The Multifaceted Role of Growth Differentiation Factor 15 (GDF15): A Narrative Review from Cancer Cachexia to Target Therapy
Abstract
1. Introduction
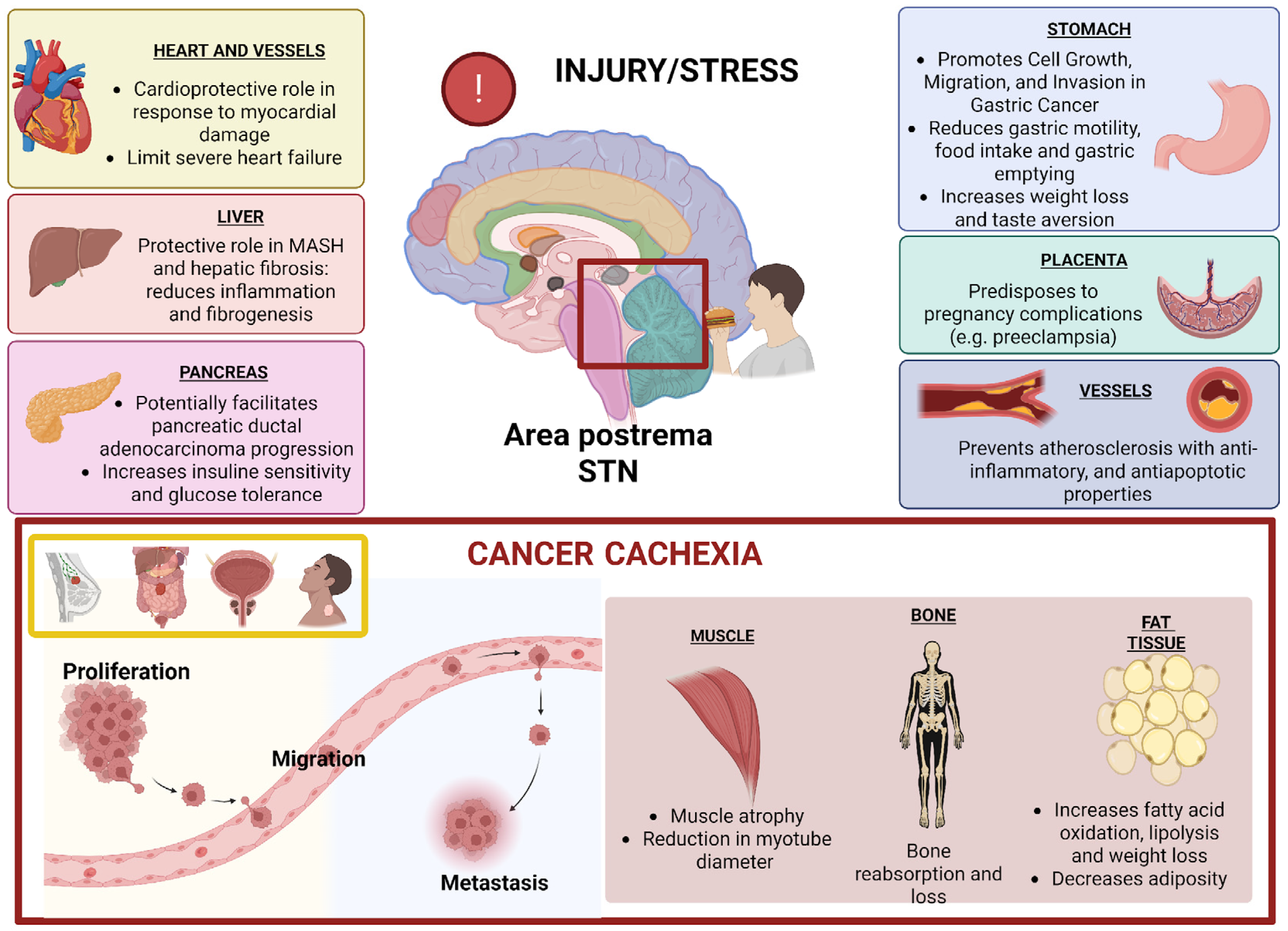
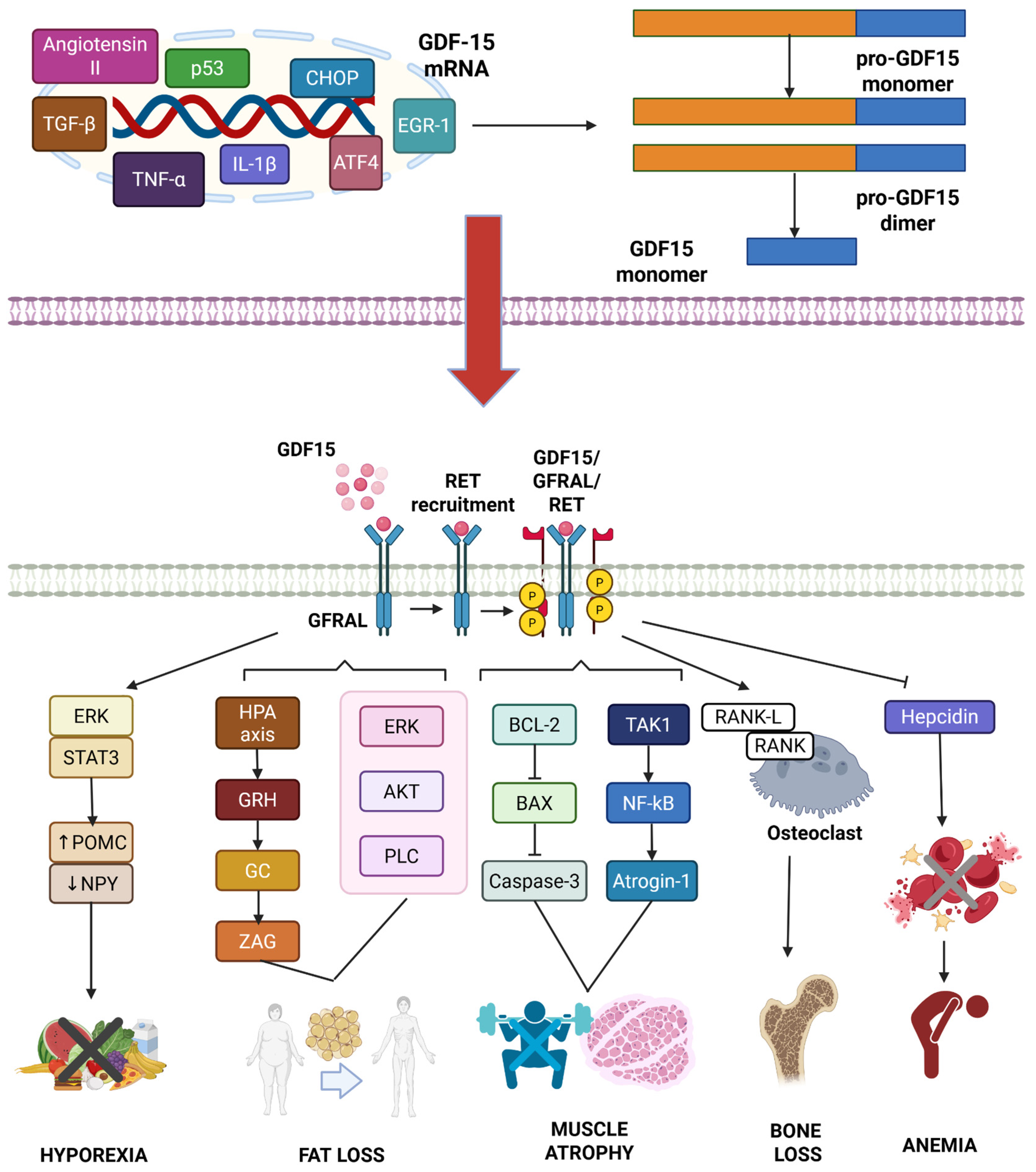
2. Physiological and Pathological Functions of GDF15
3. The Role of GDF15 in Various Tumor Types
| Tumor Type | Tumorigenic Functions | Sample Type | Diagnostic Role | Prognostic Role | Predictive Role | References |
|---|---|---|---|---|---|---|
| CRC | Reduction in tumor lymphocyte infiltration and PD-L1 positivity. Promotion of invasion, EMT, and metastasis via TGF-β, Smad2/3, and PI3K/AKT/GSK-3β pathways. Enhancement of chemoresistance and oxidative stress control through Nrf2 activation. | Serum | Biomarker for CRC diagnosis. Biomarker for detection of adenomas and CRC recurrence. Association with metastasis. | Association with poor survival outcomes. | Association with resistance to oxaliplatin. | [79,86,87,88,89,90] |
| GC | Promotion of tumor growth and invasion via STAT3 phosphorylation and ErbB2 transactivation. Enhancement of chemoresistance through antioxidant pathways. | Serum | Biomarker for GC diagnosis. | Association with poor survival outcomes. | Association with resistance to cisplatin. | [80,91,92,93,94,95] |
| Tissue | - | Association with better OS, while GFRAL and RET are associated with poor OS. | - | |||
| PC | Promotion of tumor metastasis and chemoresistance via GDF15-GFRAL, NR5A2, p38 MAPK, and AKT/CREB1 pathways. Inhibition of immune response via NF-κB pathway and TAM activity. | Serum | Biomarker for PC diagnosis. Biomarker to differentiate PC from benign disease, chronic pancreatitis, and healthy individuals. | Association with PC progression, recurrence, and poor survival outcomes. | - | [96,97,98,99,100,101,102,103,104] |
| Tissue | - | Association with PC progression. | - | |||
| BC | Promotion of tumor EMT and metastasis through downregulation of E-cadherin and upregulation of mesenchymal markers. Enhancement of chemoresistance via HER2 and p38 MAPK phosphorylation. Maintenance of cancer stem cells through the ERK1/2 pathway. Induction of hepcidin via the SMAD1-5-8 pathway. Promotion of tumor sphere formation. | Tissue | - | - | Association with resistance to trastuzumab, eribulin, and paclitaxel (in TNBC). Association with radioresistance. | [112,113,114,115,116,117,118,119] |
| OC | Promotion of tumor proliferation, invasion, and chemoresistance. Downstream factor of GPX3. | Serum | Biomarker for OC diagnosis. Biomarker for detection of OC recurrence | Association with poor survival outcomes. | Association with first-line cisplatin resistance. | [81,120,121,122,123] |
| Tissue | - | Association with poor survival outcomes. | GPX3 (promoting expression of GDF15) associated with ICI resistance. Correlation with PD-L1 expression. | |||
| CCa | Promotion of tumor proliferation through the upregulation of cyclinD1/E1 and downregulation of p21 via the PI3K/AKT and MAPK/ERK pathways in complex with ErbB2. | - | - | - | - | [124] |
| PCa | Promotion of tumor metastasis via the FAK-RhoA pathway. Enhancement of chemoresistance. Promotion of bone metastasis through osteoblastic production of CCL2 and RANKL. | Serum | Biomarker for distinguishing PCa from benign conditions. GDPP is a diagnostic biomarker for bone metastasis in castration-resistant PCa. | Biomarker for distinguishing indolent and aggressive PCa. Predictor of poor cancer-specific survival. | Association with resistance to docetaxel in metastatic castration-resistant PCa | [125,126,127,128,129,130] |
| HCC | Modulator in MASH, MAFLD, liver fibrosis, and HCC. Conflicting roles in HCC development, with evidence suggesting both pro-tumorigenic and anti-tumorigenic effects. Promotion of angiogenesis via Src. Suppression of anti-tumor immunity. | Serum | Biomarker for HCC diagnosis. Biomarker for HCC recurrence post-DAA therapy in HCV+ patients. | Association with poor survival outcomes. Correlation with the severity of MAFLD and fibrosis progression. | - | [105,106,107,108,109,110,111] |
| NSCLC | Regulation of proliferation, invasion, and migration via PTEN/PI3K/AKT signaling pathway. | Serum | Biomarker for lung cancer diagnosis. | Associated with poor survival outcomes in locally advanced NSCLC treated with chemoradiotherapy. | Association with resistance to chemotherapy and ICIs. | [84,131,132] |
| Melanoma | GDF15 overexpression is a product of the constitutively active mutant V600E B-RaF. Induction of angiogenesis, role in melanocyte differentiation and proliferation. | Serum | - | Association with poor survival outcomes. | Association with ICI resistance. | [133,134] |
| OSA | Promotion of tumor metastasis via TGF-β signaling. | Serum and tissue | - | Association with poor survival outcomes. | - | [85] |
| GBM | Correlation with a reduction in tumor lymphocyte infiltration. | Tissue | - | Association with poor survival outcomes. | - | [135] |
| OSCC | Promotion of EMT via SMAD2/3, PI3K/AKT, and MEK/ERK pathways. Anti-apoptotic effects through interaction with p53. | Serum | Biomarker for distinguishing OSCC, oral leukoplakia, and healthy controls. | Association with poor prognosis. Association with persistence of disease after surgery. | Association with resistance to TPF induction treatment. Association with radioresistance. | [136,137,138,139] |
4. A Focus on the Diagnostic, Prognostic, and Predictive Role of GDF15 in HEAD and Neck Cancer
5. Targeting GDF15 as a Therapeutic Strategy in Cancer
6. Discussion
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CRC | colorectal cancer |
| GC | gastric cancer |
| PC | pancreatic cancer |
| BC | breast cancer |
| OC | ovarian cancer |
| CCa | cervical cancer |
| PCa | prostate cancer |
| HCC | hepatocellular carcinoma |
| NSCLC | non-small cell lung cancer |
| OSA | osteosarcoma |
| GBM | glioblastoma |
| OSCC | oral squamous cell carcinoma |
| AKT/PKB | protein kinase B |
| CREB1 | cAMP response element-binding protein 1 |
| CCL2 | C-C motif chemokine ligand 2 |
| CXXC4 | CXXC finger protein 4 |
| DAAs | direct-acting antiviral agents |
| EGR1 | early growth response protein 1 |
| EMT | epithelial-to-mesenchymal transition |
| ERK1/2 | extracellular signal-regulated kinase 1/2 |
| ErbB2 | erythroblastic oncogene B2 |
| FAK-RhoA | focal adhesion kinase-Ras homolog family member A |
| GDPP | GDF15 propeptide |
| GDF15 | growth differentiation factor 15 |
| GFRAL | glial cell-derived neurotrophic factor family receptor α-like |
| GPX3 | glutathione peroxidase 3 |
| GSK-3β | glycogen synthase kinase-3β |
| HCV | hepatitis C virus |
| ICIs | immune checkpoint inhibitors |
| MAPK | mitogen-activated protein kinase |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NR5A2 | nuclear receptor subfamily 5 group A member 2 |
| Nrf2 | nuclear factor erythroid 2–related factor 2 |
| PD-L1 | programmed death-ligand 1 |
| PI3K | phosphoinositide 3-kinase |
| RANKL | receptor activator of nuclear factor κB ligand |
| STAT3 | signal transducer and activator of transcription 3 |
| TAM | tumor-associated macrophage |
| TGF-β | transforming growth factor-β |
| TNBC | triple-negative breast cancer |
| TPF | taxotere: platinum, fluorouracil |
References
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Hegde, M.; Daimary, U.D.; Girisa, S.; Kumar, A.; Kunnumakkara, A.B. Tumor cell anabolism and host tissue catabolism-energetic inefficiency during cancer cachexia. Exp. Biol. Med. 2022, 247, 713–733. [Google Scholar] [CrossRef]
- Arends, J.; Strasser, F.; Gonella, S.; Solheim, T.S.; Madeddu, C.; Ravasco, P.; Buonaccorso, L.; de van der Schueren, M.A.E.; Baldwin, C.; Chasen, M.; et al. Cancer cachexia in adult patients: ESMO Clinical Practice Guidelines. ESMO Open 2021, 6, 100092. [Google Scholar] [CrossRef]
- Associazione Italiana di Oncologia Medica (AIOM). Linee Guida AIOM per il Trattamento e la Prevenzione Della Cachessia Neoplastica. 2024. Available online: https://www.aiom.it/linee-guida-aiom-2024-trattamento-e-prevenzione-della-cachessia-neoplastica/ (accessed on 24 May 2025).
- Caillet, P.; Liuu, E.; Raynaud Simon, A.; Bonnefoy, M.; Guerin, O.; Berrut, G.; Lesourd, B.; Jeandel, C.; Ferry, M.; Rolland, Y.; et al. Association between cachexia, chemotherapy and outcomes in older cancer patients: A systematic review. Clin. Nutr. 2017, 36, 1473–1482. [Google Scholar] [CrossRef]
- Yu, Y.; Yan, L.; Huang, T.; Wu, Z.; Liu, J. Cancer cachexia reduces the efficacy of immune checkpoint inhibitors in cancer patients. Aging 2024, 16, 5354–5369. [Google Scholar] [CrossRef]
- Lis, C.G.; Gupta, D.; Lammersfeld, C.A.; Markman, M.; Vashi, P.G. Role of nutritional status in predicting quality of life outcomes in cancer--a systematic review of the epidemiological literature. Nutr. J. 2012, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Carlini, A.; Scarpi, E.; Bettini, C.; Ardizzoni, A.; Donati, C.M.; Fabbri, L.; Ghetti, F.; Martini, F.; Ricci, M.; Sansoni, E.; et al. Transdermal Fentanyl in Patients with Cachexia-A Scoping Review. Cancers 2024, 16, 3094. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef]
- Suzuki, H.; Asakawa, A.; Amitani, H.; Nakamura, N.; Inui, A. Cancer cachexia—Pathophysiology and management. J. Gastroenterol. 2013, 48, 574–594. [Google Scholar] [CrossRef]
- Brown, J.L.; Lee, D.E.; Rosa-Caldwell, M.E.; Brown, L.A.; Perry, R.A.; Haynie, W.S.; Huseman, K.; Sataranatarajan, K.; Van Remmen, H.; Washington, T.A.; et al. Protein imbalance in the development of skeletal muscle wasting in tumour-bearing mice. J. Cachexia Sarcopenia Muscle 2018, 9, 987–1002. [Google Scholar] [CrossRef]
- Honors, M.A.; Kinzig, K.P. The role of insulin resistance in the development of muscle wasting during cancer cachexia. J. Cachexia Sarcopenia Muscle 2012, 3, 5–11. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Z.; Hu, J.; Du, J.; Mitch, W.E. Insulin resistance accelerates muscle protein degradation: Activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology 2006, 147, 4160–4168. [Google Scholar] [CrossRef] [PubMed]
- Tsoli, M.; Schweiger, M.; Vanniasinghe, A.S.; Painter, A.; Zechner, R.; Clarke, S.; Robertson, G. Depletion of white adipose tissue in cancer cachexia syndrome is associated with inflammatory signaling and disrupted circadian regulation. PLoS ONE 2014, 9, 92966. [Google Scholar] [CrossRef] [PubMed]
- Bland, K.A.; Zopf, E.M.; Harrison, M.; Ely, M.; Cormie, P.; Liu, E.; Dowd, A.; Martin, P. Prognostic Markers of Overall Survival in Cancer Patients Attending a Cachexia Support Service: An Evaluation of Clinically Assessed Physical Function, Malnutrition and Inflammatory Status. Nutr. Cancer 2021, 73, 1400–1410. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Rhee, C.; Sim, J.J.; Stenvinkel, P.; Anker, S.D.; Kovesdy, C.P. Why cachexia kills: Examining the causality of poor outcomes in wasting conditions. J. Cachexia Sarcopenia Muscle 2013, 4, 89–94. [Google Scholar] [CrossRef]
- Loumaye, A.; Thissen, J.P. Biomarkers of cancer cachexia. Clin. Biochem. 2017, 50, 1281–1288. [Google Scholar] [CrossRef]
- Costelli, P.; Carbó, N.; Tessitore, L.; Bagby, G.J.; Lopez-Soriano, F.J.; Argilés, J.M.; Baccino, F.M. Tumor necrosis factor-alpha mediates changes in tissue protein turnover in a rat cancer cachexia model. J. Clin. Investig. 1993, 92, 2783–2789. [Google Scholar] [CrossRef]
- Chen, J.L.; Walton, K.L.; Qian, H.; Colgan, T.D.; Hagg, A.; Watt, M.J.; Harrison, C.A.; Gregorevic, P. Differential Effects of IL6 and Activin A in the Development of Cancer-Associated Cachexia. Cancer Res. 2016, 76, 5372–5382. [Google Scholar] [CrossRef]
- Talbert, E.E.; Lewis, H.L.; Farren, M.R.; Ramsey, M.L.; Chakedis, J.M.; Rajasekera, P.; Haverick, E.; Sarna, A.; Bloomston, M.; Pawlik, T.M.; et al. Circulating monocyte chemoattractant protein-1 (MCP-1) is associated with cachexia in treatment-naïve pancreatic cancer patients. J. Cachexia Sarcopenia Muscle 2018, 9, 358–368. [Google Scholar] [CrossRef]
- Chen, J.L.; Walton, K.L.; Winbanks, C.E.; Murphy, K.T.; Thomson, R.E.; Makanji, Y.; Qian, H.; Lynch, G.S.; Harrison, C.A.; Gregorevic, P. Elevated expression of activins promotes muscle wasting and cachexia. FASEB J. 2014, 28, 1711–1723. [Google Scholar] [CrossRef]
- Trendelenburg, A.U.; Meyer, A.; Rohner, D.; Boyle, J.; Hatakeyama, S.; Glass, D.J. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am. J. Physiol. Cell Physiol. 2009, 296, C1258–C1270. [Google Scholar] [CrossRef]
- Loumaye, A.; de Barsy, M.; Nachit, M.; Lause, P.; Frateur, L.; van Maanen, A.; Trefois, P.; Gruson, D.; Thissen, J.P. Role of Activin A and myostatin in human cancer cachexia. J. Clin. Endocrinol. Metab. 2015, 100, 2030–2038. [Google Scholar] [CrossRef] [PubMed]
- Togashi, Y.; Kogita, A.; Sakamoto, H.; Hayashi, H.; Terashima, M.; de Velasco, M.A.; Sakai, K.; Fujita, Y.; Tomida, S.; Kitano, M.; et al. Activin signal promotes cancer progression and is involved in cachexia in a subset of pancreatic cancer. Cancer Lett. 2015, 356 Pt B, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Hoda, M.A.; Rozsas, A.; Lang, E.; Klikovits, T.; Lohinai, Z.; Torok, S.; Berta, J.; Bendek, M.; Berger, W.; Hegedus, B.; et al. High circulating activin A level is associated with tumor progression and predicts poor prognosis in lung adenocarcinoma. Oncotarget 2016, 7, 13388–13399. [Google Scholar] [CrossRef] [PubMed]
- Young, V.R.; Munro, H.N. Ntau-methylhistidine (3-methylhistidine) and muscle protein turnover: An overview. Fed. Proc. 1978, 37, 2291–2300. [Google Scholar]
- Sun, S.; Henriksen, K.; Karsdal, M.A.; Armbrecht, G.; Belavý, D.L.; Felsenberg, D.; Rittweger, J.; Wang, Y.; Zheng, Q.; Nedergaard, A.F. Measurement of a MMP-2 degraded Titin fragment in serum reflects changes in muscle turnover induced by atrophy. Exp. Gerontol. 2014, 58, 83–89. [Google Scholar] [CrossRef]
- Nedergaard, A.; Dalgas, U.; Primdahl, H.; Johansen, J.; Overgaard, J.; Overgaard, K.; Henriksen, K.; Karsdal, M.A.; Lønbro, S. Collagen fragment biomarkers as serological biomarkers of lean body mass—A biomarker pilot study from the DAHANCA25B cohort and matched controls. J. Cachexia Sarcopenia Muscle 2015, 6, 335–342. [Google Scholar] [CrossRef]
- Narasimhan, A.; Ghosh, S.; Stretch, C.; Greiner, R.; Bathe, O.F.; Baracos, V.; Damaraju, S. Small RNAome profiling from human skeletal muscle: Novel miRNAs and their targets associated with cancer cachexia. J. Cachexia Sarcopenia Muscle 2017, 8, 405–416. [Google Scholar] [CrossRef]
- Chen, D.; Goswami, C.P.; Burnett, R.M.; Anjanappa, M.; Bhat-Nakshatri, P.; Muller, W.; Nakshatri, H. Cancer affects microRNA expression, release, and function in cardiac and skeletal muscle. Cancer Res. 2014, 74, 4270–4281. [Google Scholar] [CrossRef]
- Soares, R.J.; Cagnin, S.; Chemello, F.; Silvestrin, M.; Musaro, A.; De Pitta, C.; Lanfranchi, G.; Sandri, M. Involvement of microRNAs in the regulation of muscle wasting during catabolic conditions. J. Biol. Chem. 2014, 289, 21909–21925. [Google Scholar] [CrossRef]
- Ebadi, M.; Mazurak, V.C. Potential Biomarkers of Fat Loss as a Feature of Cancer Cachexia. Mediat. Inflamm. 2015, 2015, 820934. [Google Scholar] [CrossRef]
- Agustsson, T.; Rydén, M.; Hoffstedt, J.; van Harmelen, V.; Dicker, A.; Laurencikiene, J.; Isaksson, B.; Permert, J.; Arner, P. Mechanism of increased lipolysis in cancer cachexia. Cancer Res. 2007, 67, 5531–5537. [Google Scholar] [CrossRef]
- Mracek, T.; Stephens, N.A.; Gao, D.; Bao, Y.; Ross, J.A.; Rydén, M.; Arner, P.; Trayhurn, P.; Fearon, K.C.; Bing, C. Enhanced ZAG production by subcutaneous adipose tissue is linked to weight loss in gastrointestinal cancer patients. Br. J. Cancer 2011, 104, 441–447. [Google Scholar] [CrossRef]
- Lim, Y.L.; Teoh, S.E.; Yaow, C.Y.L.; Lin, D.J.; Masuda, Y.; Han, M.X.; Yeo, W.S.; Ng, Q.X. A Systematic Review and Meta-Analysis of the Clinical Use of Megestrol Acetate for Cancer-Related Anorexia/Cachexia. J. Clin. Med. 2022, 11, 3756. [Google Scholar] [CrossRef] [PubMed]
- Bootcov, M.R.; Bauskin, A.R.; Valenzuela, S.M.; Moore, A.G.; Bansal, M.; He, X.Y.; Zhang, H.P.; Donnellan, M.; Mahler, S.; Pryor, K.; et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc. Natl. Acad. Sci. USA 1997, 94, 11514–11519. [Google Scholar] [CrossRef] [PubMed]
- Mimeault, M.; Batra, S.K. Divergent molecular mechanisms underlying the pleiotropic functions of macrophage inhibitory cytokine-1 in cancer. J. Cell. Physiol. 2010, 224, 626–635. [Google Scholar] [CrossRef]
- Baek, S.J.; Horowitz, J.M.; Eling, T.E. Molecular cloning and characterization of human nonsteroidal anti-inflammatory drug-activated gene promoter. Basal transcription is mediated by Sp1 and Sp3. J. Biol. Chem. 2001, 276, 33384–33392. [Google Scholar] [CrossRef]
- Lawton, L.N.; Bonaldo, M.F.; Jelenc, P.C.; Qiu, L.; Baumes, S.A.; Marcelino, R.A.; de Jesus, G.M.; Wellington, S.; Knowles, J.A.; Warburton, D.; et al. Identification of a novel member of the TGF-beta superfamily highly expressed in human placenta. Gene 1997, 203, 17–26. [Google Scholar] [CrossRef]
- Hromas, R.; Hufford, M.; Sutton, J.; Xu, D.; Li, Y.; Lu, L. PLAB, a novel placental bone morphogenetic protein. Biochim. Biophys. Acta 1997, 1354, 40–44. [Google Scholar] [CrossRef]
- Paralkar, V.M.; Vail, A.L.; Grasser, W.A.; Brown, T.A.; Xu, H.; Vukicevic, S.; Ke, H.Z.; Qi, H.; Owen, T.A.; Thompson, D.D. Cloning and characterization of a novel member of the transforming growth factor-beta/bone morphogenetic protein family. J. Biol. Chem. 1998, 273, 13760–13767. [Google Scholar] [CrossRef]
- Filippini, D.M.; Carosi, F.; Panepinto, O.; Neri, G.; Nobili, E.; Tober, N.; Giusti, R.; Di Maio, M. Health-related quality of life assessment in head and neck cancer: A systematic review of phase II and III clinical trials. Heliyon 2024, 10, 40671. [Google Scholar] [CrossRef]
- Assadi, A.; Zahabi, A.; Hart, R.A. GDF15, an update of the physiological and pathological roles it plays: A review. Pflugers Arch. 2020, 472, 1535–1546. [Google Scholar] [CrossRef]
- Wischhusen, J.; Melero, I.; Fridman, W.H. Growth/Differentiation Factor-15 (GDF-15): From Biomarker to Novel Targetable Immune Checkpoint. Front. Immunol. 2020, 11, 951. [Google Scholar] [CrossRef]
- Molfino, A.; Amabile, M.I.; Imbimbo, G.; Rizzo, V.; Pediconi, F.; Catalano, C.; Emiliani, A.; Belli, R.; Ramaccini, C.; Parisi, C.; et al. Association between Growth Differentiation Factor-15 (GDF-15) Serum Levels, Anorexia and Low Muscle Mass among Cancer Patients. Cancers 2020, 13, 99. [Google Scholar] [CrossRef] [PubMed]
- Cimino, I.; Kim, H.; Tung, Y.C.L.; Pedersen, K.; Rimmington, D.; Tadross, J.A.; Kohnke, S.N.; Neves-Costa, A.; Barros, A.; Joaquim, S.; et al. Activation of the hypothalamic-pituitary-adrenal axis by exogenous and endogenous GDF15. Proc. Natl. Acad. Sci. USA 2021, 118, 2106868118. [Google Scholar] [CrossRef]
- Lerner, L.; Tao, J.; Liu, Q.; Nicoletti, R.; Feng, B.; Krieger, B.; Mazsa, E.; Siddiquee, Z.; Wang, R.; Huang, L.; et al. MAP3K11/GDF15 axis is a critical driver of cancer cachexia. J. Cachexia Sarcopenia Muscle 2016, 7, 467–482. [Google Scholar] [CrossRef] [PubMed]
- Burks, T.N.; Cohn, R.D. Role of TGF-β signaling in inherited and acquired myopathies. Skelet. Muscle 2011, 1, 19. [Google Scholar] [CrossRef] [PubMed]
- Falk, S.; Wurdak, H.; Ittner, L.M.; Ille, F.; Sumara, G.; Schmid, M.T.; Draganova, K.; Lang, K.S.; Paratore, C.; Leveen, P.; et al. Brain area-specific effect of TGF-beta signaling on Wnt-dependent neural stem cell expansion. Cell Stem Cell 2008, 2, 472–483. [Google Scholar] [CrossRef]
- Gordon, K.J.; Blobe, G.C. Role of transforming growth factor-beta superfamily signaling pathways in human disease. Biochim. Biophys. Acta 2008, 1782, 197–228. [Google Scholar] [CrossRef]
- Santibañez, J.F.; Quintanilla, M.; Bernabeu, C. TGF-β/TGF-β receptor system and its role in physiological and pathological conditions. Clin. Sci. 2011, 121, 233–251. [Google Scholar] [CrossRef]
- Li, J.J.; Liu, J.; Lupino, K.; Liu, X.; Zhang, L.; Pei, L. Growth Differentiation Factor 15 Maturation Requires Proteolytic Cleavage by PCSK3, -5, and -6. Mol. Cell. Biol. 2018, 38, e00249-18. [Google Scholar] [CrossRef]
- Fairlie, W.D.; Zhang, H.P.; Wu, W.M.; Pankhurst, S.L.; Bauskin, A.R.; Russell, P.K.; Brown, P.K.; Breit, S.N. The propeptide of the transforming growth factor-beta superfamily member, macrophage inhibitory cytokine-1 (MIC-1), is a multifunctional domain that can facilitate protein folding and secretion. J. Biol. Chem. 2001, 276, 16911–16918. [Google Scholar] [CrossRef]
- Weiss, A.; Attisano, L. The TGFbeta superfamily signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 47–63. [Google Scholar] [CrossRef]
- Jiang, J.; Thalamuthu, A.; Ho, J.E.; Mahajan, A.; Ek, W.E.; Brown, D.A.; Breit, S.N.; Wang, T.J.; Gyllensten, U.; Chen, M.H.; et al. A Meta-Analysis of Genome-Wide Association Studies of Growth Differentiation Factor-15 Concentration in Blood. Front. Genet. 2018, 9, 97. [Google Scholar] [CrossRef]
- Shi, M.; Zhu, J.; Wang, R.; Chen, X.; Mi, L.; Walz, T.; Springer, T.A. Latent TGF-β structure and activation. Nature 2011, 474, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Bauskin, A.R.; Brown, D.A.; Junankar, S.; Rasiah, K.K.; Eggleton, S.; Hunter, M.; Liu, T.; Smith, D.; Kuffner, T.; Pankhurst, G.J.; et al. The propeptide mediates formation of stromal stores of PROMIC-1: Role in determining prostate cancer outcome. Cancer Res. 2005, 65, 2330–2336. [Google Scholar] [CrossRef] [PubMed]
- Bauskin, A.R.; Jiang, L.; Luo, X.W.; Wu, L.; Brown, D.A.; Breit, S.N. The TGF-beta superfamily cytokine MIC-1/GDF15: Secretory mechanisms facilitate creation of latent stromal stores. J. Interferon Cytokine Res. 2010, 30, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chang, C.C.; Sun, Z.; Madsen, D.; Zhu, H.; Padkjær, S.B.; Wu, X.; Huang, T.; Hultman, K.; Paulsen, S.J.; et al. GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nat. Med. 2017, 23, 1158–1166. [Google Scholar] [CrossRef]
- Emmerson, P.J.; Wang, F.; Du, Y.; Liu, Q.; Pickard, R.T.; Gonciarz, M.D.; Coskun, T.; Hamang, M.J.; Sindelar, D.K.; Ballman, K.K.; et al. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat. Med. 2017, 23, 1215–1219. [Google Scholar] [CrossRef]
- Hsu, J.Y.; Crawley, S.; Chen, M.; Ayupova, D.A.; Lindhout, D.A.; Higbee, J.; Kutach, A.; Joo, W.; Gao, Z.; Fu, D.; et al. Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15. Nature 2017, 550, 255–259. [Google Scholar] [CrossRef]
- Mullican, S.E.; Lin-Schmidt, X.; Chin, C.N.; Chavez, J.A.; Furman, J.L.; Armstrong, A.A.; Beck, S.C.; South, V.J.; Dinh, T.Q.; Cash-Mason, T.D.; et al. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat. Med. 2017, 23, 1150–1157. [Google Scholar] [CrossRef]
- Li, Z.; Wang, B.; Wu, X.; Cheng, S.Y.; Paraoan, L.; Zhou, J. Identification, expression and functional characterization of the GRAL gene. J. Neurochem. 2005, 95, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yang, X.; Dai, J.; Lu, Y.; Zhang, J.; Keller, E.T. Prostate cancer promotes a vicious cycle of bone metastasis progression through inducing osteocytes to secrete GDF15 that stimulates prostate cancer growth and invasion. Oncogene 2019, 38, 4540–4559. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kim, S.H.; Han, D.H.; Jo, Y.S.; Lee, Y.H.; Lee, M.S. Growth differentiation factor 15 ameliorates nonalcoholic steatohepatitis and related metabolic disorders in mice. Sci. Rep. 2018, 8, 6789. [Google Scholar] [CrossRef] [PubMed]
- Breit, S.N.; Brown, D.A.; Tsai, V.W.W. The GDF15-GFRAL Pathway in Health and Metabolic Disease: Friend or Foe? Annu. Rev. Physiol. 2021, 83, 127–151. [Google Scholar] [CrossRef]
- Emmerson, P.J.; Duffin, K.L.; Chintharlapalli, S.; Wu, X. GDF15 and Growth Control. Front. Physiol. 2018, 9, 1712. [Google Scholar] [CrossRef]
- Tsai, V.W.W.; Macia, L.; Feinle-Bisset, C.; Manandhar, R.; Astrup, A.; Raben, A.; Lorenzen, J.K.; Schmidt, P.T.; Wiklund, F.; Pedersen, N.L.; et al. Serum Levels of Human MIC-1/GDF15 Vary in a Diurnal Pattern, Do Not Display a Profile Suggestive of a Satiety Factor and Are Related to BMI. PLoS ONE 2015, 10, 0133362. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Pare, G.; Hess, S.; Ford, R.J.; Sjaarda, J.; Raman, K.; McQueen, M.; Lee, S.; Haenel, H.; Steinberg, G.R.; et al. Growth Differentiation Factor 15 as a Novel Biomarker for Metformin. Diabetes Care 2017, 40, 280–283. [Google Scholar] [CrossRef]
- Kis, E.; Szatmári, T.; Keszei, M.; Farkas, R.; Esik, O.; Lumniczky, K.; Falus, A.; Sáfrány, G. Microarray analysis of radiation response genes in primary human fibroblasts. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 1506–1514. [Google Scholar] [CrossRef]
- Okazaki, R.; Moon, Y.; Norimura, T.; Eling, T. Ionizing radiation enhances the expression of the nonsteroidal anti-inflammatory drug-activated gene (NAG1) by increasing the expression of TP53 in human colon cancer cells. Radiat. Res. 2006, 165, 125–130. [Google Scholar] [CrossRef]
- Moritake, T.; Fujita, H.; Yanagisawa, M.; Nakawatari, M.; Imadome, K.; Nakamura, E.; Iwakawa, M.; Imai, T. Strain-dependent damage in mouse lung after carbon ion irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, e95–e102. [Google Scholar] [CrossRef] [PubMed]
- Tucker, J.D.; Joiner, M.C.; Thomas, R.A.; Grever, W.E.; Bakhmutsky, M.V.; Chinkhota, C.N.; Smolinski, J.M.; Divine, G.W.; Auner, G.W. Accurate gene expression-based biodosimetry using a minimal set of human gene transcripts. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Alvarez-Guaita, A.; Melvin, A.; Rimmington, D.; Dattilo, A.; Miedzybrodzka, E.L.; Cimino, I.; Maurin, A.C.; Roberts, G.P.; Meek, C.L.; et al. GDF15 Provides an Endocrine Signal of Nutritional Stress in Mice and Humans. Cell Metab. 2019, 29, 707–718.e8. [Google Scholar] [CrossRef]
- Clark, B.J.; Bull, T.M.; Benson, A.B.; Stream, A.R.; Macht, M.; Gaydos, J.; Meadows, C.; Burnham, E.L.; Moss, M.; ARDS Network Investigators. Growth differentiation factor-15 and prognosis in acute respiratory distress syndrome: A retrospective cohort study. Crit. Care 2013, 17, R92. [Google Scholar] [CrossRef]
- Ho, J.E.; Mahajan, A.; Chen, M.H.; Larson, M.G.; McCabe, E.L.; Ghorbani, A.; Cheng, S.; Johnson, A.D.; Lindgren, C.M.; Kempf, T.; et al. Clinical and genetic correlates of growth differentiation factor 15 in the community. Clin. Chem. 2012, 58, 1582–1591. [Google Scholar] [CrossRef]
- Johnen, H.; Lin, S.; Kuffner, T.; Brown, D.A.; Tsai, V.W.; Bauskin, A.R.; Wu, L.; Pankhurst, G.; Jiang, L.; Junankar, S.; et al. Tumor-induced anorexia and weight loss are mediated by the TGF-beta superfamily cytokine MIC-1. Nat. Med. 2007, 13, 1333–1340. [Google Scholar] [CrossRef]
- Kleinert, M.; Clemmensen, C.; Sjøberg, K.A.; Carl, C.S.; Jeppesen, J.F.; Wojtaszewski, J.F.P.; Kiens, B.; Richter, E.A. Exercise increases circulating GDF15 in humans. Mol. Metab. 2018, 9, 187–191. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Kong, J.; Tang, J.; Wu, Y.; Xu, E.; Zhang, H.; Lai, M. GDF15 promotes EMT and metastasis in colorectal cancer. Oncotarget 2016, 7, 860–872. [Google Scholar] [CrossRef]
- Joo, M.; Kim, D.; Lee, M.W.; Lee, H.J.; Kim, J.M. GDF15 Promotes Cell Growth, Migration, and Invasion in Gastric Cancer by Inducing STAT3 Activation. Int. J. Mol. Sci. 2023, 24, 2925. [Google Scholar] [CrossRef]
- Chang, C.; Cheng, Y.Y.; Kamlapurkar, S.; White, S.; Tang, P.W.; Elhaw, A.T.; Javed, Z.; Aird, K.M.; Mythreye, K.; Phaëton, R.; et al. GPX3 supports ovarian cancer tumor progression in vivo and promotes expression of GDF15. Gynecol. Oncol. 2024, 185, 8–16. [Google Scholar] [CrossRef]
- Huang, C.Y.; Beer, T.M.; Higano, C.S.; True, L.D.; Vessella, R.; Lange, P.H.; Garzotto, M.; Nelson, P.S. Molecular alterations in prostate carcinomas that associate with in vivo exposure to chemotherapy: Identification of a cytoprotective mechanism involving growth differentiation factor 15. Clin. Cancer Res. 2007, 13, 5825–5833. [Google Scholar] [CrossRef] [PubMed]
- Myojin, Y.; Hikita, H.; Tahata, Y.; Doi, A.; Kato, S.; Sasaki, Y.; Shirai, K.; Sakane, S.; Yamada, R.; Kodama, T.; et al. Serum growth differentiation factor 15 predicts hepatocellular carcinoma occurrence after hepatitis C virus elimination. Aliment. Pharmacol. Ther. 2022, 55, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Di Pastena, F.; Pond, G.; Tsakiridis, E.E.; Gouveia, A.; Ahmadi, E.; Biziotis, O.D.; Ali, A.; Swaminath, A.; Okawara, G.; Ellis, P.M.; et al. Growth differentiation factor 15 (GDF15) predicts relapse free and overall survival in unresected locally advanced non-small cell lung cancer treated with chemoradiotherapy. Radiat. Oncol. 2024, 19, 155. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, M.; Liu, X. GDF15 promotes osteosarcoma cell migration and invasion by regulating the TGF-β signaling pathway. Mol. Med. Rep. 2019, 20, 4262–4270. [Google Scholar] [CrossRef]
- Brown, D.A.; Hance, K.W.; Rogers, C.J.; Sansbury, L.B.; Albert, P.S.; Murphy, G.; Laiyemo, A.O.; Wang, Z.; Cross, A.J.; Schatzkin, A.; et al. Serum macrophage inhibitory cytokine-1 (MIC-1/GDF15): A potential screening tool for the prevention of colon cancer? Cancer Epidemiol. Biomark. Prev. 2012, 21, 337–346. [Google Scholar] [CrossRef]
- Wallin, U.; Glimelius, B.; Jirström, K.; Darmanis, S.; Nong, R.Y.; Pontén, F.; Johansson, C.; Påhlman, L.; Birgisson, H. Growth differentiation factor 15: A prognostic marker for recurrence in colorectal cancer. Br. J. Cancer 2011, 104, 1619–1627. [Google Scholar] [CrossRef]
- Vocka, M.; Langer, D.; Fryba, V.; Petrtyl, J.; Hanus, T.; Kalousova, M.; Zima, T.; Petruzelka, L. Growth/differentiation factor 15 (GDF-15) as new potential serum marker in patients with metastatic colorectal cancer. Cancer Biomark. 2018, 21, 869–874. [Google Scholar] [CrossRef]
- Lin, H.; Luo, Y.; Gong, T.; Fang, H.; Li, H.; Ye, G.; Zhang, Y.; Zhong, M. GDF15 induces chemoresistance to oxaliplatin by forming a reciprocal feedback loop with Nrf2 to maintain redox homeostasis in colorectal cancer. Cell Oncol. 2024, 47, 1149–1165. [Google Scholar] [CrossRef]
- Kim, K.K.; Lee, J.J.; Yang, Y.; You, K.H.; Lee, J.H. Macrophage inhibitory cytokine-1 activates AKT and ERK-1/2 via the transactivation of ErbB2 in human breast and gastric cancer cells. Carcinogenesis 2008, 29, 704–712. [Google Scholar] [CrossRef]
- Blanco-Calvo, M.; Tarrío, N.; Reboredo, M.; Haz-Conde, M.; García, J.; Quindós, M.; Figueroa, A.; Antón-Aparicio, L.; Calvo, L.; Valladares-Ayerbes, M. Circulating levels of GDF15, MMP7 and miR-200c as a poor prognostic signature in gastric cancer. Future Oncol. 2014, 10, 1187–1202. [Google Scholar] [CrossRef]
- Wang, S.F.; Chang, Y.L.; Fang, W.L.; Li, A.F.; Chen, C.F.; Yeh, T.S.; Hung, G.Y.; Huang, K.H.; Lee, H.C. Growth differentiation factor 15 induces cisplatin resistance through upregulation of xCT expression and glutathione synthesis in gastric cancer. Cancer Sci. 2023, 114, 3301–3317. [Google Scholar] [CrossRef]
- Wang, S.F.; Chang, Y.L.; Liu, T.Y.; Huang, K.H.; Fang, W.L.; Li, A.F.; Yeh, T.S.; Hung, G.Y.; Lee, H.C. Mitochondrial dysfunction decreases cisplatin sensitivity in gastric cancer cells through upregulation of integrated stress response and mitokine GDF15. FEBS J. 2024, 291, 1131–1150. [Google Scholar] [CrossRef]
- Buchholz, K.; Antosik, P.; Grzanka, D.; Gagat, M.; Smolińska, M.; Grzanka, A.; Gzil, A.; Kasperska, A.; Klimaszewska-Wiśniewska, A. Expression of the Body-Weight Signaling Players: GDF15, GFRAL and RET and their clinical relevance in Gastric Cancer. J. Cancer 2021, 12, 4698–4709. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, J.; Yin, L.; Yang, J.; Zheng, Y.; Zhang, M.; Ni, B.; Wang, H. Upregulated GDF-15 expression facilitates pancreatic ductal adenocarcinoma progression through orphan receptor GFRAL. Aging 2020, 12, 22564–22581. [Google Scholar] [CrossRef]
- Guo, F.; Zhou, Y.; Guo, H.; Ren, D.; Jin, X.; Wu, H. NR5A2 transcriptional activation by BRD4 promotes pancreatic cancer progression by upregulating GDF15. Cell Death Discov. 2021, 7, 78. [Google Scholar] [CrossRef]
- Ji, H.; Lu, H.W.; Li, Y.M.; Lu, L.; Wang, J.L.; Zhang, Y.F.; Shang, H. Twist promotes invasion and cisplatin resistance in pancreatic cancer cells through growth differentiation factor 15. Mol. Med. Rep. 2015, 12, 3841–3848. [Google Scholar] [CrossRef]
- Kalli, M.; Minia, A.; Pliaka, V.; Fotis, C.; Alexopoulos, L.G.; Stylianopoulos, T. Solid stress-induced migration is mediated by GDF15 through Akt pathway activation in pancreatic cancer cells. Sci. Rep. 2019, 9, 978. [Google Scholar] [CrossRef]
- Ratnam, N.M.; Peterson, J.M.; Talbert, E.E.; Ladner, K.J.; Rajasekera, P.V.; Schmidt, C.R.; Dillhoff, M.E.; Swanson, B.J.; Haverick, E.; Kladney, R.D.; et al. NF-κB regulates GDF-15 to suppress macrophage surveillance during early tumor development. J. Clin. Investig. 2017, 127, 3796–3809. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yan, S.; Tian, H.; Bao, Y. Macrophage inhibitory cytokine-1 versus carbohydrate antigen 19-9 as a biomarker for diagnosis of pancreatic cancer: A PRISMA-compliant meta-analysis of diagnostic accuracy studies. Medicine 2018, 97, 9994. [Google Scholar] [CrossRef] [PubMed]
- Hogendorf, P.; Durczyński, A.; Skulimowski, A.; Kumor, A.; Poznańska, G.; Strzelczyk, J. Growth differentiation factor (GDF-15) concentration combined with Ca125 levels in serum is superior to commonly used cancer biomarkers in differentiation of pancreatic mass. Cancer Biomark. 2018, 21, 505–511. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, R.S.; Emmanuel, S.; Williams, D.; Stoita, A. Macrophage inhibitory cytokine-1/growth differentiation factor-15 in premalignant and neoplastic tumours in a high-risk pancreatic cancer cohort. World, J. Gastroenterol. 2020, 26, 1660–1673. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Xu, L.X.; Huang, L.Y.; Guo, F.; Zhang, F.; He, X.Y.; Yuan, Y.Z.; Yao, W.Y. Combined detection of serum UL16-binding protein 2 and macrophage inhibitory cytokine-1 improves early diagnosis and prognostic prediction of pancreatic cancer. Oncol. Lett. 2014, 8, 2096–2102. [Google Scholar] [CrossRef]
- Gordon, E.J.; Fukuhara, D.; Weström, S.; Padhan, N.; Sjöström, E.O.; van Meeteren, L.; He, L.; Orsenigo, F.; Dejana, E.; Bentley, K.; et al. The endothelial adaptor molecule TSAd is required for VEGF-induced angiogenic sprouting through junctional c-Src activation. Sci. Signal. 2016, 9, ra72. [Google Scholar] [CrossRef]
- Du, Y.N.; Zhao, J.W. GDF15: Immunomodulatory Role in Hepatocellular Carcinoma Pathogenesis and Therapeutic Implications. J. Hepatocell. Carcinoma 2024, 11, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chi, X.; Gong, Q.; Gao, L.; Niu, Y.; Chi, X.; Cheng, M.; Si, Y.; Wang, M.; Zhong, J.; et al. Association of serum level of growth differentiation factor 15 with liver cirrhosis and hepatocellular carcinoma. PLoS ONE 2015, 10, 0127518. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Dai, W.; Zhu, C.; Liu, H.; Li, Y.; Zhang, P. Circulating levels of growth differentiation factor 15 and sex hormones in male patients with HBV-associated hepatocellular carcinoma. Biomed. Pharmacother. 2020, 121, 109574. [Google Scholar] [CrossRef]
- Chung, H.K.; Ryu, D.; Kim, K.S.; Chang, J.Y.; Kim, Y.K.; Yi, H.S.; Kang, S.G.; Choi, M.J.; Lee, S.E.; Jung, S.B.; et al. Growth differentiation factor 15 is a myomitokine governing systemic energy homeostasis. J. Cell Biol. 2017, 216, 149–165. [Google Scholar] [CrossRef]
- Xu, Q.; Xu, H.X.; Li, J.P.; Wang, S.; Fu, Z.; Jia, J.; Wang, L.; Zhu, Z.F.; Lu, R.; Yao, Z. Growth differentiation factor 15 induces growth and metastasis of human liver cancer stem-like cells via AKT/GSK-3β/β-catenin signaling. Oncotarget 2017, 8, 16972–16987. [Google Scholar] [CrossRef]
- Yu, J.; Shen, B.; Chu, E.S.H.; Teoh, N.; Cheung, K.F.; Wu, C.W.; Wang, S.; Lam, C.N.; Feng, H.; Zhao, J.; et al. Inhibitory role of peroxisome proliferator-activated receptor gamma in hepatocarcinogenesis in mice and in vitro. Hepatology 2010, 51, 2008–2019. [Google Scholar] [CrossRef]
- Peake, B.F.; Eze, S.M.; Yang, L.; Castellino, R.C.; Nahta, R. Growth differentiation factor 15 mediates epithelial mesenchymal transition and invasion of breast cancers through IGF-1R-FoxM1 signaling. Oncotarget 2017, 8, 94393–94406. [Google Scholar] [CrossRef]
- Blanchette-Farra, N.; Kita, D.; Konstorum, A.; Tesfay, L.; Lemler, D.; Hegde, P.; Claffey, K.P.; Torti, F.M.; Torti, S.V. Contribution of three-dimensional architecture and tumor-associated fibroblasts to hepcidin regulation in breast cancer. Oncogene 2018, 37, 4013–4032. [Google Scholar] [CrossRef] [PubMed]
- Sasahara, A.; Tominaga, K.; Nishimura, T.; Yano, M.; Kiyokawa, E.; Noguchi, M.; Noguchi, M.; Kanauchi, H.; Ogawa, T.; Minato, H.; et al. An autocrine/paracrine circuit of growth differentiation factor (GDF) 15 has a role for maintenance of breast cancer stem-like cells. Oncotarget 2017, 8, 24869–24881. [Google Scholar] [CrossRef] [PubMed]
- Joshi, J.P.; Brown, N.E.; Griner, S.E.; Nahta, R. Growth differentiation factor 15 (GDF15)-mediated HER2 phosphorylation reduces trastuzumab sensitivity of HER2-overexpressing breast cancer cells. Biochem. Pharmacol. 2011, 82, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, S.M.; Paplomata, E.; Peake, B.M.; Sanabria, E.; Chen, Z.; Nahta, R. P38 MAPK contributes to resistance and invasiveness of HER2-overexpressing breast cancer. Curr. Med. Chem. 2014, 21, 501–510. [Google Scholar] [CrossRef]
- Bellio, C.; Emperador, M.; Castellano, P.; Gris-Oliver, A.; Canals, F.; Sánchez-Pla, A.; Zamora, E.; Arribas, J.; Saura, C.; Serra, V.; et al. GDF15 Is an Eribulin Response Biomarker also Required for Survival of DTP Breast Cancer Cells. Cancers 2022, 14, 2562. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, X.; Hu, S.; Pan, Y.; Zhang, J.; Tai, G.; Shao, C. GDF15 Contributes to Radioresistance by Mediating the EMT and Stemness of Breast Cancer Cells. Int. J. Mol. Sci. 2022, 23, 10911. [Google Scholar] [CrossRef]
- Gkretsi, V.; Stylianou, A.; Kalli, M.; Louca, M.; Voutouri, C.; Zaravinos, A.; Stylianopoulos, T. Silencing of Growth Differentiation Factor-15 Promotes Breast Cancer Cell Invasion by Down-regulating Focal Adhesion Genes. Anticancer. Res. 2020, 40, 1375–1385. [Google Scholar] [CrossRef]
- Staff, A.C.; Bock, A.J.; Becker, C.; Kempf, T.; Wollert, K.C.; Davidson, B. Growth differentiation factor-15 as a prognostic biomarker in ovarian cancer. Gynecol. Oncol. 2010, 118, 237–243. [Google Scholar] [CrossRef]
- Zhang, Y.; Hua, W.; Niu, L.C.; Li, S.M.; Wang, Y.M.; Shang, L.; Zhang, C.; Li, W.N.; Wang, R.; Chen, B.L.; et al. Elevated growth differentiation factor 15 expression predicts poor prognosis in epithelial ovarian cancer patients. Tumour Biol. 2016, 37, 9423–9431. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, X.; Zhang, W. GDF15 predict platinum response during first-line chemotherapy and can act as a complementary diagnostic serum biomarker with CA125 in epithelial ovarian cancer. BMC Cancer 2018, 18, 328. [Google Scholar] [CrossRef]
- Izaguirre, D.I.; Ng, C.W.; Kwan, S.Y.; Kun, E.H.; Tsang, Y.T.M.; Gershenson, D.M.; Wong, K.K. The Role of GDF15 in Regulating the Canonical Pathways of the Tumor Microenvironment in Wild-Type p53 Ovarian Tumor and Its Response to Chemotherapy. Cancers 2020, 12, 3043. [Google Scholar] [CrossRef]
- Li, S.; Ma, Y.M.; Zheng, P.S.; Zhang, P. GDF15 promotes the proliferation of cervical cancer cells by phosphorylating AKT1 and Erk1/2 through the receptor ErbB2. J. Exp. Clin. Cancer Res. 2018, 37, 80. [Google Scholar] [CrossRef]
- Bansal, N.; Kumar, D.; Gupta, A.; Chandra, D.; Sankhwar, S.N.; Mandhani, A. Relevance of MIC-1 in the Era of PSA as a Serum Based Predictor of Prostate Cancer: A Critical Evaluation. Sci. Rep. 2017, 7, 16824. [Google Scholar] [CrossRef]
- Brown, D.A.; Lindmark, F.; Stattin, P.; Bälter, K.; Adami, H.O.; Zheng, S.L.; Xu, J.; Isaacs, W.B.; Grönberg, H.; Breit, S.N.; et al. Macrophage inhibitory cytokine 1: A new prognostic marker in prostate cancer. Clin. Cancer Res. 2009, 15, 6658–6664. [Google Scholar] [CrossRef]
- Senapati, S.; Rachagani, S.; Chaudhary, K.; Johansson, S.L.; Singh, R.K.; Batra, S.K. Overexpression of macrophage inhibitory cytokine-1 induces metastasis of human prostate cancer cells through the FAK-RhoA signaling pathway. Oncogene 2010, 29, 1293–1302. [Google Scholar] [CrossRef]
- Mahon, K.L.; Sutherland, S.I.; Lin, H.M.; Stockler, M.R.; Gurney, H.; Mallesara, G.; Briscoe, K.; Marx, G.; Higano, C.S.; de Bono, J.S.; et al. Clinical validation of circulating GDF15/MIC-1 as a marker of response to docetaxel and survival in men with metastatic castration-resistant prostate cancer. Prostate 2024, 84, 747–755. [Google Scholar] [CrossRef]
- Husaini, Y.; Tsai, V.W.W.; Manandhar, R.; Zhang, H.P.; Lee-Ng, K.K.M.; Lebhar, H.; Marquis, C.P.; Brown, D.A.; Breit, S.N. Growth differentiation factor-15 slows the growth of murine prostate cancer by stimulating tumor immunity. PLoS ONE 2020, 15, e0233846. [Google Scholar] [CrossRef] [PubMed]
- Yamamichi, G.; Kato, T.; Arakawa, N.; Ino, Y.; Ujike, T.; Nakano, K.; Koh, Y.; Motoyama, Y.; Outani, H.; Myoba, S.; et al. GDF15 propeptide promotes bone metastasis of castration-resistant prostate cancer by augmenting the bone microenvironment. Biomark. Res. 2024, 12, 147. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Zhang, M.; Zhang, H.; Lu, C.; Hou, G.; Feng, Y.; Fang, Z.; Lv, X. Value of Growth/Differentiation Factor 15 in Diagnosis and the Evaluation of Chemotherapeutic Response in Lung Cancer. Clin. Ther. 2021, 43, 747–759. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Sun, P.; Chung, C.; Park, D.; Lee, S.I.; Kim, N.; Lee, S.E.; Lee, J.E.; Kang, Y.E.; Kang, D.H. Plasma GDF15 levels associated with circulating immune cells predict the efficacy of PD-1/PD-L1 inhibitor treatment and prognosis in patients with advanced non-small cell lung cancer. J. Cancer Res. Clin. Oncol. 2023, 149, 159–171. [Google Scholar] [CrossRef]
- Huh, S.J.; Chung, C.Y.; Sharma, A.; Robertson, G.P. Macrophage inhibitory cytokine-1 regulates melanoma vascular development. Am. J. Pathol. 2010, 176, 2948–2957. [Google Scholar] [CrossRef]
- Weide, B.; Schäfer, T.; Martens, A.; Kuzkina, A.; Uder, L.; Noor, S.; Garbe, C.; Harter, P.N.; Mittelbronn, M.; Wischhusen, J. High GDF-15 Serum Levels Independently Correlate with Poorer Overall Survival of Patients with Tumor-Free Stage III and Unresectable Stage IV Melanoma. J. Investig. Dermatol. 2016, 136, 2444–2452. [Google Scholar] [CrossRef]
- Haake, M.; Haack, B.; Schäfer, T.; Harter, P.N.; Mattavelli, G.; Eiring, P.; Vashist, N.; Wedekink, F.; Genssler, S.; Fischer, B.; et al. Tumor-derived GDF-15 blocks LFA-1 dependent T cell recruitment and suppresses responses to anti-PD-1 treatment. Nat. Commun. 2023, 14, 4253. [Google Scholar] [CrossRef] [PubMed]
- Roth, P.; Junker, M.; Tritschler, I.; Mittelbronn, M.; Dombrowski, Y.; Breit, S.N.; Tabatabai, G.; Wick, W.; Weller, M.; Wischhusen, J.; et al. GDF-15 contributes to proliferation and immune escape of malignant gliomas. Clin. Cancer Res. 2010, 16, 3851–3859. [Google Scholar] [CrossRef]
- Ma, J.; Tang, X.; Sun, W.W.; Liu, Y.; Tan, Y.R.; Ma, H.L.; Zhu, D.W.; Wang, M.; Wang, L.Z.; Li, J.; et al. Mutant GDF15 presents a poor prognostic outcome for patients with oral squamous cell carcinoma. Oncotarget 2016, 7, 2113–2122. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Jung, S.N.; Lim, M.A.; Oh, C.; Piao, Y.; Kim, H.J.; Liu, L.; Kang, Y.E.; Chang, J.W.; Won, H.R.; et al. Transcriptional Regulation of GDF15 by EGR1 Promotes Head and Neck Cancer Progression through a Positive Feedback Loop. Int. J. Mol. Sci. 2021, 22, 11151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, X.; Chen, H.; Zhou, M.; Liu, Y.; Hou, Y.; Nie, M.; Liu, X. Identification and validation of potential novel biomarkers for oral squamous cell carcinoma. Bioengineered 2021, 12, 8845–8862. [Google Scholar] [CrossRef]
- Yang, C.Z.; Ma, J.; Luo, Q.Q.; Neskey, D.M.; Zhu, D.W.; Liu, Y.; Myers, J.N.; Zhang, C.P.; Zhang, Z.Y.; Zhong, L.P. Elevated level of serum growth differentiation factor 15 is associated with oral leukoplakia and oral squamous cell carcinoma. J. Oral. Pathol. Med. 2014, 43, 28–34. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Pan Hya et, al. Expression of growth differentiation factor 15 is positively correlated with histopathological malignant grade and in vitro cell proliferation in oral squamous cell carcinoma. Oral. Oncol. 2009, 45, 627–632. [Google Scholar] [CrossRef]
- Schiegnitz, E.; Kämmerer, P.W.; Koch, F.P.; Krüger, M.; Berres, M.; Al-Nawas, B. GDF 15 as an anti-apoptotic, diagnostic and prognostic marker in oral squamous cell carcinoma. Oral. Oncol. 2012, 48, 608–614. [Google Scholar] [CrossRef]
- Chang, J.T.C.; Chan, S.H.; Lin, C.Y.; Lin, T.Y.; Wang, H.M.; Liao, C.T.; Wang, T.H.; Lee, L.Y.; Cheng, A.J. Differentially expressed genes in radioresistant nasopharyngeal cancer cells: gp96 and GDF15. Mol. Cancer Ther. 2007, 6, 2271–2279. [Google Scholar] [CrossRef]
- Schiegnitz, E.; Kämmerer, P.W.; Rode, K.; Schorn, T.; Brieger, J.; Al-Nawas, B. Growth differentiation factor 15 as a radiation-induced marker in oral carcinoma increasing radiation resistance. J. Oral. Pathol. Med. 2016, 45, 63–69. [Google Scholar] [CrossRef]
- Li, Y.L.; Chang, J.T.; Lee, L.Y.; Fan, K.H.; Lu, Y.C.; Li, Y.C.; Chiang, C.H.; You, G.R.; Chen, H.Y.; Cheng, A.J. GDF15 contributes to radioresistance and cancer stemness of head and neck cancer by regulating cellular reactive oxygen species via a SMAD-associated signaling pathway. Oncotarget 2017, 8, 1508–1528. [Google Scholar] [CrossRef]
- Sjøberg, K.A.; Sigvardsen, C.M.; Alvarado-Diaz, A.; Andersen, N.R.; Larance, M.; Seeley, R.J.; Schjerling, P.; Knudsen, J.G.; Katzilieris-Petras, G.; Clemmensen, C.; et al. GDF15 increases insulin action in the liver and adipose tissue via a β-adrenergic receptor-mediated mechanism. Cell Metab. 2023, 35, 1327–1340.e5. [Google Scholar] [CrossRef] [PubMed]
- Akdogan, O.; Ogut, B.; Sutcuoglu, O.; Sert, A.; Gurler, F.; Akyurek, N.; Ozdemir, N.; Ozet, A.; Yazici, O. The impact of the expression level of growth differentiation factor 15 in tumor tissue on the response to immunotherapy in non-small cell lung cancer. BMC Cancer 2024, 24, 954. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Hu, Y.J.; Ju, W.T.; Fu, Y.; Sun, W.W.; Liu, Y.; Tan, Y.R.; Wang, L.Z.; Li, J.; Tu, Y.Y.; et al. Elevated growth differentiating factor 15 expression predicts long-term benefit of docetaxel, cisplatin and 5-fluorouracil induction chemotherapy in patients with oral cancer. Oncol. Lett. 2018, 15, 8118–8124. [Google Scholar] [CrossRef] [PubMed]
- Suriben, R.; Chen, M.; Higbee, J.; Oeffinger, J.; Ventura, R.; Li, B.; Mondal, K.; Gao, Z.; Ayupova, D.; Taskar, P.; et al. Antibody-mediated inhibition of GDF15-GFRAL activity reverses cancer cachexia in mice. Nat. Med. 2020, 26, 1264–1270. [Google Scholar] [CrossRef]
- Kim-Muller, J.Y.; Song, L.; LaCarubba Paulhus, B.; Pashos, E.; Li, X.; Rinaldi, A.; Joaquim, S.; Stansfield, J.C.; Zhang, J.; Robertson, A.; et al. GDF15 neutralization restores muscle function and physical performance in a mouse model of cancer cachexia. Cell Rep. 2023, 42, 111947. [Google Scholar] [CrossRef]
- Xiong, J.; Wu, G.; Ning, J.; Yan, J.; Yang, J.; Kang, J. Neutralizing antibody against GDF15 for treatment of cancer-associated cachexia. PLoS ONE 2024, 19, 0309394. [Google Scholar] [CrossRef]
- Crawford, J.; Calle, R.A.; Collins, S.M.; Weng, Y.; Lubaczewski, S.L.; Buckeridge, C.; Wang, E.Q.; Harrington, M.A.; Tarachandani, A.; Rossulek, M.I.; et al. A Phase Ib First-In-Patient Study Assessing the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of Ponsegromab in Participants with Cancer and Cachexia. Clin. Cancer Res. 2024, 30, 489–497. [Google Scholar] [CrossRef]
- Groarke, J.D.; Crawford, J.; Collins, S.M.; Lubaczewski, S.L.; Breen, D.M.; Harrington, M.A.; Jacobs, I.; Qiu, R.; Revkin, J.; Rossulek, M.I.; et al. Phase 2 study of the efficacy and safety of ponsegromab in patients with cancer cachexia: PROACC-1 study design. J. Cachexia Sarcopenia Muscle 2024, 15, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Groarke, J.D.; Crawford, J.; Collins, S.M.; Lubaczewski, S.; Roeland, E.J.; Naito, T.; Hendifar, A.E.; Fallon, M.; Takayama, K.; Asmis, T.; et al. Ponsegromab for the Treatment of Cancer Cachexia. N. Engl. J. Med. 2024, 391, 2291–2303. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, B.A.; Gbolahan, O.; Razak, A.A.; Hilton, J.F.; Lambert, A.; Hood, J.; Pluta, M.; Bragulat, V.; Sanai, E.; Kumar, R.; et al. Abstract CT100: Safety and efficacy of AZD8853, an anti-growth and differentiation factor 15 (GDF15) antibody, in patients (pts) with advanced/metastatic solid tumors: First-in-human study. Cancer Res. 2024, 84 (Suppl. 7), CT100. [Google Scholar] [CrossRef]
- Melero, I.; de Miguel Luken, M.; de Velasco, G.; Garralda, E.; Martín-Liberal, J.; Joerger, M.; Alonso, G.; Goebeler, M.E.; Schuler, M.; König, D.; et al. Neutralizing GDF-15 can overcome anti-PD-1 and anti-PD-L1 resistance in solid tumours. Nature 2025, 637, 1218–1227. [Google Scholar] [CrossRef]
- Luan, H.H.; Wang, A.; Hilliard, B.K.; Carvalho, F.; Rosen, C.E.; Ahasic, A.M.; Herzog, E.L.; Kang, I.; Pisani, M.A.; Yu, S.; et al. GDF15 Is an Inflammation-Induced Central Mediator of Tissue Tolerance. Cell 2019, 178, 1231–1244.e11. [Google Scholar] [CrossRef]
- Necchi, A.; Iacovelli, R.; Di Maio, M.; Gontero, P.; Fettes, P.; Klar, K.; Hermann, F.; Leo, E. Neutralizing GDF-15 in muscle-invasive bladder cancer (MIBC): A neoadjuvant immunotherapy trial of visugromab (CTL-002) in combination with the anti-PD1 antibody nivolumab (GDFather-Neo). J. Clin. Oncol. 2024, 42 (Suppl. 4), TPS712. [Google Scholar] [CrossRef]
- Genssler, S.; Schaetzlein, D.; Leo, E.; Haake, M.; Schuberth-Wagner, C. 1196 GDF-15 neutralizing antibody visugromab increases intratumoral immune cell infiltration to support bispecific T-cell engagers. In Regular and Young Investigator Award Abstracts; BMJ Publishing Group Ltd.: London, UK, 2023; p. A1319. [Google Scholar] [CrossRef]
- Breen, D.M.; Kim, H.; Bennett, D.; Calle, R.A.; Collins, S.; Esquejo, R.M.; He, T.; Joaquim, S.; Joyce, A.; Lambert, M.; et al. GDF-15 Neutralization Alleviates Platinum-Based Chemotherapy-Induced Emesis, Anorexia, and Weight Loss in Mice and Nonhuman Primates. Cell Metab. 2020, 32, 938–950.e6. [Google Scholar] [CrossRef]
| Study Title | Phase | Drug | Study Population | Setting | Clinical Trial Number | Status | Results |
|---|---|---|---|---|---|---|---|
| GDF15-Based TPF Induction Chemotherapy for OSCC Patients | II | TPF (in patients with high GDF15 expression) | Advanced OSCC | Induction treatment | NCT02285530 | Recruiting | NA |
| Study of the Efficacy and Safety of Ponsegromab in Patients with Cancer, Cachexia, and Elevated GDF-15 (PROACC-1) | II | Ponsegromab | NSCLC, pancreatic cancer, CRC | Cachexia and elevated serum GDF-15 concentrations | NCT05546476 | Completed | Dose-dependent weight gain at 12 weeks (primary outcome), improved appetite and cachexia symptom control, increased physical activity, trends towards increased skeletal muscle mass (secondary endpoints) |
| First-in-Human Study of the GDF-15 Neutralizing Antibody Visugromab (CTL-002) in patients with Advanced Cancer (GDFATHER) | I/II | Visugromab + anti-PD-1 | Advanced/metastatic solid tumors | Relapse/refractory to prior anti-PD-1/PD-L1 therapy | NCT04725474 | Active, not recruiting | ORR 15% in NSCLC cohort, ORR 15% in bladder cancer cohort (PR/CR if PD-L1 TPS ≥ 5%), durable responses (>1 year), good safety |
| Neoadjuvant Immunotherapy Combined With the Anti-GDF-15 Antibody Visugromab to Treat Muscle-Invasive Bladder Cancer | II | Visugromab + Nivolumab | Urothelial carcinoma ineligible for cisplatin-based CHT | Neoadjuvant | NCT06059547 | Recruiting | NA |
| A First-in-human Study to Evaluate the Safety and Tolerability of AZD8853 in Participants With Selected Advanced/Metastatic Solid Tumors | I/IIA | AZD8853 | Advanced/metastatic NSCLC, MSS-CRC, urothelial carcinoma | Second line and beyond | NCT05397171 | Completed (no further development planned) | No dose-limiting toxicity, 31.3% SD, 68.8% PD; no PR/CR. Transient GDF-15 suppression. |
| Study of NGM120 in Subjects With Advanced Solid Tumors, Pancreatic Cancer, and Prostate Cancer Using Combination Therapy | I/II | NGM120 | Metastatic pancreatic adenocarcinoma, metastatic castration-resistant prostate cancer | First line and beyond | NCT04068896 | Completed | 36% of pts experienced >3.5% lean body mass gain by Week 9. In pancreatic cancer cohort: 2 pts with PR, 4 with SD; all 6 pts had ≥5% body weight gain and ~2.9% mean increase in lean body mass by Week 16 |
| A Dose Escalation Study of AV-380 in Metastatic Cancer Patients with Cachexia | IB | AV-380 | Metastatic CRC or pancreatic cancer | Patients with cachexia and elevated GDF-15 levels in the first-line setting with SoC | NCT05865535 | Recruiting | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippini, D.M.; Romaniello, D.; Carosi, F.; Fabbri, L.; Carlini, A.; Giusti, R.; Di Maio, M.; Alfieri, S.; Lauriola, M.; Pantaleo, M.A.; et al. The Multifaceted Role of Growth Differentiation Factor 15 (GDF15): A Narrative Review from Cancer Cachexia to Target Therapy. Biomedicines 2025, 13, 1931. https://doi.org/10.3390/biomedicines13081931
Filippini DM, Romaniello D, Carosi F, Fabbri L, Carlini A, Giusti R, Di Maio M, Alfieri S, Lauriola M, Pantaleo MA, et al. The Multifaceted Role of Growth Differentiation Factor 15 (GDF15): A Narrative Review from Cancer Cachexia to Target Therapy. Biomedicines. 2025; 13(8):1931. https://doi.org/10.3390/biomedicines13081931
Chicago/Turabian StyleFilippini, Daria Maria, Donatella Romaniello, Francesca Carosi, Laura Fabbri, Andrea Carlini, Raffaele Giusti, Massimo Di Maio, Salvatore Alfieri, Mattia Lauriola, Maria Abbondanza Pantaleo, and et al. 2025. "The Multifaceted Role of Growth Differentiation Factor 15 (GDF15): A Narrative Review from Cancer Cachexia to Target Therapy" Biomedicines 13, no. 8: 1931. https://doi.org/10.3390/biomedicines13081931
APA StyleFilippini, D. M., Romaniello, D., Carosi, F., Fabbri, L., Carlini, A., Giusti, R., Di Maio, M., Alfieri, S., Lauriola, M., Pantaleo, M. A., Arribas, L., Oliva, M., Bossi, P., & Locati, L. D. (2025). The Multifaceted Role of Growth Differentiation Factor 15 (GDF15): A Narrative Review from Cancer Cachexia to Target Therapy. Biomedicines, 13(8), 1931. https://doi.org/10.3390/biomedicines13081931











