Clustering Cortical Rhythms: Monoaminergic Signatures in Time-Frequency EEG Dynamics
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Electrodes and Cannula Implantation
2.3. EEG Recording and Drug Treatment
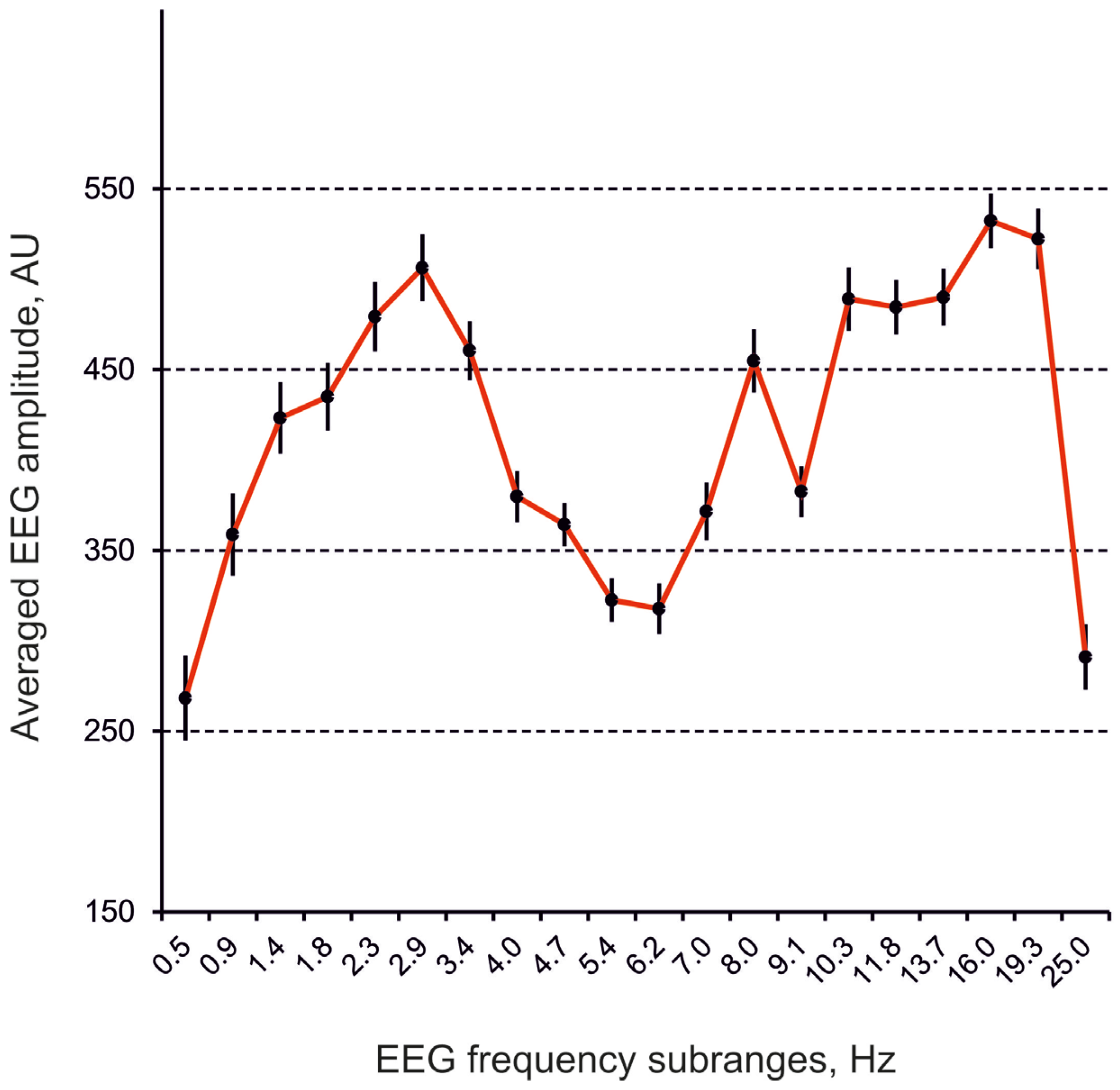
2.4. Computation of EEG Spectra
2.5. Statistics
3. Results
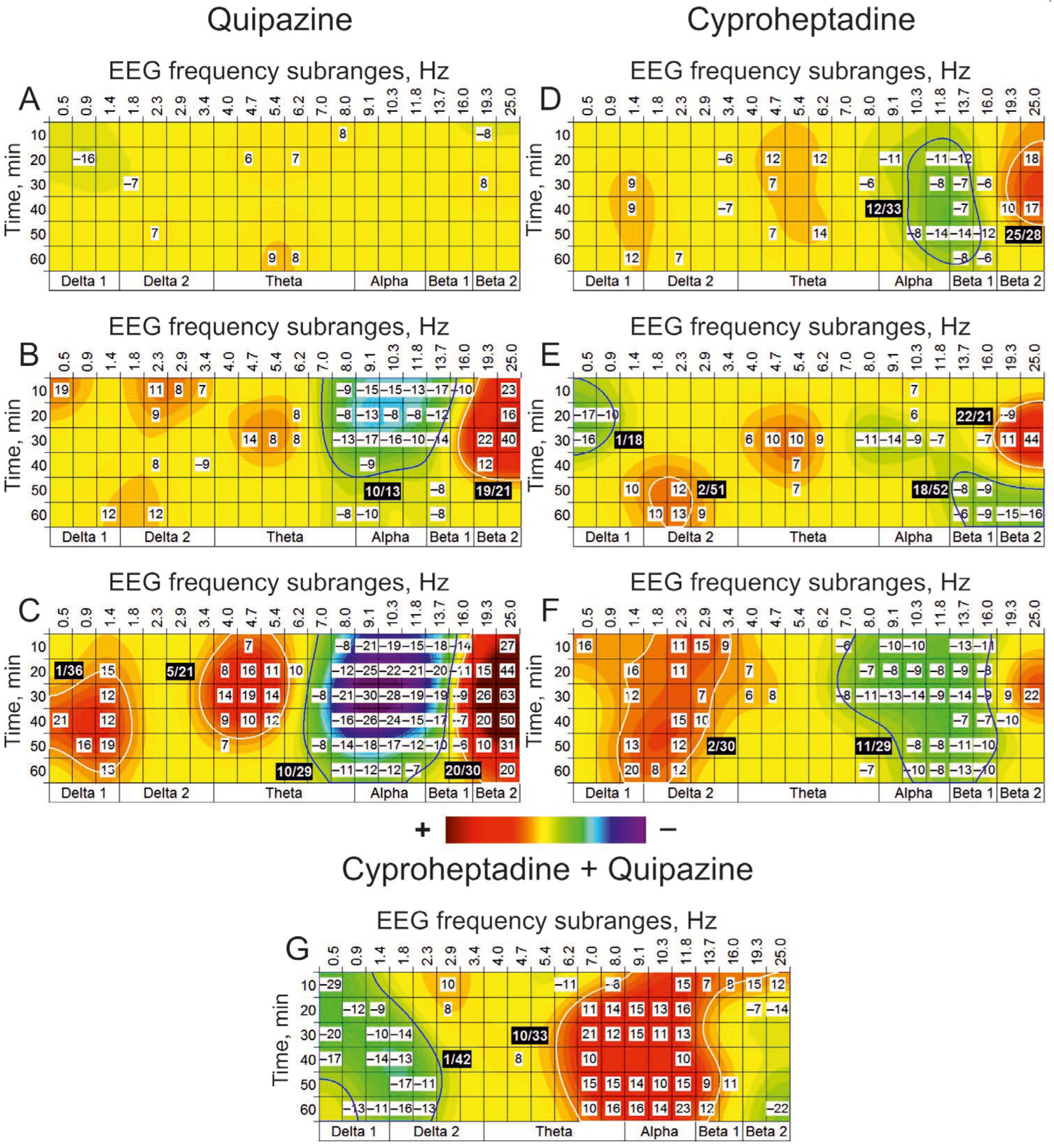
3.1. Serotoninergic Transmission
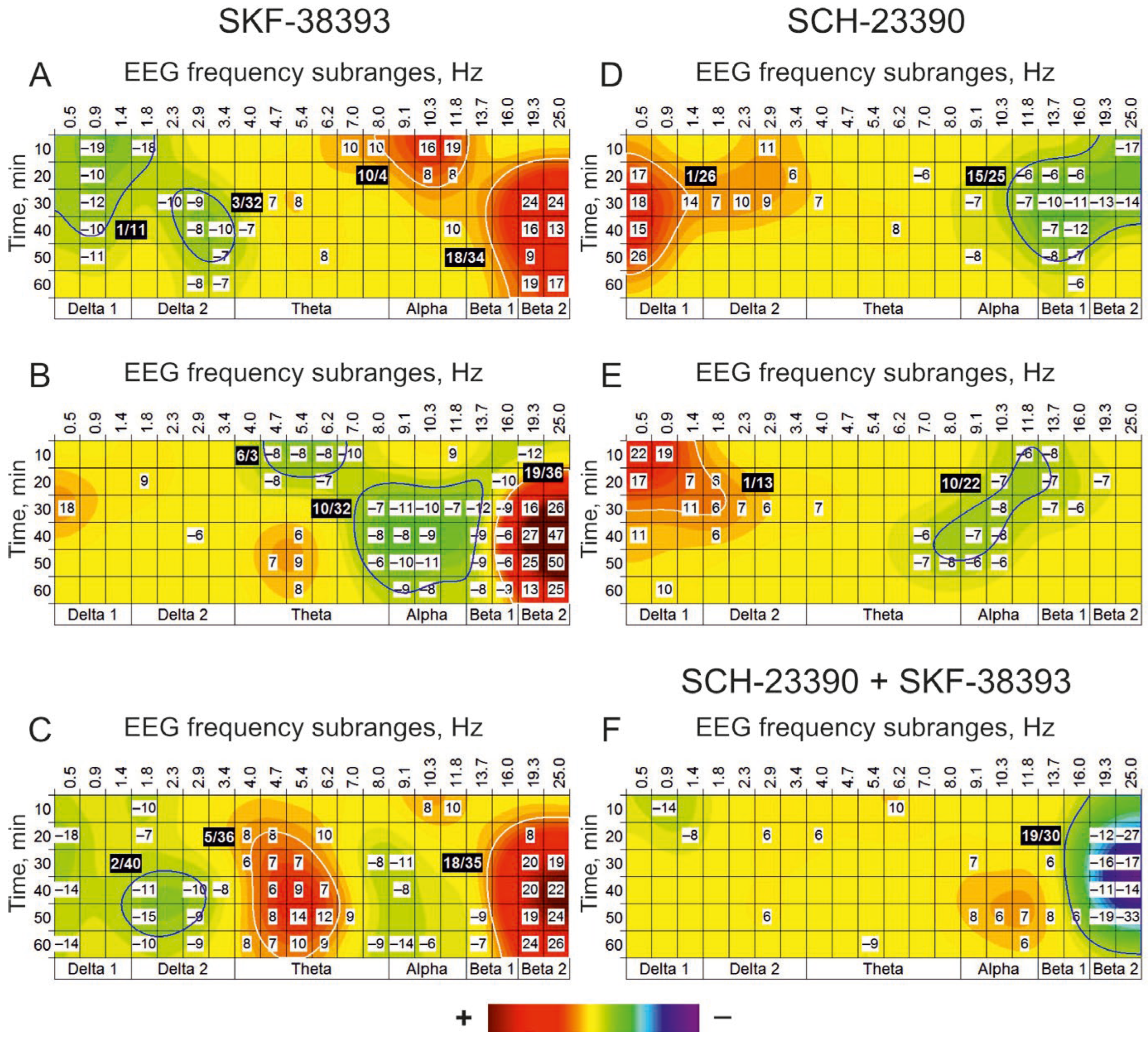
3.2. Dopaminergic Transmission
3.3. Noradrenergic Transmission
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Quipazine (100 nmol) vs. Saline | ||||||||||||||||||||
| A | Delta 1 | Delta 2 | Theta | Alpha | Beta 1 | Beta 2 | ||||||||||||||
| Hz Min | 0.5 | 0.9 | 1.4 | 1.8 | 2.3 | 2.9 | 3.4 | 4.0 | 4.7 | 5.4 | 6.2 | 7.0 | 8.0 | 9.1 | 10.3 | 11.8 | 13.7 | 16.0 | 19.3 | 25.0 |
| 10 | 16 | 10 | 6 | 0 | 1 | 4 | 2 | 4 | 7 | 3 | 0 | 0 | −8 | −21 | −19 | −15 | −18 | −14 | 4 | 27 |
| 20 | 6 | −2 | 15 | 0 | 1 | −2 | 0 | 8 | 16 | 11 | 10 | 1 | −12 | −25 | −22 | −21 | −20 | −11 | 15 | 44 |
| 30 | 5 | 2 | 12 | 0 | −3 | 1 | 2 | 14 | 19 | 14 | 1 | −8 | −21 | −30 | −28 | −19 | −19 | −9 | 26 | 63 |
| 40 | 21 | 1 | 12 | −1 | 0 | −1 | −1 | 9 | 10 | 12 | 0 | −5 | −16 | −26 | −24 | −15 | −17 | −7 | 20 | 50 |
| 50 | 7 | 16 | 19 | 1 | 0 | 1 | 0 | 7 | 4 | 4 | −1 | −8 | −14 | −18 | −17 | −12 | −10 | −6 | 10 | 31 |
| 60 | 13 | 2 | 13 | 0 | 0 | −2 | −1 | 1 | 1 | 2 | −1 | −3 | −11 | −12 | −12 | −7 | 0 | 1 | 4 | 20 |
| Cyproheptadine (10 nmol)+ Quipazine (100 nmol) vs. Saline + Saline | ||||||||||||||||||||
| B | Delta 1 | Delta 2 | Theta | Alpha | Beta 1 | Beta 2 | ||||||||||||||
| Hz Min | 0.5 | 0.9 | 1.4 | 1.8 | 2.3 | 2.9 | 3.4 | 4.0 | 4.7 | 5.4 | 6.2 | 7.0 | 8.0 | 9.1 | 10.3 | 11.8 | 13.7 | 16.0 | 19.3 | 25.0 |
| 10 | −29 | −1 | 0 | −6 | 0 | 10 | 2 | 1 | −3 | −3 | −11 | −6 | −8 | −1 | 0 | 15 | 7 | 8 | 15 | 12 |
| 20 | −13 | −12 | −9 | −4 | 2 | 8 | 1 | 0 | −1 | −2 | 0 | 11 | 14 | 15 | 13 | 16 | 5 | 0 | −7 | −14 |
| 30 | −20 | −6 | −10 | −14 | 0 | 0 | 0 | 1 | 2 | 0 | 3 | 21 | 12 | 15 | 11 | 13 | 0 | 0 | −2 | −13 |
| 40 | −17 | −1 | −14 | −13 | −2 | 0 | 2 | 1 | 8 | 0 | 1 | 10 | 2 | 0 | 0 | 10 | 0 | 0 | 8 | 2 |
| 50 | −9 | −6 | −7 | −17 | −11 | 0 | 0 | −1 | 0 | −3 | 0 | 15 | 15 | 14 | 10 | 15 | 9 | 11 | 1 | −11 |
| 60 | −1 | −13 | −11 | −16 | −13 | 0 | 1 | 0 | 0 | 0 | 0 | 10 | 16 | 16 | 14 | 23 | 12 | 3 | −1 | −22 |
| SKF-38393 (100 nmol) vs. Saline | ||||||||||||||||||||
| A | Delta 1 | Delta 2 | Theta | Alpha | Beta 1 | Beta 2 | ||||||||||||||
| Hz Min | 0.5 | 0.9 | 1.4 | 1.8 | 2.3 | 2.9 | 3.4 | 4.0 | 4.7 | 5.4 | 6.2 | 7.0 | 8.0 | 9.1 | 10.3 | 11.8 | 13.7 | 16.0 | 19.3 | 25.0 |
| 10 | −9 | −3 | −6 | −10 | 0 | 0 | 0 | 4 | 0 | 0 | 4 | 2 | 2 | 1 | 8 | 10 | 0 | 0 | 0 | −3 |
| 20 | −18 | −5 | 0 | −7 | 0 | −2 | −1 | 8 | 8 | 5 | 10 | 3 | −2 | −3 | 0 | −1 | −4 | −2 | 8 | 7 |
| 30 | −7 | −3 | 0 | −4 | −3 | −4 | 0 | 6 | 7 | 7 | 0 | −1 | −8 | −11 | −1 | −1 | −3 | 0 | 20 | 19 |
| 40 | −14 | −3 | 1 | −11 | −4 | −10 | −8 | 5 | 6 | 9 | 7 | 3 | −2 | −8 | −1 | 0 | −2 | 0 | 20 | 22 |
| 50 | −8 | −3 | −1 | −15 | −4 | −9 | −5 | 3 | 8 | 14 | 12 | 9 | 0 | −4 | −3 | −4 | −9 | −5 | 19 | 24 |
| 60 | −14 | −1 | 0 | −10 | −3 | −9 | −2 | 8 | 7 | 10 | 9 | 0 | −9 | −14 | −6 | −1 | −7 | 0 | 24 | 26 |
| SCH-23390 (10 nmol)+ SKF-38393 (100 nmol) vs. Saline + Saline | ||||||||||||||||||||
| B | Delta 1 | Delta 2 | Theta | Alpha | Beta 1 | Beta 2 | ||||||||||||||
| Hz Min | 0.5 | 0.9 | 1.4 | 1.8 | 2.3 | 2.9 | 3.4 | 4.0 | 4.7 | 5.4 | 6.2 | 7.0 | 8.0 | 9.1 | 10.3 | 11.8 | 13.7 | 16.0 | 19.3 | 25.0 |
| 10 | 0 | −14 | −4 | −3 | −1 | 0 | 1 | 4 | 0 | 0 | 10 | 7 | 1 | 1 | 0 | 2 | 0 | 1 | 0 | −1 |
| 20 | −1 | −4 | −8 | −3 | 0 | 6 | 3 | 6 | 1 | 0 | 0 | 3 | 5 | 3 | 2 | 3 | 4 | 2 | −12 | −27 |
| 30 | −1 | −3 | −5 | 0 | 1 | 1 | 1 | 1 | 0 | −1 | 3 | 1 | 3 | 7 | 2 | 5 | 6 | 3 | −16 | −17 |
| 40 | −1 | 6 | −4 | −2 | 5 | 4 | 1 | 1 | 0 | −2 | 0 | 0 | 2 | 1 | 2 | 2 | 1 | 0 | −11 | −14 |
| 50 | 0 | −3 | 0 | 0 | 3 | 6 | 3 | 0 | −5 | −5 | −1 | 0 | 4 | 8 | 6 | 7 | 8 | 6 | −19 | −33 |
| 60 | 1 | −1 | −2 | 0 | 2 | 3 | 1 | 2 | 0 | −9 | 0 | −1 | 0 | 2 | 0 | 6 | 4 | 4 | −6 | −7 |
| Clonidine (100 nmol) vs. Saline | ||||||||||||||||||||
| A | Delta 1 | Delta 2 | Theta | Alpha | Beta 1 | Beta 2 | ||||||||||||||
| Hz Min | 0.5 | 0.9 | 1.4 | 1.8 | 2.3 | 2.9 | 3.4 | 4.0 | 4.7 | 5.4 | 6.2 | 7.0 | 8.0 | 9.1 | 10.3 | 11.8 | 13.7 | 16.0 | 19.3 | 25.0 |
| 10 | 0 | −6 | −18 | −6 | −15 | −15 | −6 | 12 | 12 | 24 | 13 | 20 | 14 | 6 | 5 | 11 | 13 | 0 | −23 | −25 |
| 20 | −15 | −2 | −7 | −13 | −11 | −8 | 1 | 8 | 15 | 17 | 4 | 8 | 8 | 7 | 0 | 7 | 11 | 0 | −14 | −13 |
| 30 | −13 | −3 | −10 | −11 | −13 | −10 | 0 | 13 | 19 | 19 | 9 | 9 | 8 | 7 | 0 | 3 | 3 | 0 | −12 | −16 |
| 40 | −6 | −10 | 0 | −9 | −11 | −5 | 0 | 13 | 19 | 19 | 0 | 8 | 1 | 0 | −6 | 0 | 1 | 2 | −4 | −1 |
| 50 | 0 | −2 | −2 | −6 | −10 | −5 | 0 | 12 | 13 | 14 | 10 | 9 | 2 | 0 | −1 | 0 | 2 | −1 | −9 | −16 |
| 60 | 0 | 0 | −3 | −12 | −14 | −11 | 0 | 14 | 18 | 15 | 6 | 5 | 0 | −1 | −7 | 0 | 1 | 1 | 0 | 0 |
| Yohimbine (10 nmol)+ Clonidine (100 nmol) vs. Saline + Saline | ||||||||||||||||||||
| B | Delta 1 | Delta 2 | Theta | Alpha | Beta 1 | Beta 2 | ||||||||||||||
| Hz Min | 0.5 | 0.9 | 1.4 | 1.8 | 2.3 | 2.9 | 3.4 | 4.0 | 4.7 | 5.4 | 6.2 | 7.0 | 8.0 | 9.1 | 10.3 | 11.8 | 13.7 | 16.0 | 19.3 | 25.0 |
| 10 | 9 | 1 | 0 | 6 | 0 | 0 | 1 | 1 | 0 | −2 | 3 | 0 | −10 | −2 | −10 | −9 | −3 | −5 | 2 | 8 |
| 20 | 3 | 0 | −3 | 2 | −1 | 0 | −1 | 1 | 6 | −4 | 3 | 0 | −3 | −5 | −6 | −7 | −4 | 1 | 15 | 20 |
| 30 | −1 | 1 | 0 | 4 | 2 | 0 | 0 | 7 | 7 | −7 | 0 | 0 | −6 | −9 | −11 | −9 | −8 | 0 | 14 | 27 |
| 40 | 6 | 0 | 0 | 1 | −4 | 7 | 7 | 7 | 9 | −1 | 2 | −2 | −8 | −6 | −3 | −9 | −7 | 0 | 2 | 0 |
| 50 | 2 | −1 | 5 | 13 | 3 | 9 | 5 | 12 | 16 | 1 | 1 | 0 | −14 | −13 | −14 | −13 | −11 | −8 | 1 | 3 |
| 60 | 1 | 9 | 8 | 1 | −4 | 1 | 4 | 11 | 12 | 2 | 2 | −1 | −4 | −6 | −10 | −12 | −12 | 0 | 0 | 2 |
References
- Itil, T.M. The significance of quantitative pharmaco-EEG in the discovery and classification of psychotropic drugs. In EEG in Drug Research; Herrmann, W.M., Ed.; Gustav Fischer: Stuttgart, Germany, 1982; pp. 131–157. [Google Scholar]
- Eccles, C. EEG correlates of neurotoxicity. Neurotoxicology Teratol. 1988, 10, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Feshchenko, V.A.; Veselis, R.A.; Reinsel, R.A. Comparison of the EEG effects of midazolam, thiopental, and propofol—The role of underlying oscillatory systems. Neuropsychobiology 1997, 35, 211–220. [Google Scholar] [CrossRef]
- Kurimoto, E.; Nakashima, M.; Kimura, H.; Suzuki, M. TAK-071, a muscarinic M1 receptor positive allosteric modulator, attenuates scopolamine-induced quantitative electroencephalogram power spectral changes in cynomolgus monkeys. PLoS ONE 2019, 14, e0207969. [Google Scholar] [CrossRef]
- Masychev, K.; Ciprian, C.; Ravan, M.; Manimaran, A.; Deshmukh, A. Quantitative biomarkers to predict response to clozapine treatment using resting EEG data. Schizophr. Res. 2020, 223, 289–296. [Google Scholar] [CrossRef]
- Berro, L.F.; Overton, J.S.; Reeves-Darby, J.A.; Rowlett, J.K. Alprazolam-induced EEG spectral power changes in rhesus monkeys: A translational model for the evaluation of the behavioral effects of benzodiazepines. Psychopharmacology 2021, 238, 1373–1386. [Google Scholar] [CrossRef]
- Ongini, E.; Caporali, M.G. Differential effects of dopamine D-1 and D-2 receptor agonists on EEG activity and behavior in the rabbit. Neuropharmacology 1987, 26, 355–360. [Google Scholar] [CrossRef]
- Emilien, G. Effects of clonidine, yohimbine and eserine on the quantified EEG of rats. Arch. Int. Pharmacodyn. Ther. 1990, 304, 105–124. [Google Scholar] [PubMed]
- Kabuto, H.; Yokoi, I.; MoonSuk, S.; Yamamoto, M.; Mori, A. Effects of kainic acid, quisqualic acid, and their antagonist, pCB-PzDA, on rat electrocorticograms and monoamine metabolite levels in rat striatum. Neurochem. Res. 1994, 19, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Dringenberg, H.C.; Vanderwolf, C.H. 5-Hydroxytryptamine (5-HT) agonists: Effects on neocortical slow wave activity after combined muscarinic and serotonergic blockade. Brain Res. 1996, 728, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Hiyoshi, T.; Kambe, D.; Karasawa, J.; Chaki, S. Involvement of glutamatergic and GABAergic transmission in MK-801-increased gamma band oscillation power in rat cortical electroencephalograms. Neuroscience 2014, 280, 262–274. [Google Scholar] [CrossRef]
- Pálfi, E.; Lévay, G.; Czurkó, A.; Lendvai, B.; Kiss, T. Acute blockade of NR2C/D subunit-containing N-methyl-D-aspartate receptors modifies sleep and neural oscillations in mice. J. Sleep Res. 2021, 30, e13257. [Google Scholar] [CrossRef]
- Mustafa, N.; Afroz, R.; Batool, Z.; Salman, T.; Nawaz, S.; Haleem, D.J. Exploring serotonin-1A receptor function in the effects of buspirone on cognition by molecular receptor expression and EEG analytical studies. Eur. J. Pharmacol. 2025, 990, 177275. [Google Scholar] [CrossRef] [PubMed]
- Buzsáki, G.; Anastassiou, C.A.; Koch, C. The origin of extracellular fields and currents—EEG, ECoG, LFP and spikes. Nat. Rev. Neurosci. 2012, 13, 407–420. [Google Scholar] [CrossRef]
- Brachya, G.; Yanay, C.; Linial, M. Synaptic proteins as multi-sensor devices of neurotransmission. BMC Neurosci. 2006, 7 (Suppl. S1), S4. [Google Scholar] [CrossRef]
- Jiang, Y.; Zou, D.; Li, Y.; Gu, S.; Dong, J.; Ma, X.; Xu, S.; Wang, F.; Huang, J.H. Monoamine neurotransmitters control basic emotions and affect major depressive disorders. Pharmaceuticals 2022, 15, 1203. [Google Scholar] [CrossRef]
- Ahnaou, A.; Drinkenburg, W.H. Simultaneous changes in sleep, qEEG, physiology, behaviour and neurochemistry in rats exposed to repeated social defeat stress. Neuropsychobiology 2016, 73, 209–223. [Google Scholar] [CrossRef]
- Butler, J.J.; Ricci, D.; Aman, C.; Beyeler, A.; De Deurwaerdère, P. Classical psychedelics’ action on brain monoaminergic systems. Int. J. Biochem. Cell Biol. 2024, 176, 106669. [Google Scholar] [CrossRef] [PubMed]
- Crawford, J.L.; Berry, A.S. Examining resilience to Alzheimer’s disease through the lens of monoaminergic neuromodulator systems. Trends Neurosci. 2024, 47, 892–903. [Google Scholar] [CrossRef] [PubMed]
- Kemp, A.F.; Kinnerup, M.; Johnsen, B.; Jakobsen, S.; Nahimi, A.; Gjedde, A. EEG Frequency correlates with α2-receptor density in Parkinson’s Disease. Biomolecules 2024, 14, 209. [Google Scholar] [CrossRef]
- Fitzgerald, P.J. Frontal alpha asymmetry and its modulation by monoaminergic neurotransmitters in depression. Clin. Psychopharmacol. Neurosci. 2024, 22, 405–415. [Google Scholar] [CrossRef]
- Barnes, J.J.; O’Connell, R.G.; Nandam, L.S.; Dean, A.J.; Bellgrove, M.A. Monoaminergic modulation of behavioural and electrophysiological indices of error processing. Psychopharmacology 2014, 231, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Vakalopoulos, C. The EEG as an index of neuromodulator balance in memory and mental illness. Front. Neurosci. 2014, 8, 63. [Google Scholar] [CrossRef]
- Gener, T.; Tauste Campo, A.; Alemany-González, M.; Nebot, P.; Delgado-Sallent, C.; Chanovas, J.; Puig, M.V. Serotonin 5-HT(1A), 5-HT(2A) and dopamine D(2) receptors strongly influence prefronto-hippocampal neural networks in alert mice: Contribution to the actions of risperidone. Neuropharmacology 2019, 158, 107743. [Google Scholar] [CrossRef] [PubMed]
- Ptukha, M.; Fesenko, Z.; Belskaya, A.; Gromova, A.; Pelevin, A.; Kurzina, N.; Gainetdinov, R.R.; Volnova, A. Effects of atomoxetine on motor and cognitive behaviors and brain electrophysiological activity of dopamine transporter knockout rats. Biomolecules 2022, 12, 1484. [Google Scholar] [CrossRef]
- Tylš, F.; Vejmola, Č.; Koudelka, V.; Piorecká, V.; Kadeřábek, L.; Bochin, M.; Novák, T.; Kuchař, M.; Bendová, Z.; Brunovský, M.; et al. Underlying pharmacological mechanisms of psilocin-induced broadband desynchronization and disconnection of EEG in rats. Front. Neurosci. 2023, 17, 1152578. [Google Scholar] [CrossRef] [PubMed]
- Le, G.H.; Wong, S.; Lu, A.; Vasudeva, S.; Gill, H.; Badulescu, S.; Portelles, D.R.; Zheng, Y.J.; Teopiz, K.M.; Meshkat, S.; et al. Electroencephalography (EEG) spectral signatures of selective serotonin reuptake inhibitors (SSRIs), selective norepinephrine reuptake inhibitors (SNRIs) and vortioxetine in major depressive disorder: A systematic review. J. Affect. Disord. 2025, 368, 798–819. [Google Scholar] [CrossRef] [PubMed]
- Stigsby, B.; Walter, D.O.; Sulg, I.A. Automatic data acquisition and period-amplitude analysis of the electroencephalogram. Comput. Programs Biomed. 1973, 3, 223–236. [Google Scholar] [CrossRef]
- Ktonas, P.Y.; Gosalia, A.P. Spectral analysis vs. period-amplitude analysis of narrowband EEG activity: A comparison based on the sleep delta-frequency band. Sleep 1981, 4, 193–206. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ehlen, J.C.; Jefferson, F.; Brager, A.J.; Benveniste, M.; Paul, K.N. Period-amplitude analysis reveals wake-dependent changes in the electroencephalogram during sleep deprivation. Sleep 2013, 36, 1723–1735. [Google Scholar] [CrossRef]
- Voronina, T.A.; Molodavkin, G.M.; Chernyavskaya, L.I.; Seredenin, S.B.; Burlakova, E.B. Effects of phenazepam in ultralow doses on bioelectric activity of the brain and behavior of rats in various models of anxiety. Bull. Exp. Biol. Med. 2003, 135 (Suppl. S7), 14–16. [Google Scholar] [CrossRef]
- Vorobyov, V.; Deev, A. Abnormal EEG effects of acute apomorphine injection in 5xFAD transgenic mice are partially normalized in those chronically pretreated with apomorphine: The time-frequency clustering of EEG spectra. Biomedicines 2024, 12, 2433. [Google Scholar] [CrossRef]
- Lundqvist, M.; Miller, E.K.; Nordmark, J.; Liljefors, J.; Herman, P. Beta: Bursts of cognition. Trends Cogn. Sci. 2024, 28, 662–676. [Google Scholar] [CrossRef]
- Pellegrino, L.L.; Pellegrino, A.S.; Cushman, A.J. A Stereotaxic Atlas of the Rat Brain; Plenum Press: New York, NY, USA, 1979; p. 123. [Google Scholar]
- Hall, R.D.; Lindholm, E.P. Organization of motor and somatosensory neocortex in the albino rat. Brain Res. 1974, 66, 23–38. [Google Scholar] [CrossRef]
- Severs, W.B.; Summy-Long, J. The role of angiotensin in thirst. Life Sci. 1975, 17, 1513–1526. [Google Scholar] [CrossRef]
- Myers, R.D. Hadbook of Drug and Chemical Stimulation of the Brain: Behavioral, Pharmacological and Physiological Aspects; Van Nostr and Reinhold Company: New York, NY, USA, 1974; p. 750. [Google Scholar]
- Ghersi-Egea, J.-F.; Finnegan, W.; Chen, J.-L.; Fenstermacher, J.D. Rapid distribution of intraventricularly administered sucrose into cerebrospinal fluid cisterns via subarachnoid velae in rat. Neuroscience 1996, 75, 1271–1288. [Google Scholar] [CrossRef]
- Gal’chenko, A.A.; Vorobyov, V.V. Analysis of electroencephalograms using a modified amplitude-interval algorithm. Neurosci. Behav. Physiol. 1999, 29, 157–160. [Google Scholar] [CrossRef]
- Vorobyov, V.; Sengpiel, F. Apomorphine-induced differences in cortical and striatal EEG and their glutamatergic mediation in 6-hydroxydopamine-treated rats. Exp. Brain Res. 2008, 191, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Goodfellow, N.M.; Benekareddy, M.; Vaidya, V.A.; Lambe, E.K. Layer II/III of the prefrontal cortex: Inhibition by the serotonin 5-HT1A receptor in development and stress. J. Neurosci. 2009, 29, 10094–10103. [Google Scholar] [CrossRef] [PubMed]
- Aghajanian, G.K.; Sprouse, J.S.; Sheldon, P.; Rasmussen, K. Electrophysiology of the central serotonin system: Receptor subtypes and transducer mechanisms. Ann. N. Y. Acad. Sci. 1990, 600, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.H.T. Pharmacology of putative neurotransmitters and receptors: 5-hydroxytryptamine. In Progress in Brain Research; Fields, H.L., Besson, J.-M., Eds.; Elsevier: Amsterdam, The Netherlands, 1988; Volume 77, pp. 329–338. [Google Scholar]
- Foustoukos, G.; Lüthi, A. Monoaminergic signaling during mammalian NREM sleep—Recent insights and next-level questions. Curr. Opin. Neurobiol. 2025, 92, 103025. [Google Scholar] [CrossRef]
- Maia, G.H.; Soares, J.I.; Almeida, S.G.; Leite, J.M.; Baptista, H.X.; Lukoyanova, A.N.; Brazete, C.S.; Lukoyanov, N.V. Altered serotonin innervation in the rat epileptic brain. Brain Res. Bull. 2019, 152, 95–106. [Google Scholar] [CrossRef]
- Chikermane, M.; Weerdmeester, L.; Rajamani, N.; Köhler, R.M.; Merk, T.; Vanhoecke, J.; Horn, A.; Neumann, W.J. Cortical beta oscillations map to shared brain networks modulated by dopamine. eLife 2024, 13, RP97184. [Google Scholar] [CrossRef] [PubMed]
- De Deurwaerdère, P.; Chagraoui, A.; Di Giovanni, G. Serotonin/dopamine interaction: Electrophysiological and neurochemical evidence. Prog. Brain Res. 2021, 261, 161–264. [Google Scholar] [CrossRef]
- Liljefors, J.; Almeida, R.; Rane, G.; Lundström, J.N.; Herman, P.; Lundqvist, M. Distinct functions for beta and alpha bursts in gating of human working memory. Nat. Commun. 2024, 15, 8950. [Google Scholar] [CrossRef]
- Barnes, S.A.; Young, J.W.; Neill, J.C. D1 receptor activation improves vigilance in rats as measured by the 5-choice continuous performance test. Psychopharmacology 2012, 220, 129–141. [Google Scholar] [CrossRef]
- Timmerman, W.; Abercrombie, E.D. Amphetamine-induced release of dendritic dopamine in substantia nigra pars reticulata—D1-mediated behavioral and electrophysiological effects. Synapse 1996, 23, 280–291. [Google Scholar] [CrossRef]
- De Sarro, G.B.; Ascioti, C.; Froio, F.; Libri, V.; Nisticò, G. Evidence that locus coeruleus is the site where clonidine and drugs acting at alpha1- and alpha2-adrenoreceptors affect sleep and arousal mechanisms. Br. J. Pharmacol. 1987, 90, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Dowlatshahi, P.; Yaksh, T.L. Differential effects of two intraventricularly injected alpha 2 agonists, ST-91 and dexmedetomidine, on electroencephalogram, feeding, and electromyogram. Anesth. Analg. 1997, 84, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Washburn, M.; Moises, H.C. Electrophysiological correlates of presynapric alpha-2-receptor-mediated inhibition of norepinephrine release at locus coeruleus synapses in dentate gyrus. J. Neurosci. 1989, 9, 2131–2140. [Google Scholar] [CrossRef] [PubMed]
- Kovács, P.; Hernádi, I. Alpha2 antagonist yohimbine suppresses maintained firing of rat prefrontal neurons in vivo. Neuroreport 2003, 14, 833–836. [Google Scholar] [CrossRef]
- Arjmandi-Rad, S.; Vestergaard Nieland, J.D.; Goozee, K.G.; Vaseghi, S. The effects of different acetylcholinesterase inhibitors on EEG patterns in patients with Alzheimer’s disease: A systematic review. Neurol. Sci. 2024, 45, 417–430. [Google Scholar] [CrossRef]
- McDonald, A.J. Functional neuroanatomy of monoaminergic systems in the basolateral nuclear complex of the amygdala: Neuronal targets, receptors, and circuits. J. Neurosci. Res. 2023, 101, 1409–1432. [Google Scholar] [CrossRef]
- Mayeli, A.; Donati, F.L.; Ferrarelli, F. Altered sleep oscillations as neurophysiological biomarkers of schizophrenia. Adv. Neurobiol. 2024, 40, 351–383. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.T.; Peng, W.H.; Kan, H.W.; Wu, C.C.; Wang, D.W.; Ho, Y.C. Neurobiology of depression: Chronic stress alters the glutamatergic system in the brain-focusing on AMPA receptor. Biomedicines 2022, 10, 1005. [Google Scholar] [CrossRef] [PubMed]
- Machado-Vieira, R.; Courtes, A.C.; Zarate, C.A., Jr.; Henter, I.D.; Manji, H.K. Non-canonical pathways in the pathophysiology and therapeutics of bipolar disorder. Front. Neurosci. 2023, 17, 1228455. [Google Scholar] [CrossRef] [PubMed]

| Substances-Analyzers Used in this Study for MA Receptors | ||
|---|---|---|
| Full Name | Short Name | Affinity |
| Serotonin (5-HT) | ||
| Quipazine dimaleate | Quipazine | Agonist (5-HT1,2) |
| Cyproheptadine hydrochloride | Cyproheptadine | Antagonist (5-HT1,2) |
| Dopamine (DA) | ||
| (±)-SKF-38393 hydrochloride | SKF-38393 | Agonist (DA1) |
| (+)-SCH-23390 hydrochloride | SCH-23390 | Antagonist (DA1) |
| Norepinephrine (NE) | ||
| Clonidine hydrochloride | Clonidine | Agonist (α2) |
| Yohimbine hydrochloride | Yohimbine | Antagonist (α2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vorobyov, V.; Deev, A. Clustering Cortical Rhythms: Monoaminergic Signatures in Time-Frequency EEG Dynamics. Biomedicines 2025, 13, 1973. https://doi.org/10.3390/biomedicines13081973
Vorobyov V, Deev A. Clustering Cortical Rhythms: Monoaminergic Signatures in Time-Frequency EEG Dynamics. Biomedicines. 2025; 13(8):1973. https://doi.org/10.3390/biomedicines13081973
Chicago/Turabian StyleVorobyov, Vasily, and Alexander Deev. 2025. "Clustering Cortical Rhythms: Monoaminergic Signatures in Time-Frequency EEG Dynamics" Biomedicines 13, no. 8: 1973. https://doi.org/10.3390/biomedicines13081973
APA StyleVorobyov, V., & Deev, A. (2025). Clustering Cortical Rhythms: Monoaminergic Signatures in Time-Frequency EEG Dynamics. Biomedicines, 13(8), 1973. https://doi.org/10.3390/biomedicines13081973







