Aims and Rationale of a National Registry Integrating Clinical, Echocardiographic, and Multi-Omics Profiling to Promote Precision Medicine in Peripartum Cardiomyopathy
Abstract
1. Introduction
2. PPCM Clinical Characteristics
2.1. Epidemiology
2.2. Risk Factors and Comorbidities
2.3. Clinical Presentation
2.4. Differential Diagnosis
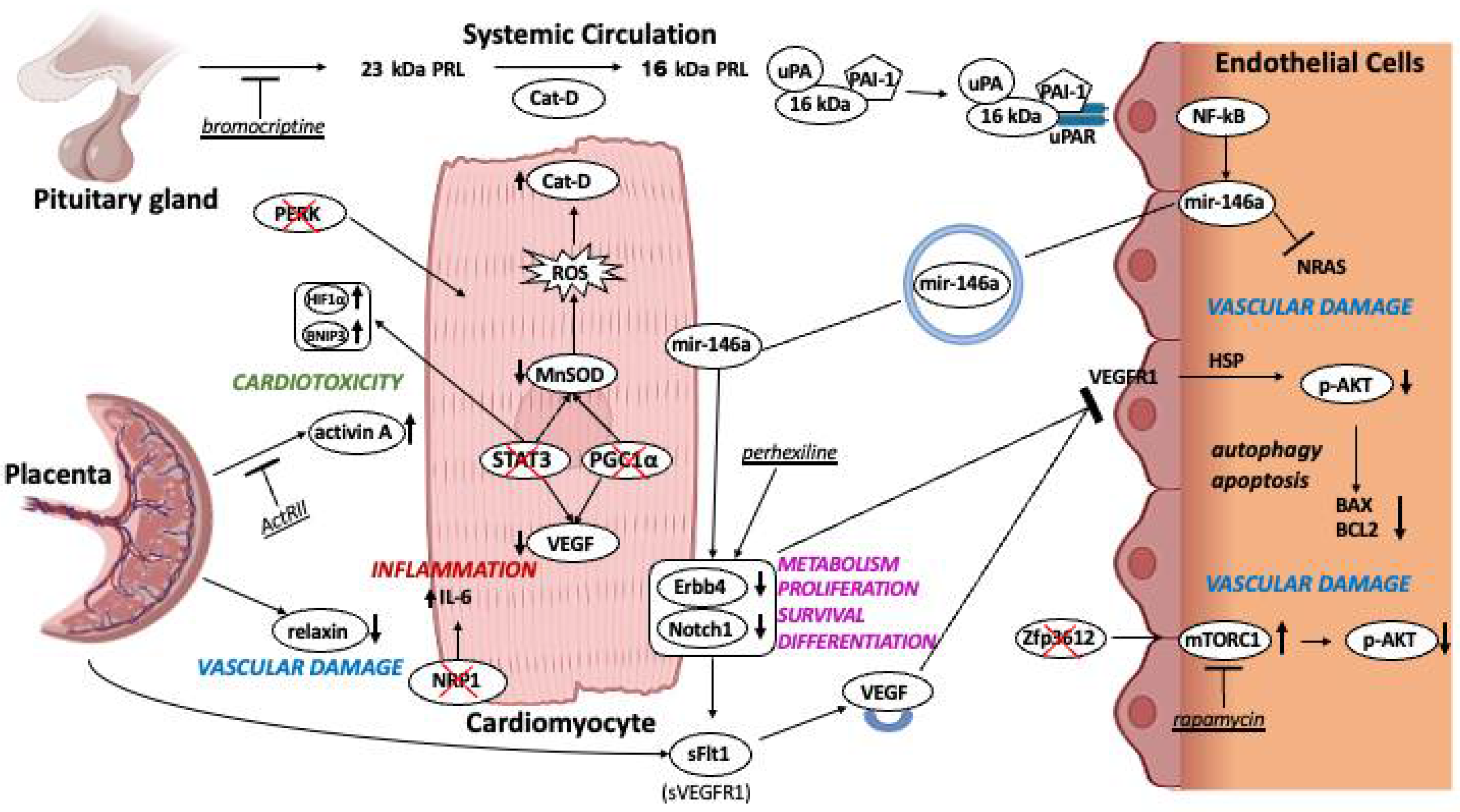
3. PPCM Etiology: An Ongoing Working Hypothesis
3.1. Cardiovascular Mechanisms Involved in PPCM
3.2. Preclinical Models of PPCM
4. Diagnostic Evaluation
4.1. Clinical Assessment and Electrocardiogram
4.2. Laboratory Tests
4.3. Cardiac Imaging
4.4. Large Scale Omics Fingerprinting and Biomarkers Discovery
5. Prognosis and Outcomes
6. Rationale, Objectives, and Expected Outcome of a Multicenter National Registry Integrating Clinical, Imaging, and Multi-Omics Profiling of PPCM Patients
6.1. Rationale
6.2. Objectives
6.3. Study Procedures
- (a)
- Multidisciplinary clinical assessment with extensive physical examination and patient interview, including medical and family history.
- (b)
- Standard 12-lead ECG.
- (c)
- Transthoracic echocardiography to primarily evaluate cardiac function. Whenever possible and if tolerated, cardiac magnetic resonance imaging will also be performed.
- (d)
- Evaluation of quality of life by Kansas City Car diomyopathy Questionnaire and the EuroQoL (EQ-5D-5L) questionnaire.
- (e)
- Blood sampling for standard laboratory tests, Peripheral Blood Mononuclear Cells (PBMC) isolation, and multi-omics profiling (whole exome sequencing; RNA sequencing; metabolomics; proteomics), as detailed in the next section.

6.4. Multi-Omics Strategy and Integration with Clinical Phenotyping
6.5. Follow-Up
6.6. Expected Outcomes, Scientific and Clinical Implications
7. Conclusions and Future Perspectives
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
| PPCM | Peripartum Cardiomyopathy |
| ESC | European Society of Cardiology |
| HF | Heart Failure |
| HFrEF | Heart Failure with Reduced Ejection Fraction |
| LVEF | Left Ventricle Ejection Fraction |
| HFpEF | Heart failure with Preserved Ejection Fraction |
| IL-6 | Interleukin-6 |
| sFlt-1 | Soluble Fms-like tyrosine kinase-1 |
| DCM | Dilated cardiomyopathy |
| VEGFR-1 | Vascular Endothelial Growth Factor Receptor 1 |
| VEGF | Vascular Endothelial Growth Factor |
| PIGF | Placental Growth Factor |
| PEACE | Peripartum Cardiomyopathy in Nigeria |
| HDL | High-Density Lipoprotein |
| SHR | Stress Hyperglycemia Ratio |
| LV | Left Ventricle |
| TNFα | Tumor Necrosis Factor α |
| sFas/Apo1 | Soluble Fas/Apoptosis Antigen 1 |
| CRP | C-Reactive Protein |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| PGC1α | PPARγ-coactivator-1α |
| cKO | Conditional Knockout |
| MnSOD | Manganese Superoxide Dismutase |
| Cat-D | Peptidase Cathepsin-D |
| PRL | Prolactin |
| ECs | Endotelial Cells |
| PAI-1 | Plasminogen Activator Inhibitor-1 |
| uPA | Urokinase-Type Plasminogen Activator |
| uPAR | Urokinase-Type Plasminogen Activator Receptor |
| NF-κB | Nuclear Factor Kappa-Light-Chain-Enhancer of activated B cells |
| MiRNA-146a | MicroRNA-146a |
| Erbb4 | Erythroblastic leukemia viral oncogene homolog 4 |
| Notch1 | Neurogenic locus notch homolog protein 1 |
| Bcl-2/Bax | B-cell lymphoma 2/Bcl-2-associated X protein |
| AKT | Protein Kinase B |
| ROS | Reactive Oxygen Species |
| ER | Endoplasmic Reticulum |
| PERK | Protein Kinase RNA-like ER kinase |
| mTORc1 | Mechanistic Target of Rapamycin Complex 1 |
| ZFP36L2 | Zinc Finger Protein 36-like 2 |
| ECG | Electrocardiogram |
| BNP | Brain Natriuretic Peptide |
| NT-proBNP | N-Terminal-proBNP |
| cMRI | Cardiac Magnetic Resonance Imaging |
| BAG3 | Bcl2-associated athanogene |
| DSP | Desmoplakin |
| TTN | Titin |
| MYH6 | Myosin Heavy Chain 6 |
| MYH7 | Myosin Heavy Chain 7 |
| VCL | Vinculin |
| MAPK | Mitogen-Activated Protein Kinase |
| PI3K | Phosphoinositide 3-Kinase-Protein Kinase |
| Th | Helper T cells |
| NYHA | New York Heart Association |
| ESC EORP PPCM | ESC EURObservational Research Programme PPCM |
| mTOR | Mammalian Target of Rapamycin |
| EDTA | Ethylenediaminetetraacetic Acid |
References
- Regitz-Zagrosek, V.; Roos-Hesselink, J.W.; Bauersachs, J.; Blomström-Lundqvist, C.; Cífková, R.; De Bonis, M.; Iung, B.; Johnson, M.R.; Kintscher, U.; Kranke, P.; et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy: The Task Force for the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 3165–3241. [Google Scholar] [CrossRef]
- Karaye, K.M.; Ishaq, N.A.; Sai’du, H.; Balarabe, S.A.; Ahmed, B.G.; Adamu, U.G.; Mohammed, I.Y.; Oboirien, I.; Umuerri, E.M.; Mankwe, A.C.; et al. Disparities in clinical features and outcomes of peripartum cardiomyopathy in high versus low prevalent regions in Nigeria. ESC Heart Fail. 2021, 8, 3257–3267. [Google Scholar] [CrossRef]
- Kamiya, C.A.; Kitakaze, M.; Ishibashi-Ueda, H.; Nakatani, S.; Murohara, T.; Tomoike, H.; Ikeda, T. Different characteristics of peripartum cardiomyopathy between patients complicated with and without hypertensive disorders—Results from the Japanese Nationwide survey of peripartum cardiomyopathy—. Circ. J. Off. J. Jpn. Circ. Soc. 2011, 75, 1975–1981. [Google Scholar] [CrossRef]
- Kolte, D.; Khera, S.; Aronow, W.S.; Palaniswamy, C.; Mujib, M.; Ahn, C.; Jain, D.; Gass, A.; Ahmed, A.; Panza, J.A.; et al. Temporal Trends in Incidence and Outcomes of Peripartum Cardiomyopathy in the United States: A Nationwide Population-Based Study. J. Am. Heart Assoc. 2014, 3, e001056. [Google Scholar] [CrossRef]
- Isezuo, S.A.; Abubakar, S.A. Epidemiologic profile of peripartum cardiomyopathy in a tertiary care hospital. Ethn. Dis. 2007, 17, 228–233. [Google Scholar] [PubMed]
- Brar, S.S.; Khan, S.S.; Sandhu, G.K.; Jorgensen, M.B.; Parikh, N.; Hsu, J.-W.Y.; Shen, A.Y.-J. Incidence, mortality, and racial differences in peripartum cardiomyopathy. Am. J. Cardiol. 2007, 100, 302–304. [Google Scholar] [CrossRef] [PubMed]
- Sliwa, K.; Mebazaa, A.; Hilfiker-Kleiner, D.; Petrie, M.C.; Maggioni, A.P.; Laroche, C.; Regitz-Zagrosek, V.; Schaufelberger, M.; Tavazzi, L.; van der Meer, P.; et al. Clinical characteristics of patients from the worldwide registry on peripartum cardiomyopathy (PPCM): EURObservational Research Programme in conjunction with the Heart Failure Association of the European Society of Cardiology Study Group on PPCM. Eur. J. Heart Fail. 2017, 19, 1131–1141. [Google Scholar] [CrossRef]
- Kuć, A.; Kubik, D.; Kościelecka, K.; Szymanek, W.; Męcik-Kronenberg, T. The Relationship Between Peripartum Cardiomyopathy and Preeclampsia—Pathogenesis, Diagnosis and Management. J. Multidiscip. Healthc. 2022, 15, 857–867. [Google Scholar] [CrossRef]
- Rana, S.; Hacker, M.R.; Modest, A.M.; Salahuddin, S.; Lim, K.-H.; Verlohren, S.; Perschel, F.H.; Karumanchi, S.A. Circulating angiogenic factors and risk of adverse maternal and perinatal outcomes in twin pregnancies with suspected preeclampsia. Hypertension 2012, 60, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Aviram, A.; Giltvedt, M.K.; Sherman, C.; Kingdom, J.; Zaltz, A.; Barrett, J.; Melamed, N. The role of placental malperfusion in the pathogenesis of preeclampsia in dichorionic twin and singleton pregnancies. Placenta 2018, 70, 41–49. [Google Scholar] [CrossRef]
- Bauersachs, J.; König, T.; van der Meer, P.; Petrie, M.C.; Hilfiker-Kleiner, D.; Mbakwem, A.; Hamdan, R.; Jackson, A.M.; Forsyth, P.; de Boer, R.A.; et al. Pathophysiology, diagnosis and management of peripartum cardiomyopathy: A position statement from the Heart Failure Association of the European Society of Cardiology Study Group on peripartum cardiomyopathy. Eur. J. Heart Fail. 2019, 21, 827–843. [Google Scholar] [CrossRef]
- Peretto, G.; Micaglio, E.; Ciconte, G.; Maia, M.; Luzzi, M.; Cariello, M.; Bonfanti, A.G.R.; Lazzeroni, D.; Anastasia, L.; Cavoretto, P.; et al. The “arrhythmic” presentation of peripartum cardiomyopathy: Case series and critical review of the literature. Front. Cardiovasc. Med. 2024, 11, 1362692. [Google Scholar] [CrossRef] [PubMed]
- Shotan, A.; Ostrzega, E.; Mehra, A.; Johnson, J.V.; Elkayam, U. Incidence of Arrhythmias in Normal Pregnancy and Relation to Palpitations, Dizziness, and Syncope. Am. J. Cardiol. 1997, 79, 1061–1064. [Google Scholar] [CrossRef]
- Vaidya, V.R.; Arora, S.; Patel, N.; Badheka, A.O.; Patel, N.; Agnihotri, K.; Billimoria, Z.; Turakhia, M.P.; Friedman, P.A.; Madhavan, M.; et al. Burden of Arrhythmia in Pregnancy. Circulation 2017, 135, 619–621. [Google Scholar] [CrossRef]
- Silversides, C.K.; Grewal, J.; Mason, J.; Sermer, M.; Kiess, M.; Rychel, V.; Wald, R.M.; Colman, J.M.; Siu, S.C. Pregnancy Outcomes in Women With Heart Disease: The CARPREG II Study. J. Am. Coll. Cardiol. 2018, 71, 2419–2430. [Google Scholar] [CrossRef]
- Silversides, C.K.; Harris, L.; Haberer, K.; Sermer, M.; Colman, J.M.; Siu, S.C. Recurrence Rates of Arrhythmias During Pregnancy in Women With Previous Tachyarrhythmia and Impact on Fetal and Neonatal Outcomes. Am. J. Cardiol. 2006, 97, 1206–1212. [Google Scholar] [CrossRef]
- Davis, M.B.; Arany, Z.; McNamara, D.M.; Goland, S.; Elkayam, U. Peripartum Cardiomyopathy: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 207–221. [Google Scholar] [CrossRef]
- Ruys, T.P.E.; Roos-Hesselink, J.W.; Hall, R.; Subirana-Domènech, M.T.; Grando-Ting, J.; Estensen, M.; Crepaz, R.; Fesslova, V.; Gurvitz, M.; De Backer, J.; et al. Heart failure in pregnant women with cardiac disease: Data from the ROPAC. Heart Br. Card. Soc. 2014, 100, 231–238. [Google Scholar] [CrossRef]
- Brooks, V.L.; Fu, Q.; Shi, Z.; Heesch, C.M. Adaptations in autonomic nervous system regulation in normal and hypertensive pregnancy. Handb. Clin. Neurol. 2020, 171, 57–84. [Google Scholar] [CrossRef] [PubMed]
- Cavoretto, P.I.; Nayak, N.R.; Odibo, A.O. Time to reconcile the dichotomy of the cardiovascular-placental axis. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2025, 65, 401–403. [Google Scholar] [CrossRef] [PubMed]
- Powe, C.E.; Levine, R.J.; Karumanchi, S.A. Preeclampsia, a disease of the maternal endothelium: The role of antiangiogenic factors and implications for later cardiovascular disease. Circulation 2011, 123, 2856–2869. [Google Scholar] [CrossRef]
- Honigberg, M.C.; Cantonwine, D.E.; Thomas, A.M.; Lim, K.-H.; Parry, S.I.; McElrath, T.F. Analysis of changes in maternal circulating angiogenic factors throughout pregnancy for the prediction of preeclampsia. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2016, 36, 172–177. [Google Scholar] [CrossRef]
- Mebazaa, A.; Seronde, M.-F.; Gayat, E.; Tibazarwa, K.; Anumba, D.O.C.; Akrout, N.; Sadoune, M.; Sarb, J.; Arrigo, M.; Motiejunaite, J.; et al. Imbalanced Angiogenesis in Peripartum Cardiomyopathy—Diagnostic Value of Placenta Growth Factor. Circ. J. Off. J. Jpn. Circ. Soc. 2017, 81, 1654–1661. [Google Scholar] [CrossRef]
- Damp, J.; Givertz, M.M.; Semigran, M.; Alharethi, R.; Ewald, G.; Felker, G.M.; Bozkurt, B.; Boehmer, J.; Haythe, J.; Skopicki, H.; et al. Relaxin-2 and Soluble Flt1 Levels in Peripartum Cardiomyopathy: Results of the Multicenter IPAC Study. JACC Heart Fail. 2016, 4, 380–388. [Google Scholar] [CrossRef]
- Monda, E.; Bakalakos, A.; Cannie, D.; O’Mahony, C.; Syrris, P.; Kaski, J.P.; Limongelli, G.; Elliott, P.M. Prevalence of Pathogenic Variants in Cardiomyopathy-Associated Genes in Acute Myocarditis: A Systematic Review and Meta-Analysis. JACC Heart Fail. 2024, 12, 1101–1111. [Google Scholar] [CrossRef]
- Soma-Pillay, P.; Nelson-Piercy, C.; Tolppanen, H.; Mebazaa, A. Physiological changes in pregnancy. Cardiovasc. J. Afr. 2016, 27, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Tibazarwa, K.; Lee, G.; Mayosi, B.; Carrington, M.; Sliwa, K. The 12-lead ECG in peripartum cardiomyopathy. Cardiovasc. J. Afr. 2012, 23, 322–329. [Google Scholar] [CrossRef]
- Mbakwem, A.C.; Bauersachs, J.; Viljoen, C.; Hoevelmann, J.; van der Meer, P.; Petrie, M.C.; Mebazaa, A.; Goland, S.; Karaye, K.; Laroche, C.; et al. Electrocardiographic features and their echocardiographic correlates in peripartum cardiomyopathy: Results from the ESC EORP PPCM registry. ESC Heart Fail. 2021, 8, 879–889. [Google Scholar] [CrossRef]
- Kaymak, D.; Oruc, S.B.B.; Davutoğlu, E.A. First-trimester inflammation and dyslipidemia in preterm delivery: The role of monocyte-to-HDL cholesterol ratio and lipid profiles. Ir. J. Med. Sci. 2025, 194, 1–6. [Google Scholar] [CrossRef]
- Ekizler, F.A.; Cay, S. A novel marker of persistent left ventricular systolic dysfunction in patients with peripartum cardiomyopathy: Monocyte count-to-HDL cholesterol ratio. BMC Cardiovasc. Disord. 2019, 19, 114. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, W.; Chen, L.; Liu, B. Stress hyperglycemia ratio: A novel predictor of left ventricular dysfunction in peripartum cardiomyopathy. J. Matern. Fetal Neonatal Med. 2025, 38, 2464181. [Google Scholar] [CrossRef]
- Stokkeland, L.M.T.; Giskeødegård, G.F.; Stridsklev, S.; Ryan, L.; Steinkjer, B.; Tangerås, L.H.; Vanky, E.; Iversen, A.-C. Serum cytokine patterns in first half of pregnancy. Cytokine 2019, 119, 188–196. [Google Scholar] [CrossRef]
- Stergiopoulos, K.; Lima, F.V. Peripartum cardiomyopathy-diagnosis, management, and long term implications. Trends Cardiovasc. Med. 2019, 29, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Patten, I.S.; Rana, S.; Shahul, S.; Rowe, G.C.; Jang, C.; Liu, L.; Hacker, M.R.; Rhee, J.S.; Mitchell, J.; Mahmood, F.; et al. Cardiac angiogenic imbalance leads to peripartum cardiomyopathy. Nature 2012, 485, 333–338. [Google Scholar] [CrossRef]
- Pfeffer, T.J.; Schlothauer, S.; Pietzsch, S.; Schaufelberger, M.; Auber, B.; Ricke-Hoch, M.; List, M.; Berliner, D.; Abou Moulig, V.; König, T.; et al. Increased Cancer Prevalence in Peripartum Cardiomyopathy. JACC CardioOncol. 2019, 1, 196–205. [Google Scholar] [CrossRef]
- Blackford, A.N.; Jackson, S.P. ATM, ATR, and DNA-PK: The Trinity at the Heart of the DNA Damage Response. Mol. Cell 2017, 66, 801–817. [Google Scholar] [CrossRef]
- Yoshioka, J. Thioredoxin Reductase 2 (Txnrd2) Regulates Mitochondrial Integrity in the Progression of Age-Related Heart Failure. J. Am. Heart Assoc. 2015, 4, e002278. [Google Scholar] [CrossRef]
- Hilfiker-Kleiner, D.; Kaminski, K.; Podewski, E.; Bonda, T.; Schaefer, A.; Sliwa, K.; Forster, O.; Quint, A.; Landmesser, U.; Doerries, C.; et al. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell 2007, 128, 589–600. [Google Scholar] [CrossRef]
- Ricke-Hoch, M.; Hoes, M.F.; Pfeffer, T.J.; Schlothauer, S.; Nonhoff, J.; Haidari, S.; Bomer, N.; Scherr, M.; Stapel, B.; Stelling, E.; et al. In peripartum cardiomyopathy plasminogen activator inhibitor-1 is a potential new biomarker with controversial roles. Cardiovasc. Res. 2020, 116, 1875–1886. [Google Scholar] [CrossRef]
- Halkein, J.; Tabruyn, S.P.; Ricke-Hoch, M.; Haghikia, A.; Nguyen, N.-Q.-N.; Scherr, M.; Castermans, K.; Malvaux, L.; Lambert, V.; Thiry, M.; et al. MicroRNA-146a is a therapeutic target and biomarker for peripartum cardiomyopathy. J. Clin. Investig. 2013, 123, 2143–2154. [Google Scholar] [CrossRef]
- Feyen, E.; Ricke-Hoch, M.; Van Fraeyenhove, J.; Vermeulen, Z.; Scherr, M.; Dugaucquier, L.; Viereck, J.; Bruyns, T.; Thum, T.; Segers, V.F.M.; et al. ERBB4 and Multiple MicroRNAs That Target ERBB4 Participate in Pregnancy-Related Cardiomyopathy. Circ. Heart Fail. 2021, 14, e006898. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.-R.; Chen, Q.; Liu, Z.-B.; Ruan, H.-G.; Wu, Q.-C.; Zhou, X.-L. Inhibition of the Notch1 pathway induces peripartum cardiomyopathy. J. Cell Mol. Med. 2020, 24, 7907–7914. [Google Scholar] [CrossRef] [PubMed]
- Ricke-Hoch, M.; Bultmann, I.; Stapel, B.; Condorelli, G.; Rinas, U.; Sliwa, K.; Scherr, M.; Hilfiker-Kleiner, D. Opposing roles of Akt and STAT3 in the protection of the maternal heart from peripartum stress. Cardiovasc. Res. 2014, 101, 587–596. [Google Scholar] [CrossRef]
- Nonhoff, J.; Ricke-Hoch, M.; Mueller, M.; Stapel, B.; Pfeffer, T.; Kasten, M.; Scherr, M.; von Kaisenberg, C.; Bauersachs, J.; Haghikia, A.; et al. Serelaxin treatment promotes adaptive hypertrophy but does not prevent heart failure in experimental peripartum cardiomyopathy. Cardiovasc. Res. 2017, 113, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, T.J.; List, M.; Müller, J.H.; Scherr, M.; Bauersachs, J.; Hilfiker-Kleiner, D.; Ricke-Hoch, M. Perhexiline treatment improves toxic effects of β-adrenergic receptor stimulation in experimental peripartum cardiomyopathy. ESC Heart Fail. 2021, 8, 3375–3381. [Google Scholar] [CrossRef]
- Stapel, B.; Kohlhaas, M.; Ricke-Hoch, M.; Haghikia, A.; Erschow, S.; Knuuti, J.; Silvola, J.M.U.; Roivainen, A.; Saraste, A.; Nickel, A.G.; et al. Low STAT3 expression sensitizes to toxic effects of β-adrenergic receptor stimulation in peripartum cardiomyopathy. Eur. Heart J. 2017, 38, 349–361. [Google Scholar] [CrossRef]
- Roh, J.D.; Castro, C.; Yu, A.; Rana, S.; Shahul, S.; Gray, K.J.; Honigberg, M.C.; Ricke-Hoch, M.; Iwamoto, Y.; Yeri, A.; et al. Placental senescence pathophysiology is shared between peripartum cardiomyopathy and preeclampsia in mouse and human. Sci. Transl. Med. 2024, 16, eadi0077. [Google Scholar] [CrossRef]
- Arany, Z.; Foo, S.-Y.; Ma, Y.; Ruas, J.L.; Bommi-Reddy, A.; Girnun, G.; Cooper, M.; Laznik, D.; Chinsomboon, J.; Rangwala, S.M.; et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature 2008, 451, 1008–1012. [Google Scholar] [CrossRef]
- Liu, G.-S.; Gardner, G.; Adly, G.; Jiang, M.; Cai, W.-F.; Lam, C.K.; Alogaili, F.; Robbins, N.; Rubinstein, J.; Kranias, E.G. A novel human S10F-Hsp20 mutation induces lethal peripartum cardiomyopathy. J. Cell Mol. Med. 2018, 22, 3911–3919. [Google Scholar] [CrossRef]
- Onusko, E.; McDermott, M.R.; Robbins, N.; Liu, G.; Kranias, E.G.; Rubinstein, J.; Koch, S.E. Probenecid treatment improves outcomes in a novel mouse model of peripartum cardiomyopathy. PLoS ONE 2020, 15, e0230386. [Google Scholar] [CrossRef]
- Fujii, J.; Homma, T.; Kobayashi, S.; Seo, H.G. Mutual interaction between oxidative stress and endoplasmic reticulum stress in the pathogenesis of diseases specifically focusing on non-alcoholic fatty liver disease. World J. Biol. Chem. 2018, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kouzu, H.; Tatekoshi, Y.; Chang, H.-C.; Shapiro, J.S.; McGee, W.A.; De Jesus, A.; Ben-Sahra, I.; Arany, Z.; Leor, J.; Chen, C.; et al. ZFP36L2 suppresses mTORc1 through a P53-dependent pathway to prevent peripartum cardiomyopathy in mice. J. Clin. Investig. 2022, 132, e154491. [Google Scholar] [CrossRef] [PubMed]
- Otani, K.; Tokudome, T.; Kamiya, C.A.; Mao, Y.; Nishimura, H.; Hasegawa, T.; Arai, Y.; Kaneko, M.; Shioi, G.; Ishida, J.; et al. Deficiency of Cardiac Natriuretic Peptide Signaling Promotes Peripartum Cardiomyopathy-Like Remodeling in the Mouse Heart. Circulation 2020, 141, 571–588. [Google Scholar] [CrossRef]
- Tanous, D.; Siu, S.C.; Mason, J.; Greutmann, M.; Wald, R.M.; Parker, J.D.; Sermer, M.; Colman, J.M.; Silversides, C.K. B-type natriuretic peptide in pregnant women with heart disease. J. Am. Coll. Cardiol. 2010, 56, 1247–1253. [Google Scholar] [CrossRef]
- Hu, C.L.; Li, Y.B.; Zou, Y.G.; Zhang, J.M.; Chen, J.B.; Liu, J.; Tang, Y.H.; Tang, Q.Z.; Huang, C.X. Troponin T measurement can predict persistent left ventricular dysfunction in peripartum cardiomyopathy. Heart Br. Card. Soc. 2007, 93, 488–490. [Google Scholar] [CrossRef]
- Marmursztejn, J.; Vignaux, O.; Goffinet, F.; Cabanes, L.; Duboc, D. Delayed-enhanced cardiac magnetic resonance imaging features in peripartum cardiomyopathy. Int. J. Cardiol. 2009, 137, e63–e64. [Google Scholar] [CrossRef]
- Webb, J.A.W.; Thomsen, H.S.; Morcos, S.K.; Members of Contrast Media Safety Committee of European Society of Urogenital Radiology (ESUR). The use of iodinated and gadolinium contrast media during pregnancy and lactation. Eur. Radiol. 2005, 15, 1234–1240. [Google Scholar] [CrossRef]
- Goli, R.; Li, J.; Brandimarto, J.; Levine, L.D.; Riis, V.; McAfee, Q.; DePalma, S.; Haghighi, A.; Seidman, J.G.; Seidman, C.E.; et al. Genetic and Phenotypic Landscape of Peripartum Cardiomyopathy. Circulation 2021, 143, 1852–1862. [Google Scholar] [CrossRef]
- Ware, J.S.; Li, J.; Mazaika, E.; Yasso, C.M.; DeSouza, T.; Cappola, T.P.; Tsai, E.J.; Hilfiker-Kleiner, D.; Kamiya, C.A.; Mazzarotto, F.; et al. Shared Genetic Predisposition in Peripartum and Dilated Cardiomyopathies. N. Engl. J. Med. 2016, 374, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Painter, T.; Li, R.; Siegfried, J.D.; Li, D.; Norton, N.; Hershberger, R.E. Rare variant mutations in pregnancy-associated or peripartum cardiomyopathy. Circulation 2010, 121, 2176–2182. [Google Scholar] [CrossRef]
- Horne, B.D.; Rasmusson, K.D.; Alharethi, R.; Budge, D.; Brunisholz, K.D.; Metz, T.; Carlquist, J.F.; Connolly, J.J.; Porter, T.F.; Lappé, D.L.; et al. Genome-wide significance and replication of the chromosome 12p11.22 locus near the PTHLH gene for peripartum cardiomyopathy. Circ. Cardiovasc. Genet. 2011, 4, 359–366. [Google Scholar] [CrossRef]
- van Spaendonck-Zwarts, K.Y.; Posafalvi, A.; van den Berg, M.P.; Hilfiker-Kleiner, D.; Bollen, I.A.E.; Sliwa, K.; Alders, M.; Almomani, R.; van Langen, I.M.; van der Meer, P.; et al. Titin gene mutations are common in families with both peripartum cardiomyopathy and dilated cardiomyopathy. Eur. Heart J. 2014, 35, 2165–2173. [Google Scholar] [CrossRef]
- Singh, A.; Irfan, H.; Ali, T.; Mughal, S.; Shaukat, A.; Jawwad, M.; Akilimali, A. Precision medicine in peripartum cardiomyopathy: Advancing diagnosis and management through genomic and phenotypic integration. Ann. Med. Surg. 2012 2024, 86, 4664–4667. [Google Scholar] [CrossRef]
- Taylor, J.; Yeung, A.C.Y.; Ashton, A.; Faiz, A.; Guryev, V.; Fang, B.; Lal, S.; Grosser, M.; Dos Remedios, C.G.; Braet, F.; et al. Transcriptomic Comparison of Human Peripartum and Dilated Cardiomyopathy Identifies Differences in Key Disease Pathways. J. Cardiovasc. Dev. Dis. 2023, 10, 188. [Google Scholar] [CrossRef]
- Li, A.; Fang, B.; Li, M.; Koay, Y.C.; Malecki, C.; Hunter, B.; Harney, D.; Dos Remedios, C.G.; Larance, M.; O’Sullivan, J.F.; et al. Myocardial Posttranscriptional Landscape in Peripartum Cardiomyopathy. Circ. Heart Fail. 2024, 17, e011725. [Google Scholar] [CrossRef]
- Lovell, J.P.; Bermea, K.; Yu, J.; Rousseau, S.; Cohen, C.D.; Bhalodia, A.; Zita, M.D.; Head, R.D.; Blumenthal, R.S.; Alharethi, R.; et al. Serum Proteomic Analysis of Peripartum Cardiomyopathy Reveals Distinctive Dysregulation of Inflammatory and Cholesterol Metabolism Pathways. JACC Heart Fail. 2023, 11, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Kodogo, V.; Viljoen, C.; Hoevelmann, J.; Chakafana, G.; Tromp, J.; Farhan, H.A.; Goland, S.; van der Meer, P.; Karaye, K.; Kryczka, K.; et al. Proteomic Profiling in Patients With Peripartum Cardiomyopathy: A Biomarker Study of the ESC EORP PPCM Registry. JACC Heart Fail. 2023, 11, 1708–1725. [Google Scholar] [CrossRef] [PubMed]
- Fulghum, K.L.; Smith, J.B.; Chariker, J.; Garrett, L.F.; Brittian, K.R.; Lorkiewicz, P.K.; McNally, L.A.; Uchida, S.; Jones, S.P.; Hill, B.G.; et al. Metabolic signatures of pregnancy-induced cardiac growth. Am. J. Physiol. Heart Circ. Physiol. 2022, 323, H146–H164. [Google Scholar] [CrossRef]
- McNamara, D.M.; Elkayam, U.; Alharethi, R.; Damp, J.; Hsich, E.; Ewald, G.; Modi, K.; Alexis, J.D.; Ramani, G.V.; Semigran, M.J.; et al. Clinical Outcomes for Peripartum Cardiomyopathy in North America: Results of the IPAC Study (Investigations of Pregnancy-Associated Cardiomyopathy). J. Am. Coll. Cardiol. 2015, 66, 905–914. [Google Scholar] [CrossRef]
- Ravi Kiran, G.; RajKumar, C.; Chandrasekhar, P. Clinical and echocardiographic predictors of outcomes in patients with peripartum cardiomyopathy: A single centre, six month follow-up study. Indian Heart J. 2021, 73, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Goland, S.; Bitar, F.; Modi, K.; Safirstein, J.; Ro, A.; Mirocha, J.; Khatri, N.; Elkayam, U. Evaluation of the Clinical Relevance of Baseline Left Ventricular Ejection Fraction as a Predictor of Recovery or Persistence of Severe Dysfunction in Women in the United States With Peripartum Cardiomyopathy. J. Card. Fail. 2011, 17, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Amos, A.M.; Jaber, W.A.; Russell, S.D. Improved outcomes in peripartum cardiomyopathy with contemporary. Am. Heart J. 2006, 152, 509–513. [Google Scholar] [CrossRef]
- Forster, O.; Hilfiker-Kleiner, D.; Ansari, A.A.; Sundstrom, J.B.; Libhaber, E.; Tshani, W.; Becker, A.; Yip, A.; Klein, G.; Sliwa, K. Reversal of IFN-gamma, oxLDL and prolactin serum levels correlate with clinical improvement in patients with peripartum cardiomyopathy. Eur. J. Heart Fail. 2008, 10, 861–868. [Google Scholar] [CrossRef]
- Li, W.; Li, H.; Long, Y. Clinical Characteristics and Long-term Predictors of Persistent Left Ventricular Systolic Dysfunction in Peripartum Cardiomyopathy. Can. J. Cardiol. 2016, 32, 362–368. [Google Scholar] [CrossRef]
- Hoevelmann, J.; Viljoen, C.A.; Manning, K.; Baard, J.; Hahnle, L.; Ntsekhe, M.; Bauersachs, J.; Sliwa, K. The prognostic significance of the 12-lead ECG in peripartum cardiomyopathy. Int. J. Cardiol. 2019, 276, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.M.; Bauersachs, J.; Petrie, M.C.; van der Meer, P.; Laroche, C.; Farhan, H.A.; Frogoudaki, A.; Ibrahim, B.; Fouad, D.A.; Damasceno, A.; et al. Outcomes at one year in women with peripartum cardiomyopathy: Findings from the ESC EORP PPCM Registry. Eur. J. Heart Fail. 2024, 26, 34–42. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmentieri, A.; Battaglia, C.; D’Alconzo, D.; Anastasia, L.; Bardi, L.; Bifulco, G.; Calanducci, M.; Carotenuto, M.; Cavoretto, P.I.; Carusone, F.; et al. Aims and Rationale of a National Registry Integrating Clinical, Echocardiographic, and Multi-Omics Profiling to Promote Precision Medicine in Peripartum Cardiomyopathy. Biomedicines 2025, 13, 2026. https://doi.org/10.3390/biomedicines13082026
Palmentieri A, Battaglia C, D’Alconzo D, Anastasia L, Bardi L, Bifulco G, Calanducci M, Carotenuto M, Cavoretto PI, Carusone F, et al. Aims and Rationale of a National Registry Integrating Clinical, Echocardiographic, and Multi-Omics Profiling to Promote Precision Medicine in Peripartum Cardiomyopathy. Biomedicines. 2025; 13(8):2026. https://doi.org/10.3390/biomedicines13082026
Chicago/Turabian StylePalmentieri, Alessia, Ciro Battaglia, Dario D’Alconzo, Luigi Anastasia, Luca Bardi, Giuseppe Bifulco, Maria Calanducci, Martina Carotenuto, Paolo Ivo Cavoretto, Federica Carusone, and et al. 2025. "Aims and Rationale of a National Registry Integrating Clinical, Echocardiographic, and Multi-Omics Profiling to Promote Precision Medicine in Peripartum Cardiomyopathy" Biomedicines 13, no. 8: 2026. https://doi.org/10.3390/biomedicines13082026
APA StylePalmentieri, A., Battaglia, C., D’Alconzo, D., Anastasia, L., Bardi, L., Bifulco, G., Calanducci, M., Carotenuto, M., Cavoretto, P. I., Carusone, F., Di Lorenzo, E., Di Santo, M., Di Spiezio Sardo, A., Ilardi, F., Ioele, D., Lanni, F., Licciardi, M., Loffredo, F., Manzo, R., ... Perrino, C. (2025). Aims and Rationale of a National Registry Integrating Clinical, Echocardiographic, and Multi-Omics Profiling to Promote Precision Medicine in Peripartum Cardiomyopathy. Biomedicines, 13(8), 2026. https://doi.org/10.3390/biomedicines13082026










