Salusins in Atherosclerosis: Dual Roles in Vascular Inflammation and Remodeling
Abstract
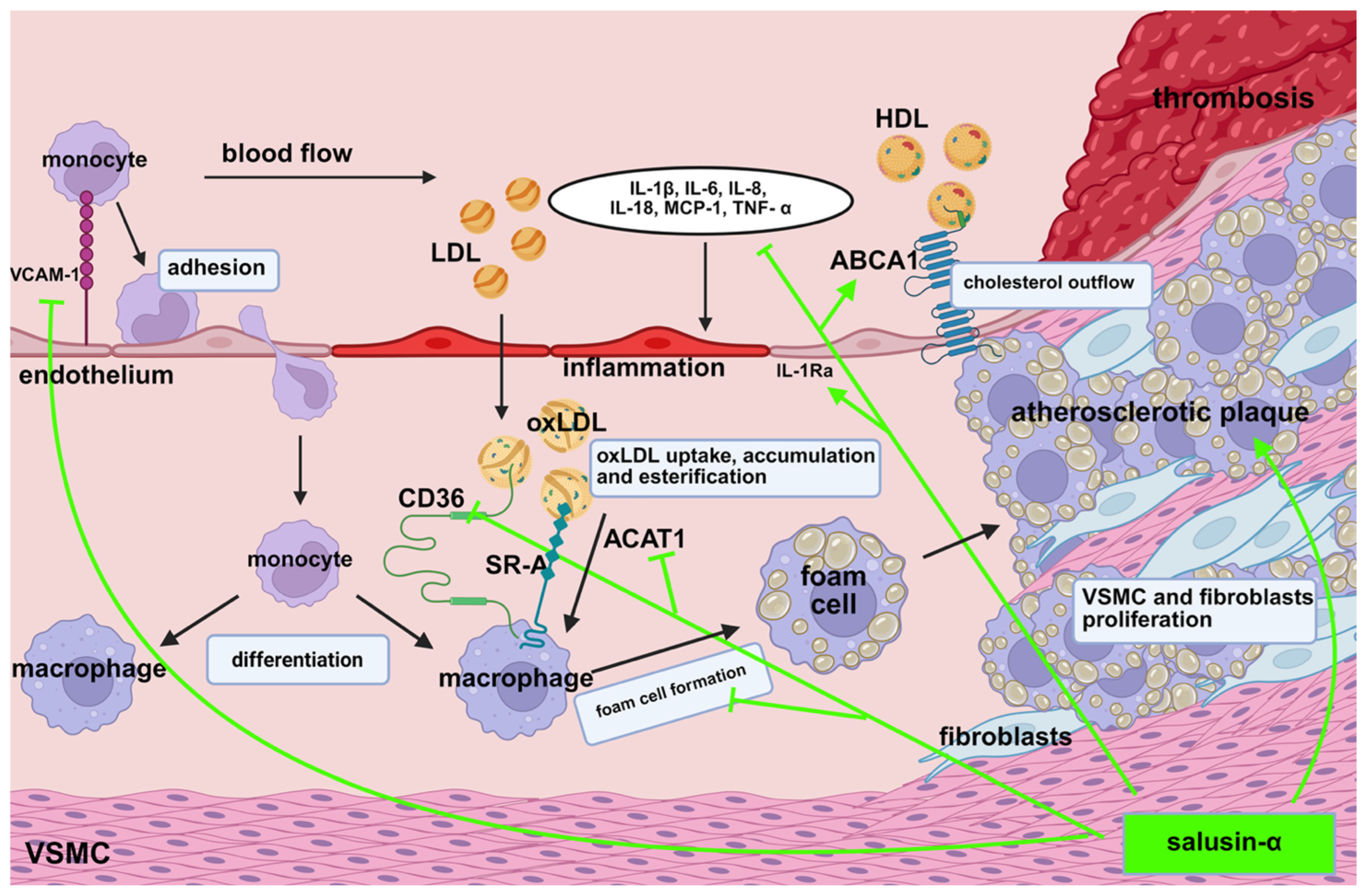
1. Introduction
2. Biosynthesis and Tissue Expression of Salusins
3. Influence of Salusins on Inflammatory Processes in Atherosclerotic Plaque
4. The Role of Salusins in Foam Cell Formation
5. Salusins and the Proliferation of Vascular Smooth Muscle Cells and Fibroblasts
6. Influence of Salusins on Lipid Infiltration in Atherosclerotic Plaque
7. Plasma Salusin Levels and the Risk of Atherosclerotic Lesions
8. Knowledge Gaps
9. Opportunities and Therapeutic Implications of Salusins
10. Summary
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABCA1 | ATP-binding cassette transporter A1 |
| ACAT1 | Acyl-CoA, cholesterol acyltransferase 1 |
| cAMP | 3′,5′-Cyclic adenosine monophosphate |
| CD36 | Cluster of differentiation 36 |
| HDL | High-density lipoprotein |
| ICAM-1 | High-density lipoprotein |
| IL-1β | Interleukin-1β |
| IL-1Ra | Interleukin-1 receptor antagonist |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| IL-18 | Interleukin-18 |
| LDL | Low-density lipoprotein |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MUAMC | Mid-upper arm muscle circumference |
| SR-A | Class A scavenger receptor |
| TNF-α | Tumor necrosis factor α |
| TSF | Triceps skinfold thickness |
| VCAM-1 | Vascular cell adhesion molecule-1 |
| VSMCs | Vascular smooth muscle cells |
References
- Shichiri, M.; Ishimaru, S.; Ota, T.; Nishikawa, T.; Isogai, T.; Hirata, Y. Salusins: Newly Identified Bioactive Peptides with Hemodynamic and Mitogenic Activities. Nat. Med. 2003, 9, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, C.; Shichiri, M.; Sato, K.; Hirata, Y. Expression of Prosalusin in Human Neuroblastoma Cells. Peptides 2009, 30, 1362–1367. [Google Scholar] [CrossRef]
- Sato, K.; Watanabe, R.; Itoh, F.; Shichiri, M.; Watanabe, T. Salusins: Potential Use as a Biomarker for Atherosclerotic Cardiovascular Diseases. Int. J. Hypertens. 2013, 2013, 965140. [Google Scholar] [CrossRef] [PubMed]
- Shichiri, M. Reply to “Salusins: Newly Identified Bioactive Peptides with Hemodynamic and Mitogenic Activities”. Nat. Med. 2007, 13, 661–662. [Google Scholar] [CrossRef]
- Sato, K.; Sato, T.; Susumu, T.; Koyama, T.; Shichiri, M. Presence of Immunoreactive Salusin-β in Human Plasma and Urine. Regul. Pept. 2009, 158, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Citil, C.; Konar, V.; Aydin, S.; Yilmaz, M.; Albayrak, S.; Ozercan, I.H.; Ozkan, Y. Brain, Liver, and Serum Salusin-Alpha and -Beta Alterations in Sprague-Dawley Rats with or Without Metabolic Syndrome. Med. Sci. Monit. 2014, 20, 1326–1333. [Google Scholar] [CrossRef]
- Suzuki, N.; Shichiri, M.; Tateno, T.; Sato, K.; Hirata, Y. Distinct Systemic Distribution of Salusin-α and Salusin-β in the Rat. Peptides 2011, 32, 805–810. [Google Scholar] [CrossRef]
- Sato, K.; Koyama, T.; Tateno, T.; Hirata, Y.; Shichiri, M. Presence of Immunoreactive Salusin-α in Human Serum and Urine. Peptides 2006, 27, 2561–2566. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, W.-M.; Zhang, X.-X.; Zhang, H.-X.; Wang, H.-C.; Zheng, F.-Y.; Zhu, F.-F. Overexpression of Salusin-β Is Associated with Poor Prognosis in Ovarian Cancer. Oncol. Rep. 2017, 37, 1826–1832. [Google Scholar] [CrossRef]
- Argun, D.; Argun, F.; Borku Uysal, B. Evaluation of Salusin-α and Salusin-β Levels in Patients with Type 2 Diabetes Mellitus and Determination of the Impact of Severity of Hyperglycemia on Salusin Levels. Ir. J. Med. Sci. 1971- 2021, 190, 1403–1411. [Google Scholar] [CrossRef]
- Sato, K.; Fujimoto, K.; Koyama, T.; Shichiri, M. Release of Salusin-β from Human Monocytes/Macrophages. Regul. Pept. 2010, 162, 68–72. [Google Scholar] [CrossRef]
- Depuydt, M.A.C.; Schaftenaar, F.H.; Prange, K.H.M.; Boltjes, A.; Hemme, E.; Delfos, L.; De Mol, J.; De Jong, M.J.M.; Bernabé Kleijn, M.N.A.; Peeters, J.A.H.M.; et al. Single-Cell T Cell Receptor Sequencing of Paired Human Atherosclerotic Plaques and Blood Reveals Autoimmune-like Features of Expanded Effector T Cells. Nat. Cardiovasc. Res. 2023, 2, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Markin, A.M.; Markina, Y.V.; Bogatyreva, A.I.; Tolstik, T.V.; Chakal, D.A.; Breshenkov, D.G.; Charchyan, E.R. The Role of Cytokines in Cholesterol Accumulation in Cells and Atherosclerosis Progression. Int. J. Mol. Sci. 2023, 24, 6426. [Google Scholar] [CrossRef] [PubMed]
- De Martin, R.; Hoeth, M.; Hofer-Warbinek, R.; Schmid, J.A. The Transcription Factor NF-Kappa B and the Regulation of Vascular Cell Function. Arterioscler. Thromb. Vasc. Biol. 2000, 20, E83–E88. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.-H.; Liu, L.; Liu, L.; Zhang, M.-X.; Guo, H.; Pan, J.; Yin, X.-X.; Ma, T.-F.; Wu, Y.-Q. Salusin-β Not Salusin-α Promotes Vascular Inflammation in ApoE-Deficient Mice via the I-κBα/NF-κB Pathway. PLoS ONE 2014, 9, e91468. [Google Scholar] [CrossRef]
- Esfahani, M.; Saidijam, M.; Najafi, R.; Goodarzi, M.T.; Movahedian, A. The Effect of Salusin-β on Expression of pro- and Anti-Inflammatory Cytokines in Human Umbilical Vein Endothelial Cells (HUVECs). ARYA Atheroscler. 2018, 14, 1–10. [Google Scholar] [CrossRef]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef]
- Koya, T.; Miyazaki, T.; Watanabe, T.; Shichiri, M.; Atsumi, T.; Kim-Kaneyama, J.; Miyazaki, A. Salusin-β Accelerates Inflammatory Responses in Vascular Endothelial Cells via NF-κB Signaling in LDL Receptor-Deficient Mice in Vivo and HUVECs in Vitro. Am. J. Physiol.-Heart Circ. Physiol. 2012, 303, H96–H105. [Google Scholar] [CrossRef]
- Zhao, M.-X.; Zhou, B.; Ling, L.; Xiong, X.-Q.; Zhang, F.; Chen, Q.; Li, Y.-H.; Kang, Y.-M.; Zhu, G.-Q. Salusin-β Contributes to Oxidative Stress and Inflammation in Diabetic Cardiomyopathy. Cell Death Dis. 2017, 8, e2690. [Google Scholar] [CrossRef]
- Harrington, J.R. The Role of MCP-1 in Atherosclerosis. Stem Cells 2000, 18, 65–66. [Google Scholar] [CrossRef]
- Esfahani, M.; Saidijam, M.; Goodarzi, M.T.; Movahedian, A.; Najafi, R. Salusin-α Attenuates Inflammatory Responses in Vascular Endothelial Cells. Biochem. Mosc. 2017, 82, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.-H.; Pan, J.; Huang, H.; Zhu, Y.; Zhang, M.; Liu, L.; Wu, Y. Salusin-β, but Not Salusin-α, Promotes Human Umbilical Vein Endothelial Cell Inflammation via the P38 MAPK/JNK-NF-κB Pathway. PLoS ONE 2014, 9, e107555. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, Y.; Ye, Y.; Jia, X.; Wang, P.; Xiong, Z.; Zhu, H. The Protective Effects of Salusin-α against Oxidative Stress and Inflammatory Response in Mice with Gestational Diabetes Mellitus (GDM). Arch. Physiol. Biochem. 2025, 131, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Nishio, K.; Kanome, T.; Matsuyama, T.; Koba, S.; Sakai, T.; Sato, K.; Hongo, S.; Nose, K.; Ota, H.; et al. Impact of Salusin-α and -β on Human Macrophage Foam Cell Formation and Coronary Atherosclerosis. Circulation 2008, 117, 638–648. [Google Scholar] [CrossRef]
- Saki, N.; Haybar, H.; Maniati, M.; Davari, N.; Javan, M.; Moghimian-Boroujeni, B. Modification Macrophage to Foam Cells in Atherosclerosis Disease: Some Factors Stimulate or Inhibit This Process. J. Diabetes Metab. Disord. 2024, 23, 1687–1697. [Google Scholar] [CrossRef]
- Qian, K.; Feng, L.; Sun, Y.; Xiong, B.; Ding, Y.; Han, P.; Chen, H.; Chen, X.; Du, L.; Wang, Y. Overexpression of Salusin- α Inhibits Vascular Intimal Hyperplasia in an Atherosclerotic Rabbit Model. BioMed Res. Int. 2018, 2018, 8973986. [Google Scholar] [CrossRef]
- Sun, H.-J.; Liu, T.-Y.; Zhang, F.; Xiong, X.-Q.; Wang, J.-J.; Chen, Q.; Li, Y.-H.; Kang, Y.-M.; Zhou, Y.-B.; Han, Y.; et al. Salusin-β Contributes to Vascular Remodeling Associated with Hypertension via Promoting Vascular Smooth Muscle Cell Proliferation and Vascular Fibrosis. Biochim. Biophys. Acta 2015, 1852, 1709–1718. [Google Scholar] [CrossRef]
- Sun, H.-J.; Zhao, M.-X.; Liu, T.-Y.; Ren, X.-S.; Chen, Q.; Li, Y.-H.; Kang, Y.-M.; Zhu, G.-Q. Salusin-β Induces Foam Cell Formation and Monocyte Adhesion in Human Vascular Smooth Muscle Cells via miR155/NOX2/NFκB Pathway. Sci. Rep. 2016, 6, 23596. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Melnichenko, A.A.; Myasoedova, V.A.; Grechko, A.V.; Orekhov, A.N. Mechanisms of Foam Cell Formation in Atherosclerosis. J. Mol. Med. 2017, 95, 1153–1165. [Google Scholar] [CrossRef]
- Nagashima, M.; Watanabe, T.; Shiraishi, Y.; Morita, R.; Terasaki, M.; Arita, S.; Hongo, S.; Sato, K.; Shichiri, M.; Miyazaki, A.; et al. Chronic Infusion of Salusin-α and -β Exerts Opposite Effects on Atherosclerotic Lesion Development in Apolipoprotein E-Deficient Mice. Atherosclerosis 2010, 212, 70–77. [Google Scholar] [CrossRef]
- Gui, Y.; Zheng, H.; Cao, R.Y. Foam Cells in Atherosclerosis: Novel Insights Into Its Origins, Consequences, and Molecular Mechanisms. Front. Cardiovasc. Med. 2022, 9, 845942. [Google Scholar] [CrossRef] [PubMed]
- Grzegorzewska, A.E.; Niepolski, L.; Sikora, J.; Janków, M.; Jagodziński, P.P.; Sowińska, A. Effect of Lifestyle Changes and Atorvastatin Administration on Dyslipidemia in Hemodialysis Patients: A Prospective Study. Pol. Arch. Med. Wewn. 2014, 124, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Xu, L.; Zhang, Y.; Yu, Q.; Li, J.; Guan, H.; Wang, X.; Cheng, D.; Liu, Y.; Bai, L.; et al. Salusin-α Inhibits Proliferation and Migration of Vascular Smooth Muscle Cell via Akt/mTOR Signaling. Cell. Physiol. Biochem. 2018, 50, 1740–1753. [Google Scholar] [CrossRef] [PubMed]
- Beręsewicz, A.; Skierczyńska, A. Miażdżyca—Choroba Całego Życia i Całej Populacji Krajów Cywilizacji Zachodniej. Chor. Serca Naczyń 2006, 3, 1–6. [Google Scholar]
- Watanabe, T.; Suguro, T.; Sato, K.; Koyama, T.; Nagashima, M.; Kodate, S.; Hirano, T.; Adachi, M.; Shichiri, M.; Miyazaki, A. Serum Salusin-Alpha Levels Are Decreased and Correlated Negatively with Carotid Atherosclerosis in Essential Hypertensive Patients. Hypertens. Res. 2008, 31, 463–468. [Google Scholar] [CrossRef]
- Niepolski, L.; Grzegorzewska, A.E. Salusins and Adropin: New Peptides Potentially Involved in Lipid Metabolism and Atherosclerosis. Adv. Med. Sci. 2016, 61, 282–287. [Google Scholar] [CrossRef]
- Liu, J.; Ren, Y.; Zhang, L.; Tong, Y.; Kang, L. Serum Salusin-β Levels Are Associated with the Presence and Severity of Coronary Artery Disease. J. Investig. Med. 2015, 63, 632–635. [Google Scholar] [CrossRef]
- Du, S.-L.; Wang, W.-J.; Wan, J.; Wang, Y.-G.; Wang, Z.-K.; Zhang, Z. Serum Salusin-α Levels Are Inversely Correlated with the Presence and Severity of Coronary Artery Disease. Scand. J. Clin. Lab. Investig. 2013, 73, 339–343. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Zhang, J.; Zhang, M.; Zhang, H.; Gong, G.; Luo, M.; Wang, T.; Mao, X. Salusin-β Is Superior to Salusin- α as a Marker for Evaluating Coronary Atherosclerosis. J. Int. Med. Res. 2020, 48, 030006052090386. [Google Scholar] [CrossRef]
- Niepolski, L.; Hełka, S.; Jęśko-Białek, S. Związek Osoczowego Stężenia Salusyny Alfa z Wybranymi Parametrami Antropometrycznymi. In Proceedings of the VII Ogólnopolska Konferencja Studenckich Kół Naukowych Badania, Innowacje i Pasje z Perspektywy Młodego Naukowca, Krosno, Poland, 28 May 2024. [Google Scholar]
- Janecka, A.; Stefanowicz, J. Use of Salusin β for Predicting Atherosclerosis and Components of the Metabolic Syndrome. Adv. Clin. Exp. Med. Off. Organ Wroclaw Med. Univ. 2024, 33, 183–192. [Google Scholar] [CrossRef]
- Li, H.-B.; Yu, X.-J.; Bai, J.; Su, Q.; Wang, M.-L.; Huo, C.-J.; Xia, W.-J.; Yi, Q.-Y.; Liu, K.-L.; Fu, L.-Y.; et al. Silencing Salusin β Ameliorates Heart Failure in Aged Spontaneously Hypertensive Rats by ROS-Relative MAPK/NF-κB Pathways in the Paraventricular Nucleus. Int. J. Cardiol. 2019, 280, 142–151. [Google Scholar] [CrossRef]
- Askin, L.; Abus, S.; Tanriverdi, O. Resistin and Cardiovascular Disease: A Review of the Current Literature Regarding Clinical and Pathological Relationships. Curr. Cardiol. Rev. 2022, 18, e290721195114. [Google Scholar] [CrossRef]


| Action | Salusin-α | Citation | Material | Salusin-β | Citation | Material |
|---|---|---|---|---|---|---|
| Development of inflammatory processes | ↓ | [21] | HUVEC | ↑ | [16] | HUVEC |
| Activation of NF-ƙB pathway | → | [21] | HUVEC | ↑ | [15] | ApoE-/- mice |
| VCAM-1 expression | →/↓ | [15,22] | ApoE-/- mice, HUVEC | ↑ | [16,18] | HUVEC |
| mRNA ICAM-1 expression | → | - | - | ↑ | [16,18] | HUVEC |
| IL-1β expression | → | - | - | ↑ | [3,18] | HUVEC |
| IL-6 expression | ↓/→ | [21,22] | HUVEC | ↑ | [16] | HUVEC |
| IL-8 expression | ↓ | [21] | HUVEC | ↑ | [16] | HUVEC |
| IL-18 expression | ↓ | [21] | HUVEC | ↑ | [16] | HUVEC |
| IL–1Ra expression | ↑ | [21] | HUVEC | ↓ | [16] | HUVEC |
| MCP-1 expression | → | [15] | ApoE-/- mice | ↑ | [3,15,18] | HUVEC; ApoE-/- mice |
| TNF-α expression | ↓/→ | [15,22] | ApoE-/- mice; HUVEC | ↑ | [19] | H9c2 or neonatal rat cardiomyocytes |
| Foam cel formation | ↓ | [24,25] | Human macrophages from monocytes | ↑ | [24,26,27] | Human macrophages from monocytes; atherosclerotic rabbit model; Human VSMCs |
| ACAT1 expression | ↓ | [24,25] | Human macrophages from monocytes | ↑ | [28,29] | Human VSMCs |
| CD36 expression | ↓ | [26,30] | Atherosclerotic rabbit model; ApoE-/- mice | ↑ | [30] | ApoE-/- mice |
| SR-A expression | → | [24,25] | Human macrophages from monocytes | ↑/→ | [3,24] | HUVEC; human macrophages from monocytes |
| ABCA1 expression | ↑/→ | [24,25,26] | Human macrophages from monocytes; Atherosclerotic rabbit model | → | [3,24] | HUVEC; human macrophages from monocytes |
| VSMC proliferation | ↑/↓ | [1] | Rat and human VSMCs | ↑ | [1] | Rat and human VSMCs |
| Fibroblast proliferation | ↑/↓ | [1] | Rat VSMCs | ↑ | [1] | Rat VSMCs |
| Presence in atherosclerotic plaque | ↑ | [24] | Human macrophages from monocytes | ↑ | [24] | Human macrophages from monocytes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niepolski, L.; Jęśko-Białek, S.; Niepolska, J.; Pendzińska, A. Salusins in Atherosclerosis: Dual Roles in Vascular Inflammation and Remodeling. Biomedicines 2025, 13, 1990. https://doi.org/10.3390/biomedicines13081990
Niepolski L, Jęśko-Białek S, Niepolska J, Pendzińska A. Salusins in Atherosclerosis: Dual Roles in Vascular Inflammation and Remodeling. Biomedicines. 2025; 13(8):1990. https://doi.org/10.3390/biomedicines13081990
Chicago/Turabian StyleNiepolski, Leszek, Szymon Jęśko-Białek, Joanna Niepolska, and Agata Pendzińska. 2025. "Salusins in Atherosclerosis: Dual Roles in Vascular Inflammation and Remodeling" Biomedicines 13, no. 8: 1990. https://doi.org/10.3390/biomedicines13081990
APA StyleNiepolski, L., Jęśko-Białek, S., Niepolska, J., & Pendzińska, A. (2025). Salusins in Atherosclerosis: Dual Roles in Vascular Inflammation and Remodeling. Biomedicines, 13(8), 1990. https://doi.org/10.3390/biomedicines13081990








