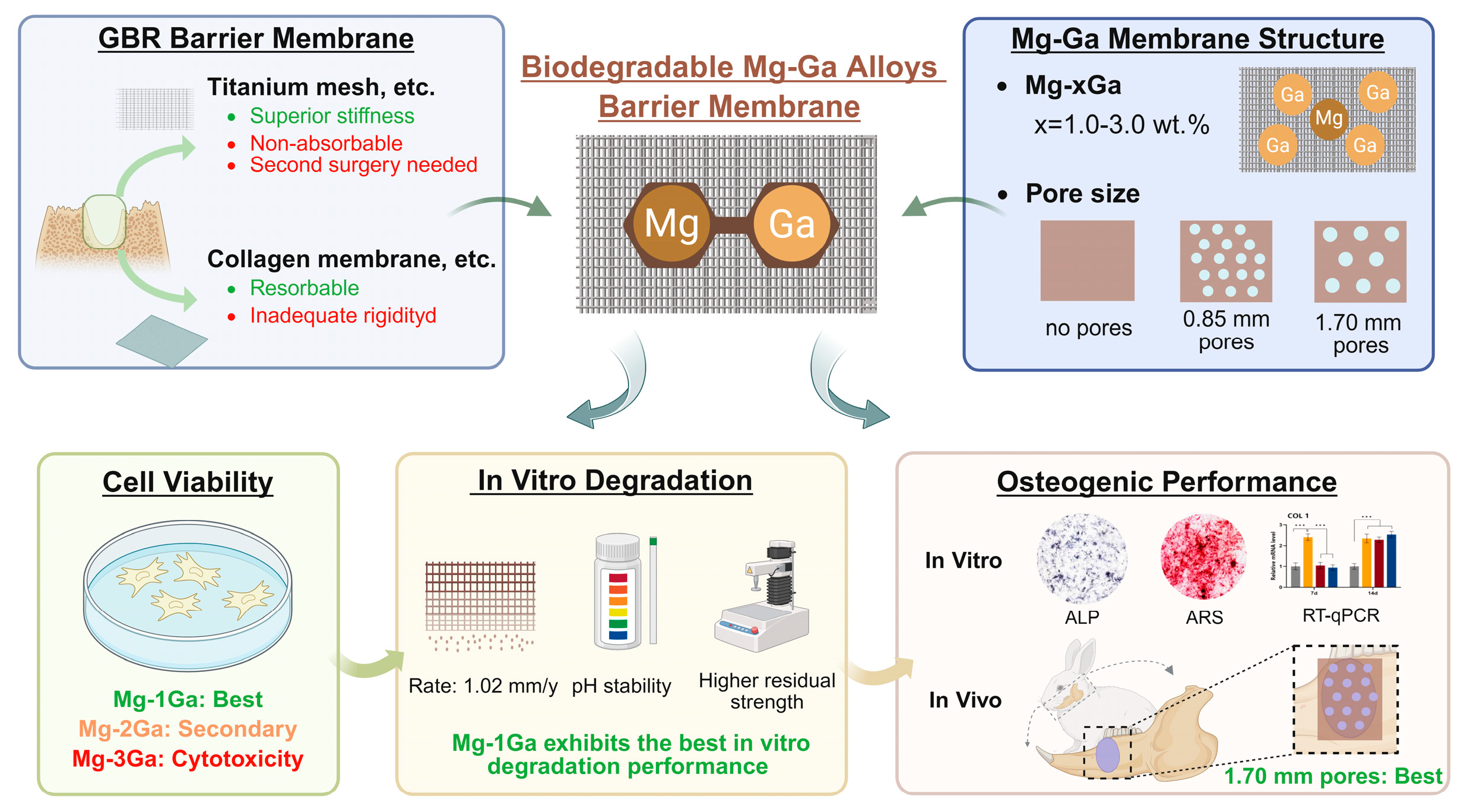
Osteogenesis Activity and Porosity Effect of Biodegradable Mg-Ga Alloys Barrier Membrane for Guided Bone Regeneration: An in Vitro and in Vivo Study in Rabbits
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation
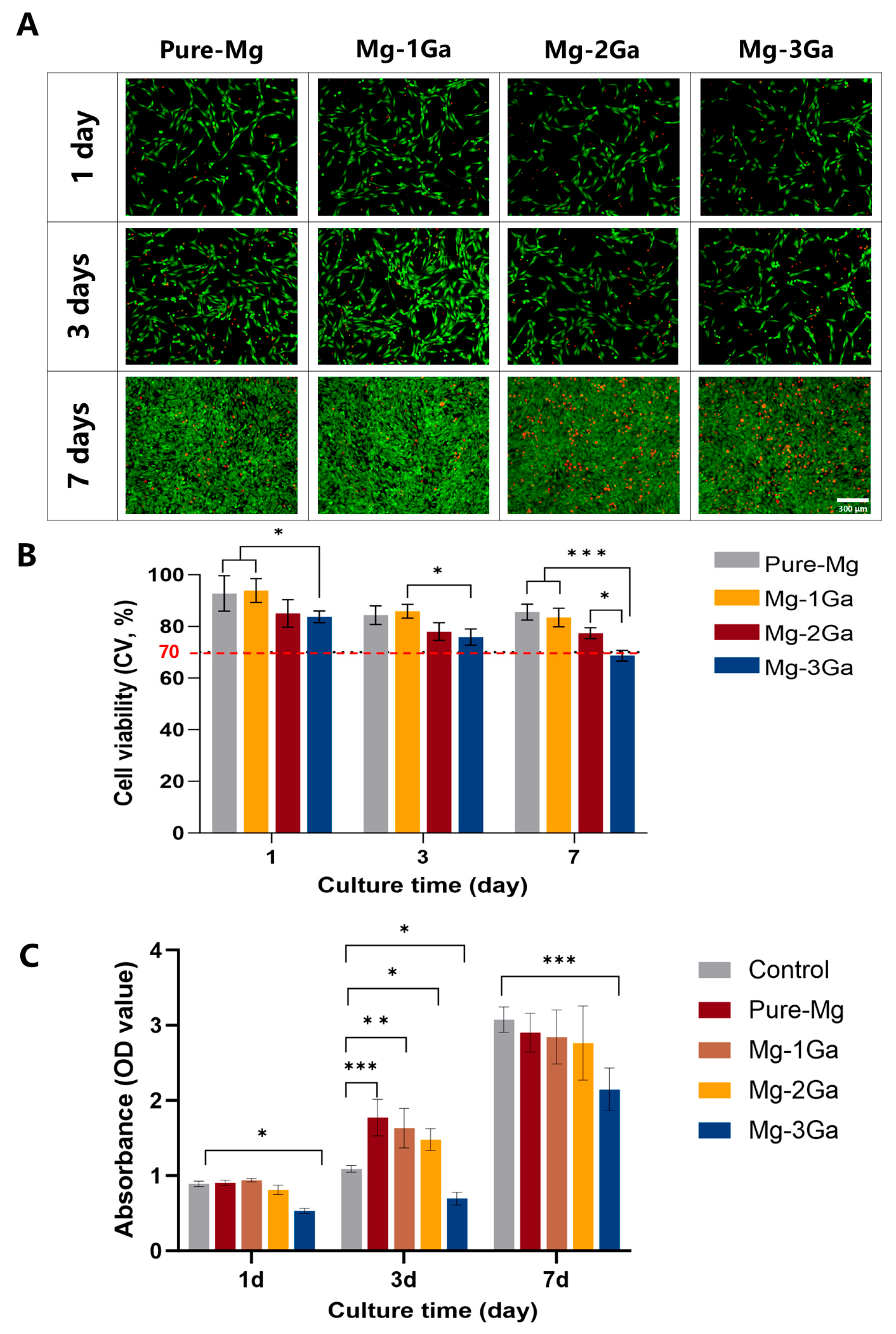
2.2. In Vitro Cell Viability Tests
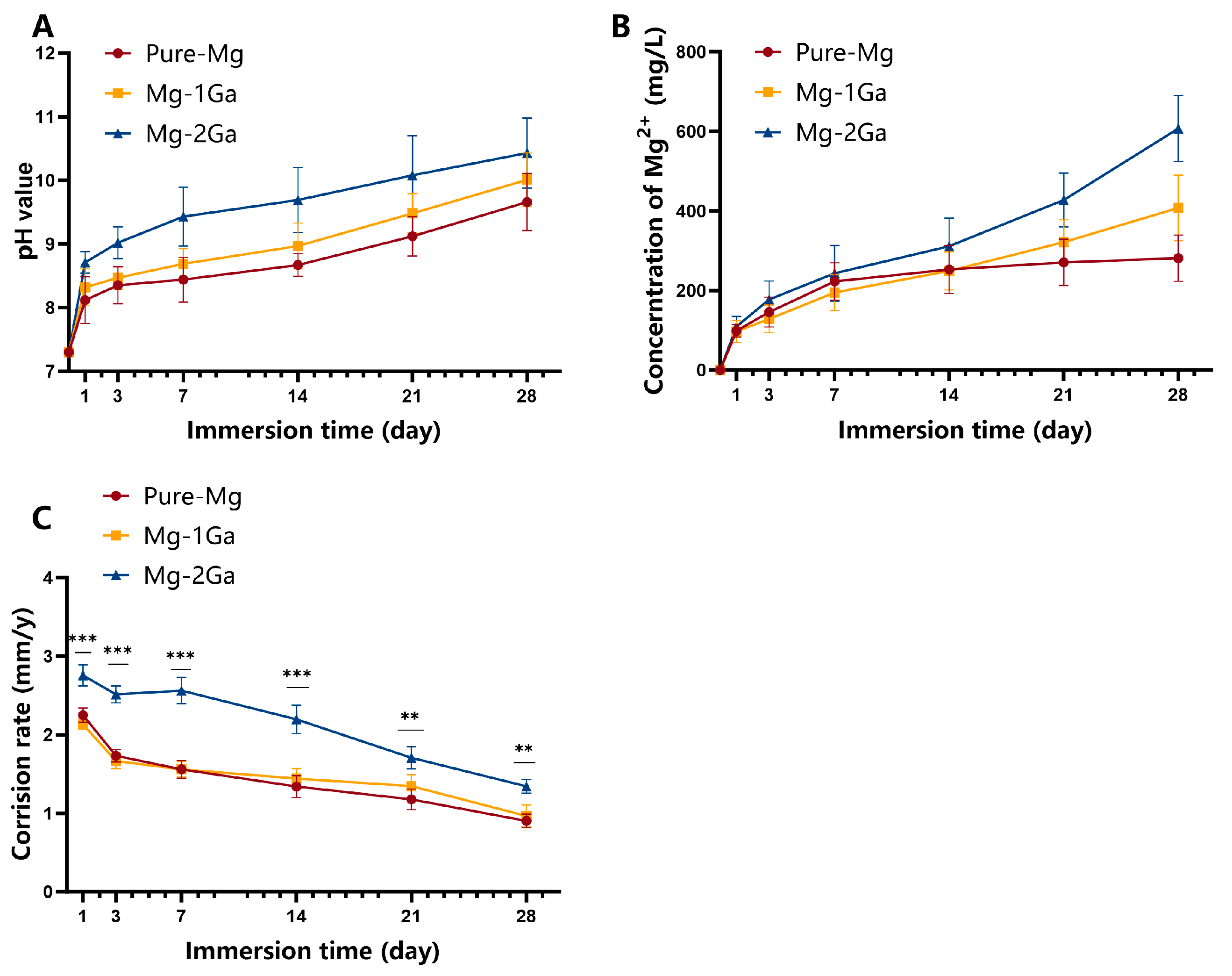
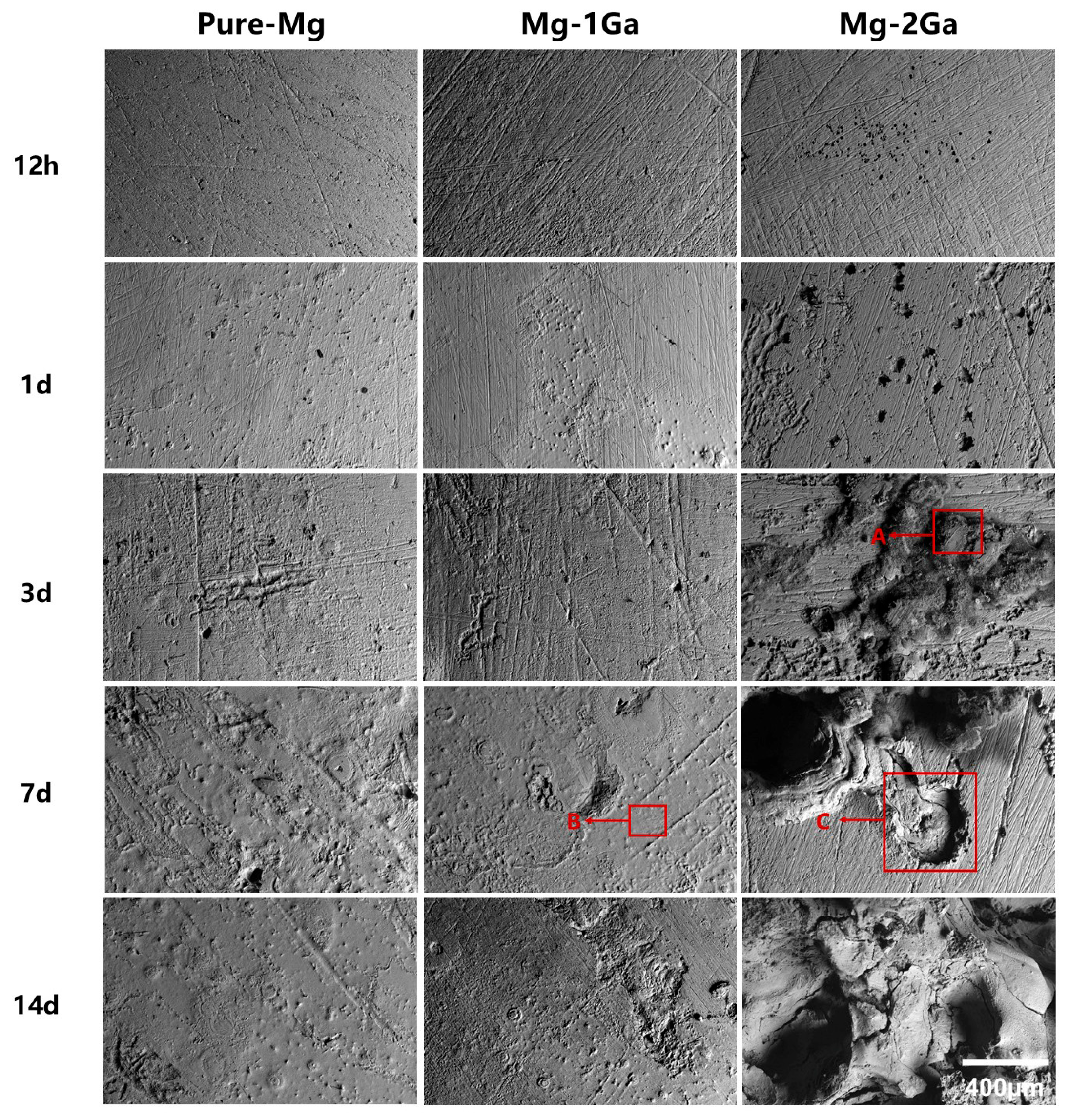
2.3. Immersion Tests
2.4. In Vitro Osteogenic Activity Assays
2.4.1. Alkaline Phosphatase Activity
2.4.2. Extracellular Matrix Mineralization
2.4.3. Quantitative Real-Time PCR
2.5. In Vivo Animal Surgery
2.5.1. Animal Model
2.5.2. Surgical Procedure
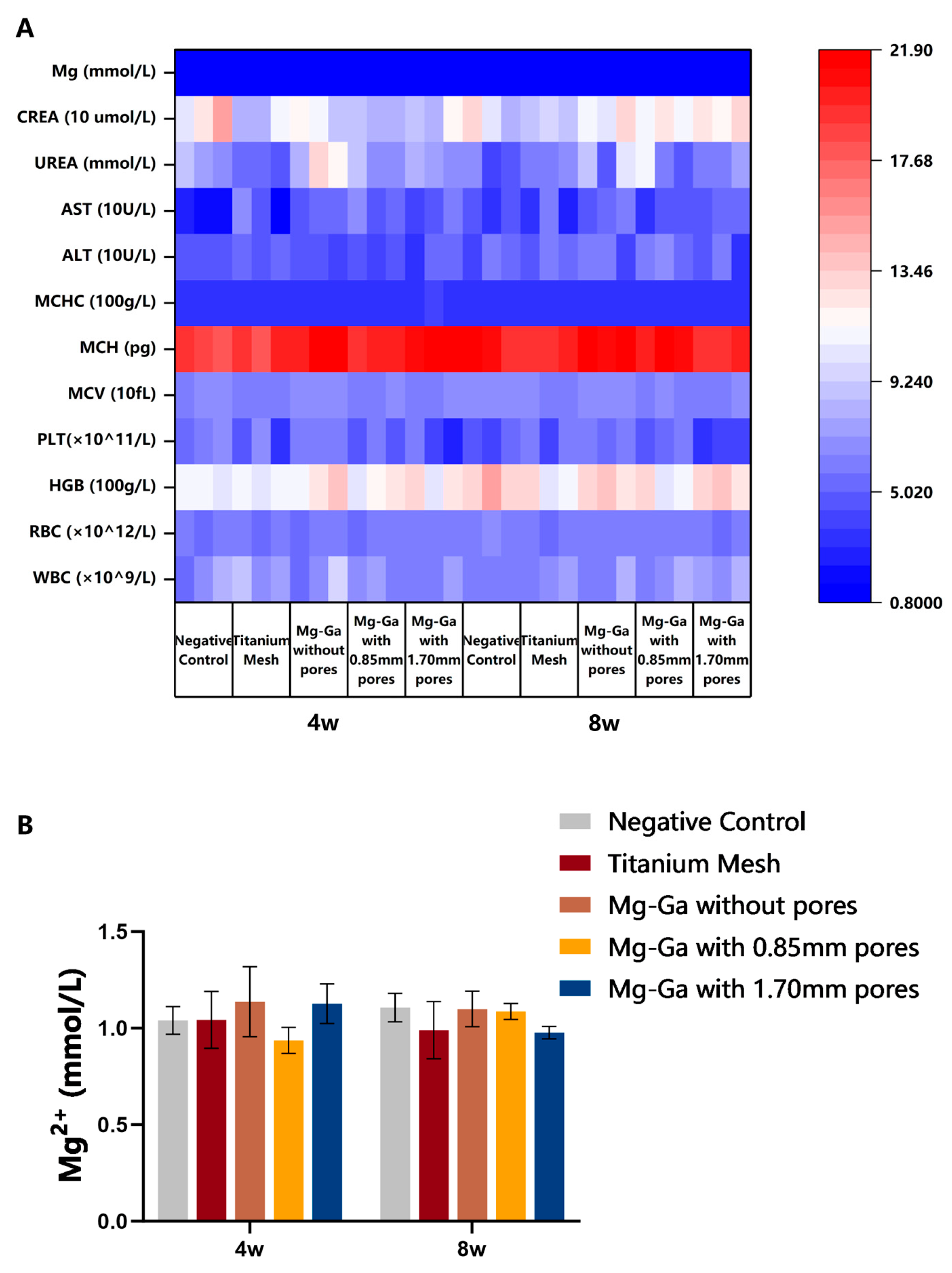
2.5.3. Blood Biochemistry Tests
2.5.4. Micro-Computed Tomography Analysis
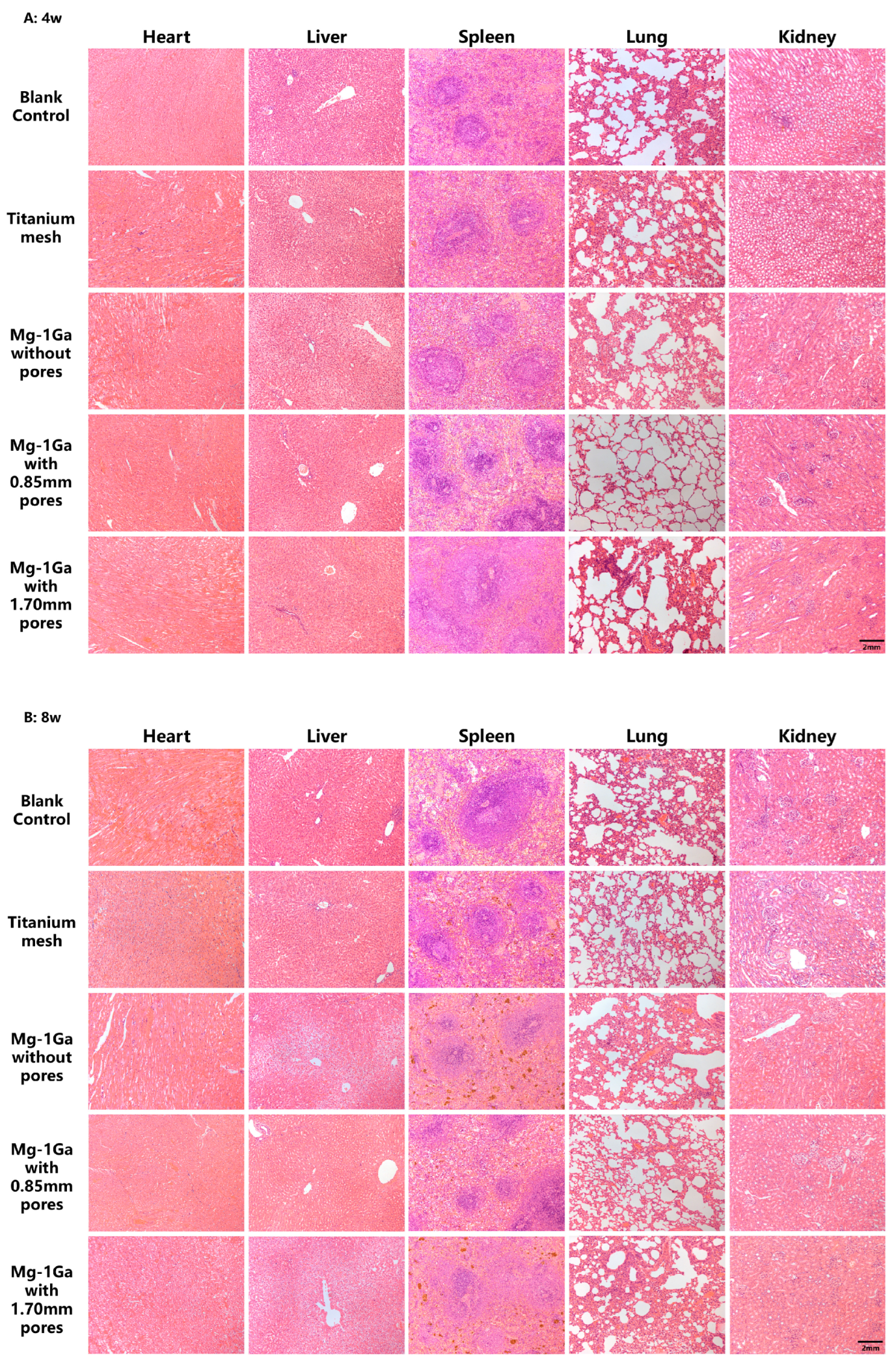
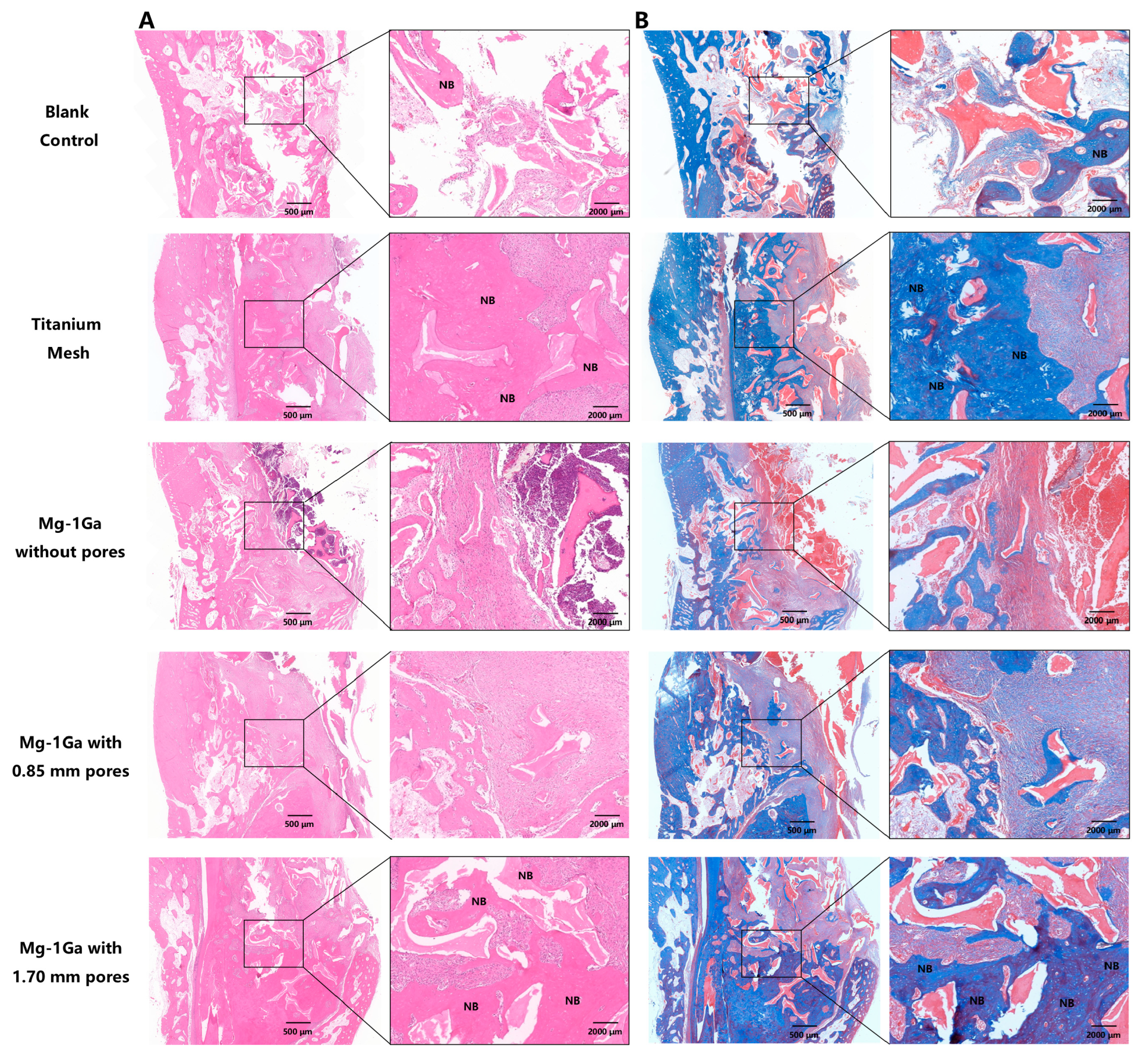
2.5.5. Histological Analysis
2.6. Statistical Analysis
3. Results
3.1. In Vitro Cell Viability Tests
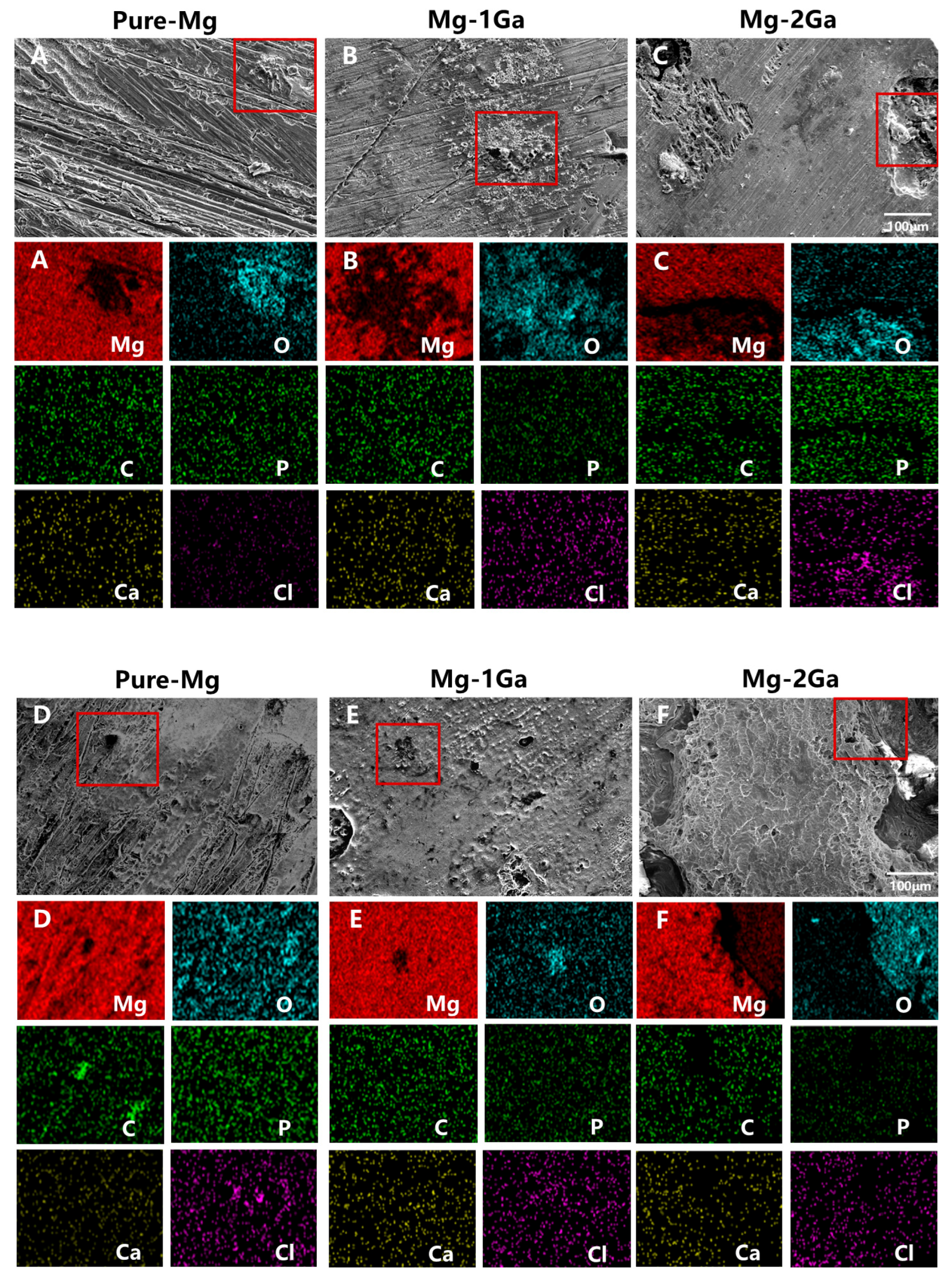
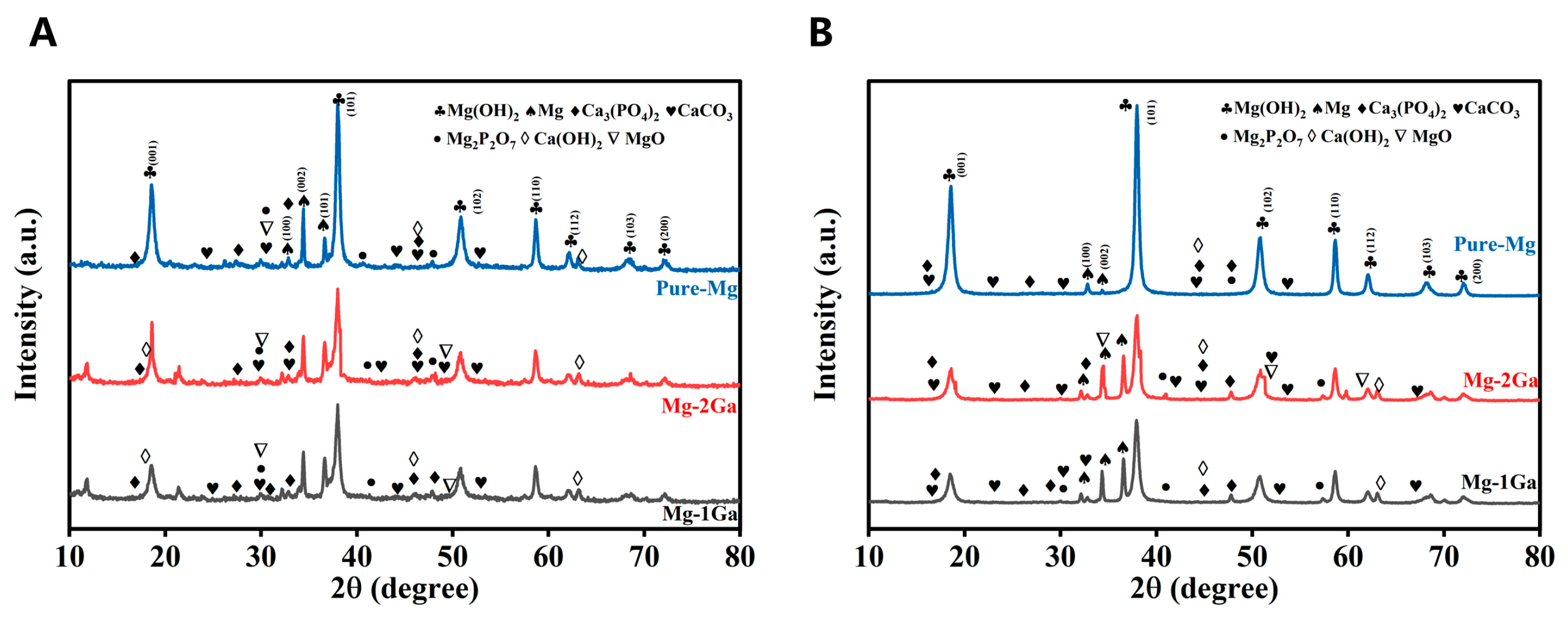
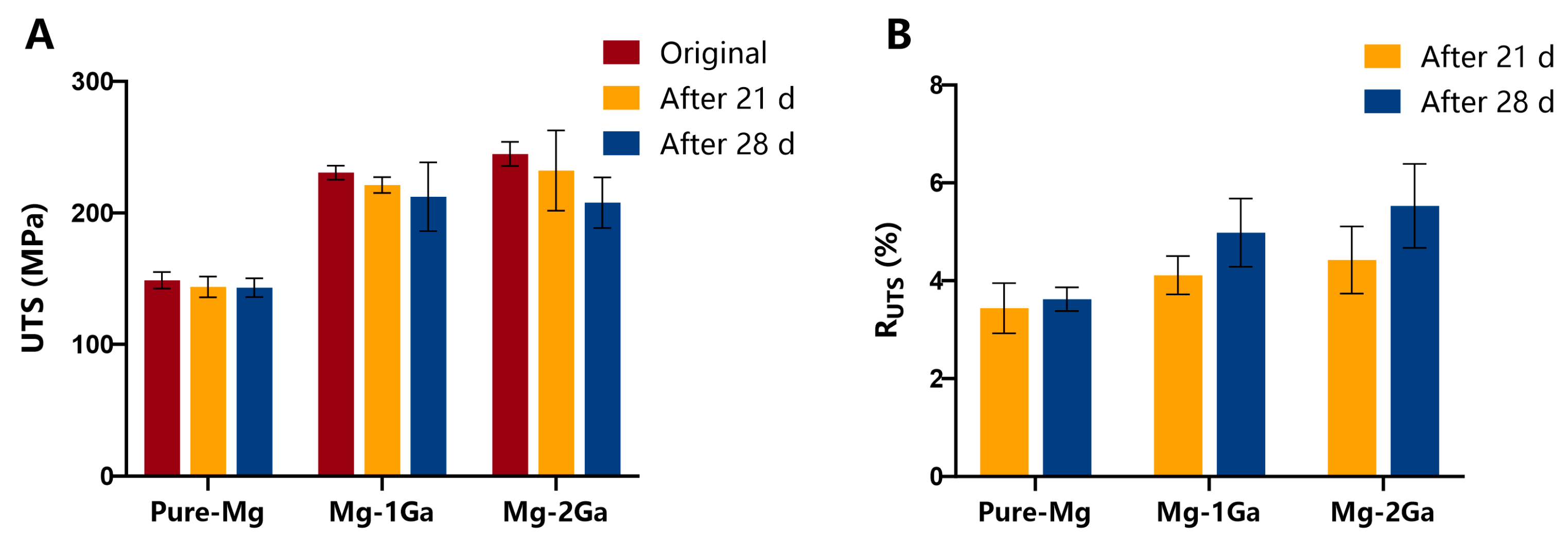
3.2. In Vitro Degradation Behavior
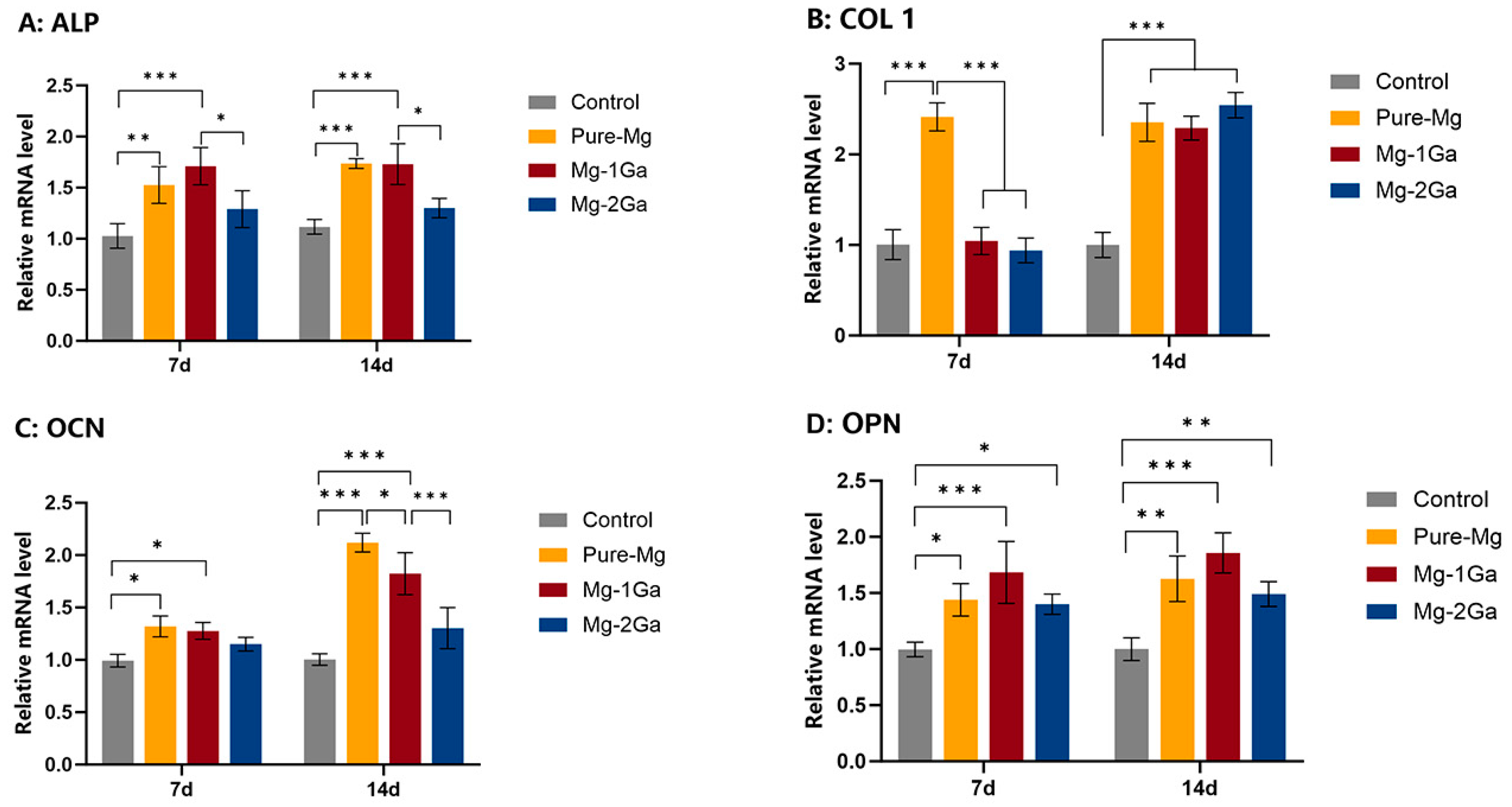
3.3. Osteogenic Differentiation
3.4. In Vivo Biocompatibility, Degradation, and Osteogenesis
3.4.1. In Vivo Biocompatibility Tests
3.4.2. Micro-CT Analysis
3.4.3. Histological Analysis of Bone Regeneration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GBR | Guided Bone Regeneration |
| Mg | Magnesium |
| Ga | Gallium |
| ALP | Alkaline Phosphatase |
| Ti | Titanium |
| PTFE | Polytetrafluoroethylene |
| rBMSCs | Rat Bone Marrow Mesenchymal Stem Cells |
| DMEM | Dulbecco’s Modified Eagle Medium |
| FBS | Fetal Bovine Serum |
| OD | Optical Density |
| SEM | Scanning Electron Microscope |
| EDS | Energy-Dispersive X-Ray Spectroscopy |
| XRD | X-Ray Diffraction |
| ARS | Alizarin Red S |
| ECM | Extracellular Matrix |
| qRT-PCR | Quantitative Real-Time Polymerase Chain Reaction |
| COL 1 | Collagen Type 1 |
| OCN | Osteocalcin |
| OPN | Osteopontin |
| GAPDH | Glyceralde- hyde 3-phosphate dehydrogenase |
| ROI | Region of Interest |
| BMD | Bone Mineral Density |
| Tb.Th | Trabecular Thickness |
| Tb.N | Trabecular Number |
| H&E | Hematoxylin and Eosin |
References
- Jensen, S.S.; Terheyden, H. Bone augmentation procedures in localized defects in the alveolar ridge: Clinical results with different bone grafts and bone-substitute materials. Int. J. Oral Maxillofac. Implant. 2009, 24, 218–236. [Google Scholar]
- Wang, H.L.; Boyapati, L. “PASS” principles for predictable bone regeneration. Implant Dent. 2006, 15, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Retzepi, M.; Donos, N. Guided Bone Regeneration: Biological principle and therapeutic applications. Clin. Oral Implant. Res. 2010, 21, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Korzinskas, T.; Jung, O.; Smeets, R.; Stojanovic, S.; Najman, S.; Glenske, K.; Hahn, M.; Wenisch, S.; Schnettler, R.; Barbeck, M. In Vivo Analysis of the Biocompatibility and Macrophage Response of a Non-Resorbable PTFE Membrane for Guided Bone Regeneration. Int. J. Mol. Sci. 2018, 19, 2952. [Google Scholar] [CrossRef]
- Cucchi, A.; Vignudelli, E.; Napolitano, A.; Marchetti, C.; Corinaldesi, G. Evaluation of complication rates and vertical bone gain after guided bone regeneration with non-resorbable membranes versus titanium meshes and resorbable membranes. A randomized clinical trial. Clin. Implant Dent. Relat. Res. 2017, 19, 821–832. [Google Scholar] [CrossRef]
- Lizio, G.; Pellegrino, G.; Corinaldesi, G.; Ferri, A.; Marchetti, C.; Felice, P. Guided bone regeneration using titanium mesh to augment 3-dimensional alveolar defects prior to implant placement. A pilot study. Clin. Oral Implant. Res. 2022, 33, 607–621. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Tonda-Turo, C.; Ferreira, A.M.; Ciardelli, G. Polymeric membranes for guided bone regeneration. Biotechnol. J. 2011, 6, 1187–1197. [Google Scholar] [CrossRef]
- Rothamel, D.; Schwarz, F.; Fienitz, T.; Smeets, R.; Dreiseidler, T.; Ritter, L.; Happe, A.; Zöller, J. Biocompatibility and biodegradation of a native porcine pericardium membrane: Results of in vitro and in vivo examinations. Int. J. Oral Maxillofac. Implant. 2012, 27, 146–154. [Google Scholar]
- Zhang, E.; Zhu, C.; Yang, J.; Sun, H.; Zhang, X.; Li, S.; Wang, Y.; Sun, L.; Yao, F. Electrospun PDLLA/PLGA composite membranes for potential application in guided tissue regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.H.; Han, Y.; Dal-Fabbro, R.; Daghrery, A.; Xu, J.; Kaigler, D.; Bhaduri, S.B.; Malda, J.; Bottino, M.C. Nanoscale β-TCP-Laden GelMA/PCL Composite Membrane for Guided Bone Regeneration. ACS Appl. Mater. Interfaces 2023, 15, 32121–32135. [Google Scholar] [CrossRef]
- Sheikh, Z.; Qureshi, J.; Alshahrani, A.M.; Nassar, H.; Ikeda, Y.; Glogauer, M.; Ganss, B. Collagen based barrier membranes for periodontal guided bone regeneration applications. Odontology 2017, 105, 1–12. [Google Scholar] [CrossRef]
- Shi, R.; Xue, J.; Wang, H.; Wang, R.; Gong, M.; Chen, D.; Zhang, L.; Tian, W. Fabrication and evaluation of a homogeneous electrospun PCL-gelatin hybrid membrane as an anti-adhesion barrier for craniectomy. J. Mater. Chem. B 2015, 3, 4063–4073. [Google Scholar] [CrossRef]
- Basler, T.; Naenni, N.; Schneider, D.; Hämmerle, C.H.F.; Jung, R.E.; Thoma, D.S. Randomized controlled clinical study assessing two membranes for guided bone regeneration of peri-implant bone defects: 3-year results. Clin. Oral Implant. Res. 2018, 29, 499–507. [Google Scholar] [CrossRef]
- Rakhmatia, Y.D.; Ayukawa, Y.; Furuhashi, A.; Koyano, K. Current barrier membranes: Titanium mesh and other membranes for guided bone regeneration in dental applications. J. Prosthodont. Res. 2013, 57, 3–14. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Díaz-Tocados, J.M.; Herencia, C.; Martínez-Moreno, J.M.; Montes de Oca, A.; Rodríguez-Ortiz, M.E.; Vergara, N.; Blanco, A.; Steppan, S.; Almadén, Y.; Rodríguez, M.; et al. Magnesium Chloride promotes Osteogenesis through Notch signaling activation and expansion of Mesenchymal Stem Cells. Sci. Rep. 2017, 7, 7839. [Google Scholar] [CrossRef]
- Wang, J.; Ma, X.Y.; Feng, Y.F.; Ma, Z.S.; Ma, T.C.; Zhang, Y.; Li, X.; Wang, L.; Lei, W. Magnesium Ions Promote the Biological Behaviour of Rat Calvarial Osteoblasts by Activating the PI3K/Akt Signalling Pathway. Biol. Trace Elem. Res. 2017, 179, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, J.; Ruan, Y.C.; Yu, M.K.; O’Laughlin, M.; Wise, H.; Chen, D.; Tian, L.; Shi, D.; Wang, J.; et al. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat. Med. 2016, 22, 1160–1169. [Google Scholar] [CrossRef]
- Riaz, U.; Shabib, I.; Haider, W. The current trends of Mg alloys in biomedical applications—A review. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 1970–1996. [Google Scholar] [CrossRef] [PubMed]
- Mohedano, M.; Blawert, C.; Yasakau, K.A.; Arrabal, R.; Matykina, E.; Mingo, B.; Scharnagl, N.; Ferreira, M.G.S.; Zheludkevich, M.L. Characterization and corrosion behavior of binary Mg-Ga alloys. Mater. Charact. 2017, 128, 85–99. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Z.; Guo, E.; Liu, X.; Kang, H.; Wang, T. The role of Ga in the microstructure, corrosion behavior and mechanical properties of as-extruded Mg–5Sn–xGa alloys. J. Alloys Compd. 2021, 863, 158762. [Google Scholar] [CrossRef]
- He, D.; Li, Y.; Zheng, Y.; Yue, X.; Wu, Y.; Xue, X.; Yu, H.; Li, W.; Li, Y. Effects of Ga content on the microstructure and mechanical properties of as-extruded Mg-xGa alloys. J. Alloys Compd. 2021, 887, 161317. [Google Scholar] [CrossRef]
- He, D.; Gong, H.; Zhang, Z.; Zheng, Y.; Zhang, H.; Li, Y. In-vitro corrosion behaviors of extruded Mg–Ga alloys in alpha minimum essential medium. Corros. Sci. 2022, 208, 110621. [Google Scholar] [CrossRef]
- Hämmerle, C.H.; Schmid, J.; Lang, N.P.; Olah, A.J. Temporal dynamics of healing in rabbit cranial defects using guided bone regeneration. J. Oral Maxillofac. Surg. 1995, 53, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kerns, D.G. Mechanisms of guided bone regeneration: A review. Open Dent. J. 2014, 8, 56–65. [Google Scholar] [CrossRef]
- Shim, J.H.; Jeong, J.H.; Won, J.Y.; Bae, J.H.; Ahn, G.; Jeon, H.; Yun, W.S.; Bae, E.B.; Choi, J.W.; Lee, S.H.; et al. Porosity effect of 3D-printed polycaprolactone membranes on calvarial defect model for guided bone regeneration. Biomed. Mater. 2017, 13, 015014. [Google Scholar] [CrossRef]
- Aprile, P.; Letourneur, D.; Simon-Yarza, T. Membranes for Guided Bone Regeneration: A Road from Bench to Bedside. Adv. Healthc. Mater. 2020, 9, e2000707. [Google Scholar] [CrossRef]
- Welsh, R.P.; Pilliar, R.M.; Macnab, I. Surgical implants. The role of surface porosity in fixation to bone and acrylic. J. Bone Jt. Surg. Am. 1971, 53, 963–977. [Google Scholar] [CrossRef]
- Rakhmatia, Y.D.; Ayukawa, Y.; Furuhashi, A.; Koyano, K. Microcomputed tomographic and histomorphometric analyses of novel titanium mesh membranes for guided bone regeneration: A study in rat calvarial defects. Int. J. Oral Maxillofac. Implant. 2014, 29, 826–835. [Google Scholar] [CrossRef]
- Her, S.; Kang, T.; Fien, M.J. Titanium mesh as an alternative to a membrane for ridge augmentation. J. Oral Maxillofac. Surg. 2012, 70, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.H.; Wang, J.; Cui, L.Y.; Zeng, R.C.; Wang, Q.Z.; Han, Q.X.; Qiu, J.; Chen, X.B.; Chen, D.C.; Guan, S.K.; et al. Corrosion resistance and antibacterial activity of zinc-loaded montmorillonite coatings on biodegradable magnesium alloy AZ31. Acta Biomater. 2019, 98, 196–214. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Qi, G. Evaluation of isolation methods and culture conditions for rat bone marrow mesenchymal stem cells. Cytotechnology 2013, 65, 323–334. [Google Scholar] [CrossRef]
- Mansoury, M.; Hamed, M.; Karmustaji, R.; Al Hannan, F.; Safrany, S.T. The edge effect: A global problem. The trouble with culturing cells in 96-well plates. Biochem. Biophys. Rep. 2021, 26, 100987. [Google Scholar] [CrossRef] [PubMed]
- ASTM G31-72; Standard Practice for Laboratory Immersion Corrosion Testing of Metals. ASTM International: West Conshohocken, PA, USA, 2004.
- Shah, S.R.; Young, S.; Goldman, J.L.; Jansen, J.A.; Wong, M.E.; Mikos, A.G. A composite critical-size rabbit mandibular defect for evaluation of craniofacial tissue regeneration. Nat. Protoc. 2016, 11, 1989–2009. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using micro–computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5:2009(E); Biological Evaluation of Medical Devices. Part 5: Tests for In Vitro Cytotoxicity; ISO: Geneva, Switzerland, 2009.
- Jamesh, M.; Kumar, S.; Sankara Narayanan, T.S.N. Corrosion behavior of commercially pure Mg and ZM21 Mg alloy in Ringer’s solution—Long term evaluation by EIS. Corros. Sci. 2011, 53, 645–654. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Lamaka, S.V.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Egorkin, V.S.; Imshinetskiy, I.M.; Zavidnaya, A.G.; Zheludkevich, M.L.; Gnedenkov, S.V. Electrochemical behaviour of the MA8 Mg alloy in minimum essential medium. Corros. Sci. 2020, 168, 108552. [Google Scholar] [CrossRef]
- Xie, Y.; Li, S.; Zhang, T.; Wang, C.; Cai, X. Titanium mesh for bone augmentation in oral implantology: Current application and progress. Int. J. Oral Sci. 2020, 12, 37. [Google Scholar] [CrossRef]
- Aludden, H.; Mordenfeld, A.; Cederlund, A.; Dahlin, C.; Spin-Neto, R.; Veiss-Pedersen, P.; Sritharan, B.; Starch-Jensen, T. Radiographic changes in height and volume after lateral GBR procedures with different ratios of deproteinized bovine bone mineral and autogenous bone at different time points. An experimental study. Clin. Oral Implant. Res. 2021, 32, 167–179. [Google Scholar] [CrossRef]
- Xin, Y.; Hu, T.; Chu, P.K. In vitro studies of biomedical magnesium alloys in a simulated physiological environment: A review. Acta Biomater. 2011, 7, 1452–1459. [Google Scholar] [CrossRef]
- Spicer, P.P.; Kretlow, J.D.; Young, S.; Jansen, J.A.; Kasper, F.K.; Mikos, A.G. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat. Protoc. 2012, 7, 1918–1929. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Soldatos, N.K.; Stylianou, P.; Koidou, V.P.; Angelov, N.; Yukna, R.; Romanos, G.E. Limitations and options using resorbable versus nonresorbable membranes for successful guided bone regeneration. Quintessence Int. 2017, 48, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Kačarević, Ž.P.; Rider, P.; Elad, A.; Tadic, D.; Rothamel, D.; Sauer, G.; Bornert, F.; Windisch, P.; Hangyási, D.B.; Molnar, B.; et al. Biodegradable magnesium fixation screw for barrier membranes used in guided bone regeneration. Bioact. Mater. 2022, 14, 15–30. [Google Scholar] [CrossRef]
- Heublein, B.; Rohde, R.; Kaese, V.; Niemeyer, M.; Hartung, W.; Haverich, A. Biocorrosion of magnesium alloys: A new principle in cardiovascular implant technology? Heart 2003, 89, 651–656. [Google Scholar] [CrossRef]
- Hänzi, A.C.; Gerber, I.; Schinhammer, M.; Löffler, J.F.; Uggowitzer, P.J. On the in vitro and in vivo degradation performance and biological response of new biodegradable Mg–Y–Zn alloys. Acta Biomater. 2010, 6, 1824–1833. [Google Scholar] [CrossRef]
- Chen, X.B.; Nisbet, D.R.; Li, R.W.; Smith, P.N.; Abbott, T.B.; Easton, M.A.; Zhang, D.H.; Birbilis, N. Controlling initial biodegradation of magnesium by a biocompatible strontium phosphate conversion coating. Acta Biomater. 2014, 10, 1463–1474. [Google Scholar] [CrossRef]
- Lee, J.W.; Han, H.S.; Han, K.J.; Park, J.; Jeon, H.; Ok, M.R.; Seok, H.K.; Ahn, J.P.; Lee, K.E.; Lee, D.H.; et al. Long-term clinical study and multiscale analysis of in vivo biodegradation mechanism of Mg alloy. Proc. Natl. Acad. Sci. USA 2016, 113, 716–721. [Google Scholar] [CrossRef]
- Kim, B.J.; Piao, Y.; Wufuer, M.; Son, W.C.; Choi, T.H. Biocompatibility and Efficiency of Biodegradable Magnesium-Based Plates and Screws in the Facial Fracture Model of Beagles. J. Oral Maxillofac. Surg. 2018, 76, 1055.e1–1055.e9. [Google Scholar] [CrossRef] [PubMed]
- Borges, C.D.; Faria, P.E.P.; Pessôa de Oliveira, P.G.F.; Sales de Melo Soares, M.; Ricoldi, M.S.T.; Costa, M.S.; Novaes Júnior, A.B.; Tambasco de Oliveira, P.; Taba Júnior, M. Influence of collagen membrane on bone quality in titanium mesh reconstructions-Study in rats. J. Periodontol. 2020, 91, 1673–1681. [Google Scholar] [CrossRef]
- Gutta, R.; Baker, R.A.; Bartolucci, A.A.; Louis, P.J. Barrier membranes used for ridge augmentation: Is there an optimal pore size? J. Oral Maxillofac. Surg. 2009, 67, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Rider, P.; Kačarević, Ž.P.; Elad, A.; Tadic, D.; Rothamel, D.; Sauer, G.; Bornert, F.; Windisch, P.; Hangyási, D.B.; Molnar, B.; et al. Biodegradable magnesium barrier membrane used for guided bone regeneration in dental surgery. Bioact. Mater. 2022, 14, 152–168. [Google Scholar] [CrossRef]
- Wang, N.; Yang, S.; Shi, H.; Song, Y.; Sun, H.; Wang, Q.; Tan, L.; Guo, S. Magnesium alloys for orthopedic applications:A review on the mechanisms driving bone healing. J. Magnes. Alloys 2022, 10, 3327–3353. [Google Scholar] [CrossRef]
- Yang, F.; Xue, Y.; Wang, F.; Guo, D.; He, Y.; Zhao, X.; Yan, F.; Xu, Y.; Xia, D.; Liu, Y. Sustained release of magnesium and zinc ions synergistically accelerates wound healing. Bioact. Mater. 2023, 26, 88–101. [Google Scholar] [CrossRef]
- Liu, J.; Liu, B.; Min, S.; Yin, B.; Peng, B.; Yu, Z.; Wang, C.; Ma, X.; Wen, P.; Tian, Y.; et al. Biodegradable magnesium alloy WE43 porous scaffolds fabricated by laser powder bed fusion for orthopedic applications: Process optimization, in vitro and in vivo investigation. Bioact. Mater. 2022, 16, 301–319. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xia, D.; Wang, S.; Gu, R.; Yang, F.; Zhao, X.; Liu, X.; Zhu, Y.; Liu, H.; Xu, Y.; et al. Photocrosslinkable Col/PCL/Mg composite membrane providing spatiotemporal maintenance and positive osteogenetic effects during guided bone regeneration. Bioact. Mater. 2022, 13, 53–63. [Google Scholar] [CrossRef] [PubMed]




| Gene | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (3′-5′) |
|---|---|---|
| ALP | ATGCTCAGGACAGGATCAAA | CGGGACATAAGCGAGTTTCT |
| COL1 | AGCTCGATACACAATGGCCT | CCTATGACTTCTGCGTCTGG |
| OCN | CAGACAAGTCCCACACAGCA | CCAGCAGAGTGAGCAGAGAG |
| OPN | ATGAGATTGGCAGTGATT TGC | GTCCATCGTCATCACCTCCT |
| GAPDH | ATGGGTGTGAACCACGAGA | CAGGGATGATGTTCTGGGCA |
| Region | Element Composition (at%) | ||||
|---|---|---|---|---|---|
| Mg | C | O | Cl | Na | |
| A | 53.44 | 29.24 | 16.59 | 0.09 | 0.09 |
| B | 49.64 | 41.45 | 8.08 | 0.12 | 0.18 |
| C | 51.02 | 25.49 | 22.47 | 0.18 | 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Q.; Gao, K.; Li, Y.; Zhang, Z.; Chen, S.; Zhou, J. Osteogenesis Activity and Porosity Effect of Biodegradable Mg-Ga Alloys Barrier Membrane for Guided Bone Regeneration: An in Vitro and in Vivo Study in Rabbits. Biomedicines 2025, 13, 1940. https://doi.org/10.3390/biomedicines13081940
Luo Q, Gao K, Li Y, Zhang Z, Chen S, Zhou J. Osteogenesis Activity and Porosity Effect of Biodegradable Mg-Ga Alloys Barrier Membrane for Guided Bone Regeneration: An in Vitro and in Vivo Study in Rabbits. Biomedicines. 2025; 13(8):1940. https://doi.org/10.3390/biomedicines13081940
Chicago/Turabian StyleLuo, Qiyue, Kang Gao, Yan Li, Ziyue Zhang, Su Chen, and Jian Zhou. 2025. "Osteogenesis Activity and Porosity Effect of Biodegradable Mg-Ga Alloys Barrier Membrane for Guided Bone Regeneration: An in Vitro and in Vivo Study in Rabbits" Biomedicines 13, no. 8: 1940. https://doi.org/10.3390/biomedicines13081940
APA StyleLuo, Q., Gao, K., Li, Y., Zhang, Z., Chen, S., & Zhou, J. (2025). Osteogenesis Activity and Porosity Effect of Biodegradable Mg-Ga Alloys Barrier Membrane for Guided Bone Regeneration: An in Vitro and in Vivo Study in Rabbits. Biomedicines, 13(8), 1940. https://doi.org/10.3390/biomedicines13081940







