Novel Therapeutic Strategies for Squamous Cell Carcinoma of the Head and Neck: Beyond EGFR and Checkpoint Blockade
Abstract
1. Introduction
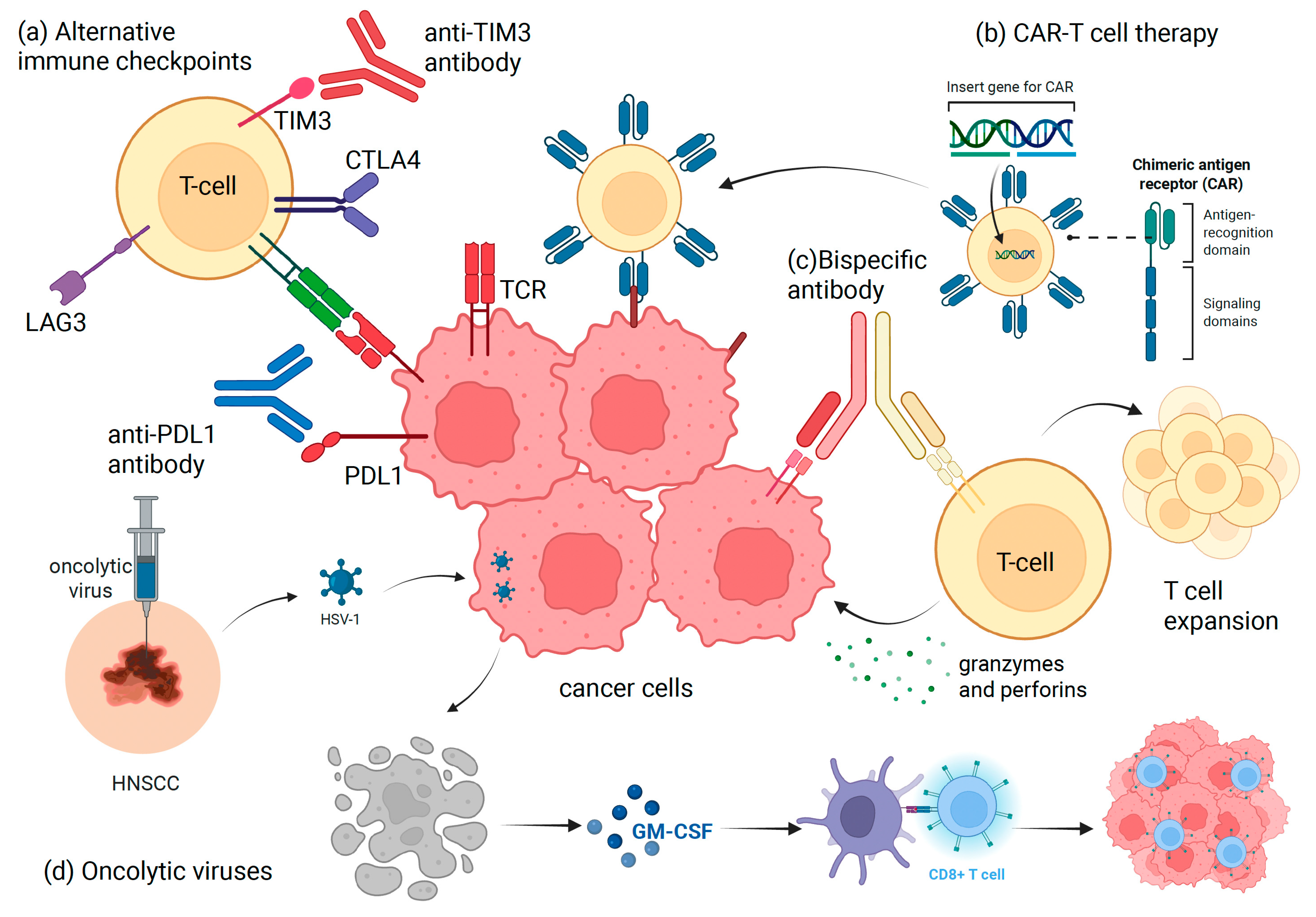
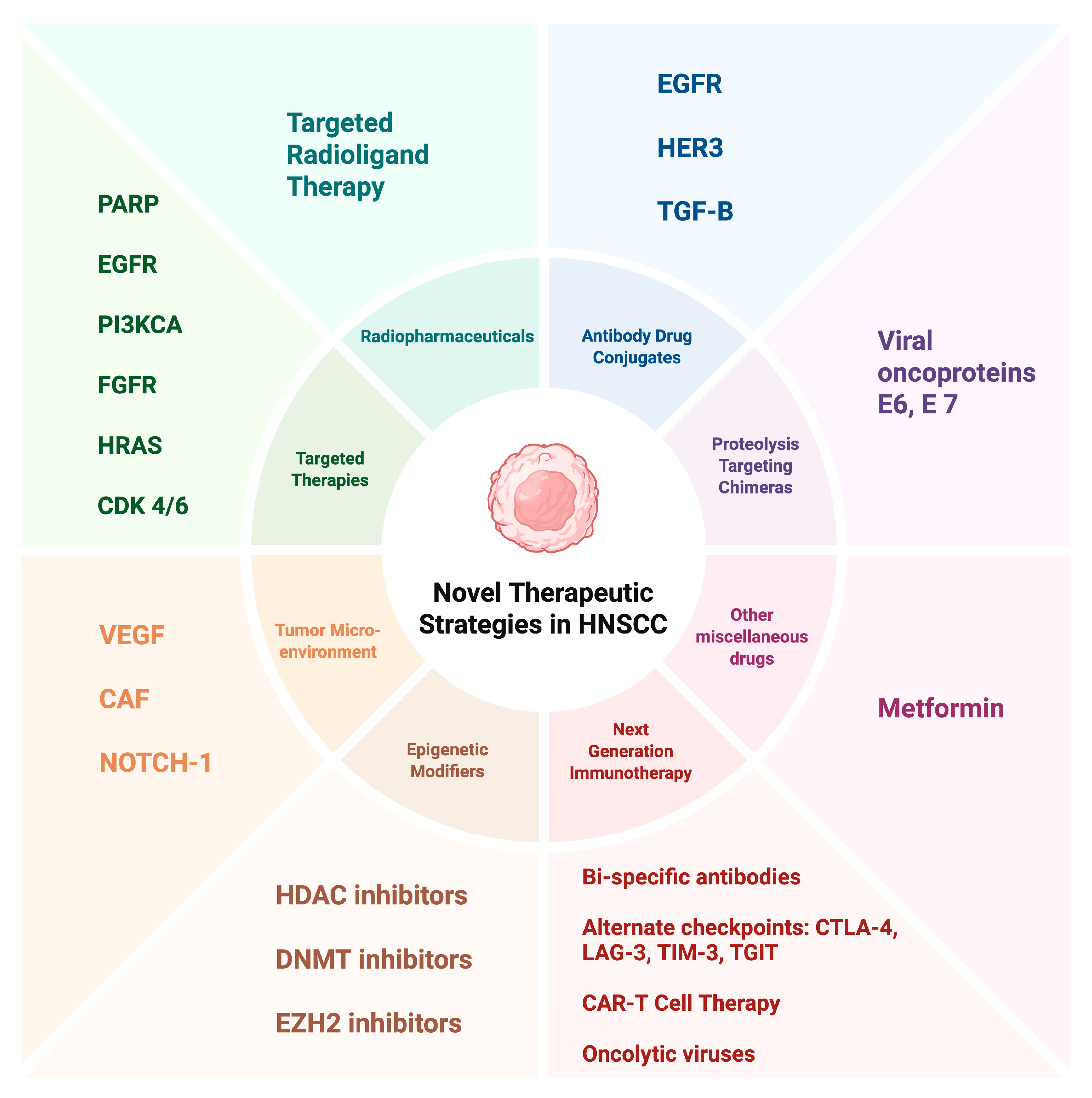
2. Next-Generation Immunotherapies
2.1. Targeting Alternative Immune Checkpoints
2.2. Bispecific Antibodies
2.3. Chimeric-Antigen Receptor (CAR) T-Cell Therapy
2.4. Cytokine-Based Therapies
2.5. Oncolytic Viruses
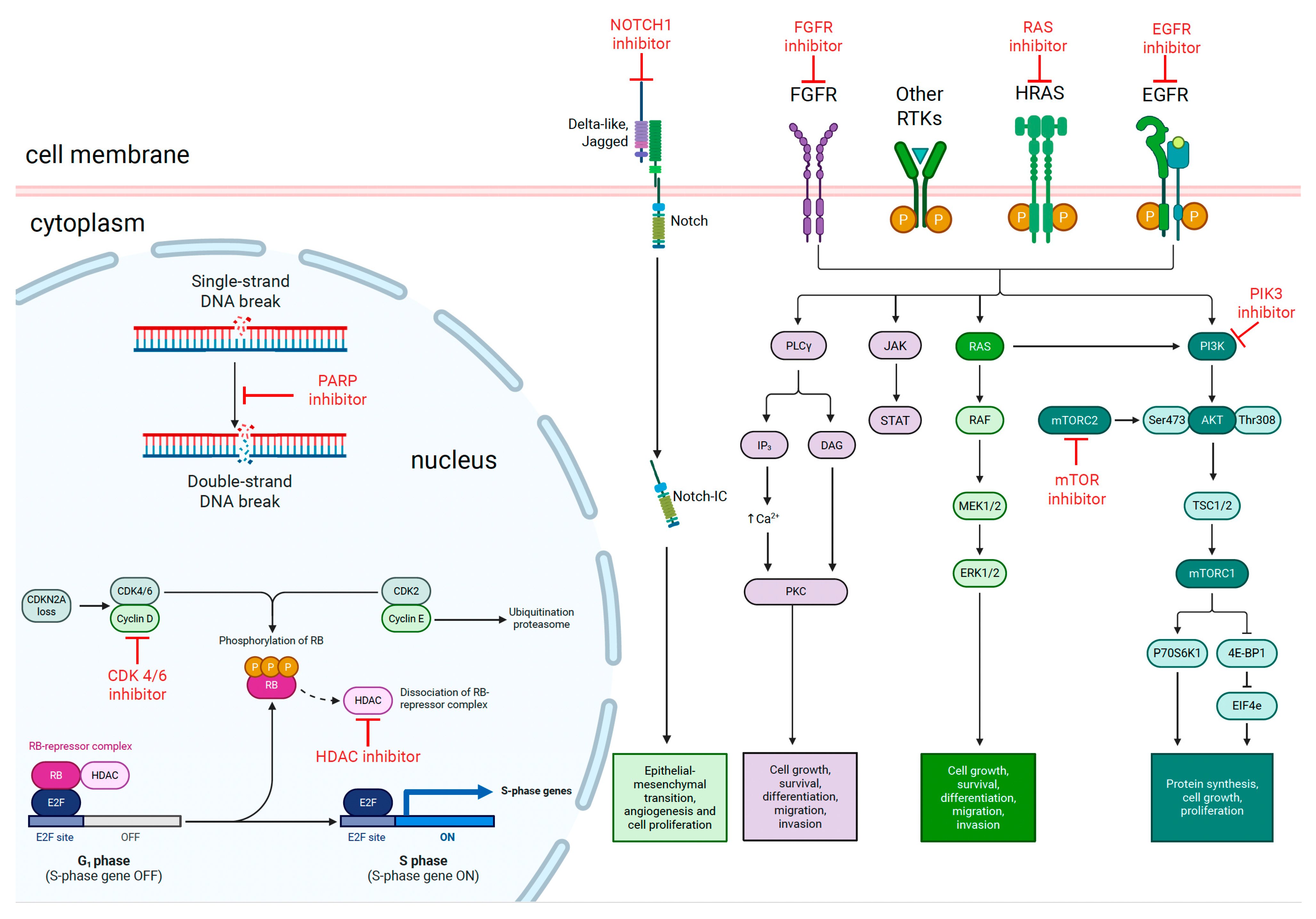
3. Targeted Therapies
3.1. EGFR Mutations
3.2. PI3K/AKT/mTOR Pathway
3.3. FGFR Alterations
3.4. HRAS Mutations
3.5. Cell Cycle Regulators
3.6. DNA Damage Response
4. Epigenetic Modifiers
4.1. Histone Deacetylase (HDAC) Inhibitors
4.2. DNA Methyltransferase (DNMT) Inhibitors
4.3. Enhancer of Zeste Homolog 2 (EZH2) Inhibitors
5. Targeting the Tumor Microenvironment (TME)
5.1. Anti-Angiogenic Agents
5.2. Cancer-Associated Fibroblasts (CAFs)
5.3. NOTCH1 Inhibition
5.4. Modulating the Immune Microenvironment
6. Other Innovative Approaches
6.1. Antibody-Drug Conjugates (ADCs)
6.2. Proteolysis Targeting Chimeras (PROTACs)
6.3. Metformin
6.4. Radiopharmaceuticals
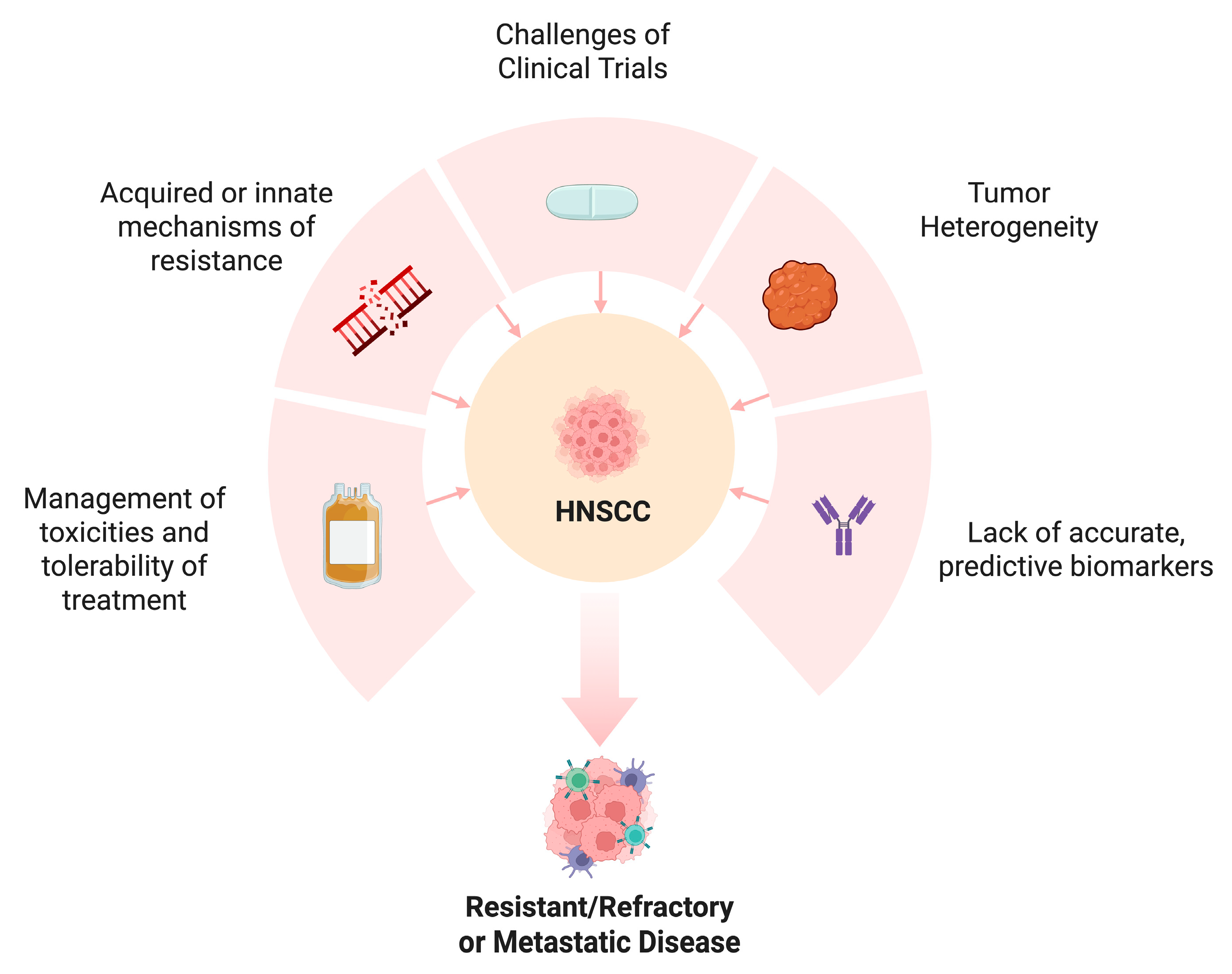
7. Challenges and Future Directions
7.1. Overcoming Resistance
7.2. Biomarker Development
7.3. Addressing Tumor Heterogeneity
7.4. Clinical Trial Design
7.5. Toxicity Management
8. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADC | Antibody drug conjugate |
| CAF | Cancer-Associated Fibroblasts |
| CAR | Chimeric-antigen receptor |
| CCND1 | Cyclin D1 |
| CDK 4/6 | Cyclin-Dependent Kinase 4/6 |
| CDKN2A | Cyclin-dependent kinase inhibitor 2A |
| CPOS | Combined positive score |
| CTLA | Cytotoxic T lymphocyte-associated antigen |
| DOR | Duration of response |
| DNMT | DNA methyltransferase |
| EBV | Epstein–Barr Virus |
| EGFR | Epidermal growth factor receptor |
| ERK | Extracellular Signal-Regulated Kinase |
| EZH2 | Enhancer of Zeste Homolog 2 |
| FDA | Food and Drug Administration |
| FGFR2 | Fibroblast growth factor receptor |
| HDAC | Histone deacetylase |
| HER3 | Human epidermal growth factor receptor 3 |
| HIF | Hypoxia inducible factor |
| HPV | Human papillomavirus |
| HRAS | Harvey rat sarcoma viral oncogene homolog |
| HRD | Homologous repair deficiency |
| HSV | Herpes simplex virus |
| IL | Interleukin |
| LAG-3 | Lymphocyte-activation gene 3 |
| LGR-5 | Leucine-rick repeat-containing G-protein |
| MAPK | Mitogen-activated protein kinase |
| MDSC | Myeloid-derived suppressor cells |
| MMAE | Monomethyl auristatin A |
| MEK | Mitogen-activated protein kinase |
| mTOR | Mammalian target of rapamycin |
| LZK | Leucine zipper-bearing kinase |
| NPC | Nasopharyngeal cancer |
| ORR | Objective response rate |
| OS | Overall survival |
| PARP | Poly ADP ribose polymerase |
| PFS | Progression free survival |
| PI3KCA | Phosphatidylinositol 3-kinase catalytic subunit alpha |
| RAF | Rapidly accelerated fibrosarcoma |
| RAS | Rat sarcoma |
| Rb | Retinoblastoma |
| R/M | Recurrent or metastatic |
| HNSCC | Head and neck squamous cell carcinoma |
| SDF1 | Stromal cell derived factor-1 |
| SSB | Single strand breaks |
| S6K | Ribosomal S6 kinase |
| TAM | Tumor associated macrophage |
| TF | Tissue factor |
| TGF-B | Transforming growth factor-beta |
| TGIT | T cell immunoreceptor with Ig and ITIM domains |
| Trop | Trophoblast cell surface antigen |
| TRT | Targeted radioligand therapy |
| TIM-3 | T-cell immunoglobulin and mucin-domain containing-3 |
| TKI | Tyrosine kinase inhibitor |
| TME | Tumor microenvionment |
| VEGF | Vascular endothelial growth factor |
| 4E-BP1 | Eukaryotic translation initiation factor 4E binding protein 1 |
References
- World Health Organization. Global Cancer Observatory, International Agency for Research on Cancer. Available online: https://gco.iarc.fr/ (accessed on 1 January 2025).
- Van den Bossche, V. Microenvironment-driven intratumoral heterogeneity in head and neck cancers: Clinical challenges and opportunities for precision medicine. Drug Resist. Updat. 2022, 60, 100806. [Google Scholar] [CrossRef] [PubMed]
- Burtness, B. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Ferris, R.L. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. New Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Mandal, R. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight 2016, 1, e89829. [Google Scholar] [CrossRef]
- Haddad, R.I. Nivolumab Plus Ipilimumab Versus EXTREME Regimen as First-Line Treatment for Recurrent/Metastatic Squamous Cell Carcinoma of the Head and Neck: The Final Results of CheckMate 651. J. Clin. Oncol. 2022, 41, 2166–2180. [Google Scholar] [CrossRef]
- Long Long, X.Z. The promising immune checkpoint LAG-3: From tumor microenvironment to cancer immunotherapy. Genes Cancer 2018, 9, 176–189. [Google Scholar] [CrossRef]
- Deng, W.-W.; Mao, L.; Yu, G.-T.; Bu, L.-L.; Ma, S.-R.; Liu, B.; Gutkind, J.S.; Kulkarni, A.B.; Zhang, W.-F.; Sun, Z.-J. LAG-3 confers poor prognosis and its blockade reshapes antitumor response in head and neck squamous cell carcinoma. Oncoimmunology 2016, 5, e1239005. [Google Scholar] [CrossRef]
- Garralda, E. A first-in-human study of the anti-LAG-3 antibody favezelimab plus pembrolizumab in previously treated, advanced microsatellite stable colorectal cancer. ESMO Open 2022, 7, 100639. [Google Scholar] [CrossRef]
- Cho, B.C. A phase 1 study of fianlimab (anti–LAG-3) in combination with cemiplimab (anti–PD-1) in patients with advanced HNSCC. J. Clin. Oncol. 2024, 42, 6038. [Google Scholar] [CrossRef]
- Brana, I. TACTI-003: A randomized phase IIb study of eftilagimod alpha (soluble LAG-3 protein) and pembrolizumab as first-line treatment of patients with recurrent or metastatic head and neck squamous cell carcinoma. J. Immunother. Cancer 2022, 10, A704. [Google Scholar] [CrossRef]
- Oweida, A. Resistance to Radiotherapy and PD-L1 Blockade Is Mediated by TIM-3 Upregulation and Regulatory T-Cell Infiltration. Clin. Cancer Res. 2018, 24, 5368–5380. [Google Scholar] [CrossRef]
- Mao, L. TIGIT/CD155 blockade enhances anti-PD-L1 therapy in head and neck squamous cell carcinoma by targeting myeloid-derived suppressor cells. Oral Oncol. 2021, 121, 105472. [Google Scholar] [CrossRef]
- Patrick Chames, D.B. Bispecific antibodies for cancer therapy. MAbs 2009, 1, 539–547. [Google Scholar] [CrossRef]
- Kalyankrishna, S.; Grandis, J.R. Epidermal growth factor receptor biology in head and neck cancer. J. Clin. Oncol. 2006, 24, 2666–2672. [Google Scholar] [CrossRef]
- Lo Muzio, L.; Farina, A.; Rubini, C.; Coccia, E.; Capogreco, M.; Colella, G.; Leonardi, R.; Campisi, G.; Carinci, F. Effect of c-Met expression on survival in head and neck squamous cell carcinoma. Tumour Biol. 2006, 27, 115–121. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, B.J.; Kim, H.S. Clinicopathological impacts of high c-Met expression in head and neck squamous cell carcinoma: A meta-analysis and review. Oncotarget 2017, 8, 113120–113128. [Google Scholar] [CrossRef]
- Lim, S.M.; Synn, C.-B.; Kang, S.-s.; Kim, D.K.; Lee, S.-H.; Baek, S.; Yang, S.M.; Han, Y.J.; Kim, M.H.; Han, H.; et al. Abstract 5865: Combinatorial activity of amivantamab and pembrolizumab in head and neck squamous cell carcinoma and lung squamous cell carcinoma expressing wild-type EGFR and MET. Cancer Res. 2023, 83 (Suppl. S7), 5865. [Google Scholar] [CrossRef]
- Hanna, G.J.; Kaczmar, J.M.; Zandberg, D.P.; Wong, D.J.L.; Yilmaz, E.; Sherman, E.J.; Hernando-Calvo, A.; Sacco, A.G.; Chung, C.H.; Bohr, D.; et al. Dose expansion results of the bifunctional EGFR/TGFβ inhibitor BCA101 with pembrolizumab in patients with recurrent, metastatic head and neck squamous cell carcinoma. J. Clin. Oncol. 2023, 41 (Suppl. S16), 6005. [Google Scholar] [CrossRef]
- Fayette, J.; Clatot, F.; Brana, I.; Saada, E.; Herpen, C.M.L.-v.; Perez, C.A.; Tabernero, J.; Tourneau, C.L.; Hollebecque, A.; Arrula, V.A.; et al. Petosemtamab (MCLA-158) with pembrolizumab as first-line (1L) treatment of recurrent/metastatic (r/m) head and neck squamous cell carcinoma (HNSCC): Phase 2 study. J. Clin. Oncol. 2024, 42 (Suppl. S16), 6014. [Google Scholar] [CrossRef]
- Rysman, B.; Mouawad, F.; Gros, A.; Lansiaux, A.; Chevalier, D.; Meignan, S. Human epidermal growth factor receptor 3 in head and neck squamous cell carcinomas. Head Neck 2016, 38 (Suppl. S1), E2412–E2418. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.L.; Huang, S.; Kruser, T.J.; Nechrebecki, M.M.; Armstrong, E.A.; Benavente, S.; Gondi, V.; Hsu, K.T.; Harari, P.M. Mechanisms of acquired resistance to cetuximab: Role of HER (ErbB) family members. Oncogene 2008, 27, 3944–3956. [Google Scholar] [CrossRef]
- Xue, L. Esults from two phase II studies of SI-B001, an EGFR×HER3 bispecific antibody, with/without chemotherapy in patients (pts) with recurrent and metastatic head and neck squamous cell carcinoma (HNSCC). J. Clin. Oncol. 2023, 41, 6037. [Google Scholar] [CrossRef]
- Newick, K.; O’Brien, S.; Moon, E.; Albelda, S.M. CAR T Cell Therapy for Solid Tumors. Annu. Rev. Med. 2017, 68, 139–152. [Google Scholar] [CrossRef]
- Dong, Y.H.; Ding, Y.M.; Guo, W.; Huang, J.W.; Yang, Z.; Zhang, Y.; Chen, X.H. The functional verification of EGFR-CAR T-cells targeted to hypopharyngeal squamous cell carcinoma. Onco Targets Ther. 2018, 11, 7053–7059. [Google Scholar] [CrossRef] [PubMed]
- Quesnelle, K.M.; Grandis, J.R. Dual kinase inhibition of EGFR and HER2 overcomes resistance to cetuximab in a novel in vivo model of acquired cetuximab resistance. Clin. Cancer Res. 2011, 17, 5935–5944. [Google Scholar] [CrossRef] [PubMed]
- Pollock, N.I.; Grandis, J.R. HER2 as a therapeutic target in head and neck squamous cell carcinoma. Clin. Cancer Res. 2015, 21, 526–533. [Google Scholar] [CrossRef]
- Warren, E.A.; Liu, H.-C.; Porter, C.E.; Liao, K.S.; Hegde, M.; Yu, W.; Castro, P.D.; Sandulache, V.; Ahmed, N.; Suzuki, M.; et al. Abstract 574: Overexpression of HER2 in head and neck cancer represents a potential target for T cell immunotherapy. Cancer Res. 2019, 79 (Suppl. S13), 574. [Google Scholar] [CrossRef]
- Du, W.; Na, J.; Zhong, L.; Zhang, P. Advances in preclinical and clinical studies of oncolytic virus combination therapy. Front. Oncol. 2025, 15, 1545542. [Google Scholar] [CrossRef]
- Park, R.; Li, J.; Slebos, R.J.C.; Chaudhary, R.; Poole, M.I.; Ferraris, C.; Farinhas, J.; Hernandez-Prera, J.; Kirtane, K.; Teer, J.K.; et al. Phase Ib trial of IRX-2 plus durvalumab in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Oral. Oncol. 2024, 154, 106866. [Google Scholar] [CrossRef] [PubMed]
- Wolf, G.T.; Liu, S.; Bellile, E.; Sartor, M.; Rozek, L.; Thomas, D.; Nguyen, A.; Zarins, K.; McHugh, J.B. Tumor infiltrating lymphocytes after neoadjuvant IRX-2 immunotherapy in oral squamous cell carcinoma: Interim findings from the INSPIRE trial. Oral. Oncol. 2020, 111, 104928. [Google Scholar] [CrossRef]
- Harrington, K.J.; Hingorani, M.; Tanay, M.A.; Hickey, J.; Bhide, S.A.; Clarke, P.M.; Renouf, L.C.; Thway, K.; Sibtain, A.; McNeish, I.A.; et al. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin. Cancer Res. 2010, 16, 4005–4015. [Google Scholar] [CrossRef]
- Vermorken, J.B.; Herbst, R.S.; Leon, X.; Amellal, N.; Baselga, J. Overview of the efficacy of cetuximab in recurrent and/or metastatic squamous cell carcinoma of the head and neck in patients who previously failed platinum-based therapies. Cancer 2008, 112, 2710–2719. [Google Scholar] [CrossRef]
- Psyrri, A.; Seiwert, T.Y.; Jimeno, A. Molecular pathways in head and neck cancer: EGFR, PI3K, and more. In American Society of Clinical Oncology Educational Book; American Society of Clinical Oncology: Alexandria, VA, USA, 2013; pp. 246–255. [Google Scholar]
- Chua, D.T.; Wei, W.I.; Wong, M.P.; Sham, J.S.; Nicholls, J.; Au, G.K. Phase II study of gefitinib for the treatment of recurrent and metastatic nasopharyngeal carcinoma. Head. Neck 2008, 30, 863–867. [Google Scholar] [CrossRef]
- Yamaoka, T.; Ohba, M.; Ohmori, T. Molecular-Targeted Therapies for Epidermal Growth Factor Receptor and Its Resistance Mechanisms. Int. J. Mol. Sci. 2017, 18, 2420. [Google Scholar] [CrossRef]
- Lui, V.W.; Hedberg, M.L.; Li, H.; Vangara, B.S.; Pendleton, K.; Zeng, Y.; Lu, Y.; Zhang, Q.; Du, Y.; Gilbert, B.R.; et al. Frequent mutation of the PI3K pathway in head and neck cancer defines predictive biomarkers. Cancer Discov. 2013, 3, 761–769. [Google Scholar] [CrossRef]
- Jiang, Q.; Xiao, J.; Hsieh, Y.C.; Kumar, N.L.; Han, L.; Zou, Y.; Li, H. The Role of the PI3K/Akt/mTOR Axis in Head and Neck Squamous Cell Carcinoma. Biomedicines 2024, 12, 1610. [Google Scholar] [CrossRef]
- Razak, A.R.A.; Wang, H.M.; Chang, J.Y.; Ahn, M.J.; Munster, P.; Blumenschein, G.; Jr Solomon, B.; Lim, D.W.; Hong, R.L.; Pfister, D.; et al. A Phase 1b/2 Study of Alpelisib in Combination with Cetuximab in Patients with Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma. Target. Oncol. 2023, 18, 853–868. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Nguyen, S.A.; Ogretmen, B.; Gutkind, J.S.; Nathan, C.A.; Day, T. mTOR inhibitor use in head and neck squamous cell carcinoma: A meta-analysis on survival, tumor response, and toxicity. Laryngoscope Investig. Otolaryngol. 2020, 5, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Anderson, K.S. Therapeutic Targeting of FGFR Signaling in Head and Neck Cancer. Cancer J. 2022, 28, 354–362. [Google Scholar] [CrossRef]
- Zhang, P.; Yue, L.; Leng, Q.; Chang, C.; Gan, C.; Ye, T.; Cao, D. Targeting FGFR for cancer therapy. J. Hematol. Oncol. 2024, 17, 39. [Google Scholar] [CrossRef] [PubMed]
- Pant, S.; Schuler, M.; Iyer, G.; Witt, O.; Doi, T.; Qin, S.; Tabernero, J.; Reardon, D.A.; Massard, C.; Minchom, A.; et al. Erdafitinib in patients with advanced solid tumours with FGFR alterations (RAGNAR): An international, single-arm, phase 2 study. Lancet Oncol. 2023, 24, 925–935. [Google Scholar] [CrossRef]
- Jagadeeshan, S.; Novoplansky, O.Z.; Cohen, O.; Kurth, I.; Hess, J.; Rosenberg, A.J.; Grandis, J.R.; Elkabets, M. New insights into RAS in head and neck cancer. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188963. [Google Scholar] [CrossRef]
- Moore, A.R.; Rosenberg, S.C.; McCormick, F.; Malek, S. RAS-targeted therapies: Is the undruggable drugged? Nat. Rev. Drug Discov. 2020, 19, 533–552. [Google Scholar] [CrossRef] [PubMed]
- Novoplansky, O.; Jagadeeshan, S.; Regev, O.; Menashe, I.; Elkabets, M. Worldwide Prevalence and Clinical Characteristics of RAS Mutations in Head and Neck Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2022, 12, 838911. [Google Scholar] [CrossRef]
- Hoxhallari, L.; Katsikis, K.; Makri, A.; Pouliou, M.; Kanaki, Z.; Vatsellas, G.; Sonou, C.; Telios, D.; Giotakis, E.; Giotakis, A.; et al. Regulation of nucleotide excision repair by wild-type HRAS signaling in head and neck cancer. Cancer Gene Ther. 2025, 32, 662–677. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.L.; Brana, I.; Haddad, R.; Bauman, J.; Bible, K.; Oosting, S.; Wong, D.J.; Ahn, M.J.; Boni, V.; Even, C.; et al. Tipifarnib in Head and Neck Squamous Cell Carcinoma with HRAS Mutations. J. Clin. Oncol. 2021, 39, 1856–1864. [Google Scholar] [CrossRef]
- Knudsen, E.S.; Wang, J.Y. Targeting the RB-pathway in cancer therapy. Clin. Cancer Res. 2010, 16, 1094–1099. [Google Scholar] [CrossRef]
- Fayette, J.; Saada-Bouzid, E.; Cropet, C.; Daste, A.; Treilleux, I.; Pissaloux, D.; Pilleul, F.; Mastier, C.; Neidhardt, E.-M.; Karabajakian, A.; et al. Abemaciclib in recurrent/metastatic head and neck squamous cell carcinoma (RM-HNSCC) harboring CDKN2A loss, and/or CCND1 and/or CDK6 amplification: A phase II multicenter trial. J. Clin. Oncol. 2023, 41 (Suppl. S16), 6044. [Google Scholar]
- van Caloen, G.; Schmitz, S.; El Baroudi, M.; Caignet, X.; Pyr Dit Ruys, S.; Roger, P.P.; Vertommen, D.; Machiels, J.P. Preclinical Activity of Ribociclib in Squamous Cell Carcinoma of the Head and Neck. Mol. Cancer Ther. 2020, 19, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Riess, C.; Irmscher, N.; Salewski, I.; Strüder, D.; Classen, C.F.; Große-Thie, C.; Junghanss, C.; Maletzki, C. Cyclin-dependent kinase inhibitors in head and neck cancer and glioblastoma-backbone or add-on in immune-oncology? Cancer Metastasis Rev. 2021, 40, 153–171. [Google Scholar] [CrossRef]
- Li, R.; Wang, Q.; Zhao, Y.; Zhu, Y.; Wang, X. Palbociclib inhibits the progression of head and neck cancer and improves the Cetuximab response under specific condition in vitro and in vivo. Mol. Biol. Rep. 2024, 51, 455. [Google Scholar] [CrossRef]
- Adkins, D.; Oppelt, P.J.; Ley, J.C.; Trinkaus, K.; Neupane, P.C.; Sacco, A.G.; Palka, K.A.; Worden, F.P.; Grilley-Olson, J.E.; Maggiore, R.J.; et al. Multicenter phase II trial of palbociclib, a selective cyclin dependent kinase (CDK) 4/6 inhibitor, and cetuximab in platinum-resistant HPV unrelated (-) recurrent/metastatic head and neck squamous cell carcinoma (RM HNSCC). J. Clin. Oncol. 2018, 36 (Suppl. S15), 6008. [Google Scholar] [CrossRef]
- Moutafi, M.; Economopoulou, P.; Rimm, D.; Psyrri, A. PARP inhibitors in head and neck cancer: Molecular mechanisms, preclinical and clinical data. Oral. Oncol. 2021, 117, 105292. [Google Scholar] [CrossRef]
- Nambiar, D.K.; Mishra, D.; Singh, R.P. Targeting DNA repair for cancer treatment: Lessons from PARP inhibitor trials. Oncol. Res. 2023, 31, 405–421. [Google Scholar] [CrossRef]
- Cohen, J.; Ley, J.C.; Liu, J.; Haselhorst, E.; Oppelt, P.J.; Adkins, D. Olaparib, a poly (ADP-ribose) polymerase (PARP) inhibitor, in combination with pembrolizumab and carboplatin as first-line treatment of recurrent or metastatic head and neck squamous-cell carcinoma (RM-HNSCC): A single-arm, phase 2 trial. J. Clin. Oncol. 2023, 41 (Suppl. S16), 6016. [Google Scholar] [CrossRef]
- Karam, S.D.; Reddy, K.; Blatchford, P.J.; Waxweiler, T.; DeLouize, A.M.; Oweida, A.; Somerset, H.; Marshall, C.; Young, C.; Davies, K.D.; et al. Final Report of a Phase I Trial of Olaparib with Cetuximab and Radiation for Heavy Smoker Patients with Locally Advanced Head and Neck Cancer. Clin. Cancer Res. 2018, 24, 4949–4959. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Hou, Y.; Li, N.; Yu, W.; Chen, L. Targeting histone deacetylases in head and neck squamous cell carcinoma: Molecular mechanisms and therapeutic targets. J. Transl. Med. 2024, 22, 418. [Google Scholar] [CrossRef]
- Ropero, S.; Esteller, M. The role of histone deacetylases (HDACs) in human cancer. Mol. Oncol. 2007, 1, 19–25. [Google Scholar] [CrossRef]
- Rodriguez, C.P.; Wu, Q.V.; Voutsinas, J.; Fromm, J.R.; Jiang, X.; Pillarisetty, V.G.; Lee, S.M.; Santana-Davila, R.; Goulart, B.; Baik, C.S.; et al. A Phase II Trial of Pembrolizumab and Vorinostat in Recurrent Metastatic Head and Neck Squamous Cell Carcinomas and Salivary Gland Cancer. Clin. Cancer Res. 2020, 26, 837–845. [Google Scholar] [CrossRef]
- Burkitt, K.; Saloura, V. Epigenetic Modifiers as Novel Therapeutic Targets and a Systematic Review of Clinical Studies Investigating Epigenetic Inhibitors in Head and Neck Cancer. Cancers 2021, 13, 5241. [Google Scholar] [CrossRef]
- Mochizuki, D.; Misawa, Y.; Kawasaki, H.; Imai, A.; Endo, S.; Mima, M.; Yamada, S.; Nakagawa, T.; Kanazawa, T.; Misawa, K. Aberrant Epigenetic Regulation in Head and Neck Cancer Due to Distinct EZH2 Overexpression and DNA Hypermethylation. Int. J. Mol. Sci. 2018, 19, 3707. [Google Scholar] [CrossRef]
- Viet, C.T.; Dang, D.; Achdjian, S.; Ye, Y.; Katz, S.G.; Schmidt, B.L. Decitabine rescues cisplatin resistance in head and neck squamous cell carcinoma. PLoS ONE 2014, 9, e112880. [Google Scholar] [CrossRef]
- Qin, T.; Mattox, A.K.; Campbell, J.S.; Park, J.C.; Shin, K.-Y.; Li, S.; Sadow, P.M.; Faquin, W.C.; Micevic, G.; Daniels, A.J.; et al. Epigenetic therapy sensitizes anti–PD-1 refractory head and neck cancers to immunotherapy rechallenge. J. Clin. Investig. 2025, 135, e181671. [Google Scholar] [CrossRef]
- von Keudell, G.; Salles, G. The role of tazemetostat in relapsed/refractory follicular lymphoma. Ther. Adv. Hematol. 2021, 12, 20406207211015882. [Google Scholar] [CrossRef]
- Puram, S.; Oppelt, P.J.; Ley, J.C.; Cohen, J.; Alberti, J.; Shannon, E.; Liu, J.; Adkins, D. Tazemetostat, a selective EZH2 inhibitor, with pembrolizumab as treatment of anti-PD-1 resistant head and neck squamous-cell carcinoma (HNSCC): A phase 1-2 trial. J. Clin. Oncol. 2023, 41 (Suppl. S16), 6022. [Google Scholar] [CrossRef]
- Lv, X.; Li, J.; Zhang, C.; Hu, T.; Li, S.; He, S.; Yan, H.; Tan, Y.; Lei, M.; Wen, M.; et al. The role of hypoxia-inducible factors in tumor angiogenesis and cell metabolism. Genes. Dis. 2017, 4, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Helbig, L.; Koi, L.; Brüchner, K.; Gurtner, K.; Hess-Stumpp, H.; Unterschemmann, K.; Baumann, M.; Zips, D.; Yaromina, A. BAY 87-2243, a novel inhibitor of hypoxia-induced gene activation, improves local tumor control after fractionated irradiation in a schedule-dependent manner in head and neck human xenografts. Radiat. Oncol. 2014, 9, 207. [Google Scholar] [CrossRef]
- Argiris, A.; Li, S.; Savvides, P.; Ohr, J.P.; Gilbert, J.; Levine, M.A.; Chakravarti, A.; Haigentz, M.; Saba, N.F., Jr.; Ikpeazu, C.V.; et al. Phase III Randomized Trial of Chemotherapy with or Without Bevacizumab in Patients with Recurrent or Metastatic Head and Neck Cancer. J. Clin. Oncol. 2019, 37, 3266–3274. [Google Scholar] [CrossRef] [PubMed]
- Elser, C.; Siu, L.L.; Winquist, E.; Agulnik, M.; Pond, G.R.; Chin, S.F.; Francis, P.; Cheiken, R.; Elting, J.; McNabola, A.; et al. Phase II trial of sorafenib in patients with recurrent or metastatic squamous cell carcinoma of the head and neck or nasopharyngeal carcinoma. J. Clin. Oncol. 2007, 25, 3766–3773. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, Y.; Wu, C.; Huang, Q. Heterogeneity of cancer-associated fibroblasts in head and neck squamous cell carcinoma: Opportunities and challenges. Cell Death Discov. 2023, 9, 124. [Google Scholar] [CrossRef]
- Grauel, A.L.; Nguyen, B.; Ruddy, D.; Laszewski, T.; Schwartz, S.; Chang, J.; Chen, J.; Piquet, M.; Pelletier, M.; Yan, Z.; et al. TGFβ-blockade uncovers stromal plasticity in tumors by revealing the existence of a subset of interferon-licensed fibroblasts. Nat. Commun. 2020, 11, 6315. [Google Scholar] [CrossRef] [PubMed]
- Fukusumi, T.; Califano, J.A. The NOTCH Pathway in Head and Neck Squamous Cell Carcinoma. J. Dent. Res. 2018, 97, 645–653. [Google Scholar] [CrossRef]
- Pickering, C.R.; Zhang, J.; Yoo, S.Y.; Bengtsson, L.; Moorthy, S.; Neskey, D.M.; Zhao, M.; Ortega Alves, M.V.; Chang, K.; Drummond, J.; et al. Integrative genomic characterization of oral squamous cell carcinoma identifies frequent somatic drivers. Cancer Discov. 2013, 3, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Ma, Y.; Zhang, Z.; Zhao, J.; Kobayashi, H.; Zhang, L.; Fu, L. Expression of Stat3 and Notch1 is associated with cisplatin resistance in head and neck squamous cell carcinoma. Oncol. Rep. 2010, 23, 671–676. [Google Scholar] [CrossRef]
- Zhao, Z.L.; Zhang, L.; Huang, C.F.; Ma, S.R.; Bu, L.L.; Liu, J.F.; Yu, G.T.; Liu, B.; Gutkind, J.S.; Kulkarni, A.B.; et al. NOTCH1 inhibition enhances the efficacy of conventional chemotherapeutic agents by targeting head neck cancer stem cell. Sci. Rep. 2016, 6, 24704. [Google Scholar] [CrossRef]
- Zaky, M.Y.; John, J.; Vashisht, M.; Singh, P.; Al-Hatamleh, M.A.I.; Siddoway, K.; Chen, Z.; Wang, J.H. Targeting Myeloid Cells in Head and Neck Squamous Cell Carcinoma: A Kinase Inhibitor Library Screening Approach. Int. J. Mol. Sci. 2024, 25, 12277. [Google Scholar] [CrossRef]
- Bisheshar, S.K.; van der Kamp, M.F.; de Ruiter, E.J.; Ruiter, L.N.; van der Vegt, B.; Breimer, G.E.; Willems, S.M. The prognostic role of tumor associated macrophages in squamous cell carcinoma of the head and neck: A systematic review and meta-analysis. Oral. Oncol. 2022, 135, 106227. [Google Scholar] [CrossRef]
- Chitu, V.; Stanley, E.R. Colony-stimulating factor-1 in immunity and inflammation. Curr. Opin. Immunol. 2006, 18, 39–48. [Google Scholar] [CrossRef]
- Chen, K.; Li, X.; Dong, S.; Guo, Y.; Luo, Z.; Zhuang, S.-M.; Liu, J.; Liu, T.; Liao, J.; Wen, W. Modulating tumor-associated macrophages through CSF1R inhibition: A potential therapeutic strategy for HNSCC. J. Transl. Med. 2025, 23, 27. [Google Scholar] [CrossRef] [PubMed]
- Tallman, M.S.; Andersen, J.W.; Schiffer, C.A.; Appelbaum, F.R.; Feusner, J.H.; Ogden, A.; Shepherd, L.; Willman, C.; Bloomfield, C.D.; Rowe, J.M.; et al. All-trans-Retinoic Acid in Acute Promyelocytic Leukemia. New Engl. J. Med. 1997, 337, 1021–1028. [Google Scholar]
- Klute, K.; Nackos, E.; Tasaki, S.; Nguyen, D.P.; Bander, N.H.; Tagawa, S.T. Microtubule inhibitor-based antibody-drug conjugates for cancer therapy. Onco Targets Ther. 2014, 7, 2227–2236. [Google Scholar]
- Conilh, L.; Sadilkova, L.; Viricel, W.; Dumontet, C. Payload diversification: A key step in the development of antibody-drug conjugates. J. Hematol. Oncol. 2023, 16, 3. [Google Scholar] [CrossRef]
- Sanders, C.; Lau, J.F.; Dietrich, D.; Strieth, S.; Brossart, P.; Kristiansen, G. Nectin-4 is widely expressed in head and neck squamous cell carcinoma. Oncotarget 2022, 13, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Swiecicki, P.L.; Yilmaz, E.; Rosenberg, A.J.; Fujisawa, T.; Bruce, J.Y.; Meng, C.; Wozniak, M.; Zhao, Y.; Mihm, M.; Kaplan, J.; et al. Phase II Trial of Enfortumab Vedotin in Patients with Previously Treated Advanced Head and Neck Cancer. J. Clin. Oncol. 2025, 43, 578–588. [Google Scholar] [CrossRef]
- Sun, L.; Fayette, J.; Salas, S.; Hong, D.S.; Adkins, D.; Dunn, L.; Ciardiello, F.; Cirauqui, B.; William, W.N.; Saba, N.F.; et al. Tisotumab vedotin in head and neck squamous cell carcinoma: Updated analysis from innovaTV 207 Part C. J. Clin. Oncol. 2024, 42 (Suppl. S16), 6012. [Google Scholar] [CrossRef]
- Kasthuri, R.S.; Taubman, M.B.; Mackman, N. Role of tissue factor in cancer. J. Clin. Oncol. 2009, 27, 4834–4838. [Google Scholar] [CrossRef]
- Cubas, R.; Zhang, S.; Li, M.; Chen, C.; Yao, Q. Trop2 expression contributes to tumor pathogenesis by activating the ERK MAPK pathway. Mol. Cancer 2010, 9, 253. [Google Scholar] [CrossRef] [PubMed]
- Michel, L.; Jimeno, A.; Sukari, A.; Beck, J.T.; Chiu, J.; Ahern, E.; Hilton, J.; Even, C.; Zanetta, S.; Mekan, S.; et al. Sacituzumab Govitecan in Patients with Relapsed/Refractory Advanced Head and Neck Squamous Cell Carcinoma: Results from the Phase II TROPiCS-03 Basket Study. Clin. Cancer Res. 2025, 31, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Ruan, D.-y.; Han, F.; Zhou, Y.; Wang, F.; Tang, L.-Q.; Li, Z.; Chen, Q.-Y.; Chen, C.; Lin, J.; Liu, F.-R.; et al. Preliminary results of phase I/II study to evaluate safety and efficacy of combination pucotenlimab with epidermal growth factor receptor-ADC (EGFR-ADC) MRG003 in patients with EGFR positive solid tumors. J. Clin. Oncol. 2024, 42 (Suppl. S16), 6013. [Google Scholar] [CrossRef]
- Qiu, M.Z.; Zhang, Y.; Guo, Y.; Guo, W.; Nian, W.; Liao, W.; Xu, Z.; Zhang, W.; Zhao, H.Y.; Wei, X.; et al. Evaluation of Safety of Treatment With Anti-Epidermal Growth Factor Receptor Antibody Drug Conjugate MRG003 in Patients with Advanced Solid Tumors: A Phase 1 Nonrandomized Clinical Trial. JAMA Oncol. 2022, 8, 1042–1046. [Google Scholar] [CrossRef]
- Moon, Y.; Jeon, S.I.; Shim, M.K.; Kim, K. Cancer-Specific Delivery of Proteolysis-Targeting Chimeras (PROTACs) and Their Application to Cancer Immunotherapy. Pharmaceutics 2023, 15, 411. [Google Scholar] [CrossRef] [PubMed]
- Katerji, M.; Bergman, K.L.; Lindberg, E.; Rubin, M.R.; Afifi, M.; Funk, A.L.; Woodroofe, C.C.; Nyswaner, K.; Karpińska, K.; Serwa, R.; et al. Discovery of potent and selective PROTACs for the protein kinase LZK for the treatment of head and neck cancer. J. Biol. Chem. 2025, 301, 108452. [Google Scholar] [CrossRef]
- Choi, Y.K.; Park, K.G. Metabolic roles of AMPK and metformin in cancer cells. Mol. Cells 2013, 36, 279–287. [Google Scholar] [CrossRef]
- Karivedu, V.; Yaniv, B.; Asman, M.; Palackdharry, S.; Gulati, S.; Jandarov, R.; Sadraei, N.H.; Takiar, V.; Wise-Draper, T.M. Metformin treatment of locally advanced head and neck squamous cell carcinoma (LAHNSCC) patients induces an anti-tumorigenic immune response. J. Clin. Oncol. 2019, 37 (Suppl. S15), 6037. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, T.; Liu, H.; Jin, Z.; Sun, X.; Zhao, H.; Shi, J.; Jia, B.; Li, F.; Wang, F. 177Lu-labeled antibodies for EGFR-targeted SPECT/CT imaging and radioimmunotherapy in a preclinical head and neck carcinoma model. Mol. Pharm. 2014, 11, 800–807. [Google Scholar] [CrossRef]
- Peng, F.; Zhang, H.; Du, Y.; Tan, P. miR-23a promotes cisplatin chemoresistance and protects against cisplatin-induced apoptosis in tongue squamous cell carcinoma cells through Twist. Oncol. Rep. 2015, 33, 942–950. [Google Scholar] [CrossRef]
- Lu, X.; Wang, Z.; Huang, H.; Wang, H. Hedgehog signaling promotes multidrug resistance by regulation of ABC transporters in oral squamous cell carcinoma. J. Oral. Pathol. Med. 2020, 49, 897–906. [Google Scholar] [CrossRef]
- Duan, M.; Ulibarri, J.; Liu, K.J.; Mao, P. Role of Nucleotide Excision Repair in Cisplatin Resistance. Int. J. Mol. Sci. 2020, 21, 9248. [Google Scholar] [CrossRef]
- Oliva, M.; Spreafico, A.; Taberna, M.; Alemany, L.; Coburn, B.; Mesia, R.; Siu, L.L. Immune biomarkers of response to immune-checkpoint inhibitors in head and neck squamous cell carcinoma. Ann. Oncol. 2019, 30, 57–67. [Google Scholar] [CrossRef]
- Weinberger, P.M.; Yu, Z.; Haffty, B.G.; Kowalski, D.; Harigopal, M.; Brandsma, J.; Sasaki, C.; Joe, J.; Camp, R.L.; Rimm, D.L.; et al. Molecular classification identifies a subset of human papillomavirus—Associated oropharyngeal cancers with favorable prognosis. J. Clin. Oncol. 2006, 24, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Spiotto, M.T.; Taniguchi, C.M.; Klopp, A.H.; Colbert, L.E.; Lin, S.H.; Wang, L.; Frederick, M.J.; Osman, A.A.; Pickering, C.R.; Frank, S.J. Biology of the Radio- and Chemo-Responsiveness in HPV Malignancies. Semin. Radiat. Oncol. 2021, 31, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, J.H.; Grandis, J.R.; Ferris, R.L. HPV-Associated Head and Neck Cancer: Unique Features of Epidemiology and Clinical Management. Annu. Rev. Med. 2016, 67, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Klussmann, J.P.; Mooren, J.J.; Lehnen, M.; Claessen, S.M.; Stenner, M.; Huebbers, C.U.; Weissenborn, S.J.; Wedemeyer, I.; Preuss, S.F.; Straetmans, J.M.; et al. Genetic signatures of HPV-related and unrelated oropharyngeal carcinoma and their prognostic implications. Clin. Cancer Res. 2009, 15, 1779–1786. [Google Scholar] [CrossRef]
- Riaz, N.; Sherman, E.; Pei, X.; Schöder, H.; Grkovski, M.; Paudyal, R.; Katabi, N.; Selenica, P.; Yamaguchi, T.N.; Ma, D.; et al. Precision Radiotherapy: Reduction in Radiation for Oropharyngeal Cancer in the 30 ROC Trial. J. Natl. Cancer Inst. 2021, 113, 742–751. [Google Scholar] [CrossRef]
- Yu, H.; Yin, X.; Mao, Y.; Chen, M.; Tang, Q.; Yan, S. The global burden of nasopharyngeal carcinoma from 2009 to 2019: An observational study based on the Global Burden of Disease Study 2019. Eur. Arch. Otorhinolaryngol. 2022, 279, 1519–1533. [Google Scholar] [CrossRef] [PubMed]
| Trial Reference No. | Phase | N | Cohort | Investigational Drug | ORR (%) | PFS (Months) | DCR (%) |
|---|---|---|---|---|---|---|---|
| NCT03005782 | I | 30 | R/M HNSCC 2 cohorts: Anti–PD-1/PD-L1-naïve (Cohort 11) or anti–PD-1/L1-experienced (Cohort 12) | Fianlimab (LAG-3 inhibitor) and Cemiplimab | 33% (Cohort 11) 7% (Cohort 12) | 2 (Cohort 11) 4 (Cohort 12) | 47% (Cohort 11) 67% (Cohort 12) |
| NCT03625323 | II | 39 | R/M HNSCC who had failed first-line platinum-based therapy, unselected for PD-L1 | Eftilagimod Alpha (LAG-3 inhibitor) with Pembrolizumab | 29.7% | 2.1 | Not reported |
| NCT04429542 | I/Ib | 39 | R/M HNSCC with a tumor PD-L1 CPS ≥ 1 with no prior systemic therapy | BCA 101 (bispecific antibody targeting TGF-B and EGFR) and Pembrolizumab | 46% | Not reached | Not reported |
| NCT03526835 | II | 26 | R/M HNSCC with no prior systemic therapy, PDL1 positive | Petosemtamab (bispecific antibody targeting EGFR and LGR-5) and Pembrolizumab | 67% | Not reported | Not reported |
| NCT05054439 | I/Ib | 29 | R/M HNSCC progressed on prior anti-PD-1/L1 with or without platinum-based chemotherapy and received no more than two lines of treatment | Group A, pts without prior exposure to paclitaxel
| Group A: 64.3% Group B: 12.5% | Group A: 5.6 Group B: 1.9 | Group A: 92.9% Group B: 62.5% |
| NCT03381183 | Ib | 16 | R/M HNSCC, may or may not have had PD1 inhibitor therapy before | IRX2 and Durvalumab | 5.3% | 6.18 | 42% |
| EudraCT2005-000777-21 | I/II | 17 | Stage III/IVA/IVB HNSCC | Intratumoral injections of oncolytic herpes simplex type-1 virus (HSV-1) encoding human granulocyte-macrophage colony-stimulating factor (GM-CSF), with chemoradiotherapy followed by surgical resection | 82.3% | Not reported | Not reported |
| NCT04083976 | II | 178 (10 HNSCC) | Advanced or metastatic solid tumors of any histology with predefined FGFR1-4 alterations, progressed on 1 or more lines of systemic therapy | Erdafitinib | 40% | 5.2 | Not reported |
| NCT02383927 | II | 30 | R/M HNSCC patients with ≥1 prior platinum-containing regimen | Tipifarnib (farnesyltransferase inhibitor that disrupts HRAS function) | 55% | 5.6 | Not reported |
| NCT02101034 | II | 30 | Platinum-resistant, cetuximab-naive HPV (−) R/M HNSCC | Palbociclib and Cetuximab | 35% | 6.4 | Not reported |
| NCT04643379 | II | 12 | R/M HNSCC with no prior treatment | Olaparib, Carboplatin and Pembrolizumab | 67% | Not reported | Not reported |
| NCT02538510 | II | 25 | Incurable HNSCC progressed on standard therapy but no prior immunotherapy | Vorinostat and Pembrolizumab | 32% | 4.5 | Not reported |
| NCT03019003 | Ib | 12 | R/M HNSCC who progressed on anti-programmed cell death protein PD-1 (anti-PD-1) therapy | Azacitidine, Durvalumab and Tremelimumab | Not reported | Not reported | Not reported |
| NCT04624113 | I-II | 12 | R/M HNSCC with PD-L1 positive tumors, progressed through standard therapies | Tazometostat with Pembrolizumab | 0% | 2.1 | 41% |
| NCT04225117 | II | 46 | R/M HNSCC progressed through platinum-based chemotherapy and PD-1/PD-L1 inhibitor therapy, with ≤2 previous lines of cytotoxic therapy | Enfortumab Vedotin (antibody drug conjugate targeting Nectin-4) | 23.9% | 3.9 | 56.5% |
| NCT03485209 | II | 40 | R/M HNSCC progressed through platinum based chemotherapy and checkpoint inhibitors | Tisotumab Vedotin (antibody drug conjugate targeting tissue factor) | 32.5% | 4.2 | Not reported |
| NCT03964727 | II | 43 | Locally advanced or metastatic HNSCC that progressed following platinum-based chemotherapy and anti-PD-(L)1 therapy | Sacituzumab Govitecan (antibody drug conjugate targeting Trop 2) | 16% | 4.1 | Not reported |
| NCT05688605 | I/II | 33 (6 HNSCC) | EGFR-positive patients with refractory advanced squamous cell carcinomas of the head and neck (HNSCC) | MRG003 (antibody drug conjugate targeting EGFR) with Pucotenlimab | 60% | Not reported | Not reported |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sim, R.H.Z.; Voon, P.J.; Cheo, S.W.; Lim, D.W.-T., on behalf of the Asian Clinical Trials Network for Cancers Project—Head and Neck Cancer Group (ATLAS-HNCG). Novel Therapeutic Strategies for Squamous Cell Carcinoma of the Head and Neck: Beyond EGFR and Checkpoint Blockade. Biomedicines 2025, 13, 1972. https://doi.org/10.3390/biomedicines13081972
Sim RHZ, Voon PJ, Cheo SW, Lim DW-T on behalf of the Asian Clinical Trials Network for Cancers Project—Head and Neck Cancer Group (ATLAS-HNCG). Novel Therapeutic Strategies for Squamous Cell Carcinoma of the Head and Neck: Beyond EGFR and Checkpoint Blockade. Biomedicines. 2025; 13(8):1972. https://doi.org/10.3390/biomedicines13081972
Chicago/Turabian StyleSim, Rachel Hui Zhen, Pei Jye Voon, Seng Wee Cheo, and Darren Wan-Teck Lim on behalf of the Asian Clinical Trials Network for Cancers Project—Head and Neck Cancer Group (ATLAS-HNCG). 2025. "Novel Therapeutic Strategies for Squamous Cell Carcinoma of the Head and Neck: Beyond EGFR and Checkpoint Blockade" Biomedicines 13, no. 8: 1972. https://doi.org/10.3390/biomedicines13081972
APA StyleSim, R. H. Z., Voon, P. J., Cheo, S. W., & Lim, D. W.-T., on behalf of the Asian Clinical Trials Network for Cancers Project—Head and Neck Cancer Group (ATLAS-HNCG). (2025). Novel Therapeutic Strategies for Squamous Cell Carcinoma of the Head and Neck: Beyond EGFR and Checkpoint Blockade. Biomedicines, 13(8), 1972. https://doi.org/10.3390/biomedicines13081972






