From “Traditional” to “Trained” Immunity: Exploring the Novel Frontiers of Immunopathogenesis in the Progression of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)
Abstract
1. Background
Global Burden of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)
2. From “Classic” to “Untraditional” MASLD Pathogenesis
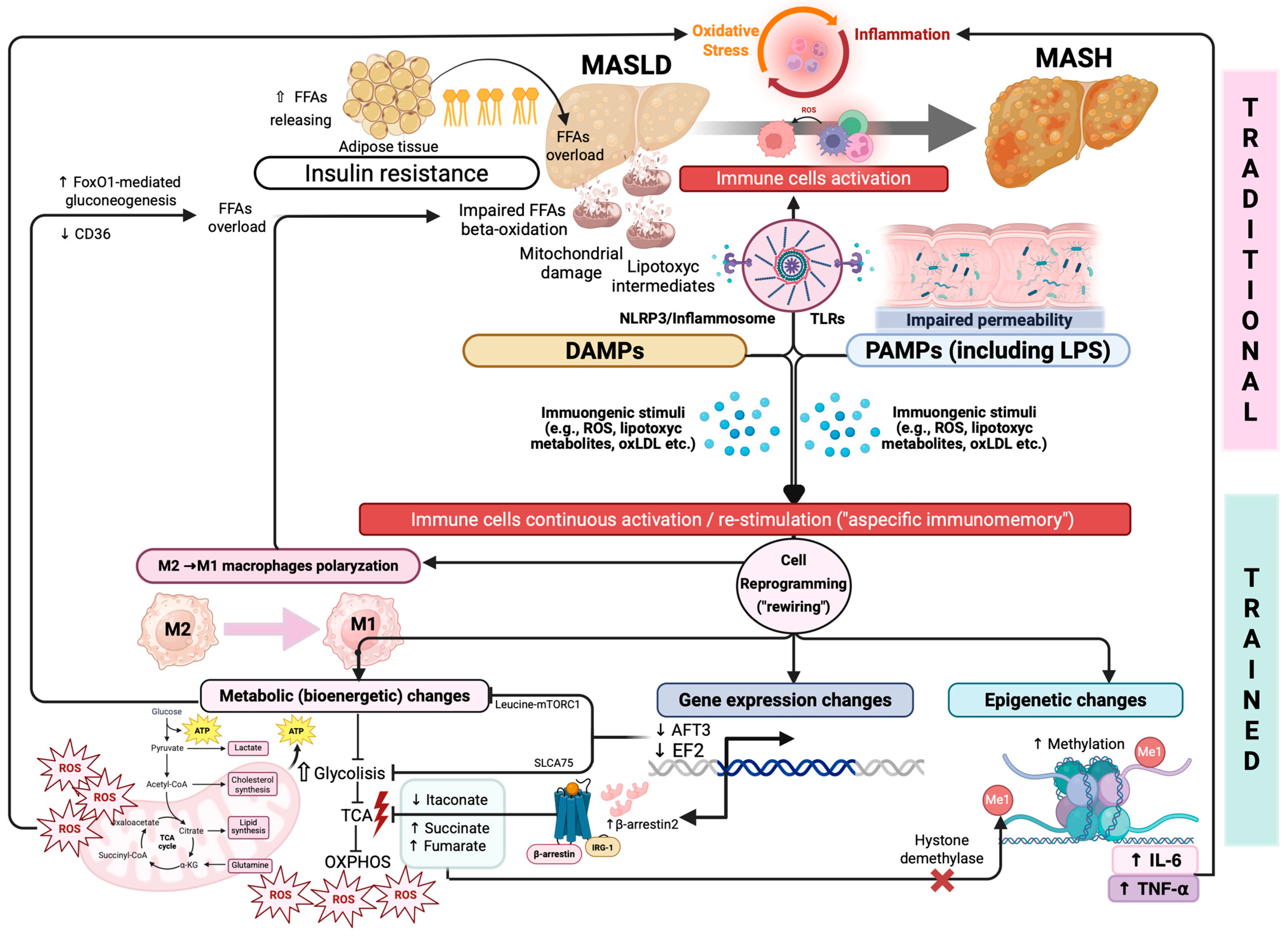
2.1. IR, Inflammation, OS, and Gut Dysbiosis as Triggers of Hepatic Immune Dysfunction
2.2. “Trained” Immunity as Revolutionary Immunologic Pathogenetic Frontier
3. “Traditional” Immunity in the Progression of MASLD
3.1. Liver as an Immunological Organ
3.1.1. Liver Sinusoidal Endothelial Cells (LSECs)
3.1.2. Kupffer Cells (KCs)
3.1.3. Hepatic Stellate Cells (HSCs)
3.1.4. Dendritic Cells (DCs)
3.1.5. Natural Killer Cells (NK Cells) and Natural Killer T Cells (NKT Cells)
3.2. “Traditional” Immunity Dysregulation in Driving the MASLD Progression
3.2.1. Role of Macrophages: Expanding the Classic “Polarization Paradigm”
3.2.2. Role of Neutrophils: A Biface Janus Mediating Liver Injury and Repair
3.2.3. Hepatic Dendritic Cells in MASLD to MASH Progression: Limited Evidence
3.2.4. Natural Killer Cells and Natural Killer T Cells in MASLD to MASH Progression
3.2.5. The Emerging Role of Adaptive Immune Cells in MASLD to MASH Progression
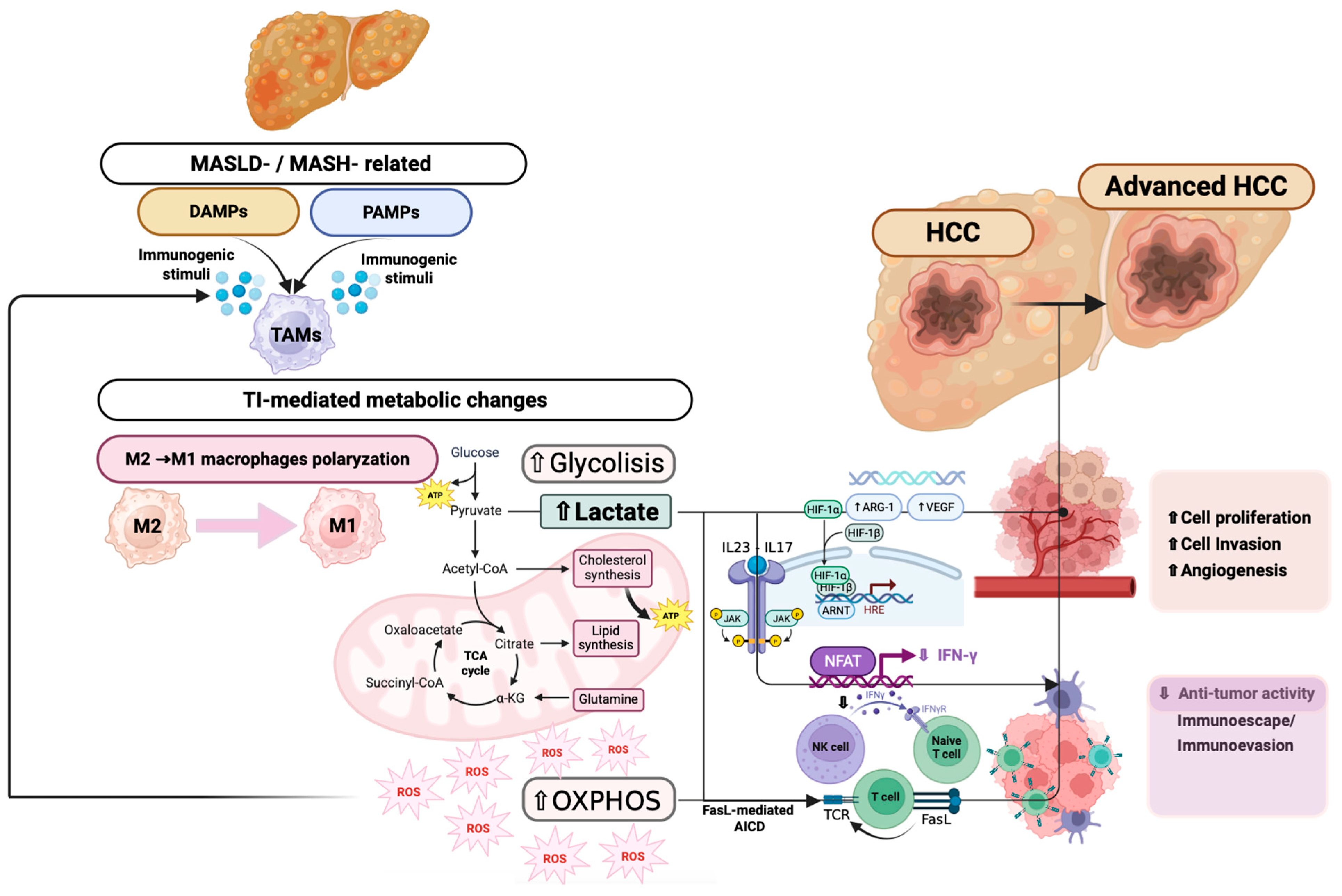
3.2.6. Innate Immune Cell Dysfunction in MASLD-Related Hepatocancerogenesis
4. “Trained” Immunity in the Progression of MASLD
4.1. Immunometabolism and Trained Immunity: New Insights in Immune Regulation
4.2. Immunometabolic Pathways Contributing to MASLD/MASH Progression
4.3. Immunometabolic Pathways Driving Hepatocellular Carcinoma Progression
5. Future Perspectives: Modulating Immunometabolism as a Promising Strategy in the Management of MASLD/MASH
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| MASH | Metabolic dysfunction-associated steatohepatitis |
| SS | Simple steatosis |
| AF | Advanced fibrosis |
| HCC | Hepatocellular carcinoma |
| IR | Insulin resistance |
| OS | Oxidative stress |
| FFAs | Free fatty acids |
| ROS | Reactive oxygen species |
| DAMPs | Damage-associated molecular patterns |
| PAMPs | Pathogen-associated molecular patterns |
| TLR | Toll-like receptor |
| TI | Trained immunity |
| SCFAs | Short-chain fatty acids |
| BAs | Bile acids |
| LPS | Lipopolysaccharide |
| KCs | Kupffer cells |
| LSECs | Liver sinusoidal endothelial cells |
| DCs | Dendritic cells |
| HSCs | Hepatic stellate cells |
| NK cells | Natural killer cells |
| NKT | Natural killer T cells |
| iNKT | Invariant natural killer T cells |
| ILCs | Innate lymphoid cells |
| ILC1 | Type 1 innate lymphoid cells |
| ILC2 | Type 2 innate lymphoid cells |
| MAIT cells | Mucosal-associated invariant T cells |
| TCR | T-cell receptor |
| IL | Interleukin |
| TNF-α | Tumor necrosis factor-alpha |
| IFNγ | Interferon gamma |
| NETs | Neutrophil extracellular traps |
| NE | Neutrophil elastase |
| MPO | Myeloperoxidase |
| miR | MicroRNA |
| CRE | Cyclic AMP response element |
| FoxO1 | Forkhead box protein O1 |
| GPCR | G protein-coupled receptor |
| IRG1 | Immune responsive gene 1 |
| OXPHOS | Oxidative phosphorylation |
| TCA | Tricarboxylic acid cycle |
| SLC7A5 | Solute carrier family 7-member 5 |
| ACLY | ATP citrate lyase |
| IDH1 | Isocitrate dehydrogenase 1 |
| PARP | Poly (ADP-ribose) polymerase |
| PD-1 | Programmed cell death protein 1 |
| BCG | Bacillus Calmette–Guérin (vaccine) |
| AFP | Alpha-fetoprotein |
| VEGF | Vascular endothelial growth factor |
| PDGF | Platelet-derived growth factor |
| STAT-3 | Signal transducer and activator of transcription 3 |
| NF-κB | Nuclear factor kappa-light-chain enhancer of activated B cells |
| HIF-1 | Hypoxia-inducible factor-1 |
| MMP-9 | Matrix metalloproteinase-9 |
| GM-CSF | Granulocyte–macrophage colony-stimulating factor |
| pDCs | Plasmacytoid dendritic cells |
| mDCs | Myeloid dendritic cells |
| IDO | Indoleamine 2,3-dioxygenase |
| CREB | cAMP response element-binding protein |
| ASCT2 | Alanine/serine/cysteine transporter 2 |
| GLS2 | Glutaminase 2 |
| EP4 | Prostaglandin E2 receptor 4 |
| TAMs | Tumor-associated macrophages |
| TANs | Tumor-associated neutrophils |
| AICD | Activation-induced cell death |
References
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The Global Epidemiology of Nonalcoholic Fatty Liver Disease (NAFLD) and Nonalcoholic Steatohepatitis (NASH): A Systematic Review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the Epidemic of Nonalcoholic Fatty Liver Disease Demonstrates an Exponential Increase in Burden of Disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef]
- Mittal, S.; El-Serag, H.B.; Sada, Y.H.; Kanwal, F.; Duan, Z.; Temple, S.; May, S.B.; Kramer, J.R.; Richardson, P.A.; Davila, J.A. Hepatocellular Carcinoma in the Absence of Cirrhosis in United States Veterans Is Associated With Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2016, 14, 124–131.e1. [Google Scholar] [CrossRef]
- Dallio, M.; Sangineto, M.; Romeo, M.; Villani, R.; Romano, A.D.; Loguercio, C.; Serviddio, G.; Federico, A. Immunity as Cornerstone of Non-Alcoholic Fatty Liver Disease: The Contribution of Oxidative Stress in the Disease Progression. Int. J. Mol. Sci. 2021, 22, 436. [Google Scholar] [CrossRef]
- Palma, R.; Pronio, A.; Romeo, M.; Scognamiglio, F.; Ventriglia, L.; Ormando, V.M.; Lamazza, A.; Pontone, S.; Federico, A.; Dallio, M. The Role of Insulin Resistance in Fueling NAFLD Pathogenesis: From Molecular Mechanisms to Clinical Implications. J. Clin. Med. 2022, 11, 3649. [Google Scholar] [CrossRef]
- Tacke, F.; Horn, P.; Wong, V.W.-S.; Ratziu, V.; Bugianesi, E.; Francque, S.; Zelber-Sagi, S.; Valenti, L.; Roden, M.; Schick, F.; et al. EASL–EASD–EASO Clinical Practice Guidelines on the Management of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A Multisociety Delphi Consensus Statement on New Fatty Liver Disease Nomenclature. J. Hepatol. 2023, 79, 1542–1556. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Zhang, E.; Narasimman, M.; Rich, N.E.; Waljee, A.K.; Hoshida, Y.; Yang, J.D.; Reig, M.; Cabibbo, G.; Nahon, P.; et al. HCC Surveillance Improves Early Detection, Curative Treatment Receipt, and Survival in Patients with Cirrhosis: A Meta-Analysis. J. Hepatol. 2022, 77, 128–139. [Google Scholar] [CrossRef]
- Bansal, S.K.; Bansal, M.B. Pathogenesis of MASLD and MASH—Role of Insulin Resistance and Lipotoxicity. Aliment. Pharmacol. Ther. 2024, 59, S10–S22. [Google Scholar] [CrossRef] [PubMed]
- Crocetto, F.; Barone, B.; Manfredi, C.; Trama, F.; Romano, L.; Romeo, M.; Russo, G.; Sicignano, E.; Persico, F.; Aveta, A.; et al. Are Insulin Resistance and Non-Alcoholic Fatty Liver Disease Associated with Peyronie’s Disease? A Pilot Study. J. Physiol. Pharmacol. 2022, 73, 53–62. [Google Scholar] [CrossRef]
- Termite, F.; Archilei, S.; D’Ambrosio, F.; Petrucci, L.; Viceconti, N.; Iaccarino, R.; Liguori, A.; Gasbarrini, A.; Miele, L. Gut Microbiota at the Crossroad of Hepatic Oxidative Stress and MASLD. Antioxidants 2025, 14, 56. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Lv, J.; Chen, X.; Shi, Y.; Chao, G.; Zhang, S. From Gut to Liver: Exploring the Crosstalk between Gut-Liver Axis and Oxidative Stress in Metabolic Dysfunction-Associated Steatotic Liver Disease. Ann. Hepatol. 2025, 30, 101777. [Google Scholar] [CrossRef] [PubMed]
- Romeo, M.; Dallio, M.; Di Nardo, F.; Napolitano, C.; Vaia, P.; Martinelli, G.; Federico, P.; Olivieri, S.; Iodice, P.; Federico, A. The Role of the Gut-Biliary-Liver Axis in Primary Hepatobiliary Liver Cancers: From Molecular Insights to Clinical Applications. J. Pers. Med. 2025, 15, 124. [Google Scholar] [CrossRef]
- Yilmaz, Y. Postbiotics as Antiinflammatory and Immune-Modulating Bioactive Compounds in Metabolic Dysfunction-Associated Steatotic Liver Disease. Mol. Nutr. Food Res. 2024, 68, 2400754. [Google Scholar] [CrossRef]
- Yu, T.; Luo, L.; Xue, J.; Tang, W.; Wu, X.; Yang, F. Gut Microbiota–NLRP3 Inflammasome Crosstalk in Metabolic Dysfunction-Associated Steatotic Liver Disease. Clin. Res. Hepatol. Gastroenterol. 2024, 48, 102458. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Xu, J.; Yang, M.; Hussain, M.; Liu, X.; Feng, F.; Guan, R. Protective Effect of Anthocyanins against Neurodegenerative Diseases through the Microbial-Intestinal-Brain Axis: A Critical Review. Nutrients 2023, 15, 496. [Google Scholar] [CrossRef] [PubMed]
- Mignini, I.; Galasso, L.; Piccirilli, G.; Calvez, V.; Termite, F.; Esposto, G.; Borriello, R.; Miele, L.; Ainora, M.E.; Gasbarrini, A.; et al. Interplay of Oxidative Stress, Gut Microbiota, and Nicotine in Metabolic-Associated Steatotic Liver Disease (MASLD). Antioxidants 2024, 13, 1532. [Google Scholar] [CrossRef] [PubMed]
- Cannito, S.; Dianzani, U.; Parola, M.; Albano, E.; Sutti, S. Inflammatory Processes Involved in NASH-Related Hepatocellular Carcinoma. Biosci. Rep. 2023, 43, BSR20221271. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Lin, Y.; Cao, C.; Chen, D.; Huang, X.; Li, C.; Xu, H.; Lai, H.; Chen, H.; et al. Roles of the Gut Microbiota in Hepatocellular Carcinoma: From the Gut Dysbiosis to the Intratumoral Microbiota. Cell Death Discov. 2025, 11, 140. [Google Scholar] [CrossRef]
- Kumar, A.R.; Nair, B.; Kamath, A.J.; Nath, L.R.; Calina, D.; Sharifi-Rad, J. Impact of Gut Microbiota on Metabolic Dysfunction-Associated Steatohepatitis and Hepatocellular Carcinoma: Pathways, Diagnostic Opportunities and Therapeutic Advances. Eur. J. Med. Res. 2024, 29, 485. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.-F.; Shi, C.-J.; Sun, L.; Gao, R.-J.; Yang, S.-P. Characterization of β-Cyclodextrin Inclusion Complexes Embedded with Lemongrass and Basil Essential Oils and Their Modified Sustained-Release Pads for Large Yellow Croaker (Larimichthys crocea) Fillet Preservation. Food Biosci. 2025, 66, 106157. [Google Scholar] [CrossRef]
- Racanelli, V.; Rehermann, B. The Liver as an Immunological Organ. Hepatology 2006, 43, S54–S62. [Google Scholar] [CrossRef] [PubMed]
- Parlar, Y.E.; Ayar, S.N.; Cagdas, D.; Balaban, Y.H. Liver Immunity, Autoimmunity, and Inborn Errors of Immunity. World J. Hepatol. 2023, 15, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; van der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining Trained Immunity and Its Role in Health and Disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef]
- Dallio, M.; Ventriglia, L.; Romeo, M.; Scognamiglio, F.; Diano, N.; Moggio, M.; Cipullo, M.; Coppola, A.; Ziogas, A.; Netea, M.G.; et al. Environmental Bisphenol A Exposure Triggers Trained Immunity-Related Pathways in Monocytes. Front. Immunol. 2023, 14, 1270391. [Google Scholar] [CrossRef]
- Netea, M.G.; Joosten, L.A.B.; Latz, E.; Mills, K.H.G.; Natoli, G.; Stunnenberg, H.G.; O’Neill, L.A.J.; Xavier, R.J. Trained Immunity: A Program of Innate Immune Memory in Health and Disease. Science 2016, 352, aaf1098. [Google Scholar] [CrossRef]
- Kountouras, J.; Kazakos, E.; Polyzos, S.A.; Papaefthymiou, A.; Zavos, C.; Tzitiridou-Chatzopoulou, M.; Chatzopoulos, D.; Vardaka, E.; Gatopoulou, A.; Kyrailidi, F.; et al. Potential Impact of Trained Innate Immunity on the Pathophysiology of Metabolic Dysfunction-Associated Fatty Liver Disease. Clin. Immunol. 2023, 256, 109776. [Google Scholar] [CrossRef] [PubMed]
- Divangahi, M.; Aaby, P.; Khader, S.A.; Barreiro, L.B.; Bekkering, S.; Chavakis, T.; van Crevel, R.; Curtis, N.; DiNardo, A.R.; Dominguez-Andres, J.; et al. Trained Immunity, Tolerance, Priming and Differentiation: Distinct Immunological Processes. Nat. Immunol. 2021, 22, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Andrés, J.; Dos Santos, J.C.; Bekkering, S.; Mulder, W.J.M.; van der Meer, J.W.M.; Riksen, N.P.; Joosten, L.A.B.; Netea, M.G. Trained Immunity: Adaptation within Innate Immune Mechanisms. Physiol. Rev. 2023, 103, 313–346. [Google Scholar] [CrossRef]
- Vuscan, P.; Kischkel, B.; Joosten, L.A.B.; Netea, M.G. Trained Immunity: General and Emerging Concepts. Immunol. Rev. 2024, 323, 164–185. [Google Scholar] [CrossRef]
- Li, Y.; Yang, P.; Ye, J.; Xu, Q.; Wu, J.; Wang, Y. Updated Mechanisms of MASLD Pathogenesis. Lipids Health Dis. 2024, 23, 117. [Google Scholar] [CrossRef]
- Riksen, N.P.; Netea, M.G. Immunometabolic Control of Trained Immunity. Mol. Asp. Med. 2021, 77, 100897. [Google Scholar] [CrossRef]
- Heeren, J.; Scheja, L. Metabolic-Associated Fatty Liver Disease and Lipoprotein Metabolism. Mol. Metab. 2021, 50, 101238. [Google Scholar] [CrossRef]
- Trefts, E.; Gannon, M.; Wasserman, D.H. The Liver. Curr. Biol. 2017, 27, R1147–R1151. [Google Scholar] [CrossRef] [PubMed]
- Stamataki, Z.; Swadling, L. The Liver as an Immunological Barrier Redefined by Single-Cell Analysis. Immunology 2020, 160, 157–170. [Google Scholar] [CrossRef]
- Wang, R.; Tang, R.; Li, B.; Ma, X.; Schnabl, B.; Tilg, H. Gut Microbiome, Liver Immunology, and Liver Diseases. Cell. Mol. Immunol. 2021, 18, 4–17. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, X.; Li, Y.; Yu, J.; Chen, Z.; Niu, Y.; Ran, S.; Wang, S.; Ye, W.; Luo, Z.; et al. Interorgan Communication with the Liver: Novel Mechanisms and Therapeutic Targets. Front. Immunol. 2023, 14, 1314123. [Google Scholar] [CrossRef]
- McConnell, M.J.; Kostallari, E.; Ibrahim, S.H.; Iwakiri, Y. The Evolving Role of Liver Sinusoidal Endothelial Cells in Liver Health and Disease. Hepatology 2023, 78, 649–669. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Shi, X.; He, X.; Gao, Y. Macrophage Phenotype and Function in Liver Disorder. Front. Immunol. 2019, 10, 3112. [Google Scholar] [CrossRef] [PubMed]
- Gola, A.; Dorrington, M.G.; Speranza, E.; Sala, C.; Shih, R.M.; Radtke, A.J.; Wong, H.S.; Baptista, A.P.; Hernandez, J.M.; Castellani, G.; et al. Commensal-Driven Immune Zonation of the Liver Promotes Host Defence. Nature 2021, 589, 131–136. [Google Scholar] [CrossRef]
- Li, W.; Yang, Y.; Yang, L.; Chang, N.; Li, L. Monocyte-Derived Kupffer Cells Dominate in the Kupffer Cell Pool during Liver Injury. Cell Rep. 2023, 42, 113164. [Google Scholar] [CrossRef]
- Peiseler, M.; Araujo David, B.; Zindel, J.; Surewaard, B.G.J.; Lee, W.-Y.; Heymann, F.; Nusse, Y.; Castanheira, F.V.S.; Shim, R.; Guillot, A.; et al. Kupffer Cell-like Syncytia Replenish Resident Macrophage Function in the Fibrotic Liver. Science 2023, 381, eabq5202. [Google Scholar] [CrossRef] [PubMed]
- Carpino, G.; Morini, S.; Ginanni Corradini, S.; Franchitto, A.; Merli, M.; Siciliano, M.; Gentili, F.; Onetti Muda, A.; Berloco, P.; Rossi, M.; et al. Alpha-SMA Expression in Hepatic Stellate Cells and Quantitative Analysis of Hepatic Fibrosis in Cirrhosis and in Recurrent Chronic Hepatitis after Liver Transplantation. Dig. Liver Dis. 2005, 37, 349–356. [Google Scholar] [CrossRef]
- Xuan, Y.; Chen, S.; Ding, X.; Wang, L.; Li, S.; Yang, G.; Lan, T. Tetrahydropalmatine Attenuates Liver Fibrosis by Suppressing Endoplasmic Reticulum Stress in Hepatic Stellate Cells. Chin. Med. J. 2022, 135, 628–630. [Google Scholar] [CrossRef]
- Kamm, D.R.; McCommis, K.S. Hepatic Stellate Cells in Physiology and Pathology. J. Physiol. 2022, 600, 1825–1837. [Google Scholar] [CrossRef]
- Klaimi, C.; Kong, W.; Blériot, C.; Haas, J.T. The Immunological Interface: Dendritic Cells as Key Regulators in Metabolic Dysfunction-Associated Steatotic Liver Disease. FEBS Lett. 2024, 599, 1971–1981. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Li, M.; Huan, C.; Lv, G. Dendritic Cells in Liver Transplantation Immune Response. Front. Cell Dev. Biol. 2023, 11, 1277743. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.; Fahey, R.; Fletcher, J.M.; Keogh, C.; Carroll, A.G.; Siddachari, R.; Geoghegan, J.; Hegarty, J.E.; Ryan, E.J.; O’Farrelly, C. CD141+ Myeloid Dendritic Cells Are Enriched in Healthy Human Liver. J. Hepatol. 2014, 60, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Highton, A.J.; Schuster, I.S.; Degli-Esposti, M.A.; Altfeld, M. The Role of Natural Killer Cells in Liver Inflammation. Semin. Immunopathol. 2021, 43, 519–533. [Google Scholar] [CrossRef]
- Pan, Z.; Ye, Y.; Liu, C.; Li, W. Role of Liver-Resident NK Cells in Liver Immunity. Hepatol. Int. 2025, 19, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, M.; Song, S.; Zhi, Y.; Huan, C.; Lv, G. The Role of Natural Killer T Cells in Liver Transplantation. Front. Cell Dev. Biol. 2023, 11, 1274361. [Google Scholar] [CrossRef]
- Barreby, E.; Chen, P.; Aouadi, M. Macrophage Functional Diversity in NAFLD—More than Inflammation. Nat. Rev. Endocrinol. 2022, 18, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, G.; Revelo, X.; Malhi, H. Pathogenesis of Nonalcoholic Steatohepatitis: An Overview. Hepatol. Commun. 2020, 4, 478–492. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Kuang, H.; Ansari, S.; Liu, T.; Gong, J.; Wang, S.; Zhao, X.-Y.; Ji, Y.; Li, C.; Guo, L.; et al. Landscape of Intercellular Crosstalk in Healthy and NASH Liver Revealed by Single-Cell Secretome Gene Analysis. Mol. Cell 2019, 75, 644–660.e5. [Google Scholar] [CrossRef] [PubMed]
- Mladenić, K.; Lenartić, M.; Marinović, S.; Polić, B.; Wensveen, F.M. The “Domino Effect” in MASLD: The Inflammatory Cascade of Steatohepatitis. Eur. J. Immunol. 2024, 54, 2149641. [Google Scholar] [CrossRef]
- Sawada, K.; Chung, H.; Softic, S.; Moreno-Fernandez, M.E.; Divanovic, S. The Bidirectional Immune Crosstalk in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Cell Metab. 2023, 35, 1852–1871. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, W.; Song, K.; Song, K.; Kolls, J.; Wu, T. YAP Activation in Liver Macrophages via Depletion of MST1/MST2 Enhances Liver Inflammation and Fibrosis in MASLD. FASEB J. 2024, 38, e70026. [Google Scholar] [CrossRef]
- Li, X.; Wang, K.; Sun, Y.; Wang, Y.; Wu, J.; Dang, Y.; Li, M.; Zhou, W. Cholesterol Overload in Macrophages Drives Metabolic Dysfunction-Associated Steatohepatitis via Inhibiting 7-Dehydrocholesterol Reductase in Mice. J. Transl. Med. 2024, 22, 1085. [Google Scholar] [CrossRef] [PubMed]
- Ganz, M.; Bukong, T.N.; Csak, T.; Saha, B.; Park, J.-K.; Ambade, A.; Kodys, K.; Szabo, G. Progression of Non-Alcoholic Steatosis to Steatohepatitis and Fibrosis Parallels Cumulative Accumulation of Danger Signals That Promote Inflammation and Liver Tumors in a High Fat-Cholesterol-Sugar Diet Model in Mice. J. Transl. Med. 2015, 13, 193. [Google Scholar] [CrossRef]
- Kazankov, K.; Jørgensen, S.M.D.; Thomsen, K.L.; Møller, H.J.; Vilstrup, H.; George, J.; Schuppan, D.; Grønbæk, H. The Role of Macrophages in Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 145–159. [Google Scholar] [CrossRef]
- Zou, Z.; Liu, X.; Yu, J.; Ban, T.; Zhang, Z.; Wang, P.; Huang, R.; Zheng, F.; Chang, Y.; Peng, W.; et al. Nuclear miR-204-3p Mitigates Metabolic Dysfunction-Associated Steatotic Liver Disease in Mice. J. Hepatol. 2024, 80, 834–845. [Google Scholar] [CrossRef]
- Blériot, C.; Barreby, E.; Dunsmore, G.; Ballaire, R.; Chakarov, S.; Ficht, X.; De Simone, G.; Andreata, F.; Fumagalli, V.; Guo, W.; et al. A Subset of Kupffer Cells Regulates Metabolism through the Expression of CD36. Immunity 2021, 54, 2101–2116.e6. [Google Scholar] [CrossRef]
- Ramachandran, P.; Dobie, R.; Wilson-Kanamori, J.R.; Dora, E.F.; Henderson, B.E.P.; Luu, N.T.; Portman, J.R.; Matchett, K.P.; Brice, M.; Marwick, J.A.; et al. Resolving the Fibrotic Niche of Human Liver Cirrhosis at Single-Cell Level. Nature 2019, 575, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Tacke, F.; Zimmermann, H.W. Macrophage Heterogeneity in Liver Injury and Fibrosis. J. Hepatol. 2014, 60, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Fodor, M.; Salcher, S.; Gottschling, H.; Mair, A.; Blumer, M.; Sopper, S.; Ebner, S.; Pircher, A.; Oberhuber, R.; Wolf, D.; et al. The Liver-Resident Immune Cell Repertoire—A Boon or a Bane during Machine Perfusion? Front. Immunol. 2022, 13, 982018. [Google Scholar] [CrossRef]
- De Oliveira, T.H.C.; Marques, P.E.; Proost, P.; Teixeira, M.M.M. Neutrophils: A Cornerstone of Liver Ischemia and Reperfusion Injury. Lab. Investig. 2018, 98, 51–62. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Wang, Y.; Brown, Z.J.; Xia, Y.; Huang, Z.; Shen, C.; Hu, Z.; Beane, J.; Ansa-Addo, E.A.; et al. Regulatory T-Cell and Neutrophil Extracellular Trap Interaction Contributes to Carcinogenesis in Non-Alcoholic Steatohepatitis. J. Hepatol. 2021, 75, 1271–1283. [Google Scholar] [CrossRef]
- van der Windt, D.J.; Sud, V.; Zhang, H.; Varley, P.R.; Goswami, J.; Yazdani, H.O.; Tohme, S.; Loughran, P.; O’Doherty, R.M.; Minervini, M.I.; et al. Neutrophil Extracellular Traps Promote Inflammation and Development of Hepatocellular Carcinoma in Nonalcoholic Steatohepatitis. Hepatology 2018, 68, 1347–1360. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, C.; He, T.; Zhang, S.; Wang, Y.; Xie, Z.; Xu, W.; Ding, C.; Shuai, Y.; Hao, H.; et al. Polyunsaturated Fatty Acids Drive Neutrophil Extracellular Trap Formation in Nonalcoholic Steatohepatitis. Eur. J. Pharmacol. 2023, 945, 175618. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yang, L.; Chang, N.; Hou, L.; Zhou, X.; Yang, L.; Li, L. Neutrophils Undergo Switch of Apoptosis to NETosis during Murine Fatty Liver Injury via S1P Receptor 2 Signaling. Cell Death Dis. 2020, 11, 379. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liang, B.; Bian, D.; Luo, Y.; Yang, J.; Li, Z.; Zhuang, Z.; Zang, S.; Shi, J. Knockout of Neutrophil Elastase Protects against Western Diet Induced Nonalcoholic Steatohepatitis in Mice by Regulating Hepatic Ceramides Metabolism. Biochem. Biophys. Res. Commun. 2019, 518, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Kageyama, S.; Kupiec-Weglinski, J.W. The Evolving Role of Neutrophils in Liver Transplant Ischemia-Reperfusion Injury. Curr. Transplant. Rep. 2019, 6, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Zang, S.; Wang, L.; Ma, X.; Zhu, G.; Zhuang, Z.; Xun, Y.; Zhao, F.; Yang, W.; Liu, J.; Luo, Y.; et al. Neutrophils Play a Crucial Role in the Early Stage of Nonalcoholic Steatohepatitis via Neutrophil Elastase in Mice. Cell Biochem. Biophys. 2015, 73, 479–487. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, M.-J.; Cai, Y.; Wang, W.; Jiang, J.X.; Varga, Z.V.; Feng, D.; Pacher, P.; Kunos, G.; Torok, N.J.; et al. Neutrophil-Hepatic Stellate Cell Interactions Promote Fibrosis in Experimental Steatohepatitis. Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 399–413. [Google Scholar] [CrossRef]
- Maretti-Mira, A.C.; Salomon, M.P.; Chopra, S.; Yuan, L.; Golden-Mason, L. Circulating Neutrophil Profiles Undergo a Dynamic Shift during Metabolic Dysfunction-Associated Steatohepatitis (MASH) Progression. Biomedicines 2024, 12, 1105. [Google Scholar] [CrossRef]
- Ariyachet, C.; Chuaypen, N.; Kaewsapsak, P.; Chantaravisoot, N.; Jindatip, D.; Potikanond, S.; Tangkijvanich, P. MicroRNA-223 Suppresses Human Hepatic Stellate Cell Activation Partly via Regulating the Actin Cytoskeleton and Alleviates Fibrosis in Organoid Models of Liver Injury. Int. J. Mol. Sci. 2022, 23, 9380. [Google Scholar] [CrossRef]
- Calvente, C.J.; Tameda, M.; Johnson, C.D.; Del Pilar, H.; Lin, Y.C.; Adronikou, N.; De Mollerat Du Jeu, X.; Llorente, C.; Boyer, J.; Feldstein, A.E. Neutrophils Contribute to Spontaneous Resolution of Liver Inflammation and Fibrosis via microRNA-223. J. Clin. Investig. 2019, 129, 4091–4109. [Google Scholar] [CrossRef]
- van der Zande, H.J.; Brombacher, E.C.; Lambooij, J.M.; Pelgrom, L.R.; Zawistowska-Deniziak, A.; Patente, T.A.; Heieis, G.A.; Otto, F.; Ozir-Fazalalikhan, A.; Yazdanbakhsh, M.; et al. Dendritic Cell-Intrinsic LKB1-AMPK/SIK Signaling Controls Metabolic Homeostasis by Limiting the Hepatic Th17 Response during Obesity. JCI Insight 2023, 8, e157948. [Google Scholar] [CrossRef]
- Song, Y.; Li, N.; Jiang, S.; Wang, K.; Lv, G.; Fan, Z.; Du, X.; Gao, W.; Lei, L.; Wang, Z.; et al. Microbiota-Derived H2S Induces c-Kit+ cDC1 Autophagic Cell Death and Liver Inflammation in Metabolic Dysfunction-Associated Steatohepatitis. Nat. Commun. 2025, 16, 2222. [Google Scholar] [CrossRef]
- Mori, T.; Yoshio, S.; Kakazu, E.; Kanto, T. Active Role of the Immune System in Metabolic Dysfunction-Associated Steatotic Liver Disease. Gastroenterol. Rep. 2024, 12, goae089. [Google Scholar] [CrossRef]
- Bourinet, M.; Anty, R.; Gual, P.; Luci, C. Roles of Innate Lymphoid Cells in Metabolic and Alcohol-Associated Liver Diseases. JHEP Rep. 2024, 6, 100962. [Google Scholar] [CrossRef]
- Nixon, B.G.; Chou, C.; Krishna, C.; Dadi, S.; Michel, A.O.; Cornish, A.E.; Kansler, E.R.; Do, M.H.; Wang, X.; Capistrano, K.J.; et al. Cytotoxic Granzyme C-Expressing ILC1s Contribute to Antitumor Immunity and Neonatal Autoimmunity. Sci. Immunol. 2022, 7, eabi8642. [Google Scholar] [CrossRef]
- Kansler, E.R.; Dadi, S.; Krishna, C.; Nixon, B.G.; Stamatiades, E.G.; Liu, M.; Kuo, F.; Zhang, J.; Zhang, X.; Capistrano, K.; et al. Cytotoxic Innate Lymphoid Cells Sense Cancer Cell-Expressed Interleukin-15 to Suppress Human and Murine Malignancies. Nat. Immunol. 2022, 23, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Chantar, M.L.; Delgado, T.C.; Beraza, N. Revisiting the Role of Natural Killer Cells in Non-Alcoholic Fatty Liver Disease. Front. Immunol. 2021, 12, 640869. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.-Y.; Park, E.-J.; Lee, C.-W. Immunological Distinctions between Nonalcoholic Steatohepatitis and Hepatocellular Carcinoma. Exp. Mol. Med. 2020, 52, 1209–1219. [Google Scholar] [CrossRef]
- Meyer, M.; Schwärzler, J.; Jukic, A.; Tilg, H. Innate Immunity and MASLD. Biomolecules 2024, 14, 476. [Google Scholar] [CrossRef]
- Wolf, M.J.; Adili, A.; Piotrowitz, K.; Abdullah, Z.; Boege, Y.; Stemmer, K.; Ringelhan, M.; Simonavicius, N.; Egger, M.; Wohlleber, D.; et al. Metabolic Activation of Intrahepatic CD8+ T Cells and NKT Cells Causes Nonalcoholic Steatohepatitis and Liver Cancer via Cross-Talk with Hepatocytes. Cancer Cell 2014, 26, 549–564. [Google Scholar] [CrossRef]
- Sutti, S.; Jindal, A.; Locatelli, I.; Vacchiano, M.; Gigliotti, L.; Bozzola, C.; Albano, E. Adaptive Immune Responses Triggered by Oxidative Stress Contribute to Hepatic Inflammation in NASH. Hepatology 2014, 59, 886–897. [Google Scholar] [CrossRef]
- Syn, W.-K.; Agboola, K.M.; Swiderska, M.; Michelotti, G.A.; Liaskou, E.; Pang, H.; Xie, G.; Philips, G.; Chan, I.S.; Karaca, G.F.; et al. NKT-Associated Hedgehog and Osteopontin Drive Fibrogenesis in Non-Alcoholic Fatty Liver Disease. Gut 2012, 61, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, T.; Takehara, T.; Uemura, A.; Nishio, K.; Shimizu, S.; Kodama, T.; Hikita, H.; Li, W.; Sasakawa, A.; Tatsumi, T.; et al. Absence of Invariant Natural Killer T Cells Deteriorates Liver Inflammation and Fibrosis in Mice Fed High-Fat Diet. J. Gastroenterol. 2010, 45, 1247–1254. [Google Scholar] [CrossRef]
- Shuai, Z.; Leung, M.W.; He, X.; Zhang, W.; Yang, G.; Leung, P.S.; Eric Gershwin, M. Adaptive Immunity in the Liver. Cell. Mol. Immunol. 2016, 13, 354–368. [Google Scholar] [CrossRef]
- Sanz, I.; Wei, C.; Jenks, S.A.; Cashman, K.S.; Tipton, C.; Woodruff, M.C.; Hom, J.; Lee, F.E.-H. Challenges and Opportunities for Consistent Classification of Human B Cell and Plasma Cell Populations. Front. Immunol. 2019, 10, 2458. [Google Scholar] [CrossRef]
- Sutti, S.; Albano, E. Adaptive Immunity: An Emerging Player in the Progression of NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Saravia, J.; Chapman, N.M.; Chi, H. Helper T Cell Differentiation. Cell. Mol. Immunol. 2019, 16, 634–643. [Google Scholar] [CrossRef]
- Raphael, I.; Nalawade, S.; Eagar, T.N.; Forsthuber, T.G. T Cell Subsets and Their Signature Cytokines in Autoimmune and Inflammatory Diseases. Cytokine 2015, 74, 5–17. [Google Scholar] [CrossRef]
- Moreno-Fernandez, M.E.; Giles, D.A.; Oates, J.R.; Chan, C.C.; Damen, M.S.M.A.; Doll, J.R.; Stankiewicz, T.E.; Chen, X.; Chetal, K.; Karns, R.; et al. PKM2-Dependent Metabolic Skewing of Hepatic Th17 Cells Regulates Pathogenesis of Non-Alcoholic Fatty Liver Disease. Cell Metab. 2021, 33, 1187–1204.e9. [Google Scholar] [CrossRef] [PubMed]
- Chackelevicius, C.M.; Gambaro, S.E.; Tiribelli, C.; Rosso, N. Th17 Involvement in Nonalcoholic Fatty Liver Disease Progression to Non-Alcoholic Steatohepatitis. World J. Gastroenterol. 2016, 22, 9096–9103. [Google Scholar] [CrossRef]
- Rau, M.; Schilling, A.-K.; Meertens, J.; Hering, I.; Weiss, J.; Jurowich, C.; Kudlich, T.; Hermanns, H.M.; Bantel, H.; Beyersdorf, N.; et al. Progression from Nonalcoholic Fatty Liver to Nonalcoholic Steatohepatitis Is Marked by a Higher Frequency of Th17 Cells in the Liver and an Increased Th17/Resting Regulatory T Cell Ratio in Peripheral Blood and in the Liver. J. Immunol. 2016, 196, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.P.; Liu, Y.; Iyer, S.S.; Liu, S.; Gupta, B.; Desai, C.; Kumar, P.; Smith, T.; Singhi, A.D.; Nusrat, A.; et al. Blocking Integrin A4β7-Mediated CD4 T Cell Recruitment to the Intestine and Liver Protects Mice from Western Diet-Induced Non-Alcoholic Steatohepatitis. J. Hepatol. 2020, 73, 1013–1022. [Google Scholar] [CrossRef]
- Koda, Y.; Teratani, T.; Chu, P.-S.; Hagihara, Y.; Mikami, Y.; Harada, Y.; Tsujikawa, H.; Miyamoto, K.; Suzuki, T.; Taniki, N.; et al. CD8+ Tissue-Resident Memory T Cells Promote Liver Fibrosis Resolution by Inducing Apoptosis of Hepatic Stellate Cells. Nat. Commun. 2021, 12, 4474. [Google Scholar] [CrossRef]
- Bhattacharjee, J.; Kirby, M.; Softic, S.; Miles, L.; Salazar-Gonzalez, R.-M.; Shivakumar, P.; Kohli, R. Hepatic Natural Killer T-Cell and CD8+ T-Cell Signatures in Mice with Nonalcoholic Steatohepatitis. Hepatol. Commun. 2017, 1, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Wabitsch, S.; McCallen, J.D.; Kamenyeva, O.; Ruf, B.; McVey, J.C.; Kabat, J.; Walz, J.S.; Rotman, Y.; Bauer, K.C.; Craig, A.J.; et al. Metformin Treatment Rescues CD8+ T-Cell Response to Immune Checkpoint Inhibitor Therapy in Mice with NAFLD. J. Hepatol. 2022, 77, 748–760. [Google Scholar] [CrossRef]
- Zhang, F.; Jiang, W.W.; Li, X.; Qiu, X.Y.; Wu, Z.; Chi, Y.J.; Cong, X.; Liu, Y.L. Role of Intrahepatic B Cells in Non-Alcoholic Fatty Liver Disease by Secreting pro-Inflammatory Cytokines and Regulating Intrahepatic T Cells. J. Dig. Dis. 2016, 17, 464–474. [Google Scholar] [CrossRef]
- Bruzzì, S.; Sutti, S.; Giudici, G.; Burlone, M.E.; Ramavath, N.N.; Toscani, A.; Bozzola, C.; Schneider, P.; Morello, E.; Parola, M.; et al. B2-Lymphocyte Responses to Oxidative Stress-Derived Antigens Contribute to the Evolution of Nonalcoholic Fatty Liver Disease (NAFLD). Free Radic. Biol. Med. 2018, 124, 249–259. [Google Scholar] [CrossRef]
- Karl, M.; Hasselwander, S.; Zhou, Y.; Reifenberg, G.; Kim, Y.O.; Park, K.-S.; Ridder, D.A.; Wang, X.; Seidel, E.; Hövelmeyer, N.; et al. Dual Roles of B Lymphocytes in Mouse Models of Diet-Induced Nonalcoholic Fatty Liver Disease. Hepatology 2022, 76, 1135–1149. [Google Scholar] [CrossRef]
- Rabiu, L.; Zhang, P.; Afolabi, L.O.; Saliu, M.A.; Dabai, S.M.; Suleiman, R.B.; Gidado, K.I.; Ige, M.A.; Ibrahim, A.; Zhang, G.; et al. Immunological Dynamics in MASH: From Landscape Analysis to Therapeutic Intervention. J. Gastroenterol. 2024, 59, 1053–1078. [Google Scholar] [CrossRef]
- Wang, H.; Herman, A.; Barrow, F.; Abdel-Ghani, A.; Draxler, M.; Fredrickson, G.; Parthiban, P.; Seelig, D.M.; Ikramuddin, S.; Revelo, X.S. Single-Cell RNA Sequencing Reveals a Reprogramming of Hepatic Immune Cells and a Protective Role for B Cells in MASH-Driven HCC. Hepatol. Commun. 2025, 9, e0668. [Google Scholar] [CrossRef]
- Ruf, B.; Heinrich, B.; Greten, T.F. Immunobiology and Immunotherapy of HCC: Spotlight on Innate and Innate-like Immune Cells. Cell. Mol. Immunol. 2021, 18, 112–127. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.-Q.; Cai, D.; Gong, J.-P.; Lai, X. Innate Immune Cells and Their Interaction with T Cells in Hepatocellular Carcinoma. Oncol. Lett. 2021, 21, 57. [Google Scholar] [CrossRef] [PubMed]
- Mattos, Â.Z.; Debes, J.D.; Boonstra, A.; Vogel, A.; Mattos, A.A. Immune Aspects of Hepatocellular Carcinoma: From Immune Markers for Early Detection to Immunotherapy. World J. Gastrointest. Oncol. 2021, 13, 1132–1143. [Google Scholar] [CrossRef]
- Gryziak, M.; Wozniak, K.; Kraj, L.; Rog, L.; Stec, R. The Immune Landscape of Hepatocellular Carcinoma-Where We Are? Oncol. Lett. 2022, 24, 410. [Google Scholar] [CrossRef]
- Ramon-Gil, E.; Geh, D.; Leslie, J. Harnessing Neutrophil Plasticity for HCC Immunotherapy. Essays Biochem. 2023, 67, 941–955. [Google Scholar] [CrossRef]
- Lu, C.; Rong, D.; Zhang, B.; Zheng, W.; Wang, X.; Chen, Z.; Tang, W. Current Perspectives on the Immunosuppressive Tumor Microenvironment in Hepatocellular Carcinoma: Challenges and Opportunities. Mol. Cancer 2019, 18, 130. [Google Scholar] [CrossRef]
- He, M.; Peng, A.; Huang, X.-Z.; Shi, D.-C.; Wang, J.-C.; Zhao, Q.; Lin, H.; Kuang, D.-M.; Ke, P.-F.; Lao, X.-M. Peritumoral Stromal Neutrophils Are Essential for C-Met-Elicited Metastasis in Human Hepatocellular Carcinoma. Oncoimmunology 2016, 5, e1219828. [Google Scholar] [CrossRef]
- Yang, L.-Y.; Luo, Q.; Lu, L.; Zhu, W.-W.; Sun, H.-T.; Wei, R.; Lin, Z.-F.; Wang, X.-Y.; Wang, C.-Q.; Lu, M.; et al. Increased Neutrophil Extracellular Traps Promote Metastasis Potential of Hepatocellular Carcinoma via Provoking Tumorous Inflammatory Response. J. Hematol. Oncol. 2020, 13, 3. [Google Scholar] [CrossRef]
- Shen, M.; Du, Y.; Ye, Y. Tumor-Associated Macrophages, Dendritic Cells, and Neutrophils: Biological Roles, Crosstalk, and Therapeutic Relevance. Med. Rev. 2021, 1, 222–243. [Google Scholar] [CrossRef]
- Warner, K.; Ohashi, P.S. ILC Regulation of T Cell Responses in Inflammatory Diseases and Cancer. Semin. Immunol. 2019, 41, 101284. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Huang, T.; Han, J. Cytokines Derived from Innate Lymphoid Cells Assist Helicobacter Hepaticus to Aggravate Hepatocellular Tumorigenesis in Viral Transgenic Mice. Gut Pathog. 2019, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Taherifard, E.; Saeed, A.; Saeed, A. MASLD-Related HCC: A Comprehensive Review of the Trends, Pathophysiology, Tumor Microenvironment, Surveillance, and Treatment Options. Curr. Issues Mol. Biol. 2024, 46, 5965–5983. [Google Scholar] [CrossRef] [PubMed]
- Jacquelot, N.; Seillet, C.; Souza-Fonseca-Guimaraes, F.; Sacher, A.G.; Belz, G.T.; Ohashi, P.S. Natural Killer Cells and Type 1 Innate Lymphoid Cells in Hepatocellular Carcinoma: Current Knowledge and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 9044. [Google Scholar] [CrossRef]
- Sachdeva, M.; Arora, S.K. Prognostic Role of Immune Cells in Hepatocellular Carcinoma. EXCLI J. 2020, 19, 718–733. [Google Scholar] [CrossRef] [PubMed]
- Daemen, S.; Schilling, J.D. The Interplay Between Tissue Niche and Macrophage Cellular Metabolism in Obesity. Front. Immunol. 2019, 10, 3133. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.H.; Dixit, V.D. Dietary Regulation of Immunity. Immunity 2020, 53, 510–523. [Google Scholar] [CrossRef] [PubMed]
- Remmerie, A.; Martens, L.; Scott, C.L. Macrophage Subsets in Obesity, Aligning the Liver and Adipose Tissue. Front. Endocrinol. 2020, 11, 259. [Google Scholar] [CrossRef]
- Trim, W.V.; Lynch, L. Immune and Non-Immune Functions of Adipose Tissue Leukocytes. Nat. Rev. Immunol. 2022, 22, 371–386. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation, Metaflammation and Immunometabolic Disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef]
- Lee, Y.S.; Olefsky, J. Chronic Tissue Inflammation and Metabolic Disease. Genes Dev. 2021, 35, 307–328. [Google Scholar] [CrossRef]
- Daemen, S.; Gainullina, A.; Kalugotla, G.; He, L.; Chan, M.M.; Beals, J.W.; Liss, K.H.; Klein, S.; Feldstein, A.E.; Finck, B.N.; et al. Dynamic Shifts in the Composition of Resident and Recruited Macrophages Influence Tissue Remodeling in NASH. Cell Rep. 2021, 34, 108626. [Google Scholar] [CrossRef]
- Jaitin, D.A.; Adlung, L.; Thaiss, C.A.; Weiner, A.; Li, B.; Descamps, H.; Lundgren, P.; Bleriot, C.; Liu, Z.; Deczkowska, A.; et al. Lipid-Associated Macrophages Control Metabolic Homeostasis in a Trem2-Dependent Manner. Cell 2019, 178, 686–698.e14. [Google Scholar] [CrossRef]
- Ryan, D.G.; O’Neill, L.A.J. Krebs Cycle Reborn in Macrophage Immunometabolism. Annu. Rev. Immunol. 2020, 38, 289–313. [Google Scholar] [CrossRef]
- Prasun, P. Mitochondrial Dysfunction in Metabolic Syndrome. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165838. [Google Scholar] [CrossRef]
- Carli, F.; Della Pepa, G.; Sabatini, S.; Vidal Puig, A.; Gastaldelli, A. Lipid Metabolism in MASLD and MASH: From Mechanism to the Clinic. JHEP Rep. 2024, 6, 101185. [Google Scholar] [CrossRef]
- Zhu, L.; Han, Y.; Xi, X.; Li, L.; Yan, B. Completion of Metal-Damaged Traces Based on Deep Learning in Sinogram Domain for Metal Artifacts Reduction in CT Images. Sensors 2021, 21, 8164. [Google Scholar] [CrossRef]
- Xu, Z.; Kombe Kombe, A.J.; Deng, S.; Zhang, H.; Wu, S.; Ruan, J.; Zhou, Y.; Jin, T. NLRP Inflammasomes in Health and Disease. Mol. Biomed. 2024, 5, 14. [Google Scholar] [CrossRef]
- Bekkering, S.; Arts, R.J.W.; Novakovic, B.; Kourtzelis, I.; van der Heijden, C.D.C.C.; Li, Y.; Popa, C.D.; Ter Horst, R.; van Tuijl, J.; Netea-Maier, R.T.; et al. Metabolic Induction of Trained Immunity through the Mevalonate Pathway. Cell 2018, 172, 135–146.e9. [Google Scholar] [CrossRef] [PubMed]
- Bhargavi, G.; Subbian, S. The Causes and Consequences of Trained Immunity in Myeloid Cells. Front. Immunol. 2024, 15, 1365127. [Google Scholar] [CrossRef] [PubMed]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.-A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage Plasticity, Polarization, and Function in Health and Disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Wang, S.; Liu, G.; Li, Y.; Pan, Y. Metabolic Reprogramming Induces Macrophage Polarization in the Tumor Microenvironment. Front. Immunol. 2022, 13, 840029. [Google Scholar] [CrossRef] [PubMed]
- Viola, A.; Munari, F.; Sánchez-Rodríguez, R.; Scolaro, T.; Castegna, A. The Metabolic Signature of Macrophage Responses. Front. Immunol. 2019, 10, 1462. [Google Scholar] [CrossRef]
- O’Neill, L.A.J.; Pearce, E.J. Immunometabolism Governs Dendritic Cell and Macrophage Function. J. Exp. Med. 2016, 213, 15–23. [Google Scholar] [CrossRef]
- Arts, R.J.W.; Novakovic, B.; Ter Horst, R.; Carvalho, A.; Bekkering, S.; Lachmandas, E.; Rodrigues, F.; Silvestre, R.; Cheng, S.-C.; Wang, S.-Y.; et al. Glutaminolysis and Fumarate Accumulation Integrate Immunometabolic and Epigenetic Programs in Trained Immunity. Cell Metab. 2016, 24, 807–819. [Google Scholar] [CrossRef]
- Sangineto, M.; Ciarnelli, M.; Colangelo, T.; Moola, A.; Bukke, V.N.; Duda, L.; Villani, R.; Romano, A.; Giandomenico, S.; Kanwal, H.; et al. Monocyte Bioenergetics: An Immunometabolic Perspective in Metabolic Dysfunction-Associated Steatohepatitis. Cell Rep. Med. 2024, 5, 101564. [Google Scholar] [CrossRef]
- Bertoli, C.; Skotheim, J.M.; de Bruin, R.A.M. Control of Cell Cycle Transcription during G1 and S Phases. Nat. Rev. Mol. Cell Biol. 2013, 14, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, H.; Liang, Y.; Liu, M.; Huang, Q.; Wang, M.; Zhou, J.; Bu, Q.; Zhou, H.; Lu, L. E2F2 Reprograms Macrophage Function By Modulating Material and Energy Metabolism in the Progression of Metabolic Dysfunction-Associated Steatohepatitis. Adv. Sci. 2024, 11, e2410880. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Li, R.; Gong, D.; Hu, P.; Xu, J.; Ai, Y.; Zhao, X.; Hu, C.; Xu, M.; Liu, C.; et al. Atf3-Mediated Metabolic Reprogramming in Hepatic Macrophage Orchestrates Metabolic Dysfunction-Associated Steatohepatitis. Sci. Adv. 2024, 10, eado3141. [Google Scholar] [CrossRef]
- Ku, H.-C.; Cheng, C.-F. Master Regulator Activating Transcription Factor 3 (ATF3) in Metabolic Homeostasis and Cancer. Front. Endocrinol. 2020, 11, 556. [Google Scholar] [CrossRef] [PubMed]
- Cahill, T.J.; Thomsen, A.R.B.; Tarrasch, J.T.; Plouffe, B.; Nguyen, A.H.; Yang, F.; Huang, L.-Y.; Kahsai, A.W.; Bassoni, D.L.; Gavino, B.J.; et al. Distinct Conformations of GPCR-β-Arrestin Complexes Mediate Desensitization, Signaling, and Endocytosis. Proc. Natl. Acad. Sci. USA 2017, 114, 2562–2567. [Google Scholar] [CrossRef]
- Georgescu, D.; Lighezan, D.F.; Rosca, C.I.; Nistor, D.; Ancusa, O.E.; Suceava, I.; Iancu, M.A.; Kundnani, N.R. NASH/NAFLD-Related Hepatocellular Carcinoma: An Added Burden. Life 2024, 14, 25. [Google Scholar] [CrossRef]
- Groh, L.A.; Ferreira, A.V.; Helder, L.; van der Heijden, C.D.C.C.; Novakovic, B.; van de Westerlo, E.; Matzaraki, V.; Moorlag, S.J.C.F.M.; de Bree, L.C.; Koeken, V.A.C.M.; et al. oxLDL-Induced Trained Immunity Is Dependent on Mitochondrial Metabolic Reprogramming. Immunometabolism 2021, 3, e210025. [Google Scholar] [CrossRef]
- Ferreira, A.V.; Koeken, V.A.C.M.; Matzaraki, V.; Kostidis, S.; Alarcon-Barrera, J.C.; de Bree, L.C.J.; Moorlag, S.J.C.F.M.; Mourits, V.P.; Novakovic, B.; Giera, M.A.; et al. Glutathione Metabolism Contributes to the Induction of Trained Immunity. Cells 2021, 10, 971. [Google Scholar] [CrossRef]
- Findeisen, H.M.; Voges, V.C.; Braun, L.C.; Sonnenberg, J.; Schwarz, D.; Körner, H.; Reinecke, H.; Sohrabi, Y. LXRα Regulates oxLDL-Induced Trained Immunity in Macrophages. Int. J. Mol. Sci. 2022, 23, 6166. [Google Scholar] [CrossRef]
- Park, S.; Hall, M.N. Metabolic Reprogramming in Hepatocellular Carcinoma: Mechanisms and Therapeutic Implications. Exp. Mol. Med. 2025, 57, 515–523. [Google Scholar] [CrossRef]
- Xia, Y.; Brown, Z.J.; Huang, H.; Tsung, A. Metabolic Reprogramming of Immune Cells: Shaping the Tumor Microenvironment in Hepatocellular Carcinoma. Cancer Med. 2021, 10, 6374–6383. [Google Scholar] [CrossRef]
- Huang, J.; Wu, Q.; Geller, D.A.; Yan, Y. Macrophage Metabolism, Phenotype, Function, and Therapy in Hepatocellular Carcinoma (HCC). J. Transl. Med. 2023, 21, 815. [Google Scholar] [CrossRef]
- Wculek, S.K.; Dunphy, G.; Heras-Murillo, I.; Mastrangelo, A.; Sancho, D. Metabolism of Tissue Macrophages in Homeostasis and Pathology. Cell. Mol. Immunol. 2022, 19, 384–408. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Wang, Q.; Peng, H. Tumor-Associated Macrophages: New Insights on Their Metabolic Regulation and Their Influence in Cancer Immunotherapy. Front. Immunol. 2023, 14, 1157291. [Google Scholar] [CrossRef]
- Yuan, Y.; Wu, D.; Li, J.; Huang, D.; Zhao, Y.; Gao, T.; Zhuang, Z.; Cui, Y.; Zheng, D.-Y.; Tang, Y. Mechanisms of Tumor-Associated Macrophages Affecting the Progression of Hepatocellular Carcinoma. Front. Pharmacol. 2023, 14, 1217400. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, J.; Lin, H.; Lin, B.; Zhu, M.; Li, M. Glucose Metabolism Reprogramming Promotes Immune Escape of Hepatocellular Carcinoma Cells. Explor. Target. Antitumor Ther. 2023, 4, 519–536. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, N.; Yan, T.; Wei, J.; Hao, L.; Sun, C.; Zhao, H.; Jiang, S. Lactate-Mediated Metabolic Reprogramming of Tumor-Associated Macrophages: Implications for Tumor Progression and Therapeutic Potential. Front. Immunol. 2025, 16, 1573039. [Google Scholar] [CrossRef] [PubMed]
- Bannister, M.E.; Chatterjee, D.A.; Shetty, S.; Patten, D.A. The Role of Macrophages in Hepatocellular Carcinoma and Their Therapeutic Potential. Int. J. Mol. Sci. 2024, 25, 13167. [Google Scholar] [CrossRef]
- Shime, H.; Yabu, M.; Akazawa, T.; Kodama, K.; Matsumoto, M.; Seya, T.; Inoue, N. Tumor-Secreted Lactic Acid Promotes IL-23/IL-17 Proinflammatory Pathway. J. Immunol. 2008, 180, 7175–7183. [Google Scholar] [CrossRef]
- Maciver, N.J.; Jacobs, S.R.; Wieman, H.L.; Wofford, J.A.; Coloff, J.L.; Rathmell, J.C. Glucose Metabolism in Lymphocytes Is a Regulated Process with Significant Effects on Immune Cell Function and Survival. J. Leukoc. Biol. 2008, 84, 949–957. [Google Scholar] [CrossRef]
- Xia, L.; Oyang, L.; Lin, J.; Tan, S.; Han, Y.; Wu, N.; Yi, P.; Tang, L.; Pan, Q.; Rao, S.; et al. The Cancer Metabolic Reprogramming and Immune Response. Mol. Cancer 2021, 20, 28. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Arakaki, R.; Saito, M.; Kudo, Y.; Ishimaru, N. Dual Role of Fas/FasL-Mediated Signal in Peripheral Immune Tolerance. Front. Immunol. 2017, 8, 403. [Google Scholar] [CrossRef]
- Punyawatthananukool, S.; Matsuura, R.; Wongchang, T.; Katsurada, N.; Tsuruyama, T.; Tajima, M.; Enomoto, Y.; Kitamura, T.; Kawashima, M.; Toi, M.; et al. Prostaglandin E2-EP2/EP4 Signaling Induces Immunosuppression in Human Cancer by Impairing Bioenergetics and Ribosome Biogenesis in Immune Cells. Nat. Commun. 2024, 15, 9464. [Google Scholar] [CrossRef]
- Zheng, Y.; Xu, R.; Chen, X.; Lu, Y.; Zheng, J.; Lin, Y.; Lin, P.; Zhao, X.; Cui, L. Metabolic Gatekeepers: Harnessing Tumor-Derived Metabolites to Optimize T Cell-Based Immunotherapy Efficacy in the Tumor Microenvironment. Cell Death Dis. 2024, 15, 775. [Google Scholar] [CrossRef]
- Chen, J.; Cui, L.; Lu, S.; Xu, S. Amino Acid Metabolism in Tumor Biology and Therapy. Cell Death Dis. 2024, 15, 42. [Google Scholar] [CrossRef]
- Suzuki, S.; Venkatesh, D.; Kanda, H.; Nakayama, A.; Hosokawa, H.; Lee, E.; Miki, T.; Stockwell, B.R.; Yokote, K.; Tanaka, T.; et al. GLS2 Is a Tumor Suppressor and a Regulator of Ferroptosis in Hepatocellular Carcinoma. Cancer Res. 2022, 82, 3209–3222. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Tang, R.; Zhou, K.; Chang, J.; Wang, H.; Zhang, Q.; Shi, J.; Sun, C. An Asparagine Metabolism-Based Classification Reveals the Metabolic and Immune Heterogeneity of Hepatocellular Carcinoma. BMC Med. Genom. 2022, 15, 222. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-C.; Yeh, H.; Shiu, R.-F.; Chin, W.-C.; Yen, T.-H. Impact of Microplastics and Nanoplastics on Liver Health: Current Understanding and Future Research Directions. World J. Gastroenterol. 2024, 30, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Mao, X.; Chen, Y.; Yan, L.; Ye, L.; Li, S. Reactive Oxygen Species Induce Fatty Liver and Ischemia-Reperfusion Injury by Promoting Inflammation and Cell Death. Front. Immunol. 2022, 13, 870239. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, Y.; Yang, L.; Wang, R. Insights into the Role of Oxidative Stress in Hepatocellular Carcinoma Development. Front. Biosci. 2023, 28, 286. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, Y.; Guo, A.; Xia, Z.; Peng, L. Progress in the Correlation Between Inflammasome NLRP3 and Liver Fibrosis. J. Clin. Transl. Hepatol. 2024, 12, 191–200. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, M.; Xia, S.; Zeng, F.; Liu, Q. Systematic and Comprehensive Insights into HIF-1 Stabilization under Normoxic Conditions: Implications for Cellular Adaptation and Therapeutic Strategies in Cancer. Cell. Mol. Biol. Lett. 2025, 30, 2. [Google Scholar] [CrossRef]
- Du, J.-Y.; Zhang, C.-T.; Li, T.; Li, Y.-P. Targeting Hypoxia and Angiogenesis in Hepatocellular Carcinoma: New Insights and Therapeutic Strategies. World J. Hepatol. 2024, 16, 1371–1376. [Google Scholar] [CrossRef]
- Pinkosky, S.L.; Newton, R.S.; Day, E.A.; Ford, R.J.; Lhotak, S.; Austin, R.C.; Birch, C.M.; Smith, B.K.; Filippov, S.; Groot, P.H.E.; et al. Liver-Specific ATP-Citrate Lyase Inhibition by Bempedoic Acid Decreases LDL-C and Attenuates Atherosclerosis. Nat. Commun. 2016, 7, 13457. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, C.M.; Bays, H.; Catapano, A.L.; Goldberg, A.; Ray, K.K.; Saseen, J.J. Role of Bempedoic Acid in Clinical Practice. Cardiovasc. Drugs Ther. 2021, 35, 853–864. [Google Scholar] [CrossRef]
- Granchi, C. ATP-Citrate Lyase (ACLY) Inhibitors as Therapeutic Agents: A Patenting Perspective. Expert. Opin. Ther. Pat. 2022, 32, 731–742. [Google Scholar] [CrossRef]
- Casak, S.J.; Pradhan, S.; Fashoyin-Aje, L.A.; Ren, Y.; Shen, Y.-L.; Xu, Y.; Chow, E.C.Y.; Xiong, Y.; Zirklelbach, J.F.; Liu, J.; et al. FDA Approval Summary: Ivosidenib for the Treatment of Patients with Advanced Unresectable or Metastatic, Chemotherapy Refractory Cholangiocarcinoma with an IDH1 Mutation. Clin. Cancer Res. 2022, 28, 2733–2737. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.T.; Borad, M.J.; Bridgewater, J.A.; et al. Final Overall Survival Efficacy Results of Ivosidenib for Patients With Advanced Cholangiocarcinoma With IDH1 Mutation. JAMA Oncol. 2021, 7, 1669–1677. [Google Scholar] [CrossRef]
- Ruff, S.M.; Dillhoff, M. Review of IDH Mutations and Potential Therapies for Intrahepatic Cholangiocarcinoma. Hepatoma Res. 2023, 9, 37. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Y.; Hu, Y.; Yang, J. Clinical Research Progress of Targeted Therapy Combined with Immunotherapy for Advanced Cholangiocarcinoma. Cancer Treat. Res. Commun. 2023, 37, 100771. [Google Scholar] [CrossRef] [PubMed]
- Ochando, J.; Mulder, W.J.M.; Madsen, J.C.; Netea, M.G.; Duivenvoorden, R. Trained Immunity—Basic Concepts and Contributions to Immunopathology. Nat. Rev. Nephrol. 2023, 19, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, F.; Setayesh, T.; Hu, Y.; Ravindran, R.; Wei, D.; Wan, Y.-J.Y. BCG as an Innovative Option for HCC Treatment: Repurposing and Mechanistic Insights. Adv. Sci. 2024, 11, e2308242. [Google Scholar] [CrossRef] [PubMed]
- Yarchoan, M.; Gane, E.J.; Marron, T.U.; Perales-Linares, R.; Yan, J.; Cooch, N.; Shu, D.H.; Fertig, E.J.; Kagohara, L.T.; Bartha, G.; et al. Personalized Neoantigen Vaccine and Pembrolizumab in Advanced Hepatocellular Carcinoma: A Phase 1/2 Trial. Nat. Med. 2024, 30, 1044–1053. [Google Scholar] [CrossRef]
- Hsieh, C.-H.; Chuang, P.-C.; Liu, Y.-W. Beyond Adaptive Immunity: Trained Innate Immune Responses as a Novel Frontier in Hepatocellular Carcinoma Therapy. Cancers 2025, 17, 1250. [Google Scholar] [CrossRef]
- Xing, R.; Gao, J.; Cui, Q.; Wang, Q. Strategies to Improve the Antitumor Effect of Immunotherapy for Hepatocellular Carcinoma. Front. Immunol. 2021, 12, 783236. [Google Scholar] [CrossRef]
| Feature | Traditional Immunity | Trained Immunity | References |
|---|---|---|---|
| Definition | Immediate, non-specific immune response to pathogens or damage | Long-lasting functional reprogramming of innate immune cells after initial stimulus | [4,24,26] |
| Memory Formation | No immunological memory | Epigenetic and metabolic memory-like features | [26,29] |
| Response Specificity | Non-specific, same response to repeated stimuli | Enhanced response upon re-exposure to similar or unrelated stimuli | [30] |
| Duration of Effect | Short-lived | Persistent (weeks to months) | [4,30,31] |
| Mechanism of Activation | Pattern recognition receptors (PRRs) detecting PAMPs/DAMPs | PRRs plus metabolic and epigenetic reprogramming | [4,27] |
| Role in MASLD | Initial inflammation, cytokine release, immune cell recruitment | Sustained inflammation, fibrosis, progression to MASH and HCC | [4,27] |
| Therapeutic Implications | Targeting acute inflammation | Modulating trained immunity to prevent chronic liver damage | [14,32] |
| Cell Type | Principal Implications in MASLD/MASH Pathogenesis | References |
|---|---|---|
| Macrophages | Promote hepatic inflammation (via cytokines, ROS) and fibrosis; recruitment of monocyte-derived macrophages (MDMs) exacerbates liver injury. YAP and miR-204-3p represent the main implicated pathways. | [37,40,41,42] |
| Neutrophils | Dual role: exacerbate inflammation via NE, MPO, and NETs; contribute to insulin resistance, steatosis, and fibrosis; miR-223 mediates protective effects. | [12,43,44,45,46,47,48,49,50,51] |
| Dendritic cells (DCs) | Shift toward proinflammatory phenotype with lipid overload; c-kit+ cDC1 cells exert protective effects; LKB1-AMPK/SIK pathway restrains Th17 cells. | [30,37,52,53,54] |
| Natural killer (NK) cells | Increase in NK activation during MASLD; phenotypic shift toward ILC1-like cells influences inflammation and disease progression. | [37,55,56,57,58,59,60] |
| Natural killer T (NKT) cells | Dual role: exacerbate inflammation and fibrosis in steatohepatitis but may protect against fibrosis in certain models. | [61,62,63,64,65] |
| CD4+ T cells | Polarization toward Th1/Th17 phenotypes drives MASLD progression; IFNγ and IL-17 production promote inflammation and fibrosis. | [69,70,71,72,73,74] |
| CD8+ T cells | Amplify liver inflammation via IFNγ and TNF; cytotoxic activity drives hepatocellular damage; aid in resolution during regression phases. | [62,75,76,77] |
| B cells | Promote inflammation through cytokines (IL-6, TNF); elevated anti-OSE IgG; regulatory B cell loss exacerbates disease. | [78,79,80] |
| Cell Type | Principal Involvement in HCC Pathogenesis | References |
|---|---|---|
| Macrophages (TAMs) | M2 TAMs promote tumor growth via cytokines (CSF-1, VEGF, CCL2) and STAT-3, NF-κB, and HIF-1; M1 macrophages may upregulate PD-L1; Kupffer cells (KCs) release IL-6, IL-1β, VEGF, and PDGF and support tumor progression via TLR signaling. | [85,92] |
| Neutrophils (TANs) | Promote angiogenesis via MMP-9; infiltrate via CXCL1/CXCL5; produce HGF (stimulated by GM-CSF), enhancing metastasis via HGF/c-Met axis; induce DNA damage via ROS; form NETs that support inflammation and metastasis. | [82,83,84,85,86,87,88] |
| Dendritic Cells (DCs) | Reduced mature CD83+ DCs linked to prognosis; IL-12 enhances and IL-10 inhibits DC function; pDCs and regulatory DCs promote immunosuppression via IL-10 and IDO. | [85] |
| Innate-like T Cells (ILCs) | ILC-derived IFNγ promotes hepatocarcinogenesis; NK cells show reduced function but correlate with good prognosis when infiltrating; NKT cells reduced in HCC tissue; TGF-β suppresses NK/NKT activity. | [82,85,89,90,91,92,93] |
| MAIT Cells | Reduced in tumor core; high intratumoral density linked to poor prognosis; may gain protumor functions during HCC development. | [82,94] |
| γδ T Cells | Involved in early surveillance; low numbers linked to recurrence; may promote tumor growth via IL-17. | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romeo, M.; Silvestrin, A.; Senese, G.; Di Nardo, F.; Napolitano, C.; Vaia, P.; Coppola, A.; Federico, P.; Dallio, M.; Federico, A. From “Traditional” to “Trained” Immunity: Exploring the Novel Frontiers of Immunopathogenesis in the Progression of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Biomedicines 2025, 13, 2004. https://doi.org/10.3390/biomedicines13082004
Romeo M, Silvestrin A, Senese G, Di Nardo F, Napolitano C, Vaia P, Coppola A, Federico P, Dallio M, Federico A. From “Traditional” to “Trained” Immunity: Exploring the Novel Frontiers of Immunopathogenesis in the Progression of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Biomedicines. 2025; 13(8):2004. https://doi.org/10.3390/biomedicines13082004
Chicago/Turabian StyleRomeo, Mario, Alessia Silvestrin, Giusy Senese, Fiammetta Di Nardo, Carmine Napolitano, Paolo Vaia, Annachiara Coppola, Pierluigi Federico, Marcello Dallio, and Alessandro Federico. 2025. "From “Traditional” to “Trained” Immunity: Exploring the Novel Frontiers of Immunopathogenesis in the Progression of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)" Biomedicines 13, no. 8: 2004. https://doi.org/10.3390/biomedicines13082004
APA StyleRomeo, M., Silvestrin, A., Senese, G., Di Nardo, F., Napolitano, C., Vaia, P., Coppola, A., Federico, P., Dallio, M., & Federico, A. (2025). From “Traditional” to “Trained” Immunity: Exploring the Novel Frontiers of Immunopathogenesis in the Progression of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Biomedicines, 13(8), 2004. https://doi.org/10.3390/biomedicines13082004









