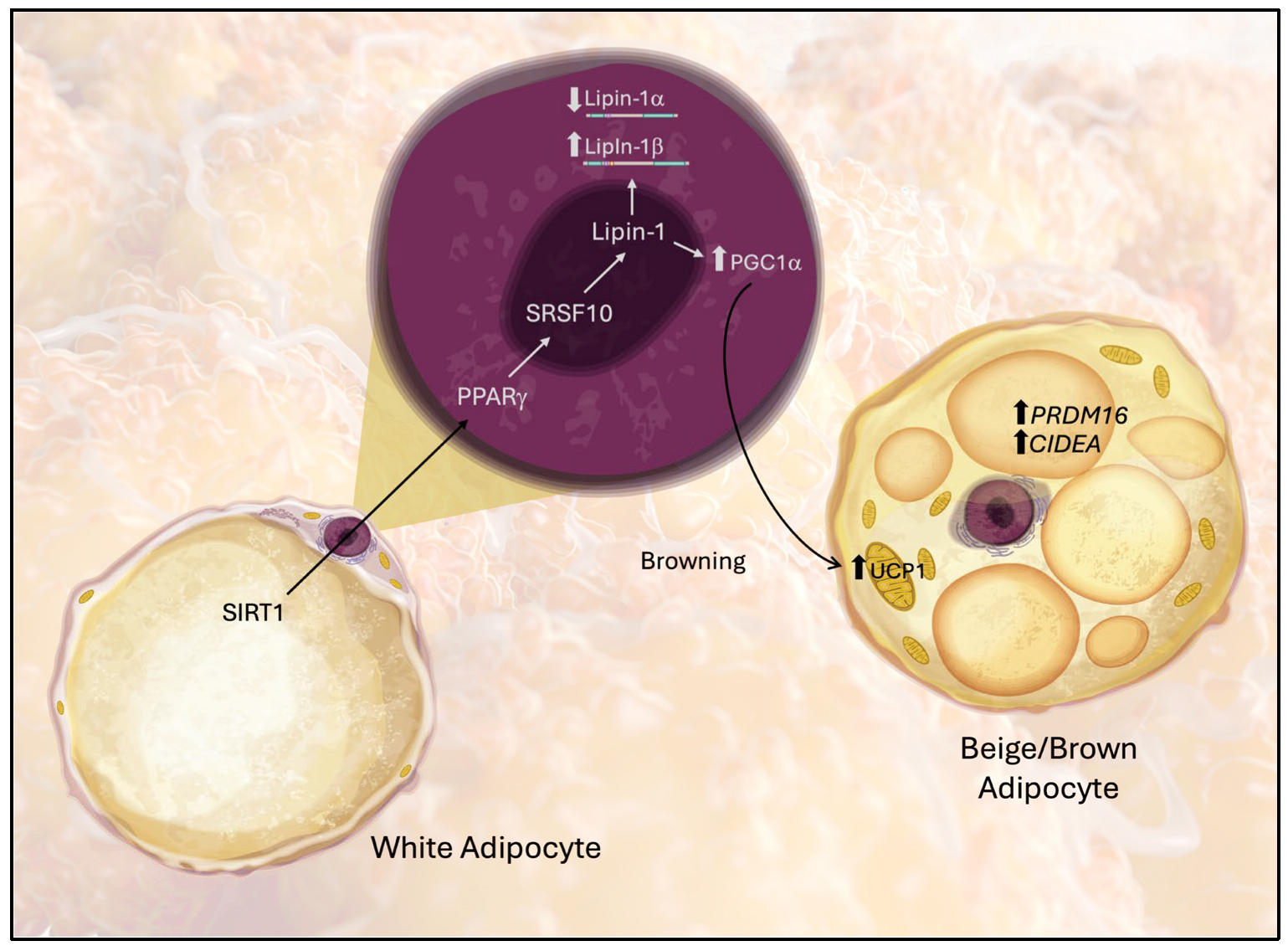
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [PubMed]
- Baldelli, S.; Aiello, G.; Mansilla Di Martino, E.; Campaci, D.; Muthanna, F.M.S.; Lombardo, M. The Role of Adipose Tissue and Nutrition in the Regulation of Adiponectin. Nutrients 2024, 16, 2436. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Beltrán-Velasco, A.I.; Martín-Rodríguez, A.; Martínez-Guardado, I.; Navarro-Jiménez, E.; Laborde-Cárdenas, C.C.; Tornero-Aguilera, J.F. The Role of Adipokines in Health and Disease. Biomedicines 2023, 11, 1290. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, A.; Virbasius, J.V.; Puri, V.; Czech, M.P. Adipocyte Dysfunctions Linking Obesity to Insulin Resistance and Type 2 Diabetes. Nat. Rev. Mol. Cell Biol. 2008, 9, 367–377. [Google Scholar] [CrossRef]
- Goossens, G.H. The Role of Adipose Tissue Dysfunction in the Pathogenesis of Obesity-Related Insulin Resistance. Physiol. Behav. 2008, 94, 206–218. [Google Scholar] [CrossRef]
- Kozak, L.P. Brown Fat and the Myth of Diet-Induced Thermogenesis. Cell Metab. 2010, 11, 263–267. [Google Scholar] [CrossRef]
- Schirinzi, V.; Poli, C.; Berteotti, C.; Leone, A. Browning of Adipocytes: A Potential Therapeutic Approach to Obesity. Nutrients 2023, 15, 2229. [Google Scholar] [CrossRef]
- Machado, S.A.; Pasquarelli-do-Nascimento, G.; da Silva, D.S.; Farias, G.R.; de Oliveira Santos, I.; Baptista, L.B.; Magalhães, K.G. Browning of the White Adipose Tissue Regulation: New Insights into Nutritional and Metabolic Relevance in Health and Diseases. Nutr. Metab. 2022, 19, 61. [Google Scholar] [CrossRef]
- Qiang, L.; Wang, L.; Kon, N.; Zhao, W.; Lee, S.; Zhang, Y.; Rosenbaum, M.; Zhao, Y.; Gu, W.; Farmer, S.R.; et al. Brown Remodeling of White Adipose Tissue by SirT1-Dependent Deacetylation of Pparγ. Cell 2012, 150, 620–632. [Google Scholar] [CrossRef]
- Schug, T.T.; Li, X. Sirtuin 1 in Lipid Metabolism and Obesity. Ann. Med. 2011, 43, 198–211. [Google Scholar] [CrossRef]
- Ye, X.; Li, M.; Hou, T.; Gao, T.; Zhu, W.; Yang, Y. Sirtuins in Glucose and Lipid Metabolism. Oncotarget 2016, 8, 1845–1859. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Koya, D. SIRT1 in Type 2 Diabetes: Mechanisms and Therapeutic Potential. Diabetes Metab. J. 2013, 37, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Phan, J.; Reue, K. Lipin, a Lipodystrophy and Obesity Gene. Cell Metab. 2005, 1, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; O’Loughlin, L.; Brindley, D.N.; Reue, K. Regulation of Lipin-1 Gene Expression by Glucocorticoids during Adipogenesis. J. Lipid Res. 2008, 49, 1519–1528. [Google Scholar] [CrossRef]
- Zhang, P.; Takeuchi, K.; Csaki, L.S.; Reue, K. Lipin-1 Phosphatidic Phosphatase Activity Modulates Phosphatidate Levels to Promote Peroxisome Proliferator-Activated Receptor γ (PPARγ) Gene Expression during Adipogenesis. J. Biol. Chem. 2012, 287, 3485–3494. [Google Scholar] [CrossRef]
- Reue, K.; Zhang, P. The Lipin Protein Family: Dual Roles in Lipid Biosynthesis and Gene Expression. FEBS Lett. 2008, 582, 90–96. [Google Scholar] [CrossRef]
- Reue, K.; Dwyer, J.R. Lipin Proteins and Metabolic Homeostasis. J. Lipid Res. 2009, 50, S109–S114. [Google Scholar] [CrossRef]
- Reue, K.; Brindley, D.N. Thematic Review Series: Glycerolipids. Multiple Roles for Lipins/Phosphatidate Phosphatase Enzymes in Lipid Metabolism. J. Lipid Res. 2008, 49, 2493–2503. [Google Scholar] [CrossRef]
- Bou Khalil, M.; Sundaram, M.; Zhang, H.-Y.; Links, P.H.; Raven, J.F.; Manmontri, B.; Sariahmetoglu, M.; Tran, K.; Reue, K.; Brindley, D.N.; et al. The Level and Compartmentalization of Phosphatidate Phosphatase-1 (Lipin-1) Control the Assembly and Secretion of Hepatic VLDL. J. Lipid Res. 2009, 50, 47–58. [Google Scholar] [CrossRef]
- Csaki, L.S.; Reue, K. Lipins: Multifunctional Lipid Metabolism Proteins. Annu. Rev. Nutr. 2010, 30, 257–272. [Google Scholar] [CrossRef]
- Reue, K. The Lipin Family: Mutations and Metabolism. Curr. Opin. Lipidol. 2009, 20, 165–170. [Google Scholar] [CrossRef] [PubMed]
- The Role of Lipin-1 in the Pathogenesis of Alcoholic Fatty Liver—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/25595739/ (accessed on 12 September 2024).
- Li, Y.; Zhou, J. Roles of Silent Information Regulator 1–Serine/Arginine-Rich Splicing Factor 10–Lipin 1 Axis in the Pathogenesis of Alcohol Fatty Liver Disease. Exp. Biol. Med. 2017, 242, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Hu, M.; Liang, X.; Ajmo, J.M.; Li, X.; Bataller, R.; Odena, G.; Stevens, S.M.; You, M. Deletion of SIRT1 from Hepatocytes in Mice Disrupts Lipin-1 Signaling and Aggravates Alcoholic Fatty Liver. Gastroenterology 2014, 146, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhang, M.; Webster, N.J.G. Alternative RNA Splicing in Fatty Liver Disease. Front. Endocrinol. 2021, 12, 613213. [Google Scholar] [CrossRef]
- Zhou, F.; Fan, X.; Miao, Y. LPIN1 Promotes Triglycerides Synthesis and Is Transcriptionally Regulated by PPARG in Buffalo Mammary Epithelial Cells. Sci. Rep. 2022, 12, 2390. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.-J.; Kim, J.M.; Lee, S.Y.; Bae, M.-A.; Ahn, J.H.; Han, D.C.; Kwon, B.-M. PPARγ Agonists Induce Adipocyte Differentiation by Modulating the Expression of Lipin-1, Which Acts as a PPARγ Phosphatase. Int. J. Biochem. Cell. Biol. 2016, 81, 57–66. [Google Scholar] [CrossRef]
- Rocha, A.L.; Guerra, B.A.; Boucher, J.; Mori, M.A. A Method to Induce Brown/Beige Adipocyte Differentiation from Murine Preadipocytes. Bio-Protocol 2021, 11, e4265. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual; Markossian, S., Grossman, A., Baskir, H., Arkin, M., Auld, D., Austin, C., Baell, J., Brimacombe, K., Chung, T.D.Y., Coussens, N.P., et al., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004. [Google Scholar]
- Wang, Y.-M.; Huang, T.-L.; Meng, C.; Zhang, J.; Fang, N.-Y. SIRT1 Deacetylates Mitochondrial Trifunctional Enzyme α Subunit to Inhibit Ubiquitylation and Decrease Insulin Resistance. Cell Death Dis. 2020, 11, 821. [Google Scholar] [CrossRef]
- Sormunen, A.; Koivulehto, E.; Alitalo, K.; Saksela, K.; Laham-Karam, N.; Ylä-Herttuala, S. Comparison of Automated and Traditional Western Blotting Methods. Methods Protoc. 2023, 6, 43. [Google Scholar] [CrossRef]
- Huang, S.; Huang, S.; Wang, X.; Zhang, Q.; Liu, J.; Leng, Y. Downregulation of Lipin-1 Induces Insulin Resistance by Increasing Intracellular Ceramide Accumulation in C2C12 Myotubes. Int. J. Biol. Sci. 2017, 13, 1–12. [Google Scholar] [CrossRef]
- Pal, P.; Kanaujiya, J.K.; Lochab, S.; Tripathi, S.B.; Sanyal, S.; Behre, G.; Trivedi, A.K. Proteomic Analysis of Rosiglitazone and Guggulsterone Treated 3T3-L1 Preadipocytes. Mol. Cell. Biochem. 2013, 376, 81–93. [Google Scholar] [CrossRef]
- Sakers, A.; De Siqueira, M.K.; Seale, P.; Villanueva, C.J. Adipose-Tissue Plasticity in Health and Disease. Cell 2022, 185, 419–446. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yu, T.; Ma, G.; Zheng, L.; Jiang, X.; Yang, F.; Wang, Z.; Li, N.; He, Z.; Song, X.; et al. Berberine Modulates Deacetylation of PPARγ to Promote Adipose Tissue Remodeling and Thermogenesis via AMPK/SIRT1 Pathway. Int. J. Biol. Sci. 2021, 17, 3173–3187. [Google Scholar] [CrossRef] [PubMed]
- Mayoral, R.; Osborn, O.; McNelis, J.; Johnson, A.M.; Oh, D.Y.; Izquierdo, C.L.; Chung, H.; Li, P.; Traves, P.G.; Bandyopadhyay, G.; et al. Adipocyte SIRT1 Knockout Promotes PPARγ Activity, Adipogenesis and Insulin Sensitivity in Chronic-HFD and Obesity. Mol. Metab. 2015, 4, 378–391. [Google Scholar] [CrossRef] [PubMed]
- Naing, Y.T.; Sun, L. The Role of Splicing Factors in Adipogenesis and Thermogenesis. Mol. Cells 2023, 46, 268–277. [Google Scholar] [CrossRef]
- Li, H.; Cheng, Y.; Wu, W.; Liu, Y.; Wei, N.; Feng, X.; Xie, Z.; Feng, Y. SRSF10 Regulates Alternative Splicing and Is Required for Adipocyte Differentiation. Mol. Cell. Biol. 2014, 34, 2198–2207. [Google Scholar] [CrossRef]
- Pihlajamäki, J.; Lerin, C.; Itkonen, P.; Boes, T.; Floss, T.; Schroeder, J.; Dearie, F.; Crunkhorn, S.; Burak, F.; Jimenez-Chillaron, J.C.; et al. Expression of the Splicing Factor Gene SFRS10 Is Reduced in Human Obesity and Contributes to Enhanced Lipogenesis. Cell Metab. 2011, 14, 208–218. [Google Scholar] [CrossRef]
- Martinez, L.; Berenguer, M.; Bruce, M.C.; Le Marchand-Brustel, Y.; Govers, R. Rosiglitazone Increases Cell Surface GLUT4 Levels in 3T3-L1 Adipocytes through an Enhancement of Endosomal Recycling. Biochem. Pharmacol. 2010, 79, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Renes, J.; Bouwman, F.; Bunschoten, A.; Mariman, E.; Keijer, J. Absence of an Adipogenic Effect of Rosiglitazone on Mature 3T3-L1 Adipocytes: Increase of Lipid Catabolism and Reduction of Adipokine Expression. Diabetologia 2007, 50, 654–665. [Google Scholar] [CrossRef]
- Li, Y.; Pan, Y.; Zhao, X.; Wu, S.; Li, F.; Wang, Y.; Liu, B.; Zhang, Y.; Gao, X.; Wang, Y.; et al. Peroxisome Proliferator-Activated Receptors: A Key Link between Lipid Metabolism and Cancer Progression. Clin. Nutr. 2024, 43, 332–345. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Mao, S.; Chen, S.; Zhang, W.; Liu, C. PPARs-Orchestrated Metabolic Homeostasis in the Adipose Tissue. Int. J. Mol. Sci. 2021, 22, 8974. [Google Scholar] [CrossRef] [PubMed]
- Boutant, M.; Cantó, C. SIRT1 Metabolic Actions: Integrating Recent Advances from Mouse Models. Mol. Metab. 2014, 3, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Simmons, G.E.; Pruitt, W.M.; Pruitt, K. Diverse Roles of SIRT1 in Cancer Biology and Lipid Metabolism. Int. J. Mol. Sci. 2015, 16, 950–965. [Google Scholar] [CrossRef]
- Wang, B.; Hu, Z.; Cui, L.; Zhao, M.; Su, Z.; Jiang, Y.; Liu, J.; Zhao, Y.; Hou, Y.; Yang, X.; et al. βAR-mTOR-Lipin1 Pathway Mediates PKA-RIIβ Deficiency-Induced Adipose Browning. Theranostics 2024, 14, 5316–5335. [Google Scholar] [CrossRef]
- Kroon, T.; Harms, M.; Maurer, S.; Bonnet, L.; Alexandersson, I.; Lindblom, A.; Ahnmark, A.; Nilsson, D.; Gennemark, P.; O’Mahony, G.; et al. PPARγ and PPARα Synergize to Induce Robust Browning of White Fat in Vivo. Mol. Metab. 2020, 36, 100964. [Google Scholar] [CrossRef]
- Majeed, Y.; Halabi, N.; Madani, A.Y.; Engelke, R.; Bhagwat, A.M.; Abdesselem, H.; Agha, M.V.; Vakayil, M.; Courjaret, R.; Goswami, N.; et al. SIRT1 Promotes Lipid Metabolism and Mitochondrial Biogenesis in Adipocytes and Coordinates Adipogenesis by Targeting Key Enzymatic Pathways. Sci. Rep. 2021, 11, 8177. [Google Scholar] [CrossRef]
- Seale, P.; Kajimura, S.; Yang, W.; Chin, S.; Rohas, L.; Uldry, M.; Tavernier, G.; Langin, D.; Spiegelman, B.M. Transcriptional Control of Brown Fat Determination by PRDM16. Cell Metab. 2007, 6, 38–54. [Google Scholar] [CrossRef]
- Puri, V.; Ranjit, S.; Konda, S.; Nicoloro, S.M.C.; Straubhaar, J.; Chawla, A.; Chouinard, M.; Lin, C.; Burkart, A.; Corvera, S.; et al. Cidea Is Associated with Lipid Droplets and Insulin Sensitivity in Humans. Proc. Natl. Acad. Sci. USA 2008, 105, 7833–7838. [Google Scholar] [CrossRef]
- Barneda, D.; Planas-Iglesias, J.; Gaspar, M.L.; Mohammadyani, D.; Prasannan, S.; Dormann, D.; Han, G.-S.; Jesch, S.A.; Carman, G.M.; Kagan, V.; et al. The Brown Adipocyte Protein CIDEA Promotes Lipid Droplet Fusion via a Phosphatidic Acid-Binding Amphipathic Helix. eLife 2015, 4, e07485. [Google Scholar] [CrossRef]





| RT-qPCR Primer | Sequence |
|---|---|
| Ucp1 forward | 5′-GGC ATT CAG AGG CAA ATC AGC T-3′ |
| Ucp1 reverse | 5′-CAA TGA ACA CTG CCA CAC CTC-3′ |
| Sirt1 forward | 5′-TGC CAT CAT GAA GCC AGA GA-3′ |
| Sirt1 reverse | 5′-AAC ATC GCA GTC TCC AAG GA-3′ |
| Lpin1a forward | 5′-GGT CCC CCA GCC CCA GTC CTT-3′ |
| Lpin1a reverse | 5′-GCA GCC TGT GGC AAT TCA-3′ |
| Srsf10 forward | 5′-GTC CCA CTT GAT TTC TAC ACT CG-3′ |
| Srsf10 reverse | 5′-TTC TCC TTT CAT AAC TCC GGC T-3′ |
| Pgc1a forward | 5′-TGA GGA CTG CTA GCA AGT TTG-3′ |
| Pgc1a reverse | 5′-AGT GAC CAA TCA GAA ATA ATA TCC AAT C-3′ |
| Lpin1 forward | 5′-TCC CAG TTC GGA CAG AGA AT-3′ |
| Lpin1 reverse | 5′-GCC AGA GCA TTT CCA GGT TA-3′ |
| Lpin1b forward | 5′-CAG CCT GGT AGA TTG CCA GA-3′ |
| Lpin1b reverse | 5′-GCA GCC TGT GGC AAT TCA-3′ |
| Pparg forward | 5′-CCA GAG CAT GGT GCC TTC GCT-3′ |
| Pparg reverse | 5′-CAG CAA CCA TTG GGT CAG CTC-3′ |
| Actb forward | 5′-GGC ACC ACA CCT TCT ACA ATG-3′ |
| Actb reverse | 5′-GGG GTG TTG AAG GTC TCA AAC-3′ |
| PRDM16 forward | 5′-GAT GGG AGA TGCT GAC GGAT-3′ |
| PRDM16 reverse | 5′-TGA TCT GAC ACA TGG CGA GG-3′ |
| Cidea forward | 5′-GTG TTA AGG AAT CTG CTG AG-3′ |
| Cidea reverse | 5′-CTA TAA CAG AGA GCA GGG TC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamzah, S.S.; Zamri, L.A.; Abdul Azar, S.A.; Abdul Aziz, S.M.; Abdullah, S.R.; Abu Seman, N. Lipin-1 Drives Browning of White Adipocytes via Promotion of Brown Phenotype Markers. Biomedicines 2025, 13, 2069. https://doi.org/10.3390/biomedicines13092069
Hamzah SS, Zamri LA, Abdul Azar SA, Abdul Aziz SM, Abdullah SR, Abu Seman N. Lipin-1 Drives Browning of White Adipocytes via Promotion of Brown Phenotype Markers. Biomedicines. 2025; 13(9):2069. https://doi.org/10.3390/biomedicines13092069
Chicago/Turabian StyleHamzah, Siti Sarah, Liyana Ahmad Zamri, Siti Azrinnah Abdul Azar, Siti Mastura Abdul Aziz, Shazana Rifham Abdullah, and Norhashimah Abu Seman. 2025. "Lipin-1 Drives Browning of White Adipocytes via Promotion of Brown Phenotype Markers" Biomedicines 13, no. 9: 2069. https://doi.org/10.3390/biomedicines13092069
APA StyleHamzah, S. S., Zamri, L. A., Abdul Azar, S. A., Abdul Aziz, S. M., Abdullah, S. R., & Abu Seman, N. (2025). Lipin-1 Drives Browning of White Adipocytes via Promotion of Brown Phenotype Markers. Biomedicines, 13(9), 2069. https://doi.org/10.3390/biomedicines13092069





