Efficacy of Nurse-Led and Multidisciplinary Self-Management Programmes for Heart Failure with Reduced Ejection Fraction: An Umbrella Systematic Review
Abstract
1. Introduction
Aims
2. Methods
2.1. Eligibility Criteria
2.2. Search Strategy
2.2.1. Study Characteristics
2.2.2. Electronic Search
2.2.3. Searching Other Resources and Information Sources
2.3. Data Collection and Analysis
2.3.1. Study Selection
2.3.2. Data Extraction and Management
- Study quality and risk of bias;
- Review characteristics (e.g., number and type of included studies);
- Population and setting;
- CDSM intervention characteristics;
- Outcomes and findings relevant to CHF.
2.3.3. Data Items
2.3.4. Quality Appraisal and Risk of Bias
- AMSTAR 2 for assessing the quality of systematic reviews [12];
2.3.5. Certainty of Evidence
- Risk of bias;
- Inconsistency;
- Indirectness;
- Imprecision;
- Publication bias.
2.3.6. Data Analysis, Assessment of Heterogeneity, and Publication Bias
- Planned methods for study analysis and statistical methodology
- Risk of bias in individual studies
2.4. Measures of Treatment Effect
2.5. Ethics and Dissemination
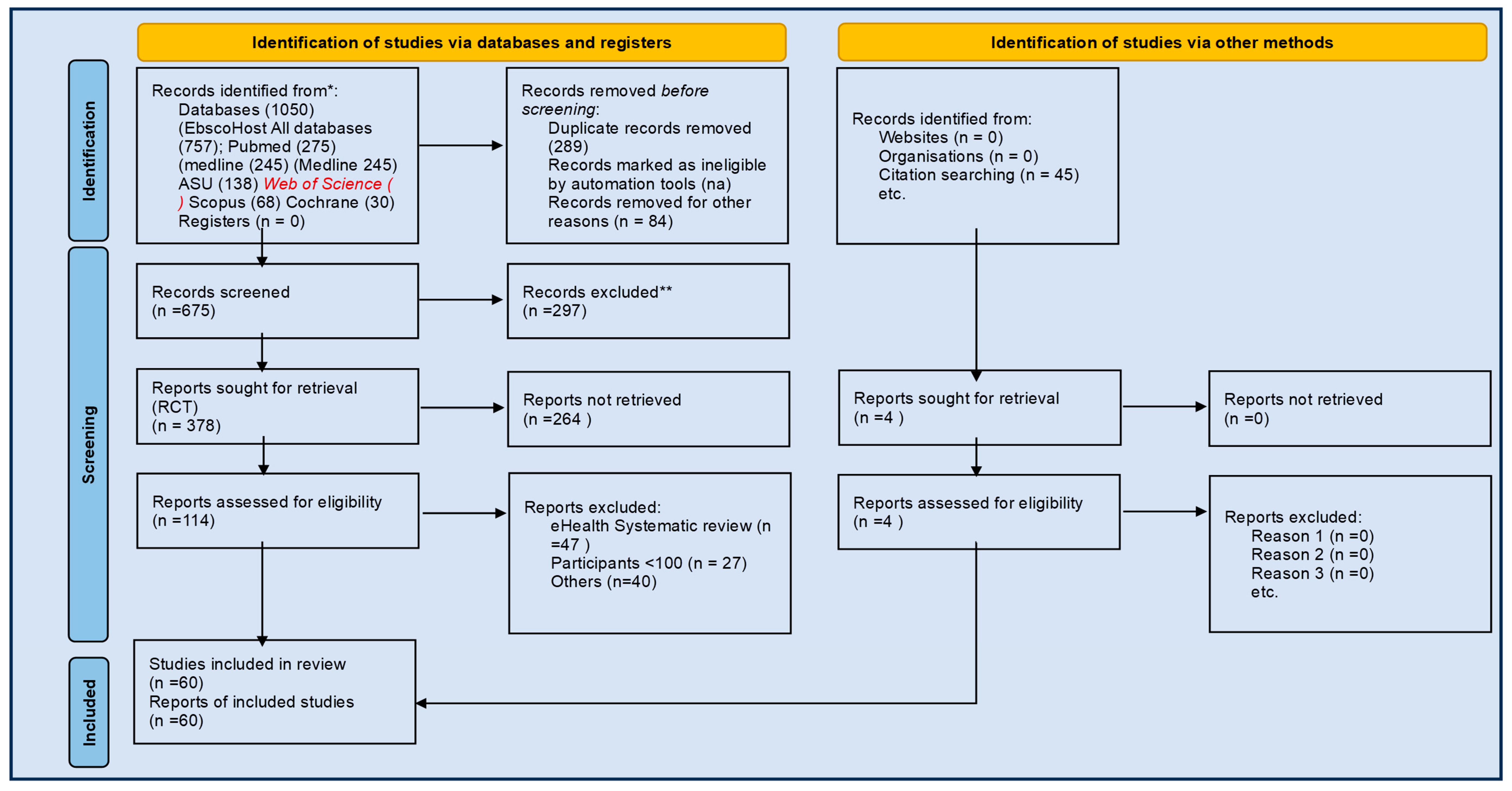
3. Results
- Did the research questions and inclusion criteria for the review include the components of PICO?
- Did the report of the review contain an explicit statement that the review methods were established prior to the conduct of the review, and did the report justify any significant deviations from the protocol?
- Did the review authors explain their selection of the study designs for inclusion in the review?
- Did the review authors use a comprehensive literature search strategy?
- Did the review authors perform study selection in duplicate?
- Did the review authors perform data extraction in duplicate?
- Did the review authors provide a list of excluded studies and justify the exclusions?
- Did the review authors describe the included studies in adequate detail?
- Did the review authors use a satisfactory technique for assessing the risk of bias (RoB) in individual studies that were included in the review? (RCTs; NRSI)
- Did the review authors report on the sources of funding for the studies included in the review?
- If meta-analysis was performed, did the review authors use appropriate methods for statistical combination of results? (RCTs; NRSI)
- If meta-analysis was performed, did the review authors assess the potential impact of RoB in individual studies on the results of the meta-analysis or other evidence synthesis?
- Did the review authors account for RoB in individual studies when interpreting/discussing the results of the review?
- Did the review authors provide a satisfactory explanation for, and discussion of, any heterogeneity observed in the results of the review?
- If they performed quantitative synthesis, did the review authors carry out an adequate investigation of publication bias (small study bias) and discuss its likely impact on the results of the review?
- Did the review authors report any potential sources of conflict of interest, including any funding they received for conducting the review?
- High—Zero or one non-critical weakness: The systematic review provides an accurate and comprehensive summary of the results of the available studies that address the question of interest.
- Moderate—More than one non-critical weakness: The systematic review has more than one weakness but no critical flaws. It may provide an accurate summary of the results of the available studies that were included in the review.
- Low—One critical flaw with or without non-critical weaknesses: The review has a critical flaw and may not provide an accurate and comprehensive summary of the available studies that address the question of interest.
- Critically low—More than one critical flaw with or without non-critical weaknesses: The review has more than one critical flaw and should not be relied on to provide an accurate and comprehensive summary of the available studies.
- Note: Multiple non-critical weaknesses may diminish confidence in the review, and it may be appropriate to move the overall appraisal down from moderate to low confidence.
- i.
- Effects by cardiovascular outcomes
- a.
- Mortality
- Significant mortality reduction: [43].
- ii.
- Hospital readmissions
- Negative findings: None reported.
- Negative: [48].
- b.
- Health-related quality of life
- c.
- Self-management behaviours
- Negative outcome: [62].
- Not reported: [33].
- iii.
- Effects by intervention on health services
- iv.
- Results synthesis and summary
4. Discussion
- Nurse-led CDSM should be embedded into routine HFrEF care as part of standard, multidisciplinary disease management programmes.
- Future trials should adopt a core outcomes set that includes HRQoL, hospital readmissions, self-management behaviours, and cost-effectiveness.
- Co-design and cultural tailoring should be prioritised to enhance relevance and patient engagement across diverse settings.
- Economic evaluations must be incorporated into programme design to support scale-up and policy adoption.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Study | Reason for Exclusion |
|---|---|
| Kent B, Cull E, Phillips N. A systematic review of the effectiveness of current interventions to assist adults with heart failure to comply with therapy and enhance self-care behaviours. JBI Library of Systematic Reviews. 2010;8:1–12. | Protocol |
| Inglis SC, Clark RA, Dierckx R, Prieto-Merino D, Cleland JGF. Structured telephone support or non-invasive telemonitoring for patients with heart failure. The Cochrane database of systematic reviews. 2015;(10):CD007228. | Update in 2015 |
| Buck HG, Harkness K, Wion R, et al. Caregivers’ contributions to heart failure self-care: a systematic review. European journal of cardiovascular nursing. 2015;14(1):79–89. | No outcome data |
| Lancey, A.; Slater, C.E. Heart failure self-management: A scoping review of interventions implemented by allied health pro-fessionals. Disabil. Rehabil. 2023, 46, 4848–4859. | Scoping Review |
| McBain H, Shipley M, Newman S. The impact of self-monitoring in chronic illness on healthcare utilisation: a systematic review of reviews. BMC health services research. 2015;15:565. | Telemonitoring |
| Ballester M, Orrego C, Heijmans M, et al. Comparing the effectiveness and cost-effectiveness of self-management interventions in four high-priority chronic conditions in Europe (COMPAR-EU): a research protocol. BMJ open. 2020;10(1):e034680. | Not SR |
| Riegel B, Westland H, Iovino P, Barelds I, Bruins Slot J, Stawnychy MA, Osokpo O, Tarbi E, Trappenburg JCA, Vellone E, Strömberg A, Jaarsma T. Characteristics of self-care interventions for patients with a chronic condition: A scoping review. Int J Nurs Stud. 2021 Apr;116:103713 | Scoping Review |
| Salahodinkolah MK, Ganji J, Moghadam SH, Shafipour V, Jafari H, Salari S. Educational intervention for improving self-care behaviors in patients with heart failure: A narrative review. Journal of Nursing & Midwifery Sciences. 2020;7(1):60–68. | Not SR |
| Noonan MC, Wingham J, Dalal HM, Taylor RS. Involving caregivers in self-management interventions for patients with heart failure and chronic obstructive pulmonary disease. A systematic review and meta-analysis. J Adv Nurs. 2019 Dec;75(12):3331–3345. | No MACE data |
| Kyriakou M, Middleton N, Ktisti S, Philippou K, Lambrinou E. Supportive Care Interventions to Promote Health-Related Quality of Life in Patients Living With Heart Failure: A Systematic Review and Meta-Analysis. Heart Lung Circ. 2020 Nov;29(11):1633–1647. | No MACE data |
| Bayly J, Bone AE, Ellis-Smith C, et al. Common elements of service delivery models that optimise quality of life and health service use among older people with advanced progressive conditions: a tertiary systematic review. BMJ open. 2021;11(12):e048417. | No MACE data |
| WANG Sixiong, ZHU Meiyi. Influence of motivational interviewing on self-care behavior in patients with chronic heart failure: a Meta-analysis. Chinese Evidence-based Nursing. 2021;5:598–603. | Article not accessible |
| Pinto Braga P, Barbosa de Castro EA, de Medeiros Souza T, Rocha Raimundo Leone D, Sabrina de Souza M, Lara da Silva K. Costs and Benefits of Home Care for People with Complex Chronic Conditions: An Integrative Review. Ciencia, Cuidado e Saude. 2022;21:1–11. | Integrative review |
| LIU Mengdie, XIONG Xiaoyun, SUN Xinglan, et al. Summary of the best evidence for self-management in patients with chronic heart failure. Chinese Journal of Nursing. 2022;57(23):2937–2944. | No relevant data |
| Ali AA, Sagheer S. A Review to Access Knowledge, Attitudes, and Practices among Nurses of Cardiac Medicine for Heart Failure Patients to Prevent Readmission in Hospitals. i-manager’s Journal on Nursing. 2023;12(4):42–48. | Not relevant to this review |
| McGreal MH, Hogan MJ, Walsh-Irwin C, Maggio NJ, Jurgens CY. Heart failure self-care interventions to reduce clinical events and symptom burden. Research Reports in Clinical Cardiology. 2014;5:243–257. | No relevant data |
| Koirala B, Himmelfarb CD, Budhathoki C, Tankumpuan T, Asano R, Davidson PM. Factors affecting heart failure self-care: An integrative review. Heart & Lung. 2018;47(6):539–545. | Integrative review |
| King AJL, Johnson R, Cramer H, Purdy S, Huntley AL. Community case management and unplanned hospital admissions in patients with heart failure: A systematic review and qualitative evidence synthesis. Journal of advanced nursing. 2018;74(7):1463–1473. | Only qualitative data |
| Purdey S, Huntley A. Predicting and preventing avoidable hospital admissions: a review. The journal of the Royal College of Physicians of Edinburgh. 2013;43(4):340–344. | No relevant data |
| Imran HM, Baig M, Erqou S, Taveira TH, Shah NR, Morrison A, Choudhary G, Wu WC. Home-Based Cardiac Rehabilitation Alone and Hybrid With Center-Based Cardiac Rehabilitation in Heart Failure: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2019 Aug 20;8(16):e012779. | CDSM not described |
| Son Y-J, Lee Y, Lee H-J. Effectiveness of Mobile Phone-Based Interventions for Improving Health Outcomes in Patients with Chronic Heart Failure: A Systematic Review and Meta-Analysis. International journal of environmental research and public health. 2020;17(5). | Telemonitoring |
References
- Allegrante, J.P.; Wells, M.T.; Peterson, J.C. Interventions to Support Behavioral Self-Management of Chronic Diseases. Annu. Rev. Public Health 2019, 40, 127–146. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.M.; Savard, L.A.; Thompson, D.R. What Is the Strength of Evidence for Heart Failure Disease-Management Programs? J. Am. Coll. Cardiol. 2009, 54, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Krumholz, H.M.; Currie, P.M.; Riegel, B.; Phillips, C.O.; Peterson, E.D.; Smith, R.; Yancy, C.W.; Faxon, D.P.; American Heart Association Disease Management Taxonomy Writing Group. A taxonomy for disease management. Circulation 2006, 114, 1432–1445. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Fonarow, G.C.; Breathett, K.; Jurgens, C.Y.; Pisani, B.A.; Pozehl, B.J.; Spertus, J.A.; Taylor, K.G.; Thibodeau, J.T.; Yancy, C.W.; et al. 2020 ACC/AHA Clinical Performance and Quality Measures for Adults with Heart Failure. J. Am. Coll. Cardiol. 2020, 76, 2527–2564. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary. J. Am. Coll. Cardiol. 2022, 79, 1757–1780. [Google Scholar] [CrossRef]
- Iyngkaran, P.; Liew, D.; Neil, C.; Driscoll, A.; Marwick, T.H.; Hare, D.L. Moving From Heart Failure Guidelines to Clinical Practice: Gaps Contributing to Readmissions in Patients with Multiple Comorbidities and Older Age. Clin. Med. Insights Cardiol. 2018, 12, 1179546818809358. [Google Scholar] [CrossRef]
- Fonarow, G.C.; Abraham, W.T.; Albert, N.M.; Stough, W.G.; Gheorghiade, M.; Greenberg, B.H.; O’Connor, C.M.; Pieper, K.; Sun, J.L.; Yancy, C.; et al. Association between performance measures and clinical outcomes for patients hospitalized with heart failure. JAMA 2007, 297, 61–70. [Google Scholar] [CrossRef]
- Toukhsati, S.R.; Jaarsma, T.; Babu, A.S.; Driscoll, A.; Hare, D.L. Self-Care Interventions That Reduce Hospital Readmissions in Patients With Heart Failure; Towards the Identification of Change Agents. Clinical Medicine Insights. Cardiology 2019, 13, 1179546819856855. [Google Scholar] [CrossRef]
- Iyngkaran, P.; Buhler, M.; de Courten, M.; Hanna, F. Effectiveness of self-management programmes for heart failure with reduced ejection fraction: A systematic review protocol. BMJ Open 2024, 14, e079830. [Google Scholar] [CrossRef]
- Iyngkaran, P.; Fazli, F.; Nguyen, H.; Patel, T.; Hanna, F. Historical Gaps in the Integration of Patient-Centric Self-Management Components in HFrEF Interventions: An Umbrella Narrative Review. J. Clin. Med. 2025, 14, 2832. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iyngkaran, P.; Toukhsati, S.R.; Harris, M.; Connors, C.; Kangaharan, N.; Ilton, M.; Nagel, T.; Moser, D.K.; Battersby, M. Self Managing Heart Failure in Remote Australia—Translating Concepts into Clinical Practice. Curr. Cardiol. Rev. 2016, 12, 270–284. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Hartling, L.; Ospina, M.; Liang, Y.; Dryden, D.M.; Hooton, N.; Krebs Seida, J.; Klassen, T.P. Risk of bias versus quality assessment of randomised controlled trials: Cross sectional study. BMJ 2009, 339, b4012. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions, version 6.2 (updated February 2021); Cochrane: London, UK, 2021; Available online: https://www.training.cochrane.org/handbook (accessed on 12 July 2025).
- Cochrane Effective Practice and Organisation of Care Group. Available online: http://www.epoc.cochrane.org (accessed on 10 July 2025).
- Freund, T. Improving the Quality of Quality Assessment in Systematic Reviews. Rapid Response to: Risk of Bias Versus Quality Assessment of Randomised Controlled Trials: Cross Sectional Study. BMJ 2009, 339, b4012. Available online: https://www.bmj.com/rapid-response/2011/11/02/improving-quality-quality-assessment-systematic-reviews (accessed on 10 July 2025).
- Freund, T.; Kayling, F.; Miksch, A.; Szecsenyi, J.; Wensing, M. Effectiveness and efficiency of primary care based case management for chronic diseases: Rationale and design of a systematic review and meta-analysis of randomized and non-randomized trials [CRD32009100316]. BMC Health Serv. Res. 2010, 10, 112. [Google Scholar] [CrossRef]
- Shepperd, S.; Lewin, S.; Straus, S.; Clarke, M.; Eccles, M.P.; Fitzpatrick, R.; Wong, G.; Sheikh, A. Can we systematically review studies that evaluate complex interventions? PLoS Med. 2009, 6, e1000086. [Google Scholar] [CrossRef]
- Belbasis, L.; Bellou, V.; Ioannidis, J.P.A. Conducting umbrella reviews. BMJ Med. 2022, 1, e000071. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef]
- Aromataris, E.; Fernandez, R.; Godfrey, C.; Holly, C.; Khalil, H.; Tungpunkom, P. Chapter 10: Umbrella Reviews. In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; JBI: Adelaide, Australia, 2020; Available online: https://synthesismanual.jbi.global (accessed on 17 April 2025).
- Zhao, X.; Wu, S.; Luo, N.; Lin, Q.; Zhao, X.; Li, K. Care models for patients with heart failure at home: A systematic review. J. Clin. Nurs. 2024, 33, 1295–1305. [Google Scholar] [CrossRef]
- Chen, C.W.; Lee, M.-C.; Wu, S.-F.V. Effects of a collaborative health management model on people with congestive heart failure: A systematic review and meta-analysis. J. Adv. Nurs. 2023, 80, 2290–2307. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y. Effect of Transitional Care Strategies on Health-Related Quality of Life (HRQoL) in Heart Failure with Reduced Ejection Fraction (HFrEF): A Systematic Review and Meta-analysis of Randomized Controlled Trails. Altern. Ther. Health Med. 2023, 29, AT8909. [Google Scholar]
- Yang, Y.-F.; Hoo, J.-X.; Tan, J.-Y.; Lim, L.-L. Multicomponent integrated care for patients with chronic heart failure: Systematic review and meta-analysis. ESC Heart Fail. 2023, 10, 791–807. [Google Scholar] [CrossRef] [PubMed]
- Hafkamp, F.J.; Tio, R.A.; Otterspoor, L.C.; de Greef, T.; van Steenbergen, G.J.; van de Ven, A.R.; Smits, G.; Post, H.; van Veghel, D. Optimal effectiveness of heart failure management—An umbrella review of meta-analyses examining the effectiveness of interventions to reduce (re)hospitalizations in heart failure. Heart Fail. Rev. 2022, 27, 1683–1748. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-F.; Lee, S.-Y.; Hsu, T.-F.; Li, J.-Y.; Tung, H.-H. Patient Navigators for Transition Care of Heart Failure: A Systematic Review and Meta-Analysis. Int. J. Gerontol. 2022, 16, 76–82. [Google Scholar]
- Toback, M.; Clark, N. Strategies to improve self-management in heart failure patients. Contemp. Nurse 2017, 53, 105–120. [Google Scholar] [CrossRef]
- Taylor, S.J.; Bestall, J.C.; Cotter, S.; Falshaw, M.; Hood, S.G.; Parsons, S.; Wood, L.; Underwood, M. Clinical service organization for heart failure. Cochrane Database Syst. Rev. 2005, 2, CD002752. [Google Scholar]
- Roccaforte, R.; Demers, C.; Baldassarre, F.; Teo, K.K.; Yusuf, S. Effectiveness of comprehensive disease management programmes in improving clinical outcomes in heart failure patients. A meta-analysis. Eur. J. Heart Fail. 2005, 7, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Gonseth, J.; Guallar-Castillón, P.; Banegas, J.R.; Rodríguez-Artalejo, F. The effectiveness of disease management programmes in reducing hospital re-admission in older patients with heart failure: A systematic review and meta-analysis of published reports. Eur. Heart J. 2004, 25, 1570–1595. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, T.; Gao, R.; Chair, S.Y. Effects of nurse-led self-care interventions on health outcomes among people with heart failure: A systematic review and meta-analysis. J. Clin. Nurs. 2023, 33, 1282–1294. [Google Scholar] [CrossRef]
- Nwosu, W.O.; Rajani, R.; McDonaugh, T.; Goulder, A.; Smith, D.; Hughes, L. The impact of nurse-led patient education on quality of life in patients with heart failure. Br. J. Card. Nurs. 2023, 18, 1–13. [Google Scholar] [CrossRef]
- Checa, C.; Canelo-Aybar, C.; Suclupe, S.; Ginesta-López, D.; Berenguera, A.; Castells, X.; Brotons, C.; Posso, M. Effectiveness and Cost-Effectiveness of Case Management in Advanced Heart Failure Patients Attended in Primary Care: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 13823. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, T.; Chair, S.Y. Effectiveness of nurse-led self-care interventions on self-care behaviors, self-efficacy, depression and illness perceptions in people with heart failure: A systematic review and meta-analysis. Int. J. Nurs. Stud. 2022, 132, 104255. [Google Scholar] [CrossRef]
- do Céu Sá, M.; Nabais, A. How to care for patients with heart failure—A systematic review of nursing interventions. New Trends Qual. Res. 2022, 11, e557. [Google Scholar]
- Tonapa, S.I.; Inayati, A.; Sithichoksakulchai, S.; Daryanti Saragih, I.; Efendi, F.; Chou, F.H. Outcomes of nurse-led telecoaching intervention for patients with heart failure: A systematic review and meta-analysis of randomised controlled trials. J. Clin. Nurs. 2022, 31, 1125–1135. [Google Scholar] [CrossRef]
- Son, Y.J.; Choi, J.; Lee, H.J. Effectiveness of Nurse-Led Heart Failure Self-Care Education on Health Outcomes of Heart Failure Patients: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 6559. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.C. A Nurse Led Clinic’s contribution to Patient Education and Promoting Self-care in Heart Failure Patients: A Systematic Review. Int. J. Integr. Care 2017, 17, 492. [Google Scholar] [CrossRef]
- Alnomasy, N.; Still, C.H. Nonpharmacological Interventions for Preventing Rehospitalization Among Patients with Heart Failure: A Systematic Review and Meta-Analysis. SAGE Open Nurs. 2023, 9, 23779608231209220. [Google Scholar] [CrossRef]
- Mhanna, M.; Sauer, M.C.; Al-Abdouh, A.; Jabri, A.; Abusnina, W.; Safi, M.; Beran, A.; Mansour, S. Cognitive behavioral therapy for depression in patients with heart failure: A systematic review and metanalysis of randomized control trials. Heart Fail. Rev. 2023, 28, 1091–1100. [Google Scholar] [CrossRef]
- Olano-Lizarraga, M.; Wallström, S.; Martín-Martín, J.; Wolf, A. Interventions on the social dimension of people with chronic heart failure: A systematic review of randomized controlled trials. Eur. J. Cardiovasc. Nurs. 2023, 22, 113–125. [Google Scholar] [CrossRef]
- Nso, N.M.; Emmanuel, K.; Nassar, M.M.; Bookani, K.R.; Antwi-Amoabeng, D.M.; Alshamam, M.; Kondaveeti, R.; Kompella, R.; Lakhdar, S.; Rizzo, V.M.; et al. Efficacy of Cognitive Behavioral Therapy in Heart Failure Patients: A Systematic Review and Meta-Analysis. Cardiol. Rev. 2023, 31, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Balata, M.; Gbreel, M.I.; Elrashedy, A.A.; Westenfeld, R.; Pfister, R.; Zimmer, S.; Nickenig, G.; Becher, M.U.; Sugiura, A. Clinical effects of cognitive behavioral therapy in heart failure patients: A meta-analysis of randomized controlled trials. BMC Complement. Med. Ther. 2023, 23, 280. [Google Scholar] [CrossRef]
- Koikai, J.; Khan, Z. The Effectiveness of Self-Management Strategies in Patients with Heart Failure: A Narrative Review. Cureus 2023, 15, e41863. [Google Scholar] [CrossRef]
- Feng, C.; Wang, Y.; Li, S.; Qu, Z.; Zheng, S. Effect of self-management intervention on prognosis of patients with chronic heart failure: A meta-analysis. Nurs. Open 2023, 10, 2015–2029. [Google Scholar] [CrossRef]
- Nahlén Bose, C. A meta-review of systematic reviews and meta-analyses on outcomes of psychosocial interventions in heart failure. Front. Psychiatry 2023, 14, 1095665. [Google Scholar] [CrossRef]
- Lee, C.S.; Westland, H.; Faulkner, K.M.; Iovino, P.; Thompson, J.H.; Sexton, J.; Farry, E.; Jaarsma, T.; Riegel, B. The effectiveness of self-care interventions in chronic illness: A meta-analysis of randomized controlled trials. Int. J. Nurs. Stud. 2022, 134, 104322. [Google Scholar] [CrossRef]
- Villero-Jiménez, A.I.; Martínez-Torregrosa, N.; Olano Lizarraga, M.; Garai-López, J.; Vázquez-Calatayud, M. Dyadic self-care interventions in chronic heart failure in hospital settings: A systematic review. An. Sis. Sanit. Navar. 2022, 45, e1001. [Google Scholar] [CrossRef] [PubMed]
- Ghizzardi, G.; Arrigoni, C.; Dellafiore, F.; Vellone, E.; Caruso, R. Efficacy of motivational interviewing on enhancing self-care behaviors among patients with chronic heart failure: A systematic review and meta-analysis of randomized controlled trials. Heart Fail. Rev. 2022, 27, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Suksatan, W.; Tankumpuan, T. The Effectiveness of Transition Care Interventions from Hospital to Home on Rehospitalization in Older Patients with Heart Failure: An Integrative Review. Home Health Care Manag. Pract. 2022, 34, 63–71. [Google Scholar] [CrossRef]
- Meng, X.; Wang, Y.; Tang, X.; Gu, J.; Fu, Y. Self-management on heart failure: A meta-analysis. Diabetes Metab. Syndr. 2021, 15, 102176. [Google Scholar] [CrossRef]
- de Melo Vellozo Pereira Tinoco, J.; da Silva Figueiredo, L.; Flores, P.V.P.; de Padua, B.L.R.; Mesquita, E.T.; Cavalcanti, A.C.D. Effectiveness of health education in the self-care and adherence of patients with heart failure: A meta-analysis. Rev. Latino-Am. Enferm. 2021, 29, e3389. [Google Scholar]
- Aghajanloo, A.; Negarandeh, R.; Janani, L.; Tanha, K.; Hoseini-Esfidarjani, S.S. Self-care status in patients with heart failure: Systematic review and meta-analysis. Nurs. Open 2021, 8, 2235–2248. [Google Scholar] [CrossRef]
- Cañon-Montañez, W.; Duque-Cartagena, T.; Rodríguez-Acelas, A.L. Effect of Educational Interventions to Reduce Readmissions due to Heart Failure Decompensation in Adults: A Systematic Review and Meta-analysis. Investig. Educ. Enferm. 2021, 39, e05. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.A.; Clemett, V. What impact do specialist and advanced-level nurses have on people living with heart failure compared to physician-led care? A literature review. J. Res. Nurs. 2021, 26, 229–249. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Chen, C.; Zhang, J.; Ye, Y.; Fan, X. Effects of self-management interventions on heart failure: Systematic review and meta-analysis of randomized controlled trials. Int. J. Nurs. Stud. 2021, 116, 103909. [Google Scholar] [CrossRef] [PubMed]
- Poudel, N.; Kavookjian, J.; Scalese, M.J. Motivational Interviewing as a Strategy to Impact Outcomes in Heart Failure Patients: A Systematic Review. Patient 2020, 13, 43–55. [Google Scholar] [CrossRef]
- Świątoniowska-Lonc, N.A.; Sławuta, A.; Dudek, K.; Jankowska, K.; Jankowska-Polańska, B.K. The impact of health education on treatment outcomes in heart failure patients. Adv. Clin. Exp. Med. 2020, 29, 481–492. [Google Scholar] [CrossRef]
- Peng, Y.; Fang, J.; Huang, W.; Qin, S. Efficacy of Cognitive Behavioral Therapy for Heart Failure. Int. Heart J. 2019, 60, 665–670. [Google Scholar] [CrossRef]
- Parajuli, D.R.; Kourbelis, C.; Franzon, J.; Newman, P.; Mckinnon, R.A.; Shakib, S.; Whitehead, D.; Clark, R.A. Effectiveness of the Pharmacist-Involved Multidisciplinary Management of Heart Failure to Improve Hospitalizations and Mortality Rates in 4630 Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Card. Fail. 2019, 25, 744–756. [Google Scholar] [CrossRef]
- Shanbhag, D.; Graham, I.D.; Harlos, K.; Haynes, R.B.; Gabizon, I.; Connolly, S.J.; Van Spall, H.G.C. Effectiveness of implementation interventions in improving physician adherence to guideline recommendations in heart failure: A systematic review. BMJ Open 2018, 8, e017765. [Google Scholar] [CrossRef]
- Sterling, M.R.; Shaw, A.L.; Leung, P.B.; Safford, M.M.; Jones, C.D.; Tsui, E.K.; Delgado, D. Home care workers in heart failure: A systematic review. J. Multidiscip. Healthc. 2018, 11, 481–492. [Google Scholar] [CrossRef]
- Jiang, Y.; Shorey, S.; Seah, B.; Chan, W.X.; Tam, W.W.S.; Wang, W. The effectiveness of psychological interventions on self-care, psychological and health outcomes in patients with chronic heart failure-A systematic review and meta-analysis. Int. J. Nurs. Stud. 2018, 78, 16–25. [Google Scholar] [CrossRef]
- Jonkman, N.H.; Westland, H.; Groenwold, R.H.; Ågren, S.; Anguita, M.; Blue, L.; de la Porte, P.W.B.-A.; DeWalt, D.A.; Hebert, P.L.; Heisler, M.; et al. What Are Effective Program Characteristics of Self-Management Interventions in Patients with Heart Failure? An Individual Patient Data Meta-analysis. J. Card. Fail. 2016, 22, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Ruppar, T.M.; Cooper, P.S.; Mehr, D.R.; Delgado, J.M.; Dunbar-Jacob, J.M. Medication Adherence Interventions Improve Heart Failure Mortality and Readmission Rates: Systematic Review and Meta-Analysis of Controlled Trials. J. Am. Heart Assoc. 2016, 5, e002606. [Google Scholar] [CrossRef]
- Jonkman, N.H.; Westland, H.; Groenwold, R.H.; Ågren, S.; Atienza, F.; Blue, L.; Bruggink-André de la Porte, P.W.; DeWalt, D.A.; Hebert, P.L.; Heisler, M.; et al. Do Self-Management Interventions Work in Patients with Heart Failure? An Individual Patient Data Meta-Analysis. Circulation 2016, 133, 1189–1198. [Google Scholar] [CrossRef]
- Srisuk, N.; Cameron, J.; Ski, C.F.; Thompson, D.R. Heart failure family-based education: A systematic review. Patient Educ. Couns. 2016, 99, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Ha Dinh, T.T.; Bonner, A.; Clark, R.; Ramsbotham, J.; Hines, S. The effectiveness of the teach-back method on adherence and self-management in health education for people with chronic disease: A systematic review. JBI Database Syst. Rev. Implement. Rep. 2016, 14, 210–247. [Google Scholar] [CrossRef]
- Inglis, S.C.; Clark, R.A.; Dierckx, R.; Prieto-Merino, D.; Cleland, J.G.F. Structured telephone support or non-invasive telemonitoring for patients with heart failure. Cochrane Database Syst. Rev. 2015, 10, CD007228. [Google Scholar] [CrossRef]
- Ruppar, T.M.; Delgado, J.M.; Temple, J. Medication adherence interventions for heart failure patients: A meta-analysis. Eur. J. Cardiovasc. Nurs. 2015, 14, 395–404. [Google Scholar] [CrossRef]
- Casimir, Y.E.; Williams, M.M.; Liang, M.Y.; Pitakmongkolkul, S.; Slyer, J.T. The effectiveness of patient-centered self-care education for adults with heart failure on knowledge, self-care behaviors, quality of life, and readmissions: A systematic review. JBI Database Syst. Rev. Implement. Rep. 2014, 12, 188–262. [Google Scholar] [CrossRef]
- Wakefield, B.J.; Boren, S.A.; Groves, P.S.; Conn, V.S. Heart failure care management programs: A review of study interventions and meta-analysis of outcomes. J. Cardiovasc. Nurs. 2013, 28, 8–19. [Google Scholar] [CrossRef]
- Barnason, S.; Zimmerman, L.; Young, L. An integrative review of interventions promoting self-care of patients with heart failure. J. Clin. Nurs. 2012, 21, 448–475. [Google Scholar] [CrossRef] [PubMed]
- Boyde, M.; Turner, C.; Thompson, D.R.; Stewart, S. Educational interventions for patients with heart failure: A systematic review of randomized controlled trials. J. Cardiovasc. Nurs. 2011, 26, E27–E35. [Google Scholar] [CrossRef] [PubMed]
- Dickson, V.V.; Buck, H.; Riegel, B. A qualitative meta-analysis of heart failure self-care practices among individuals with multiple comorbid conditions. J. Card. Fail. 2011, 17, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Yehle, K.S.; Plake, K.S. Self-efficacy and educational interventions in heart failure: A review of the literature. J. Cardiovasc. Nurs. 2010, 25, 175–188. [Google Scholar] [CrossRef]
- Ditewig, J.B.; Blok, H.; Havers, J.; van Veenendaal, H. Effectiveness of self-management interventions on mortality, hospital readmissions, chronic heart failure hospitalization rate and quality of life in patients with chronic heart failure: A systematic review. Patient Educ. Couns. 2010, 78, 297–315. [Google Scholar] [CrossRef]
- Boren, S.A.; Wakefield, B.J.; Gunlock, T.L.; Wakefield, D.S. Heart failure self-management education: A systematic review of the evidence. Int. J. Evid. Based Healthc. 2009, 7, 159–168. [Google Scholar] [CrossRef]
- Jovicic, A.; Holroyd-Leduc, J.M.; Straus, S.E. Effects of self-management intervention on health outcomes of patients with heart failure: A systematic review of randomized controlled trials. BMC Cardiovasc. Disord. 2006, 6, 43. [Google Scholar] [CrossRef]
- McAlister, F.A.; Stewart, S.; Ferrua, S.; McMurray, J.J. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: A systematic review of randomized trials. J. Am. Coll. Cardiol. 2004, 44, 810–819. [Google Scholar]
- Available online: https://www.emro.who.int/uhc-health-systems/access-health-services/phc-oriented-models-of-care.html (accessed on 22 March 2025).
- Son, Y.-J.; Lee, Y.; Lee, H.-J. Effectiveness of Mobile Phone-Based Interventions for Improving Health Outcomes in Patients with Chronic Heart Failure: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 1749. [Google Scholar] [CrossRef]
- Imran, H.M.; Baig, M.; Erqou, S.; Taveira, T.H.; Shah, N.R.; Morrison, A.; Choudhary, G.; Wu, W.C. Home-Based Cardiac Rehabilitation Alone and Hybrid with Center-Based Cardiac Rehabilitation in Heart Failure: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2019, 8, e012779. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.flindersprogram.com.au (accessed on 17 April 2025).
- Lenferink, A.; Brusse-Keizer, M.; van der Valk, P.D.; A Frith, P.; Zwerink, M.; Monninkhof, E.M.; van der Palen, J.; Effing, T.W. Self-management interventions including action plans for exacerbations versus usual care in patients with chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2017, 8, CD011682. [Google Scholar] [CrossRef]
- Moreno-Ligero, M.; Moral-Munoz, J.A.; Salazar, A.; Failde, I. mHealth Intervention for Improving Pain, Quality of Life, and Functional Disability in Patients with Chronic Pain: Systematic Review. JMIR mHealth uHealth 2023, 11, e40844. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.K.; Kim, M.Y. Self-Management Nursing Intervention for Controlling Glucose among Diabetes: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 12750. [Google Scholar] [CrossRef] [PubMed]
| Author (Year); Country | Study Details | Study Intervention | Summary of Intervention | |||
|---|---|---|---|---|---|---|
| Type/Number | Participants | F/U | Database | |||
| Zhao et al., 2024 China [23] | RCT 20 | P = 4681 R = 10–1518 | NA | Em, Md, Pb, SD, WS [In to 2022] | Case Management | 4 different MDT care models |
| Chen et al., 2023 Taiwan [24] | RCT + MA 13 | P = 2666 R = 28–197 | 2–24 m | CI, CL, Pb, Md [2002–2022] | Case Management | Collaborative health Management |
| Li 2023 China [25] | RCT + MA 10 | P = NA R = NA | 3–12 m | CE, CK, Eb, Pb, WoS [NA] | Case Management | Transitional care |
| Yang Mly 2023 [26] | RCT + MA 105 | P = 37,607 R = NA | 6–12 m | CL, Em, Ov, Pb [In–2022] | Case Management | Multicomponent integrated care |
| Hafkamp 2022 Holland [27] | RCT + MA 44/186 | P = 6101 R = 40–1650 | 3–34 m | CI, CL, Pb, PsI WoS [2011–2021] | Case Management | Care pathways |
| Hsu 2022 Taiwan [28] | RCT-Co + MA 6/1 | N = 2346 R = 40–1937 | NA | CI, CL, CP, Em, Pb [2020] | Case Management | Patient navigators |
| Toback 2017 Canada [29] | RCT-NRT 26 | NA | NA | Pb, UP [1999–2016] | Case Management | Multiple self-management support |
| Taylor 2005 UK [30] | RCT 21 | P = 1627 | NA | Am, D, CI, CL, Em, Med; NHS, NRR, SCI; Si [2003] | Case Management | Disease management (MDT, CMM, CM) |
| Roccaforte 2005 [31] | RCT + MA 33 | P = 7538 R = 34 to 1518 | 3–22 m | CL; Em; Med; Pb [1980–2004] | Case Management | Disease management programmes |
| Gonseth 2004 [32] | RCT-Co + MA 54 | NA R = 34–1966 | 1–50.4 m | CL, Em, Med [1966–2003] | Case Management | Disease management programmes—elderly |
| Huang 2023 [33] | RCT + MA 25 | P = 2746 R 40 to 228 | 1 w–12 m | CE, CI, Em, Med, PsI, WoS (In to 2022) | Nurse-Led | Self-management |
| Nwosu 2023 [34] | SR 18 | P = 2413 | NA | CI, Med, PsI, WoS (In to 2022) | Nurse-Led | Patient education |
| Checa 2022 [35] | RCT-QE-Co + MA 30 | P = 8209 R-24 to 1894 | 30 d–12 m | CE, CL, CT, Em, ICTRP, Med, WHO (In to 2022) | Nurse-Led | Case management primary care |
| Huang 2022 [36] | RCT + MA 24 | P = 2488 R-36 to 382 | 30 d-12 m | CE, CI, Em, Med, PsI, WoS (In to 2021) | Nurse-Led | Self-management |
| Ceu 2022 [37] | RCT-NRT 9 | NA | NA | CI, Med | Nurse-Led | Variable nursing interventions |
| Imanuel Tonapa 2022 [38] | RCT + MA 12 | P = 1938 R 36–1437 | 2 m–12 m | CI, CL, Em, Med, Ov, Pb, WoS (In to 2020) | Nurse-Led | Telecoaching |
| Son 2020 [39] | RCT + MA 8 | P = 1979 R 88–412 | 3 m–12 m | CI, CL, Em, Pb, WoS (2000 to 2019) | Nurse-Led | Self-management education |
| Walsh 2017 Abs Conf [40] | RCT-NRT 68 | NA | NA | CI, Pb, Med, SC (2006 to 2016) | Nurse-Led | Clinic-based self-management education |
| Alnomasy 2023 [41] | RCT + MA 14 | P = 2035 R: 40–767 | 30 d–12 m | CL; NAHL; Pb; WoS (NA) | Non-Pharmacological | Ambulatory—home visits, phone calls, digital platforms, technologies |
| Mhanna 2023 [42] | RCT + MA 6 | P = 489 R: 41–158 | 30 d-12 m | CL; Em; Med; Pb (In to 2022) | Non-Pharmacological | CBT |
| Olano-Lizarraga 2023 [43] | RCT 8 | P = 1623 R: 64–468 | 30 d-12 m | CI; CL; Pb; PsI; SC; (2010 to 2022) | Non-Pharmacological | Interventions targeting the social dimension |
| Nso 2023 [44] | RCT + MA 9 | P = 1070 | 3–6 m | Pb; Sco (NA) | Non-Pharmacological | CBT |
| Balata 2023 [45] | RCT + MA 7 | P = 611 R: 26–158 | 4–32 wk | CL, Pb, SC, WoS (In to 2022) | Non-Pharmacological | CBT |
| Koikai 2023 [46] | RCT-NCT N = 30 | P = 7685 R: 50–1223 | NA | CL, Em, GS Pb, SD (2012 to 2022) | Non-Pharmacological | Self-management education strategies |
| Feng 2023 [47] | RCT + MA 20 | P = 3459 R: 39–317 | 3–12 m | CK, Pb, WoS, VIP (1999 to 2022) | Non-Pharmacological | Self-management intervention strategies |
| Nahlen Bose 2023 [48] | Meta-review SR = 7, RCT = 67 | P = 10,132 R = 320 to 3837 | NA | CI, CL Pb, PsI (NA) | Non-Pharmacological | Psychosocial interventions |
| Lee/Reigel 2022 USA [49] | RCT + MA 27 | P = 6950 R = NA | NA | CI, Em, Pb, PsI (2008–2019) | Non-Pharmacological | Self-management intervention |
| Villero-Jimenez 2022 Spain [50] L-SP-ENG | RCT-NCT 12 | P = 1380 R = 19–369 | NA | CI, Pb, PsI (NA) | Non-Pharmacological | Dyadic self-management interventions |
| Ghizzardi 2022 [51] | RCT + MA 9 | P = 1214 R- 30 to 510 | 1–16 m | CI, Em, Pb PsI, SC (In to 2020) | Non-Pharmacological | Motivational interviewing on self-management |
| Suksatan 2022 Thailand [52] | RCT + NCT 15 | P = 10,701 R-36–2494 | 30 d | CI, CL, Pb, PsI, SC (2011 to 2022) | Non-Pharmacological | Transitional care intervention—elderly |
| Meng 2021 China [53] | RCT + MA 8 | P = 1707 R: 20–902 | 6 m-42 m | CL, CNKI, Em, Pb (2000 to 2020) | Non-Pharmacological | SM intervention in Knowledge, Attitude, Practice |
| Tinoco 2021 Brazil [54] | RCT-NRT + MA 19 | N = 1841 | NA | CI, LI, Pb, SC (2012 to 2019) | Non-Pharmacological | Health education and self-management |
| Aghajanloo 2021 Iran [55] | RCT-NRT + MA 39 | P = 8958 R: 17–2082 | NA | Em, GS, Ma, Pb, SID, WoS, (2004 to 2018) | Non-Pharmacological | Self-management behaviours with SCHFI |
| Cañon-Montañez 2021 Colombia [56] | RCT + RCT 45 | P = 9688 R: 37–1049 | 3–18 m | CI, CL, Em, Li, Pb, SC, WoS (In to 2019) | Non-Pharmacological | Educational Intervention |
| Anderson 2021 UK [57] | RCT + QE 12 | P = 3887 R: 25–1023 | 3–24 m | BNI, CI, Em, Med (2008 to 2020) | Non-Pharmacological | Advanced-level nurses specialist nurses vs. physician led |
| Zhao 2021 China [58] | RCT + MA 15 | P = 2630 R: 28–475 | 2 w-12 m | CL, Em, Pb, WoS (In to 2019) | Non-Pharmacological | Self-management interventions |
| Poudel 2020 USA [59] | RCT + NRT 8 | P = 758 R: 30–241 | NA | CI, CL, GS, HS, Med, PsI (1990 to 2019) | Non-Pharmacological | Motivational interviewing |
| Świątoniowska-Lonc 2020 [60] | RCT + MA 16 | P = 944 60–1160 | 1–18 m | Med, Pb, SC (2010 to 2019) | Non-Pharmacological | Health education |
| Peng 2019 [61] | RCT + MA 8 | P = 480 R: 17–158 | NA | CL, Em, Pb (In to 2018) | Non-Pharmacological | CBT |
| Parajuli 2019 Australia [62] | RCT + MA 18 | P = 4630 R: 34 to 2169 | 6 w-55 m | CI, CL, Em, Med, Pb, SC, WoS (In to 2017) | Non-Pharmacological | Pharmacist-involved MDT |
| Shanbhag 2018 Canada [63] | RCT-NRT 38 | P = 76,582 R = 68–50,678 | NA | CI, CL, Em, Med (1990–2017) | Non-Pharmacological | Interventions improving physician adherence to guideline |
| Sterling 2018 USA [64] | RCT-NRT N = 6 | P = NA R-NA | NA | AgeLine, CI, CL, Em, Med (In-2017) | Non-Pharmacological | Home care workers |
| Jiang 2018 Taiwan [65] | RCT + MA 29 | P = 3837 | 1–48 m | CI, CL, Em, Pb, PsI, SC, WoS, ProQuest Dissertation (16 February 2006) | Non-Pharmacological | Psychological interventions on self-management |
| Jonkman 2016 Holland [66] | RCT + MA 20 | P = 5624 R: 42–1023 | 3–18 | CI, CL, Em, Pb, PsI (1985–2013) | Non-Pharmacological | Self-management and programme characteristics |
| Ruppar 2016 USA [67] | RCT-NCT + MA N = 57 | P = 4527 R:10–1518 | NA | CI, CL, D, IPA, Highwire Med, SC, PQ (In–2013) | Non-Pharmacological | Medication adherence interventions |
| Jonkman 2016 Holland [68] | RCT + RCT 20 | P = 5624 R: 42–1023 | 3–18 | CI, CL, Em, Pb, PsI (1985–2013) | Non-Pharmacological | Self-management interventions |
| Srisuk 2016 Thailand [69] | RCT 9 | P = 666 R: 61–155 | NA | CI, CL, Em, Med, Pb, PsI, SC, WoS (2005–2015) | Non-Pharmacological | Family-based education |
| Ha Dinh 2016 Vietnam [70] | RCT + NCT 12 (2 HF) | P = 467 (3 HF) R: 88–276 | 12–15 | CI, CL, Em Med, WoS (In–2013) | Non-Pharmacological | Teach-back method and self-management |
| Inglis 2015 Australia [71] | RCT + MA 41 | P = 9332 | 38% < 6 m | CENRAL, DARE, HTA, Med, Em, CI, SCI, AMED (In–2015) | Non-Pharmacological | Structured telephone support, non-invasive telemonitoring |
| Ruppar 2015 [72] | RCT + NRT 29 | P = 4285 | 11 d-24 m | CI, CL, Em, Med (In–2013) | Non-Pharmacological | Medication adherence interventions |
| Casimir 2014 [73] | RCT 7 | P: NA R: 121–314 | NA | CINAHL, Pb, PsychINFO, EMBASE, CENTRAL, ERIC, SC, DynaMed. (1990–2013) | Non-Pharmacological | Patient centred self-management |
| Wakefield 2013 USA [74] | RCT + MA 43 | P = 8071 R: 48–1518 | 10 d-549 d | CI, CL, Med (1995–2008) | Non-Pharmacological | Care management programme |
| Barnason 2012 USA [75] | IR-RCT 19 | P:NA R: 18–902 | NA | CI, CL, Med, PsI (2000–2010) | Non-Pharmacological | Self-management interventions |
| Boyde 2011 USA [76] | RCT 19 | P = 2686 R: 36–314 | 3 m-288 d | CI, CL, Em, Med, PsI (1998–2008) | Non-Pharmacological | Educational interventions |
| Dickson 2011 USA [77] | RCT + MA N = 3 | P = 99 NA | NA | NA | Non-Pharmacological | Self-care practices |
| Yehle 2010 USA [78] | RCT + NRT 12 | P = 1360 R: 20–151 | NA | Pb, CL, CINAHL, Med, ERIC, Academic Search Premier, Health Source (1966–2009) | Non-Pharmacological | Educational interventions |
| Ditewig 2010 Holland [79] | RCT 19 | P = 4011 R: 50–766 | 6–24 m | CI, CL, Em, Med (1996–2009) | Non-Pharmacological | Self-management interventions |
| Boren 2009 USA [80] | RCT 35 | P = 7413 R: 36–713 | 3–18 m | CI, CL, Med (1966–2007) | Non-Pharmacological | Self-management education |
| Jovicic 2006 Canada [81] | RCT 6 | P = 857 R: 70–223 | 3–12 m | ACP, CI, CL, Em, Med (1966–2005) | Non-Pharmacological | Self-management intervention |
| McAlister 2004 Canada [82] | RCT 29 | P = 5039 R: 34–1396 | 1–12 | AMED, CI, CL, Em, Med (1966–2003) | Non-Pharmacological | Multidisciplinary strategies |
| Author (Year); Country | Death/HFM | Readmission | Quality of Life (Depression/Anxiety) | Self-Management Behaviour Ability | A&E Use | Length of Stay | Cost | Strength of Evidence |
|---|---|---|---|---|---|---|---|---|
| Zhao et al., 2024 China [23] | + | + | + | + + | NA | NA | NA | L |
| Chen et al., 2023 Taiwan [24] | + | + + | + | NA | NA | NA | NA | L |
| Li 2023 China [25] | NA | NA | + + | + | NA | NA | NA | L |
| Yang Mly 2023 [26] | + | + − | NA | + | + | NA | NA | L |
| Hafkamp 2022 Holland [27] | − | + + | NA | NA | NA | NA | NA | H |
| Hsu 2022 Taiwan [28] | − | − | − | + | − | + | + | L |
| Toback 2017 Canada [29] | NA | + | + | + | − | − | + | VL |
| Taylor 2005 UK [30] | ~ | ~ | NA | NA | NA | NA | NA | H |
| Roccaforte 2005 [31] | + | + + | ~ | NA | NA | NA | NA | H |
| Gonseth 2004 [32] | + | + + | ~ + | ~+ | NA | NA | NA | H |
| Huang 2023 [33] | ~ | ~ | + | NA | ~ | NA | NA | M |
| Nwosu 2023 [34] | ~ | ~ | + | NA | NA | NA | NA | L |
| Checa 2022 [35] | − | + + | + | ~ | NA | NA | + | H |
| Huang 2022 [36] | NA | NA | NA | + | NA | NA | NA | M |
| Ceu 2022 [37] | NA | ~ | + | ~ | NA | NA | NA | VL |
| Imanuel Tonapa 2022 [38] | NA | NA | + | + | NA | NA | NA | M |
| Son 2020 [39] | + | + | − | - | NA | NA | NA | M |
| Walsh 2017 Abs Conf [40] | NA | NA | ~ | ~ | NA | NA | NA | L |
| Alnomasy 2023 [41] | NA | + | NA | ~ | NA | NA | NA | M |
| Mhanna 2023 [42] | NA | NA | ~ | + | NA | NA | NA | H |
| Olano-Lizarraga 2023 [43] | + | + | + | + | NA | NA | NA | M |
| Nso 2023 [44] | − | − | + | + | NA | NA | NA | L |
| Balata 2023 [45] | NA | NA | + | − | NA | NA | NA | M |
| Koikai 2023 [46] | + | + | + | + | NA | NA | + | M |
| Feng 2023 [47] | − | + | + | + | NA | NA | NA | M |
| Nahlen Bose 2023 [48] | − | − | ~+ | − | NA | NA | NA | M |
| Lee/Reigel 2022 USA [49] | ~ | ~ | ~ | + | NA | NA | NA | H |
| Villero-Jimenez 2022 Spain [50] L-SP-ENG | NA | NA | + | + | NA | NA | NA | H |
| Ghizzardi 2022 [51] | NA | NA | − | + | NA | NA | NA | M |
| Suksatan 2022 Thailand [52] | NA | + | NA | NA | NA | NA | + | H |
| Meng 2021 China [53] | NA | NA | NA | + | NA | NA | NA | M |
| Tinoco 2021 Brazil [54] | NA | NA | NA | ~ | NA | NA | NA | M |
| Aghajanloo 2021 Iran [55] | NA | NA | NA | ~ | NA | NA | NA | L |
| Cañon-Montañez 2021 Colombia [56] | NA | + | ~ | ~ | NA | + | + | M |
| Anderson 2021 UK [57] | + | + | + | + | + | + | + | L |
| Zhao 2021 China [58] | NA | + | + | + | NA | NA | NA | M |
| Poudel 2020 USA [59] | NA | + | + | + | NA | NA | NA | M |
| Świątoniowska-Lonc 2020 [60] | NA | NA | − | + | NA | NA | NA | L |
| Peng 2019 [61] | NA | NA | + | − | NA | NA | NA | L |
| Parajuli 2019 Australia [62] | − | + | ~ | − | NA | NA | − | H |
| Shanbhag 2018 Canada [63] | ~ + | ~ + | ~ + | ~ + | ~ + | ~ + | ~ + | L |
| Sterling 2018 USA [64] | ~ | ~ | ~ | ~ | ~ | ~ | ~ | L |
| Jiang 2018 Taiwan [65] | NA | NA | + | ~ + | NA | NA | NA | M |
| Jonkman 2016 Holland [66] | ~ + | + | + | NA | NA | NA | NA | H |
| Ruppar 2016 USA [67] | + | + | + | + | NA | NA | NA | H |
| Jonkman 2016 Holland [68] | + | + | + | ~ + | NA | + | NA | H |
| Srisuk 2016 Thailand [69] | NA | NA | + | + | NA | NA | NA | L |
| Ha Dinh 2016 Vietnam [70] | NA | NA | + | + | NA | NA | NA | L |
| Inglis 2015 Australia [71] | + | + | + | + | NA | + | + | H |
| Ruppar 2015 [72] | NA | NA | NA | ~ | NA | NA | NA | H |
| Casimir 2014 [73] | − | + | + | + | NA | NA | NA | M |
| Wakefield 2013 USA [74] | NA | + | + | + | NA | NA | + | H |
| Barnason 2012 USA [75] | NA | NA | NA | + | NA | NA | NA | M |
| Boyde 2011 USA [76] | ~ | ~ | ~ | + | NA | NA | NA | M |
| Dickson 2011 USA [77] | NA | NA | NA | + | NA | NA | NA | H |
| Yehle 2010 USA [78] | NA | NA | NA | + | NA | NA | NA | H |
| Ditewig 2010 Holland [79] | ~ + | ~ + | ~ + | ~ + | NA | NA | NA | H |
| Boren 2009 USA [80] | + | NA | + | + | NA | NA | + | H |
| Jovicic 2006 Canada [81] | − | + | ~ − | ~ − | NA | NA | + | H |
| McAlister 2004 Canada [82] | − | + | ~ + | ~ + | NA | NA | + | H |
| Author (Year); Country | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zhao et al., 2024 China [23] | Y | PY | Y | PY | Y | Y | N | PY | Y | N | N-MA | N-MA | N | N | N-MA | Y | L |
| Chen et al., 2023 Taiwan [24] | Y | PY | Y | PY | Y | Y | N | Y | Y | N | Y RCT | Y | Y | Y | Y | Y | L |
| Li 2023 China [25] | Y | PY | Y | PY | Y | Y | N | Y | Y RCT | N | Y RCT | Y | Y | Y | N | Y | L |
| Yang Mly 2023 [26] | Y | PY | Y | PY | Y | Y | N | Y | Y RCT | N | Y RCT | Y | Y | Y | Y | Y | L |
| Hafkamp 2022 Holland [27] | Y | PY | Y | Y | Y | Y | Y | Y | Y RCT | Y | Y RCT | Y | Y | Y | Y | Y | H |
| Hsu 2022 Taiwan [28] | Y | PY | Y | PY | Y | Y | Y | PY | PY | Y | Y | PY | PY | PY | N | Y | L |
| Toback 2017 Canada [29] | Y | PY | Y | PY | Y | Y | N | Y | Y RCT | N | Y RCT | Y | Y | Y | Y | Y | L |
| Taylor 2005 UK [30] | Y | PY | Y | Y | Y | Y | PY | PY | Y RCT | Y | Y RCT | Y | Y | Y | Y | Y | H |
| Roccaforte 2005 [31] | Y | PY | Y | PY | Y | Y | PY | PY | Y RCT | Y | Y RCT | Y | Y | Y | Y | Y | H |
| Gonseth 2004 [32] | Y | PY | Y | Y | Y | Y | Y | Y | Y RCT | Y | Y RCT | Y | Y | Y | Y | Y | H |
| Huang 2023 [33] | Y | PY | Y | PY | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | M |
| Nwosu 2023 [34] | Y | PY | Y | PY | Y | Y | Y | PY | PY | Y | Y | PY | PY | PY | N | Y | L |
| Checa 2022 [35] | Y | Y | Y | Y | Y | Y | Y | Y | Y RCT | Y | Y RCT | Y | Y | Y | Y | Y | H |
| Huang 2022 [36] | Y | PY | Y | PY | Y | Y | Y | Y | Y RCT | N | Y RCT | Y | Y | Y | Y | Y | M |
| Ceu 2022 [37] | Y | PY | Y | PY | Y | Y | N | Y | N BOTH | N | N BOTH | N-MA | N | N | N-MA | N | VL |
| Imanuel Tonapa 2022 [38] | Y | PY | Y | PY | Y | Y | PY | Y | PY RCT | N | Y RCT | N | Y | Y | Y | Y | M |
| Son 2020 [39] | Y | PY | Y | PY | Y | Y | PY | Y | Y RCT | Y | Y RCT | Y | Y | Y | N | Y | M |
| Walsh 2017 Abs Conf [40] | Y | PY | Y | PY | Y | N | N | PY | N BOTH | N | N-MA | N-MA | N | N | N-MA | N | L |
| Alnomasy 2023 [41] | Y | Y | Y | PY | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | M |
| Mhanna 2023 [42] | Y | PY | Y | Y | Y | Y | PY | PY | Y RCT | Y | Y RCT | Y | Y | Y | Y | Y | H |
| Olano-Lizarraga 2023 [43] | Y | PY | Y | PY | Y | Y | N | Y | Y RCT | N | Y RCT | Y | Y | Y | N | Y | M |
| Nso 2023 [44] | Y | PY | Y | PY | Y | Y | N | Y | Y RCT | N | Y RCT | Y | Y | Y | Y | Y | L |
| Balata 2023 [45] | Y | Y | Y | PY | Y | Y | N | Y | Y RCT | N | Y RCT | Y | Y | Y | Y | Y | M |
| Koikai 2023 [46] | Y | PY | Y | PY | Y | Y | N | Y | Y BOTH | N | N | N-MA | N | N | N | Y | M |
| Feng 2023 [47] | Y | PY | Y | PY | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | M |
| Nahlen Bose 2023 [48] | Y | PY | Y | PY | Y | N | Y | Y | Y RCT | Y | Y RCT | Y | Y | Y | Y | Y | M |
| Lee/Reigel 2022 USA [49] | Y | PY | Y | PY | Y | Y | N | PY | Y RCT | N | Y RCT | Y | Y | Y | Y | Y | H |
| Villero-Jimenez 2022 Spain [50] L-SP-ENG | Y | PY | Y | PY | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | M |
| Ghizzardi 2022 [51] | Y | PY | Y | Y | Y | Y | Y | Y | Y RCT | Y | Y RCT | Y | Y | Y | Y | Y | H |
| Suksatan 2022 Thailand [52] | Y | PY | Y | Y | Y | Y | Y | Y | Y BOTH | Y | Y RCT | Y | Y | Y | Y | Y | M |
| Meng 2021 China [53] | Y | PY | Y | PY | Y | Y | Y | PY | PY RCT | Y | Y RCT | N | Y | Y | Y | Y | M |
| Tinoco 2021 Brazil [54] | Y | PY | Y | PY | Y | Y | Y | PY | PY RCT | N | Y RCT | Y | Y | Y | Y | Y | L |
| Aghajanloo 2021 Iran [55] | Y | PY | Y | PY | Y | Y | Y | PY | Y NRSI | N | Y BOTH | Y | Y | Y | Y | Y | M |
| Cañon-Montañez 2021 Colombia [56] | Y | Y | Y | Y | Y | Y | Y | Y | PY RCT | Y | Y RCT | Y | Y | Y | Y | Y | M |
| Anderson 2021 UK [57] | Y | PY | PY | Y | Y | PY | Y | Y BOTH | N | N BOTH | N MA | Y | Y | Y | Y | L | |
| Zhao 2021 China [58] | Y | PY | Y | PY | Y | Y | N | Y | PY RCT | N | Y RCT | N | Y | Y | Y | Y | M |
| Poudel 2020 USA [59] | Y | Y | Y | Y | Y | Y | Y | Y | PY RCT | Y | Y RCT | Y | Y | Y | Y | Y | M |
| Świątoniowska-Lonc 2020 [60] | Y | PY | Y | PY | Y | Y | PY | Y | PY RCT | Y | Y RCT | Y | Y | Y | N-MA | Y | VL |
| Peng 2019 [61] | Y | PY | Y | PY | Y | Y | PY | PY | Y RCT | Y | Y RCT | Y | Y | Y | N | Y | L |
| Parajuli 2019 Australia [62] | Y | PY | Y | Y | Y | Y | Y | Y | Y RCT | Y | Y RCT | Y | Y | Y | Y | Y | H |
| Shanbhag 2018 Canada [63] | Y | Y | Y | PY | Y | Y | Y | Y | Y BOTH | N | N-MA | N-MA | Y | Y | N-MA | Y | L |
| Sterling 2018 USA [64] | Y | Y | Y | PY | Y | Y | PY | Y | Y NRSI | Y | N-MA | N-MA | Y | Y | N | Y | L |
| Jiang 2018 Taiwan [65] | Y | PY | Y | PY | Y | Y | PY | PY | Y RCT | Y | Y RCT | Y | Y | Y | Y | Y | M |
| Jonkman 2016 Holland [66] | Y | PY | Y | PY | Y | Y | PY | Y | Y RCT | Y | Y RCT | Y | Y | Y | Y | Y | H |
| Ruppar 2016 USA [67] | Y | PY | Y | PY | Y | Y | N | Y | Y RCT | N | Y RCT | Y | Y | Y | Y | Y | H |
| Jonkman 2016 Holland [68] | Y | PY | Y | PY | Y | Y | PY | Y | Y RCT | Y | Y RCT | Y | Y | Y | Y | Y | H |
| Srisuk 2016 Thailand [69] | Y | PY | Y | PY | Y | Y | PY | Y | Y RCT | Y | N-MA | N-MA | Y | Y | N-MA | Y | L |
| Ha Dinh 2016 Vietnam [70] | Y | PY | Y | PY | Y | Y | Y | Y | Y BOTH | N | N MA | N MA | Y | Y | N-MA | Y | L |
| Inglis 2015 Australia [71] | Y | Y | Y | PY | Y | Y | PY | PY | Y RCT | Y RCT | Y | Y | Y | Y | Y | H | |
| Ruppar 2015 [72] | Y | Y | Y | PY | Y | Y | PY | Y | Y RCT | Y | Y RCT | Y | Y | Y | Y | Y | H |
| Casimir 2014 [73] | Y | PY | Y | Y | Y | Y | PY | PY | Y RCT | N | N-MA | N-MA | Y | N | N-MA | Y | M |
| Wakefield 2013 USA [74] | Y | PY | Y | Y | Y | Y | Y | Y | Y RCT | Y | Y RCT | Y | Y | Y | Y | Y | H |
| Barnason 2012 USA [75] | Y | Y | Y | Y | Y | Y | N | Y | Y RCT/PY NRSI | Y | Y BOTH | Y | Y | N | N | Y | M |
| Boyde 2011 USA [76] | Y | PY | Y | PY | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | M |
| Dickson 2011 USA [77] | Y | PY | Y | Y | Y | Y | Y | Y | Y RCT | Y | Y RCT | Y | Y | Y | Y | Y | H |
| Yehle 2010 USA [78] | Y | PY | Y | Y | Y | Y | Y | Y | Y RCT | Y | Y RCT | Y | Y | Y | Y | Y | H |
| Ditewig 2010 Holland [79] | Y | PY | Y | PY | Y | Y | PY | Y | Y RCT | N | N-MA | N-MA | Y | Y | N-MA | Y | H |
| Boren 2009 USA [80] | Y | PY | Y | Y | Y | Y | Y | Y | Y RCT | Y | Y RCT | Y | Y | Y | Y | Y | H |
| Jovicic 2006 Canada [81] | Y | PY | Y | Y | Y | Y | PY | Y | Y RCT | Y | Y RCT | Y | Y | Y | Y | Y | H |
| McAlister 2004 Canada [82] | Y | PY | Y | PY | Y | Y | PY | Y | Y RCT | Y | Y RCT | Y | Y | Y | Y | Y | H |
| (Author/Year/Country) | Study Aim/Background | Study Intervention | MA + Summary | Outcomes |
|---|---|---|---|---|
| Hafkamp 2022 Holland [27] | A: umbrella systematic review and meta-analyses on effectiveness of interventions in reducing HF-related (re)hospitalization | DF: pharmaceutical; device; rehabilitation multidisciplinary—variable haemodynamic, m-health, nurse-led, STS, CR. DCP |
|
|
| Taylor 2005 UK [30] | A: effectiveness of disease management interventions for patients with CHF SF: inpatient, outpatient, or community based (clinical service intervention: multidisciplinary models; case management models; clinic models) EG: IP: patient; IC: care package (enhanced or novel) | DF: telephone follow-up DC: education; SM; weight-monitoring; sodium diet advice/restriction; exercise recommendation; medication review; social and psychological support |
|
|
| Roccaforte 2005 Spain [31] | A: re-evaluate the effectiveness of HFDMP on MACE and outcomes. SF: in hospital/post discharge EG: IP: patient, carer; IC: primarily education based excluded | DF: education, discharge plan, pre-planned outpatient clinic visits, home visits, tele, education session, counselling, therapy, close follow-up. Therapy optimisation, status monitoring DC: multidisciplinary approach (cardiologist, physician); case management (nurse, pharmacist, case manager) |
|
|
| Gonseth 2004 Spain [32] | A: evaluate DMPs reducing hospital re-admissions among elderly SF: In hospital or post discharge; Home or outpatient visits | DF: nurse, dDischarge planning, care co-ord DC: education, counselling, and monitoring to enhance self-control mechanisms, timely medical visits, diet, and drug therapy compliance. Individualised and comprehensive patient and family HF education, follow-up and surveillance, promotion of optimal HF medications and medication doses |
|
|
| Checa 2022 Spain [35] | A: effect of nurse-led case management models on an advanced HF (NYHA III, IV) SF: intensive vs. basic telemedicine; home-visit interventions were intensive programmes, and basic were clinical consultations and phone calls | DF: Hosp, Comm Intensive vs. Basic DC: telemedicine, home-visit |
|
|
| Mhanna 2023 Spain [42] | A: efficacy of adjunctive CBT vs. standard care in HF patients with major depression SP: Hosp, post 1–2/week up to 32 weeks; EF—N/A EG: CBT including SC intervention | DF: 30–60 m, F2F, tele DP: nurse; other not stated |
|
|
| Lee 2022 USA [49] | A: effectiveness of self-care interventions on relevant outcomes SP: six chronic conditions. OP, home. EF-NA EG: IR: patients, carers; IC: see @ | DF: face-to-face individual/group, web, media, print, phone, tools DP: multiple see @ |
|
|
| Villero-Jimenez 2022 Spain [50] | Identify dyadic self-management interventions in CHF in hospital settings. Hosp SP: hospital HF. NYHA I-IV EG: IR: patient carers; IC: cognitive-attitudinal, affective-emotional and behavioural | DF: variable delivery format and strategies (cognitive-attitudinal, affective-emotional, and behavioural); providers and recipients; measurement instruments used; and effectiveness. DP: nurse |
|
|
| Suksatan 2022 Thailand [52] | A: effect of TCI on rehospitalization before discharge from hospital to home SP: Hosp, older patients, EF-NA EG: IR: HF pt; IC: discharge planning, clinic appointments, medication reconciliation, early follow-up telephone calls, HF knowledge, and SM education. TCI components: 1 = screening; 2 = staffing; 3 = maintaining relationships; 4 = engaging patients and caregivers; 5 = assessing/managing risks and symptoms; 6 = educating/promoting SM; 7 = collaborating; 8 = promoting continuity; 9 = fostering coordination | DF: F2F, home visit, tele, mob app DP: nurses, pharmacists, and multidisciplinary teams |
|
|
| Parajuli 2019 Australia [62] | A: evidence for the role of the pharmacist within the multidisciplinary team for HF management to improve clinical outcomes SG: hospital, outpatient clinic, or family medical practice, or under multidisciplinary HF-specialist care; NYHA II-IV EG: IR: patient; IC: pharmacist(s) working in collaboration, at a minimum, with a physician within the intervention model | DF: medication reconciliation, discharge counselling, patient education, collaborative medication management, telephone follow-up, home medication review, self-adjustment of diuretic DP: pharmacist, physician (± MDT) |
|
|
| Jonkman 2016 Holland [66] | A: characteristics of SM interventions effective in influencing HRQol, mortality, and hospitalizations SG: Hosp, Op, Ab; EF: 39.2 EG: IR: patient, family/carer/partner; IC: SMI | DF: F2F, Tele, TM, EMep, HV DP: N, Phy, Pha, HW |
|
|
| Ruppar 2016 USA [67] | A: interventions to improve medication adherence SG: Hosp, Op, Ab; EG: IR: patient, carers; IC: medication education and disease education; 11 SC | DF: F2F, Tele, TM, txt, web, Media, PM DP: MD, nurses, phar, phy, diet, SW, CM, HCW |
|
|
| Jonkman 2016 Holland [68] | A: characteristics of SM interventions effective in influencing MACE and HRQol SG: Hosp, Op, Ab; EF: 39.2 EG: IR: patient, family/carer/partner; IC: SMI | DF: F2F, Tele, TM, EMep, HV DP: N, Phy, Pha, HW |
|
|
| Inglis 2015 Australia [71] | A: HF management via structured telesupport SG: Hosp, Op, Am EG: IR: patient IC: disease management | DF: all options DP: all options |
|
|
| Ruppar 2015 USA [72] | A: quantify the effect of interventions to improve adherence to HF medications SG: post discharge, Op, Ab EG: IR: patients; IC: medication, disease education EG: IR: patients; IC: medication, disease education | DF: verbal (F2F, Tele) and written/electronic instructions DP: pharmacist, nurse |
|
|
| Wakefield 2013 USA [74] | A: quantify individual interventions used in multicomponent outpatient HF management programme SG: Op, Ab EG: IR: patient; IC: patient education, symptom monitoring, medication adherence strategies | DF: F2F, tele, devices, interactive videophone, scales, organiser DP: nurse |
|
|
| Dickson 2011 USA [77] | A: explore how comorbidity influences HF self-care SG: NA EG: IR: patients; IC: HF education | DF: F2F, tele, group, nixed DP: NA |
|
|
| Yehle 2010 USA [78] | A: how to structure educational interventions for HF patients to improve self-efficacy and SM behaviours SG: discharge, Op, Ab EG: IR: patients; IC: SM and HF education | DF: F2F, tele, group, media nixed DP: nurses, pharmacists, health educators, peer mentors |
|
|
| Ditewig 2010 Holland [79] | A: effectiveness of self-management interventions compared to usual care SG: Hosp, Op, Ab; EG: IR: patient; IC: structured HF, SC education | DF: Tele, Media, Video, pEM DP: Nurse, Phar, CM, MDT |
|
|
| Boren 2009 USA [80] | A: identify educational content and techniques that lead to successful patient SM and improved outcome SG: inpatient, acute discharge, Op. Ab EG: IR: patient and carer; IC: structured education knowledge, social interaction and support, fluid management, diet and activity | DF: F2F, written format, health organisers, audio, video DP: nurses, pharmacists, dieticians, health educators, physician |
|
|
| Jovicic 2006 Canada [81] | A: effectiveness of SM interventions SG: Hosp, Op, Ab. EG: IR: patient, family IC: structured education | DF: F2F, Tele, SMS, Media DP: Nurse, Physician |
|
|
| McAlister 2004 Canada [82] | A: multidisciplinary strategies improve outcomes for heart failure SG: Hosp, Op, Ab; EG: IR: patient: IC: structured patient education | DF: Tele, TM, Media DP: MDT, Nurse, Phar, CM |
|
|
| (a) | ||
| Outcome | Number of Studies | References |
| Significant improvement | 25 | [23,25,26,28,29,36,38,42,43,44,46,47,49,50,51,53,57,58,59,60,67,69,70,71,73,74,75,76,77,78,80] |
| Positive trend (not significant) | 4 | [63,65,68,79] |
| Equivocal findings | 10 | [35,37,40,41,54,55,56,64,67,81] |
| Negative findings | 4 | [39,45,48,61] |
| Not reported | 9 | [24,27,30,31,32,33,34,52,66] |
| (b) | ||
| Study Quality | Outcome Type | References |
| High-quality studies | Significant improvement | [42,49,50,67,68,72,74,77,78,80] |
| Equivocal | [35,68,72,79,81,82] | |
| Negative | [62] | |
| Not reported | [27,30,31,32,52,66] | |
| Moderate-quality studies | Significant improvement | [33,36,38,43,46,47,51,53,58,59,73,75,76] |
| Equivocal | [41,54,56,65] | |
| Negative | [39,45,48] | |
| Not reported | [33] | |
| (a) | ||
| Outcome | Number of Studies | References |
| Significant reduction | 25 | [23,24,27,29,31,32,35,39,41,43,46,47,52,56,57,58,59,62,66,67,68,71,73,74,81,82] |
| Positive trend (not significant) | 3 | [26,63,79] |
| Equivocal findings | 7 | [30,33,34,37,49,64,76] |
| Negative findings | 3 | [28,44,48] |
| Not reported | 21 | [25,33,38,42,45,50,51,53,54,55,60,61,65,69,70,72,75,77,78,80] |
| (b) | ||
| Study Quality | Outcome Type | References |
| High-quality studies | Significant reduction | [27,31,32,35,62,66,67,68,71,74,81,82] |
| Equivocal | [30,49,80] | |
| Negative | None reported | |
| Not reported | [42,50,67,77,78,80] | |
| Moderate-quality | Significant reduction | [39,41,43,46,47,56,58,59,73] |
| Equivocal | [33,76] | |
| Negative | [48] | |
| Not reported | [36,38,45,51,53,54,65,75] | |
| Outcome | Significant Improvement | Positive Trend | Equivocal | Negative | Not Reported |
|---|---|---|---|---|---|
| HRQoL | 23 studies [23,24,25,29,33,34,35,37,38,43,44,45,46,47,50,57,58,59,65,66,67,68,69,70,71,72,73,75,80] | 7 studies [32,48,61,62,63,79,82] | 9 studies [31,40,42,49,56,60,64,76,81] | 4 studies [28,39,51,60] | 14 studies [26,27,30,36,41,52,53,54,55,73,75,77,78] |
| Self-Management | 25 studies [23,25,26,28,29,36,38,42,43,44,46,47,49,50,51,53,57,58,59,60,67,69,70,71,73,74,75,76,77,78,80] | 4 studies [63,65,68,80] | 10 studies [35,37,40,41,54,56,64,67,81] | 4 studies [39,45,48,61] | 9 studies [23,27,30,31,32,33,34,52,66] |
| Mortality | 12 studies [23,24,26,31,32,39,43,46,57,67,68,80] | 3 studies [63,66,79] | 6 studies [30,33,34,49,64,76] | 10 studies [27,28,35,44,47,48,49,62,81,82] | 28 studies [25,29,36,37,38,40,41,42,45,50,51,52,53,54,55,56,58,59,60,61,65,69,70,72,74,75,77,78] |
| Readmissions | 25 studies [23,24,27,29,31,32,35,39,41,43,46,47,52,56,57,58,59,62,66,67,68,71,73,74,81,82] | 3 studies [26,63,80] | 7 studies [30,33,34,37,49,64,76] | 3 studies [28,44,48] | 21 studies [25,33,38,42,45,50,51,53,54,55,60,61,65,69,70,72,75,77,78,80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iyngkaran, P.; Patel, T.; Asadi, D.; Siddique, I.; Gupta, B.; de Courten, M.; Hanna, F. Efficacy of Nurse-Led and Multidisciplinary Self-Management Programmes for Heart Failure with Reduced Ejection Fraction: An Umbrella Systematic Review. Biomedicines 2025, 13, 1955. https://doi.org/10.3390/biomedicines13081955
Iyngkaran P, Patel T, Asadi D, Siddique I, Gupta B, de Courten M, Hanna F. Efficacy of Nurse-Led and Multidisciplinary Self-Management Programmes for Heart Failure with Reduced Ejection Fraction: An Umbrella Systematic Review. Biomedicines. 2025; 13(8):1955. https://doi.org/10.3390/biomedicines13081955
Chicago/Turabian StyleIyngkaran, Pupalan, Taksh Patel, Diana Asadi, Iqra Siddique, Bhawna Gupta, Maximilian de Courten, and Fahad Hanna. 2025. "Efficacy of Nurse-Led and Multidisciplinary Self-Management Programmes for Heart Failure with Reduced Ejection Fraction: An Umbrella Systematic Review" Biomedicines 13, no. 8: 1955. https://doi.org/10.3390/biomedicines13081955
APA StyleIyngkaran, P., Patel, T., Asadi, D., Siddique, I., Gupta, B., de Courten, M., & Hanna, F. (2025). Efficacy of Nurse-Led and Multidisciplinary Self-Management Programmes for Heart Failure with Reduced Ejection Fraction: An Umbrella Systematic Review. Biomedicines, 13(8), 1955. https://doi.org/10.3390/biomedicines13081955








