Leadless vs. Transvenous Pacemakers in Patients with End-Stage Renal Disease: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Search Strategy
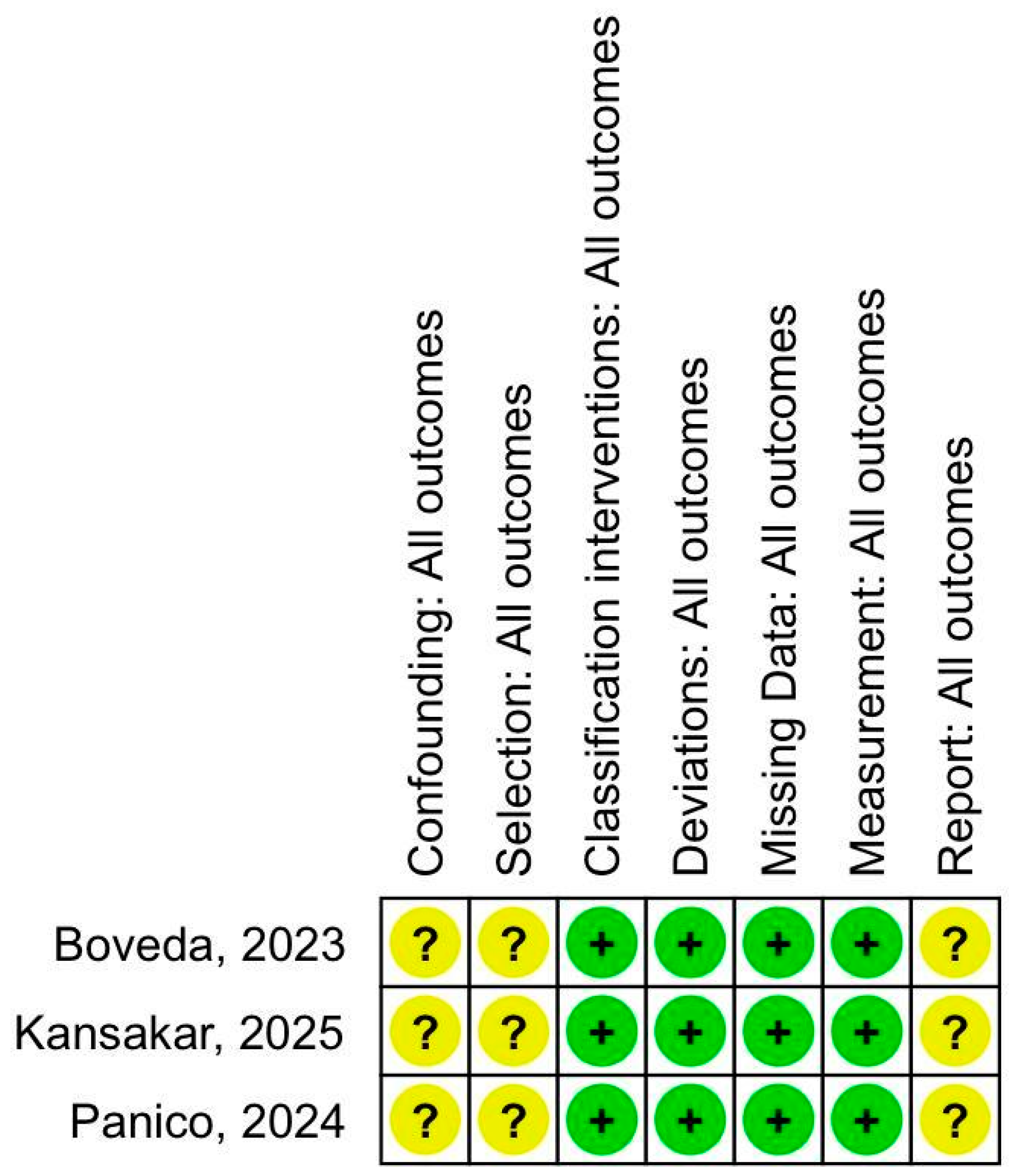
2.3. Risk of Bias
2.4. Statistical Analysis
3. Results
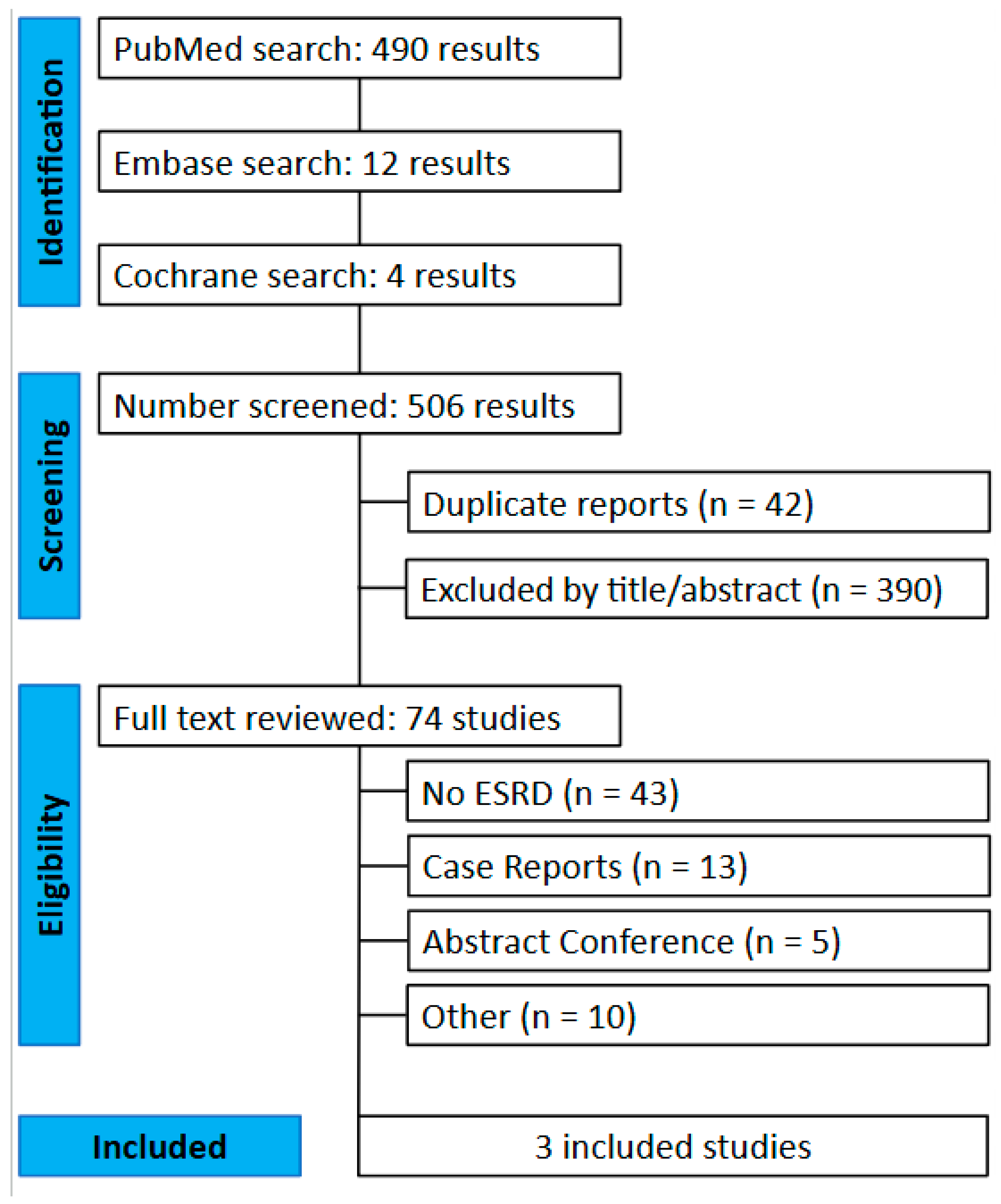
3.1. Study Selection and Baseline Characteristics
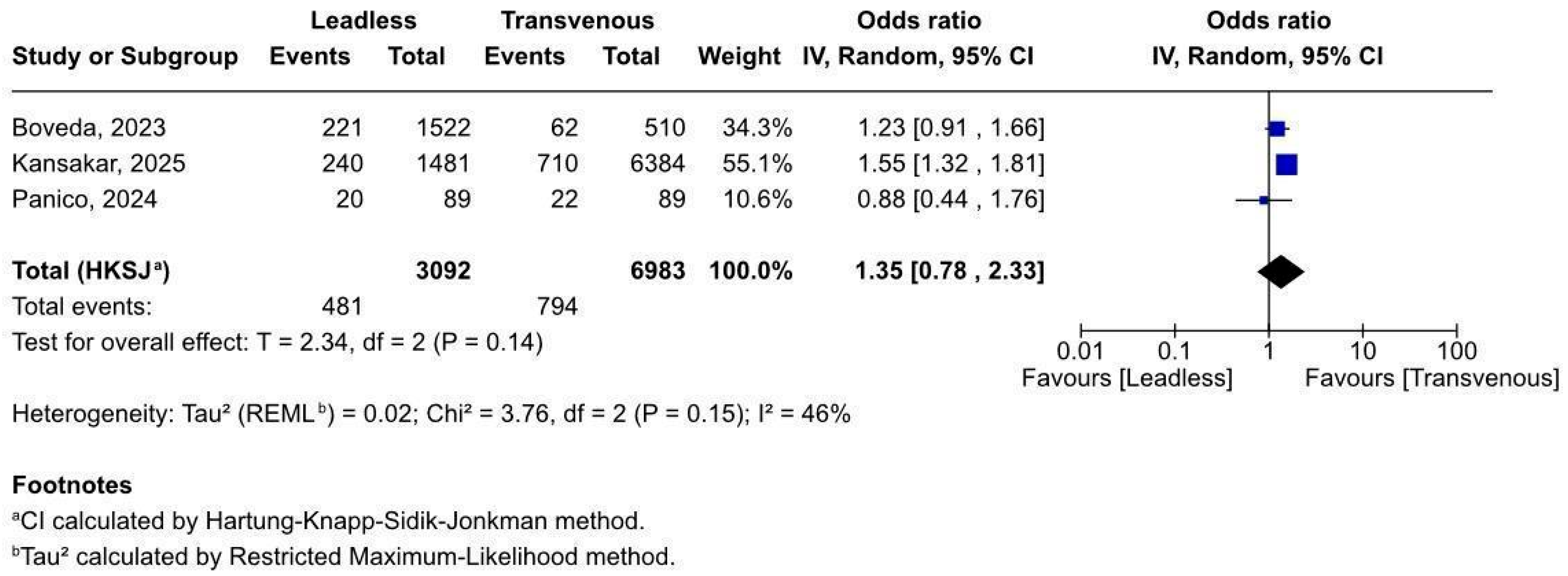
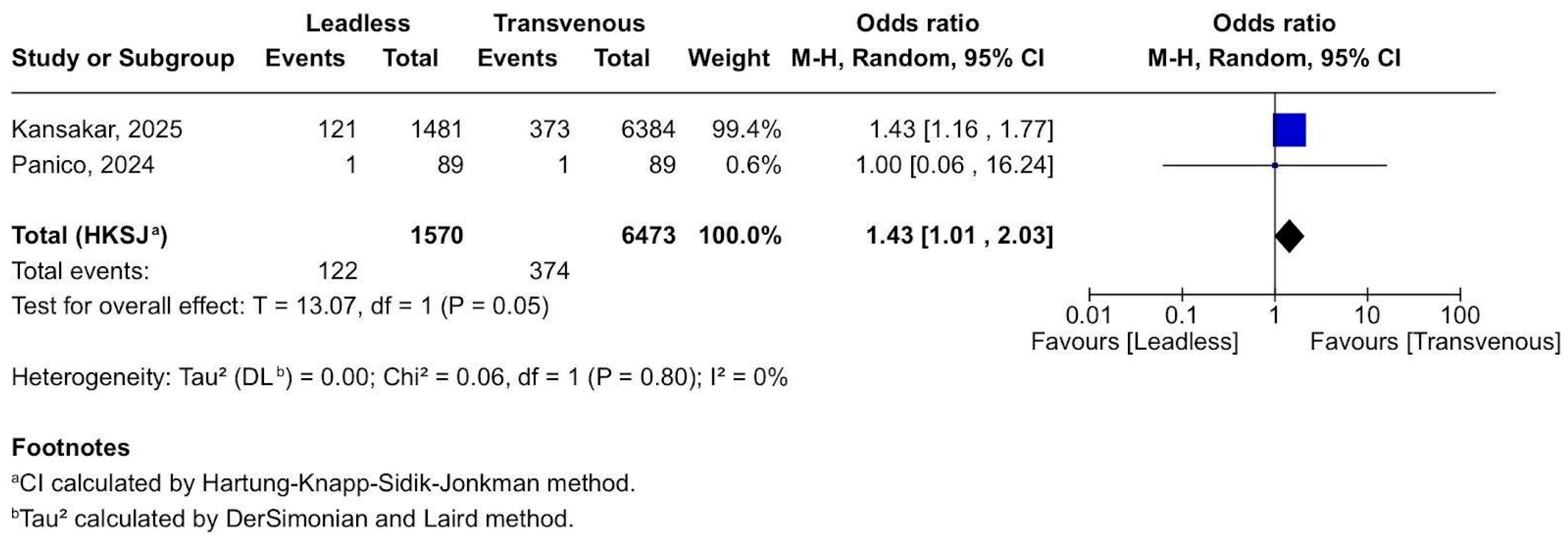
3.2. Forest Plots
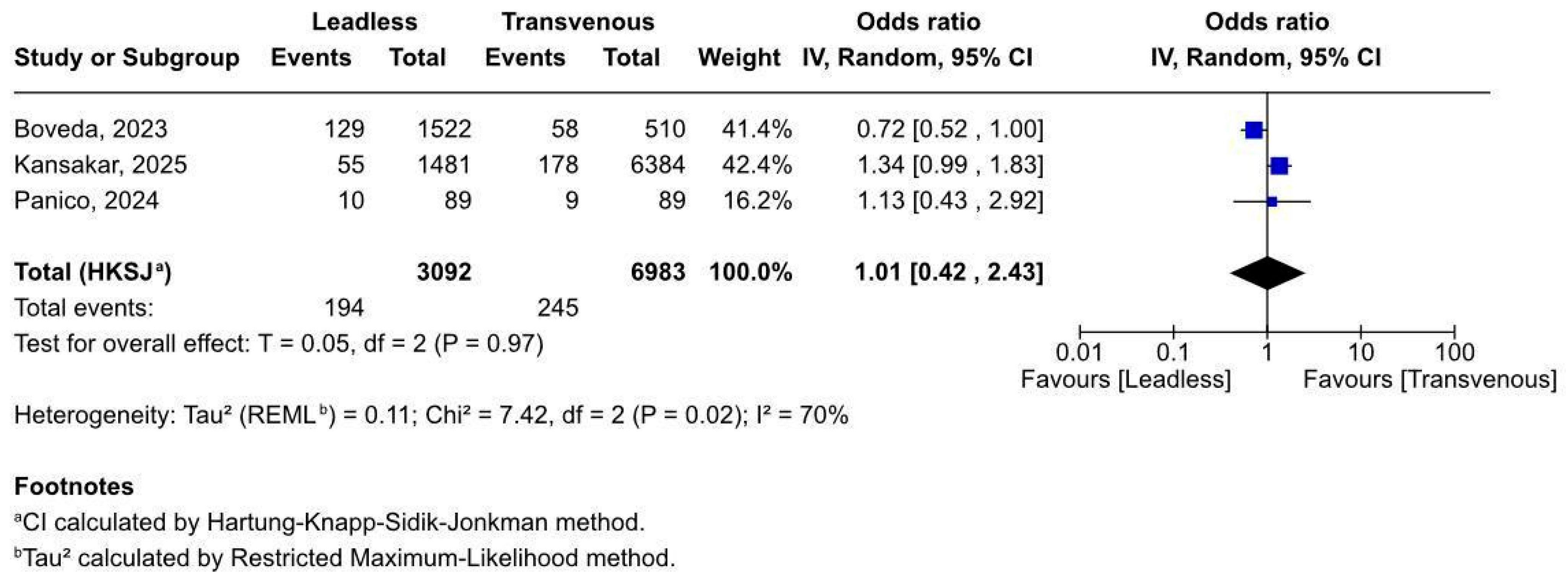
3.2.1. Overall Complications After Pacemaker Implant
3.2.2. Early Mortality Within 30 Days
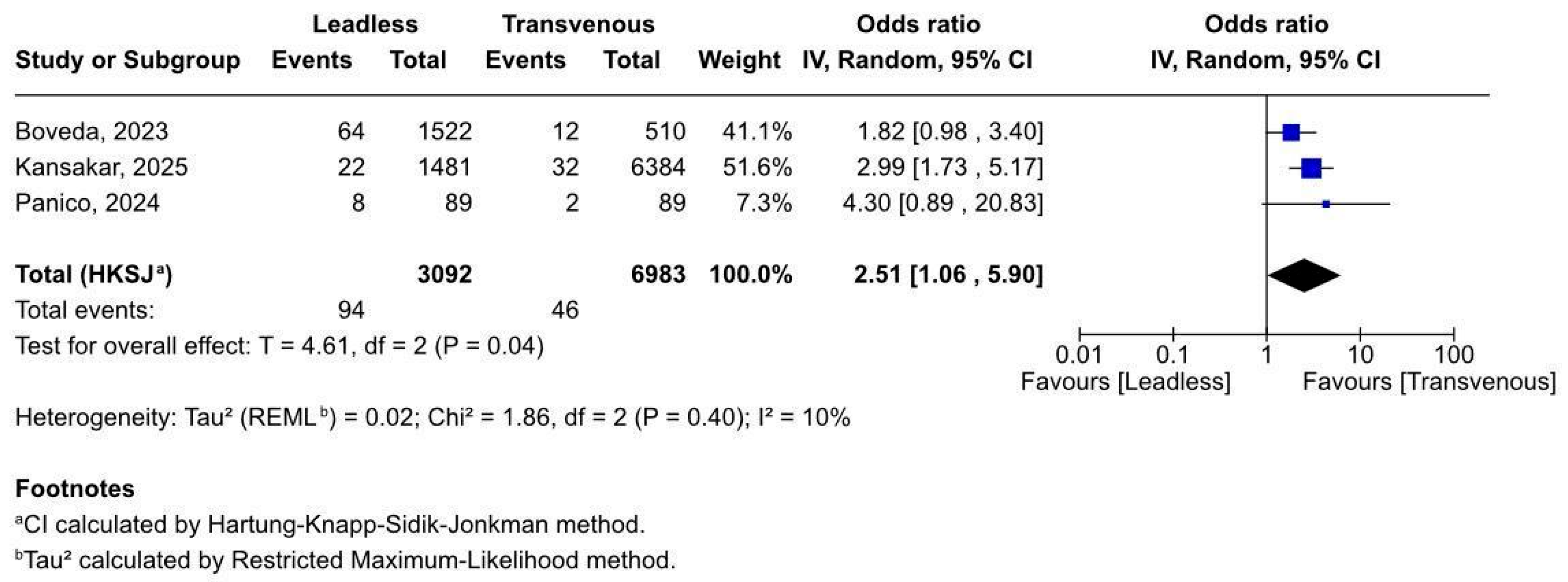
3.2.3. Access Site Complications
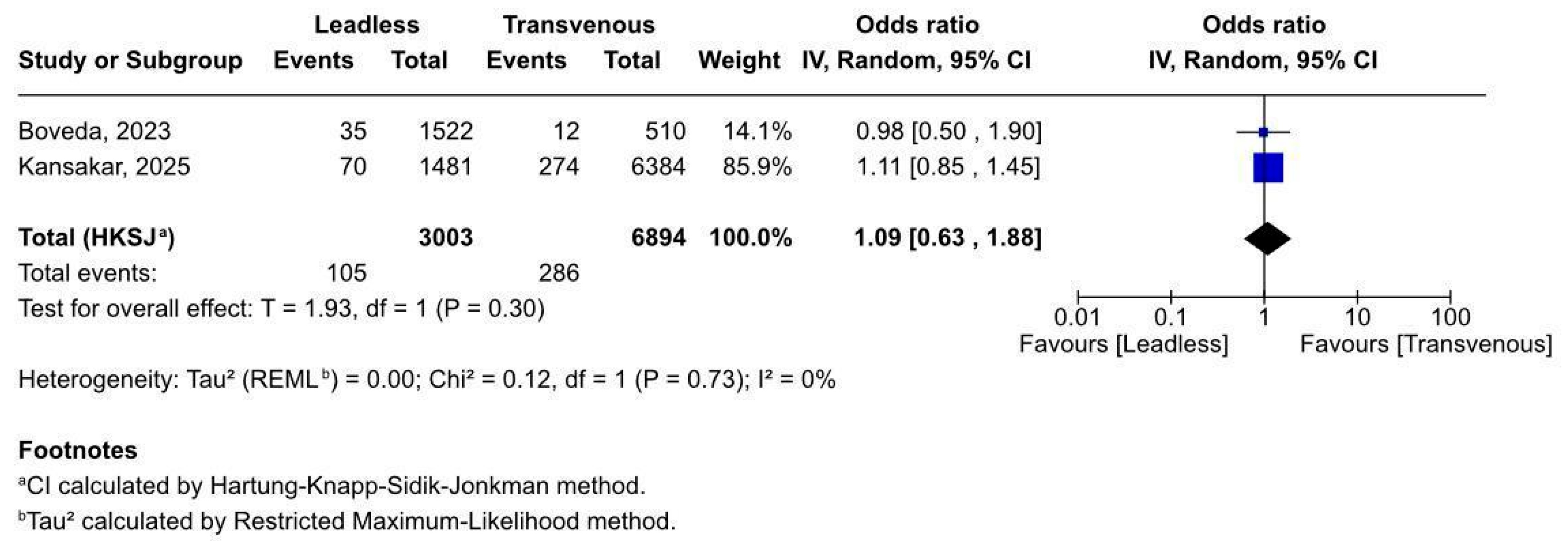
3.2.4. Device-Related Complications
3.2.5. Cardiac Thrombosis Complications
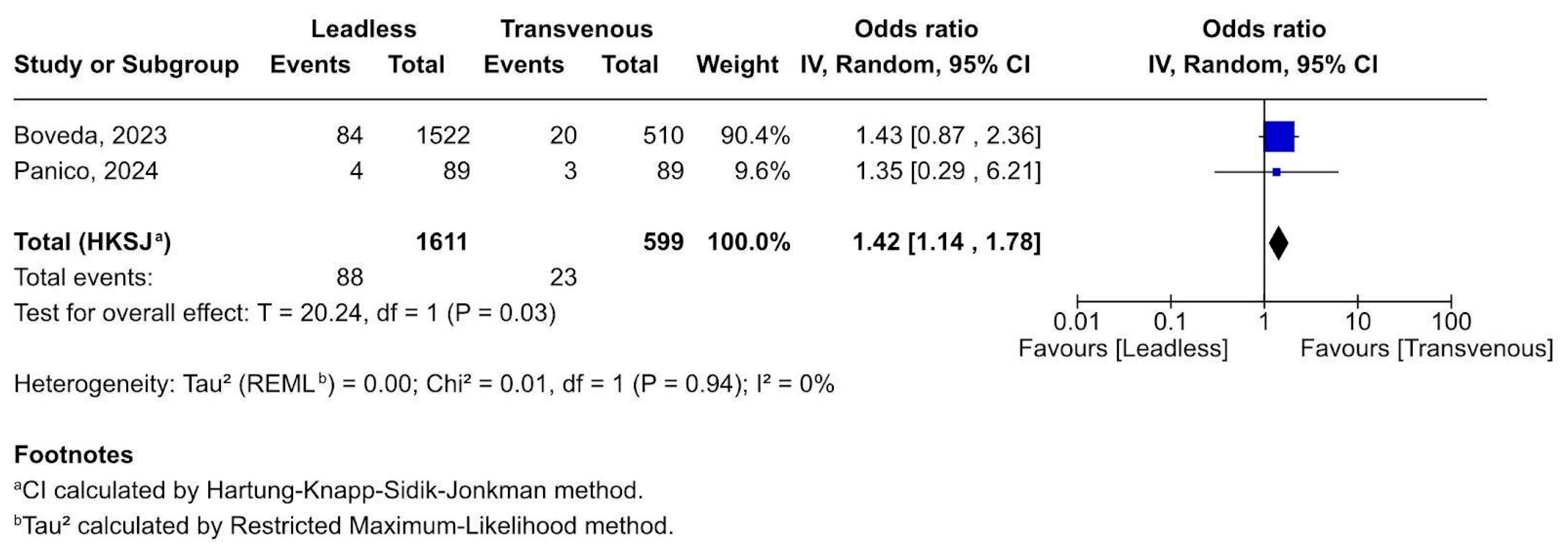
3.2.6. Respiratory Complications
3.3. Quality Assessment
4. Discussion
4.1. Limitations, Strengths, and Future Directions
4.1.1. Limitations
4.1.2. Strengths
4.1.3. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AV | Atrioventricular synchronous |
| CED | Coverage with evidence development |
| CI | Confidence interval |
| CKD | Chronic kidney disease |
| ESRD | End-stage renal disease |
| Fr | French (unit of catheter diameter) |
| LPM | Leadless pacemaker |
| NRD | National Readmissions Database |
| OR | Odds ratio |
| PICM | Pacing-induced cardiomyopathy |
| TVP | Transvenous pacemaker |
References
- Garweg, C.; Duchenne, J.; Vandenberk, B.; Mao, Y.; Ector, J.; Haemers, P.; Poels, P.; Voigt, J.U.; Willems, R. Evolution of ventricular and valve function in patients with right ventricular pacing—A randomized controlled trial comparing leadless and conventional pacing. Pacing Clin. Electrophysiol. PACE 2023, 46, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Piccini, J.P.; El-Chami, M.; Wherry, K.; Crossley, G.H.; Kowal, R.C.; Stromberg, K.; Longacre, C.; Hinnenthal, J.; Bockstedt, L. Contemporaneous Comparison of Outcomes Among Patients Implanted with a Leadless vs Transvenous Single-Chamber Ventricular Pacemaker. JAMA Cardiol. 2021, 6, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Crossley, G.H.; El-Chami, M.F.; Piccini, J.P.; Pokorney, S.D.; Longacre, C.; Stromberg, K.; El-Chami, M.F. Leadless versus transvenous single-chamber ventricular pacemakers: 3-year follow-up of the Micra CED study. J. Cardiovasc. Electrophysiol. 2023, 34, 1015–1023. [Google Scholar] [CrossRef]
- Palmisano, P.; Facchin, D.; Ziacchi, M.; Nigro, G.; Nicosia, A.; Bongiorni, M.G.; Tomasi, L.; Rossi, A.; De Filippo, P.; Sgarito, G.; et al. Rate and nature of complications with leadless transcatheter pacemakers compared with transvenous pacemakers: Results from an Italian multicentre large population analysis. EP Eur. 2023, 25, 112–120. [Google Scholar] [CrossRef]
- Zucchelli, G.; Tolve, S.; Barletta, V.; Di Cori, A.; Parollo, M.; De Lucia, R.; Della Tommasina, V.; Santoro, M.G.; Viani, S.; Cellamaro, T.; et al. Comparison between leadless and transvenous single-chamber pacemaker therapy in a referral centre for lead extraction. J. Interv. Card. Electrophysiol. 2021, 61, 395–404. [Google Scholar] [CrossRef]
- Martinez-Sande, J.L.; Garcia-Seara, J.; Gonzalez-Melchor, L.; Varela-Roman, A.; Gonzalez-Juanatey, J.R. Conventional single-chamber pacemakers versus transcatheter pacing systems in a “real world” cohort of patients: A comparative prospective single-center study. Indian. Pacing Electrophysiol. J. 2021, 21, 89–94. [Google Scholar] [CrossRef]
- Crossley, G.H.; Longacre, C.; Higuera, L.; Stromberg, K.; Pokorney, S.D.; El-Chami, M.F. Outcomes of patients implanted with an atrioventricular synchronous leadless ventricular pacemaker in the Medicare population. Heart Rhythm. 2024, 21, 66–73. [Google Scholar] [CrossRef]
- Khan, M.Z.; Nguyen, A.; Khan, M.U.; Sattar, Y.; Alruwaili, W.; Gonuguntla, K.; Hayat, H.M.S.; Mendez, M.; Nassar, S.; Asad, Z.U.A.; et al. Association of chronic kidney disease and end-stage renal disease with procedural complications and inpatient outcomes of leadless pacemaker implantations across the United States. Heart Rhythm. 2024, 21, 1695–1702. [Google Scholar] [CrossRef] [PubMed]
- Haddadin, F.; Majmundar, M.; Jabri, A.; Pecha, L.; Scott, C.; Daher, M.; Kumar, A.; Kalra, A.; Fram, R.; Haddadin, F.; et al. Clinical outcomes and predictors of complications in patients undergoing leadless pacemaker implantation. Heart Rhythm. 2022, 19, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Bodin, A.; Clementy, N.; Bisson, A.; Pierre, B.; Herbert, J.; Babuty, D.; Fauchier, L. Leadless or conventional transvenous ventricular permanent pacemakers: A nationwide matched control study. J. Am. Heart Assoc. 2022, 11, e02533. [Google Scholar] [CrossRef]
- Ohta, Y.; Goda, A.; Daimon, A.; Manabe, E.; Masai, K.; Kishima, H.; Mine, T.; Asakura, M.; Ishihara, M. The differences between conventional lead, thin lead, and leadless pacemakers regarding effects on tricuspid regurgitation in the early phase. J. Med. Ultrason. 2023, 50, 51–56. [Google Scholar] [CrossRef]
- Sanchez, R.; Nadkarni, A.; Buck, B.; Daoud, G.; Koppert, T.; Okabe, T.; Houmsse, M.; Weiss, R.; Augostini, R.; Hummel, J.D.; et al. Incidence of pacing-induced cardiomyopathy in pacemaker-dependent patients is lower with leadless pacemakers compared to transvenous pacemakers. J. Cardiovasc. Electrophysiol. 2021, 32, 477–483. [Google Scholar] [CrossRef] [PubMed]
- El-Chami, M.F.; Higuera, L.; Longacre, C.; Stromberg, K.; Crossley, G.; Piccini, J.P. Two-year outcomes of Micra AV leadless pacemakers in the Micra AV CED study. EP Eur. 2024, 26, euae273. [Google Scholar]
- Bertelli, M.; Toniolo, S.; Ziacchi, M.; Gasperetti, A.; Schiavone, M.; Arosio, R.; Capobianco, C.; Mitacchione, G.; Statuto, G.; Angeletti, A.; et al. Is Less Always More? A Prospective Two-Centre Study Addressing Clinical Outcomes in Leadless versus Transvenous Single-Chamber Pacemaker Recipients. J. Clin. Med. 2022, 11, 6071. [Google Scholar] [CrossRef]
- Mitacchione, G.; Schiavone, M.; Gasperetti, A.; Tripepi, G.L.; Cerini, M.; Montemerlo, E.; Del Monte, A.; Bontempi, L.; Moltrasio, M.; Breitenstein, A.; et al. Leadless pacemakers in patients with different stages of chronic kidney disease: Real-world data from the updated i-LEAPER registry. Heart Rhythm. 2025, 22, 325–331. [Google Scholar] [CrossRef] [PubMed]
- El-Chami, M.F.; Clementy, N.; Garweg, C.; Omar, R.; Duray, G.Z.; Gornick, C.C.; Leyva, F.; Sagi, V.; Piccini, J.P.; Soejima, K.; et al. Leadless Pacemaker Implantation in Hemodialysis Patients: Experience with the Micra Transcatheter Pacemaker. JACC Clin. Electrophysiol. 2019, 5, 162–170. [Google Scholar] [CrossRef]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: Developed by the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Eur. Heart J. 2021, 42, 3427–3520. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Boveda, S.; Higuera, L.; Longacre, C.; Wolff, C.; Wherry, K.; Stromberg, K.; El-Chami, M.F. Two-year outcomes of leadless vs. transvenous single-chamber ventricular pacemaker in high-risk subgroups. EP Eur. 2023, 25, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Kansakar, S.; Naeem, A.; Moskovits, N.; Shrestha, D.B.; Shtembari, J.; Biswas, M.; Shantha, G.; Basyal, B.; Storey, J.; Katz, D. Leadless Pacemaker vs. Transvenous Pacemaker in End Stage Kidney Disease: Insights from the Nationwide Readmission Database. J. Clin. Med. 2025, 14, 202. [Google Scholar] [CrossRef]
- Panico, A.; Flahault, A.; Guillemin, F.; Varlet, E.; Couchoud, C.; Bauwens, M.; Marijon, E.; Roueff, S.; Lazareth, H. Improved outcomes with leadless vs. single-chamber transvenous pacemaker in haemodialysis patients. EP Eur. 2024, 26. [Google Scholar] [CrossRef]
- Mauriello, A.; Rago, A.; Amore, D.; Sica, G.; D’Andrea, A.; Russo, V. Migration of an Implantable Loop Recorder: A Meta-summary of Case Reports. J. Innov. Card Rhythm. Manag. 2025, 16, 6292–6296. [Google Scholar] [CrossRef]
- Gharehdaghi, S.; Engh, M.; Veres, B.; Vigh, N.; Fehervari, P.; Hegyi, P.; Zima, E.; Duray, G. Intracardiac leadless pacemaker: Long-term safety over traditional pacemakers. A systematic review and meta-analysis. Eur. Heart J. 2024, 45 (Suppl. S1), ehae666.419. [Google Scholar] [CrossRef]
- Gangannapalle, M.; Monday, O.; Rawat, A.; Nwoko, U.A.; Mandal, A.K.; Babur, M.; Khan, T.J.; Palleti, S.K. Comparison of Safety of Leadless Pacemakers and Transvenous Pacemakers: A Meta-Analysis. Cureus 2023, 15, e45086. [Google Scholar] [CrossRef]
- Mhasseb, C.; Kiwan, M.; Merhi, M.-E.; Moussallem, N.; Moussalli, J.; Zeid, M.A.; Daher, S.A.; Nabbout, G.; Azar, S.; Kanaan, A.; et al. Comparative safety of transvenous and leadless pacemakers in patients with cardiovascular diseases: A meta-analysis study. Heliyon 2024, 11, e40982. [Google Scholar] [CrossRef] [PubMed]
- Habboush, S.; Elmoursi, A.; Gadelmawla, A.F.; Masoud, A.T.M.; Khalil, M.; Sheashaa, H.; Merza, N.; Massoud, A.T. Transvenous Compared with Leadless Pacemakers: A meta-analysis comparing TP versus LP. Cardiol. Rev. 2024. [Google Scholar] [CrossRef] [PubMed]
- Ohn, M.H.; Luen, N.P.; Jouhra, F.; Ahmed, F.Z. Comparative safety outcomes of leadless versus transvenous cardiac pacemaker: A systematic review and meta-analysis. Eur. Heart J. 2024, 45 (Suppl. S1), ehae666.748. [Google Scholar] [CrossRef]
- Knops, R.E.; Reddy, V.Y.; Ip, J.E.; Doshi, R.; Exner, D.V.; Defaye, P.; Canby, R.; Bongiorni, M.G.; Shoda, M.; Hindricks, G.; et al. A Dual-Chamber Leadless Pacemaker. N. Engl. J. Med. 2023, 388, 2360–2370. [Google Scholar] [CrossRef]
- La Fazia, V.M.; Lepone, A.; Pierucci, N.; Gianni, C.; Barletta, V.; Mohanty, S.; Della Rocca, D.G.; La Valle, C.; Torlapati, P.G.; Al-Ahmad, M.; et al. Low prevalence of new-onset severe tricuspid regurgitation following leadless pacemaker implantation in a large series of consecutive patients. Hear. Rhythm. 2024, 21, 2603–2604. [Google Scholar] [CrossRef] [PubMed]
- Lamas, G.A.; Lee, K.L.; Sweeney, M.O.; Silverman, R.; Leon, A.; Yee, R.; Marinchak, R.A.; Flaker, G.; Schron, E.; Orav, E.J.; et al. Ventricular pacing or dual-chamber pacing for sinus-node dysfunction. N. Engl. J. Med. 2002, 346, 1854–1862. [Google Scholar] [CrossRef]
- Vouliotis, A.I.; Roberts, P.R.; Dilaveris, P.; Gatzoulis, K.; Yue, A.; Tsioufis, K. Leadless Pacemakers: Current Achievements and Future Perspectives. Eur. Cardiol. Rev. 2023, 18, e49. [Google Scholar] [CrossRef] [PubMed]
- Abbott Medical. FDA Approves World’s First Dual Chamber Leadless Pacemaker System. 2023. Available online: https://www.cardiovascular.abbott/us/en/hcp/products/cardiac-rhythm-management/blog/fda-approves-worlds-first-dual-chamber-leadless-pacemaker-system.html (accessed on 23 July 2025).
- Abbott Medical 2025. Device Coding and Pricing Overview for Aveir DR Leadless Pacemaker System. 2025. Available online: https://www.cardiovascular.abbott/content/dam/cv/cardiovascular/hcp/reimbursement/crm/coding-coverage/AVEIR%20Medicare%20Facility%20Coding%20Guide.pdf (accessed on 23 July 2025).
- Solomon, A.; Tzuberi, M.; Berkovitch, A.; Hoch, E.; Beinart, R.; Nof, E. Leadless Pacemaker Implantation During Extraction in Patients with Active Infection: A Comprehensive Analysis of Safety, Patient Benefits and Costs. J. Clin. Med. 2025, 14, 5450. [Google Scholar] [CrossRef]
| Boveda, 2023 [19] (n = 2032) | Kansakar, 2025 [20] (n = 7865) | Panico, 2024 [21] (n = 178) | ||||
|---|---|---|---|---|---|---|
| Characteristics | Leadless (n = 1522) | Transvenous (n = 510) | Leadless (n = 1481) | Transvenous (n = 6384) | Leadless (n = 89) | Transvenous (n = 89) |
| Study design | Retrospective | Retrospective | Retrospective | |||
| Country | United States | United States | France | |||
| Study duration | 2017–2019 | 2016–2021 | 2015–2021 | |||
| Follow-up (Days) * | 728 | 30 | 728 | |||
| Age (Years) ** | 71.2 ± 11.2 | 75.5 ± 10.1 | 71.6 ± 11.1 | 72.3 ± 11.1 | 77.3 ± 9.8 | 77.9 ± 8.6 |
| Female (n, %) | 659 (43.3) | 193 (37.8) | 550 (37.1) | 2566 (40.2) | 31 (35.0) | 27 (30.0) |
| Diabetes (n, %) | 1213 (79.7) | 389 (76.3) | 1061 (71.6) | 4579 (71.7) | 68 (76.0) | 66 (74.0) |
| Atrial fibrillation (n, %) | 1018 (66.9) | 419 (82.2) | 420 (28.3) | 1574 (24.7) | 61 (69.0) | 60 (67.0) |
| Peripheral vascular disease (n, %) | 677 (44.5) | 241 (47.3) | 150 (10.1) | 784 (12.3) | 45 (51.0) | 52 (58.0) |
| Chronic Heart Failure (n, %) | 1182 (77.7) | 415 (81.4) | 867 (58.5) | 3295 (51.6) | 62 (70.0) | 56 (63.0) |
| Supraventricular arrhythmia (n, %) | 170 (11.2) | 41 (8.0) | N/A | N/A | 9 (10) | 8 (9.0) |
| Ventricular arrhythmia (n, %) | 302 (19.8) | 91 (17.8) | N/A | N/A | 3 (3.4) | 4 (4.5) |
| Tricuspid Valve Disease (n, %) | 508 (33.4) | 204 (40.0) | N/A | N/A | 13 (15) | 11 (12.0) |
| Pulmonary Diseases (n, %) *** | 607 (39.9) | 205 (40.2) | 369 (24.9) | 1480 (23.2) | 29 (33) | 20 (22.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogdan, Ș.; Bistriceanu, M.I.A.; Ursu, C.G.; Anghel, A.C.; Andreescu, D.I.; Ababei, A.; Deaconu, S.; Deaconu, A. Leadless vs. Transvenous Pacemakers in Patients with End-Stage Renal Disease: A Systematic Review and Meta-Analysis. Biomedicines 2025, 13, 1952. https://doi.org/10.3390/biomedicines13081952
Bogdan Ș, Bistriceanu MIA, Ursu CG, Anghel AC, Andreescu DI, Ababei A, Deaconu S, Deaconu A. Leadless vs. Transvenous Pacemakers in Patients with End-Stage Renal Disease: A Systematic Review and Meta-Analysis. Biomedicines. 2025; 13(8):1952. https://doi.org/10.3390/biomedicines13081952
Chicago/Turabian StyleBogdan, Ștefan, Mircea Ioan Alexandru Bistriceanu, Cosmin Gabriel Ursu, Andrei Constantin Anghel, Darie Ioan Andreescu, Alexandru Ababei, Silvia Deaconu, and Alexandru Deaconu. 2025. "Leadless vs. Transvenous Pacemakers in Patients with End-Stage Renal Disease: A Systematic Review and Meta-Analysis" Biomedicines 13, no. 8: 1952. https://doi.org/10.3390/biomedicines13081952
APA StyleBogdan, Ș., Bistriceanu, M. I. A., Ursu, C. G., Anghel, A. C., Andreescu, D. I., Ababei, A., Deaconu, S., & Deaconu, A. (2025). Leadless vs. Transvenous Pacemakers in Patients with End-Stage Renal Disease: A Systematic Review and Meta-Analysis. Biomedicines, 13(8), 1952. https://doi.org/10.3390/biomedicines13081952