Model for End-Stage Liver Disease Excluding INR Is Associated with Poor Prognosis in Elderly Patients with Decompensated Heart Failure
Abstract
1. Introduction
2. Material and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Butrous, H.; Hummel, S.L. Heart failure in older adults. Can. J. Cardiol. 2016, 32, 1140–1147. [Google Scholar] [CrossRef]
- Pulignano, G.; Scherillo, M.; Del Sindaco, D.; Giulivi, A.; Giovannini, E. Qualità delle cure e modelli di assistenza per i pazienti anziani con scompenso cardiaco. Ital. Heart J. 2004, 5 (Suppl. S10), 74S–86S. [Google Scholar] [PubMed]
- Dunlay, S.M.; Chamberlain, A.M. Multimorbidity in older patients with cardiovascular disease. Curr. Cardiovasc. Risk Rep. 2016, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Stolfo, D.; Sinagra, G.; Savarese, G. Evidence-based therapy in older patients with heart failure with reduced ejection fraction. Card. Fail. Rev. 2022, 8, e16. [Google Scholar] [CrossRef] [PubMed]
- Masoudi, F.A.; Havranek, E.P.; Wolfe, P.; Gross, C.P.; Rathore, S.S.; Steiner, J.F.; Ordin, D.L.; Krumholz, H.M. Most hospitalized older persons do not meet the enrollment criteria for clinical trials in heart failure. Am. Heart J. 2003, 146, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Barsheshet, A.; Shotan, A.; Cohen, E.; Garty, M.; Goldenberg, I.; Sandach, A.; Behar, S.; Zimlichman, E.; Lewis, B.S.; Gottlieb, S.; et al. Predictors of long-term (4-year) mortality in elderly and young patients with acute heart failure. Eur. J. Heart Fail. 2010, 12, 833–840. [Google Scholar] [CrossRef]
- Szlagor, M.; Dybiec, J.; Młynarska, E.; Rysz, J.; Franczyk, B. Chronic kidney disease as a comorbidity in heart failure. Int. J. Mol. Sci. 2023, 24, 2988. [Google Scholar] [CrossRef]
- Schefold, J.C.; Filippatos, G.; Hasenfuss, G.; Anker, S.D.; von Haehling, S. Heart failure and kidney dysfunction: Epidemiology, mechanisms and management. Nat. Rev. Nephrol. 2016, 12, 610–623. [Google Scholar] [CrossRef]
- Fried, L.F.; Shlipak, M.G.; Crump, C.; Bleyer, A.J.; Gottdiener, J.S.; Kronmal, R.A.; Kuller, L.H.; Newman, A.B. Renal insufficiency as a predictor of cardiovascular outcomes and mortality in elderly individuals. J. Am. Coll. Cardiol. 2003, 41, 1364–1372. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, M.; Parissis, J.; Yilmaz, M.B.; Seronde, M.F.; Kivikko, M.; Laribi, S.; Paugam-Burtz, C.; Cai, D.; Pohjanjousi, P.; Laterre, P.F.; et al. Liver function abnormalities, clinical profile, and outcome in acute decompensated heart failure. Eur. Heart J. 2013, 34, 742–749. [Google Scholar] [CrossRef]
- Okano, T.; Motoki, H.; Minamisawa, M.; Kimura, K.; Kanai, M.; Yoshie, K.; Higuchi, S.; Saigusa, T.; Ebisawa, S.; Okada, A.; et al. Cardio-renal and cardio-hepatic interactions predict cardiovascular events in elderly patients with heart failure. PLoS ONE 2020, 15, e0241003. [Google Scholar] [CrossRef] [PubMed]
- Heuman, D.M.; Mihas, A.A.; Habib, A.; Gilles, H.S.; Stravitz, R.T.; Sanyal, A.J.; Fisher, R.A. MELD-XI: A rational approach to “sickest first” liver transplantation in cirrhotic patients requiring anticoagulant therapy. Liver Transplant. 2007, 13, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.A.; Kato, T.S.; Shulman, B.P.; Takayama, H.; Farr, M.; Jorde, U.P.; Mancini, D.M.; Naka, Y.; Schulze, P.C. Liver dysfunction as a predictor of outcomes in patients with advanced heart failure requiring ventricular assist device support: Use of the MELD and MELD-XI scoring system. J. Heart Lung Transplant. 2012, 31, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Szczurek, W.; Szyguła-Jurkiewicz, B.; Zakliczyński, M.W.; Król, B.; Gąsior, M.; Zembala, M. Prognostic value of selected risk scales in patients with end-stage heart failure. Kardiol. Pol. 2018, 76, 1320–1326. [Google Scholar] [CrossRef]
- Lambert, D.S.; Picó, A.M.; Vincent, J.D.; Deych, E.; Coglianese, E.; Schilling, J.D.; Vader, J.M.; Yang, B.Q. Model for End Stage Liver Disease Excluding International Normalized Ratio Predicts Severe Right Ventricular Failure After HeartMate 3 Implantation in a Contemporary Cohort. J. Am. Heart Assoc. 2025, 14, e037553. [Google Scholar] [CrossRef]
- Curcio, F.; Amarelli, C.; Chiappetti, R.; Mattucci, I.; Flocco, V.; Rammal, M.I.; Abete, C.; Mazzella, F.; Maiello, C.; Abete, P.; et al. MELD score predicts outcomes in patients with advanced heart failure: A longitudinal evaluation. ESC Heart Fail. 2025, 12, 839–847. [Google Scholar] [CrossRef]
- Ho, K.K.; Pinsky, J.L.; Kannel, W.B.; Levy, D. The epidemiology of heart failure: The Framingham Study. J. Am. Coll. Cardiol. 1993, 22, 6A–13A. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, X.; Xiao, L.; Li, Y.; Qi, C.; Song, S.; Zhao, Y.; Zou, L. The MELD-XI score predicts 3-year mortality in patients with chronic heart failure. Front. Cardiovasc. Med. 2022, 9, 985503. [Google Scholar] [CrossRef]
- Kawahira, M.; Tamaki, S.; Yamada, T.; Watanabe, T.; Morita, T.; Furukawa, Y.; Kawasaki, M.; Kikuchi, A.; Kawai, T.; Seo, M.; et al. Prognostic value of impaired hepato-renal function and liver fibrosis in patients admitted for acute heart failure. ESC Heart Fail. 2021, 8, 1274–1283. [Google Scholar] [CrossRef]
- Mizobuchi, S.; Saito, Y.; Fujito, H.; Miyagawa, M.; Kitano, D.; Toyama, K.; Fukamachi, D.; Okumura, Y. Prognostic importance of improving hepatorenal function during hospitalization in acute decompensated heart failure. ESC Heart Fail. 2022, 9, 3113–3123. [Google Scholar] [CrossRef]
- Biegus, J.; Zymliński, R.; Sokolski, M.; Siwołowski, P.; Gajewski, P.; Nawrocka-Millward, S.; Poniewierka, E.; Jankowska, E.A.; Banasiak, W.; Ponikowski, P. Impaired hepato-renal function defined by the MELD-XI score as prognosticator in acute heart failure. Eur. J. Heart Fail. 2016, 18, 1518–1521. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Nakai, T.; Ikeya, Y.; Kogawa, R.; Otsuka, N.; Wakamatsu, Y.; Kurokawa, S.; Ohkubo, K.; Nagashima, K.; Okumura, Y. Prognostic value of the MELD-XI score in patients undergoing cardiac resynchronization therapy. ESC Heart Fail. 2022, 9, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Vasques-Nóvoa, F.; Ferreira, J.P.; Marques, P.; Neves, J.S.; Vale, C.; Ribeirinho-Soares, P.; Marques, J.; Martins, S.; Guimarães, J.T.; Barros, A.S.; et al. Interleukin-6, infection and cardiovascular outcomes in acute heart failure: Findings from the EDIFICA registry. Cytokine 2022, 160, 156053. [Google Scholar] [CrossRef] [PubMed]
- Palazzuoli, A.; Beltrami, M.; Nodari, S.; McCullough, P.A.; Ronco, C. Clinical impact of renal dysfunction in heart failure. Rev. Cardiovasc. Med. 2011, 12, 186–199. [Google Scholar] [CrossRef]
- Virzì, G.M.; Breglia, A.; Brocca, A.; de Cal, M.; Bolin, C.; Vescovo, G.; Ronco, C. Levels of proinflammatory cytokines, oxidative stress, and tissue damage markers in patients with acute heart failure with and without cardiorenal syndrome type 1. Cardiorenal Med. 2018, 8, 321–331. [Google Scholar] [CrossRef]
- Metra, M.; Voors, A.A. The puzzle of kidney dysfunction in heart failure: An introduction. Heart Fail. Rev. 2012, 17, 129–131. [Google Scholar] [CrossRef]
- Giallourakis, C.C.; Rosenberg, P.M.; Friedman, L.S. The liver in heart failure. Clin. Liver Dis. 2002, 6, 947–967. [Google Scholar] [CrossRef]
- Myers, R.P.; Cerini, R.; Sayegh, R.; Moreau, R.; Degott, C.; Lebrec, D.; Lee, S.S. Cardiac hepatopathy: Clinical, hemodynamic, and histologic characteristics and correlations. Hepatology 2003, 37, 393–400. [Google Scholar] [CrossRef]
- Durante-Mangoni, E.; Parrella, A.; Pafundi, P.C.; Vitrone, M.; Ragone, E.; De Rosa, I.; Amarelli, C.; Zampino, R. Liver histopathological findings in advanced heart failure: A reappraisal of cardiac cirrhosis concept. Intern. Emerg. Med. 2019, 14, 931–940. [Google Scholar] [CrossRef]
- Møller, S.; Bernardi, M. Interactions of the heart and the liver. Eur. Heart J. 2013, 34, 2804–2811. [Google Scholar] [CrossRef]
- Abe, S.; Yoshihisa, A.; Takiguchi, M.; Shimizu, T.; Nakamura, Y.; Yamauchi, H.; Iwaya, S.; Owada, T.; Miyata, M.; Sato, T.; et al. Liver dysfunction assessed by model for end-stage liver disease excluding INR (MELD-XI) scoring system predicts adverse prognosis in heart failure. PLoS ONE 2014, 9, e100618. [Google Scholar] [CrossRef] [PubMed]
- Aspromonte, N.; Fumarulo, I.; Petrucci, L.; Biferali, B.; Liguori, A.; Gasbarrini, A.; Massetti, M.; Miele, L. The liver in heart failure: From biomarkers to clinical risk. Int. J. Mol. Sci. 2023, 24, 15665. [Google Scholar] [CrossRef] [PubMed]
- Formiga, F.; Chivite, D.; Montero, A.; Petit, I.; Moreno-González, R.; Franco, J.; Corbella, X. Association between diabetes and mortality in elderly patients admitted for a first episode of acute heart failure. Geriatr. Gerontol. Int. 2018, 18, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.G.; Jang, S.Y.; Jang, J.; Cho, H.-J.; Lee, S.; Lee, S.E.; Kim, K.H.; Yoo, B.-S.; Kang, S.-M.; Baek, S.H.; et al. Impact of diabetes mellitus on mortality in patients with acute heart failure: A prospective cohort study. Cardiovasc. Diabetol. 2020, 19, 49. [Google Scholar] [CrossRef]
- Dei Cas, A.; Fonarow, G.C.; Gheorghiade, M.; Butler, J. Concomitant diabetes mellitus and heart failure. Curr. Probl. Cardiol. 2015, 40, 7–43. [Google Scholar] [CrossRef]
- Targher, G.; Dauriz, M.; Laroche, C.; Temporelli, P.L.; Hassanein, M.; Seferovic, P.M.; Drozdz, J.; Ferrari, R.; Anker, S.; Coats, A.; et al. Long-Term Registry investigators. In-hospital and 1-year mortality associated with diabetes in patients with acute heart failure: Results from the ESC-HFA Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2017, 19, 54–65. [Google Scholar] [CrossRef]
- Dauriz, M.; Mantovani, A.; Bonapace, S.; Verlato, G.; Zoppini, G.; Bonora, E.; Targher, G. Prognostic Impact of Diabetes on Long-term Survival Outcomes in Patients With Heart Failure: A Meta-analysis. Diabetes Care 2017, 40, 1597–1605. [Google Scholar] [CrossRef]
- MacDonald, M.R.; Petrie, M.C.; Varyani, F.; Östergren, J.; Michelson, E.L.; Young, J.B.; Solomon, S.D.; Granger, C.B.; Swedberg, K.; Yusuf, S.; et al. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: An analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur. Heart J. 2008, 29, 1377–1385. [Google Scholar] [CrossRef]
- Cunha, F.M.; Pereira, J.; Ribeiro, A.; Amorim, M.; Silva, S.; Araújo, J.P.; Leite-Moreira, A.; Bettencourt, P.; Lourenço, P. Age affects the prognostic impact of diabetes in chronic heart failure. Acta Diabetol. 2018, 55, 271–278. [Google Scholar] [CrossRef]
- Domanski, M.; Krause-Steinrauf, H.; Deedwania, P.; Follmann, D.; Ghali, J.K.; Gilbert, E.; Haffner, S.; Katz, R.; Lindenfeld, J.; Lowes, B.D.; et al. The effect of diabetes on outcomes of patients with advanced heart failure in the BEST trial. J. Am. Coll. Cardiol. 2003, 42, 914–922. [Google Scholar] [CrossRef]
- Kosiborod, M.; Inzucchi, S.E.; Spertus, J.A.; Wang, Y.; Masoudi, F.A.; Havranek, E.P.; Krumholz, H.M. Elevated admission glucose and mortality in elderly patients hospitalized with heart failure. Circulation 2009, 119, 1899–1907. [Google Scholar] [CrossRef]
- Romiti, G.F.; Nabrdalik, K.; Corica, B.; Bucci, T.; Proietti, M.; Qian, M.; Chen, Y.; Thompson, J.L.P.; Homma, S.; Lip, G.Y.H.; et al. Diabetes mellitus in patients with heart failure and reduced ejection fraction: A post hoc analysis from the WARCEF trial. Intern. Emerg. Med. 2024, 19, 931–939. [Google Scholar] [CrossRef]
- Dunlay, S.M.; Givertz, M.M.; Aguilar, D.; Allen, L.A.; Chan, M.; Desai, A.S.; Deswal, A.; Dickson, V.V.; Kosiborod, M.N.; Lekavich, C.L.; et al. Type 2 diabetes mellitus and heart failure a scientific statement from the American Heart Association and the Heart Failure Society of America. Circulation 2019, 140, E294–E324. [Google Scholar] [CrossRef]

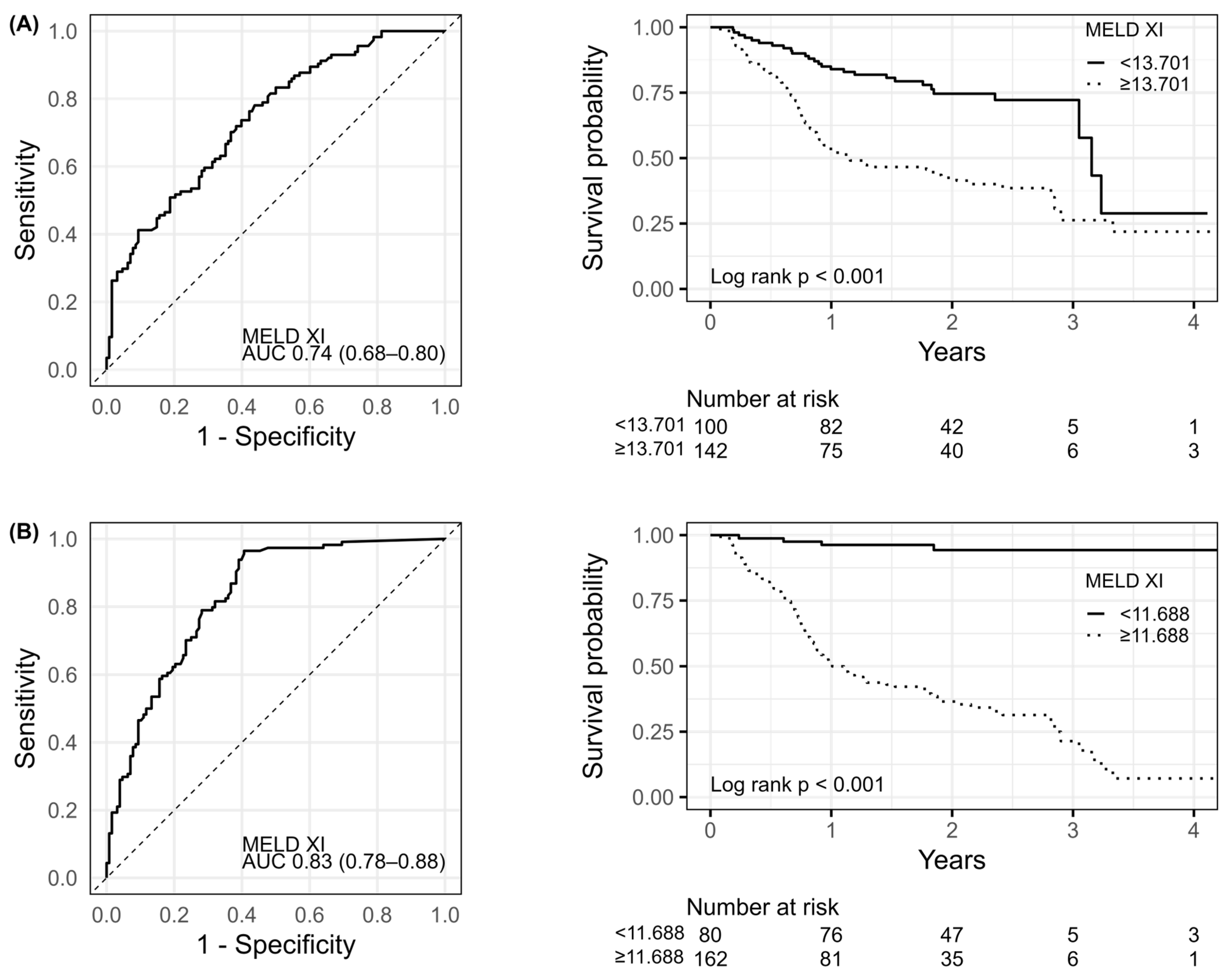
| Parameter | MEDL-XI < 11.69 n = 80 1 | MEDL-XI ≥ 11.69 n = 162 1 | p Value 2 |
|---|---|---|---|
| Socioclinical Characteristics | |||
| Age, years | 67.0 (65.0–74.6) | 68.5 (66.0–74.7) | 0.104 |
| Female, n (%) | 17 (21.3%) | 34 (21.0%) | 0.962 |
| Ischemic etiology of HF, n (%) | 48 (60.0%) | 92 (56.8%) | 0.634 |
| BMI, kg/m2 | 26.3 (22.8–29.9) | 26.4 (24.2–30.2) | 0.251 |
| Hypertension, n (%) | 30 (37.5%) | 105 (64.8%) | <0.001 |
| Type 2 diabetes, n (%) | 22 (27.5%) | 70 (43.2%) | 0.018 |
| Persistent AF, n (%) | 37 (46.3%) | 111 (68.5%) | <0.001 |
| COPD, n (%) | 4 (5.0%) | 13 (8.0%) | 0.386 |
| Analytical Parameters | |||
| Total bilirubin on admission, µmol/L | 14.7 (10.2–22.4) | 23.4 (17.3–31.2) | <0.001 |
| Total bilirubin on discharge, µmol/L | 11.4 (8.5–16.2) | 19.5 (14.7–25.5) | <0.001 |
| Creatinine on admission, µmol/L | 96.0 (80.8–115.5) | 132.0 (110.3–166.8) | <0.001 |
| Creatinine at discharge, µmol/L | 86.0 (76.8–97.3) | 131.0 (113.0–153.0) | <0.001 |
| MELD-XI on admission | 11.1 (9.4–13.8) | 16.1 (13.7–19.2) | <0.001 |
| MELD-XI at discharge | 9.5 (9.4–10.6) | 15.5 (13.4–17.7) | <0.001 |
| MELD-XI differences | 1.2 (1.0–3.4) | 0.3 (−1.8–2.9) | 0.001 |
| APTT, s | 36.6 (31.6–41.5) | 37.2 (32.5–44.4) | 0.188 |
| Uric acid, µmol/L | 418.0 (316.0–559.3) | 422.0 (340.0–527.0) | 0.642 |
| Cholesterol, mmol/L | 3.6 (2.9–4.5) | 3.5 (2.7–4.3) | 0.181 |
| LDL, mmol/L | 2.2 (1.6–2.7) | 1.9 (1.3–2.5) | 0.131 |
| Hemoglobin, mmol/L | 9.2 (8.2–11.3) | 9.9 (8.4–12.7) | 0.171 |
| WBC, ×109/L | 8.0 (6.2–9.0) | 7.0 (5.6–9.0) | 0.014 |
| Platelets | 203.0 (158.5–273.5) | 181.0 (142.0–218.0) | 0.002 |
| NT-proBNP, pg/mL | 4652.5 (1876.0–9421.0) | 7562.0 (3545.0–12,962.3) | 0.002 |
| Sodium, mmol/L | 138.0 (135.0–140.0) | 136.0 (133.0–139.0) | 0.017 |
| LVEDd, mm | 66.0 (61.0–73.0) | 69.0 (65.0–76.0) | 0.003 |
| LA, mm | 49.0 (46.0–54.0) | 52.0 (48.0–57.0) | 0.005 |
| LVEF, % | 20.0 (14.0–26.0) | 18.5 (15.0–21.0) | 0.129 |
| Therapy | |||
| ICD/CRT-D, n (%) | 80 (100%) | 162 (100%) | 1.0 |
| B-blockers, n (%) | 74 (92.5%) | 151 (93.2%) | 0.839 |
| Ivabradine, n (%) | 16 (20%) | 22 (13.6%) | 0.194 |
| MRA, n (%) | 65 (81.3%) | 134 (82.7%) | 0.779 |
| ACEI/ARB/ARNI, n (%) | 63 (78.8%) | 126 (77.8%) | 0.863 |
| Dapagliflosin/Empagliflosin, n (%) | 56 (70.0%) | 106 (65.4%) | 0.477 |
| Loop diuretics, n (%) | 73 (91.3%) | 157 (96.9%) | 0.066 |
| Inotropic at admission, n (%) | 20 (25.0%) | 50 (30.9%) | 0.344 |
| VKA, n (%) | 20 (25.0%) | 54 (33.3%) | 0.186 |
| Digoxin, n (%) | 11 (13.8%) | 55 (34.0%) | <0.001 |
| Statin, n (%) | 40 (50.0%) | 89 (54.9%) | 0.469 |
| Acetylsalicylic acid, n (%) | 23 (28.8%) | 40 (24.7%) | 0.498 |
| NOAC, n (%) | 31 (38.8%) | 51 (31.5%) | 0.261 |
| Parameter | On Admission | At Discharge | p |
|---|---|---|---|
| Bilirubin | 21.1 (14.6–29.8) | 16.8 (11.9–23.0) | <0.001 |
| Creatinine | 121.5 (95.0–149.0) | 113.0 (94.3–143.8) | 0.011 |
| MELD-XI | 14.6 (11.7–17.9) | 13.4 (10.7–16.5) | <0.001 |
| NT-proBNP | 6352.5 (2887.3–11,403.3) | 4538.0 (2209.0–8991.0) | <0.001 |
| Sodium | 137.0 (134.0–140.0) | 136.0 (134.0–138.0) | 0.132 |
| Hemoglobin | 9.6 (8.33–12.5) | 10.2 (9.7–10.8) | 0.775 |
| AUC [±95 CI] | Cut-Off | Sens. [±95 CI] | Spec. [±95 CI] | Accuracy | |
|---|---|---|---|---|---|
| MELD-XI on admission | 0.742 (0.681–0.803) | 13.70 | 0.781 (0.694–0.853) | 0.562 (0.472–0.65) | 0.665 |
| MELD-XI at discharge | 0.827 (0.776–0.878) | 11.69 | 0.965 (0.913–0.99) | 0.594 (0.503–0.68) | 0.769 |
| Parameter | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Arterial hypertension | 1.975 [1.328–2.937] | <0.001 | ||
| Diabetes mellitus | 1.517 [1.046–2.200] | 0.028 | 1.656 [1.113–2.463] | 0.013 |
| Bilirubin on admission | 1.035 [1.023–1.047] | <0.001 | ||
| Bilirubin at discharge | 1.029 [1.021–1.038] | <0.001 | ||
| Creatinine on admission | 1.005 [1.003–1.007] | <0.001 | ||
| Creatinine at discharge | 1.014 [1.010–1.018] | <0.001 | ||
| MELD-XI on admission | 1.114 [1.077–1.152] | <0.001 | ||
| MELD-XI at discharge | 1.251 [1.193–1.312] | <0.001 | 1.267 [1.210–1.327] | <0.001 |
| Platelets ↓ * | 1.003 [1.000–1.006] | 0.030 | ||
| Sodium ↓ | 1.062 [1.021–1.103] | 0.002 | ||
| logNT-proBNP on admission | 1.324 [1.075–1.629] | <0.008 | ||
| Left atrium | 1.029 [1.005–1.053] | 0.016 | ||
| VKA | 1.468 [1.002–2.150] | 0.049 | ||
| The lack of NOAC use | 2.033 [1.302–3.165] | 0.002 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurkiewicz, M.; Szczurek-Wasilewicz, W.; Skrzypek, M.; Jóźwiak, J.J.; Gąsior, M.; Szyguła-Jurkiewicz, B. Model for End-Stage Liver Disease Excluding INR Is Associated with Poor Prognosis in Elderly Patients with Decompensated Heart Failure. Biomedicines 2025, 13, 2000. https://doi.org/10.3390/biomedicines13082000
Jurkiewicz M, Szczurek-Wasilewicz W, Skrzypek M, Jóźwiak JJ, Gąsior M, Szyguła-Jurkiewicz B. Model for End-Stage Liver Disease Excluding INR Is Associated with Poor Prognosis in Elderly Patients with Decompensated Heart Failure. Biomedicines. 2025; 13(8):2000. https://doi.org/10.3390/biomedicines13082000
Chicago/Turabian StyleJurkiewicz, Michał, Wioletta Szczurek-Wasilewicz, Michał Skrzypek, Jacek J. Jóźwiak, Mariusz Gąsior, and Bożena Szyguła-Jurkiewicz. 2025. "Model for End-Stage Liver Disease Excluding INR Is Associated with Poor Prognosis in Elderly Patients with Decompensated Heart Failure" Biomedicines 13, no. 8: 2000. https://doi.org/10.3390/biomedicines13082000
APA StyleJurkiewicz, M., Szczurek-Wasilewicz, W., Skrzypek, M., Jóźwiak, J. J., Gąsior, M., & Szyguła-Jurkiewicz, B. (2025). Model for End-Stage Liver Disease Excluding INR Is Associated with Poor Prognosis in Elderly Patients with Decompensated Heart Failure. Biomedicines, 13(8), 2000. https://doi.org/10.3390/biomedicines13082000






