Co-Evaluation of Lactate, Base Excess, and Albumin as Predictors of Mortality in Sepsis by Excluding the Factors That Affect Their Levels: An Observational Study †
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
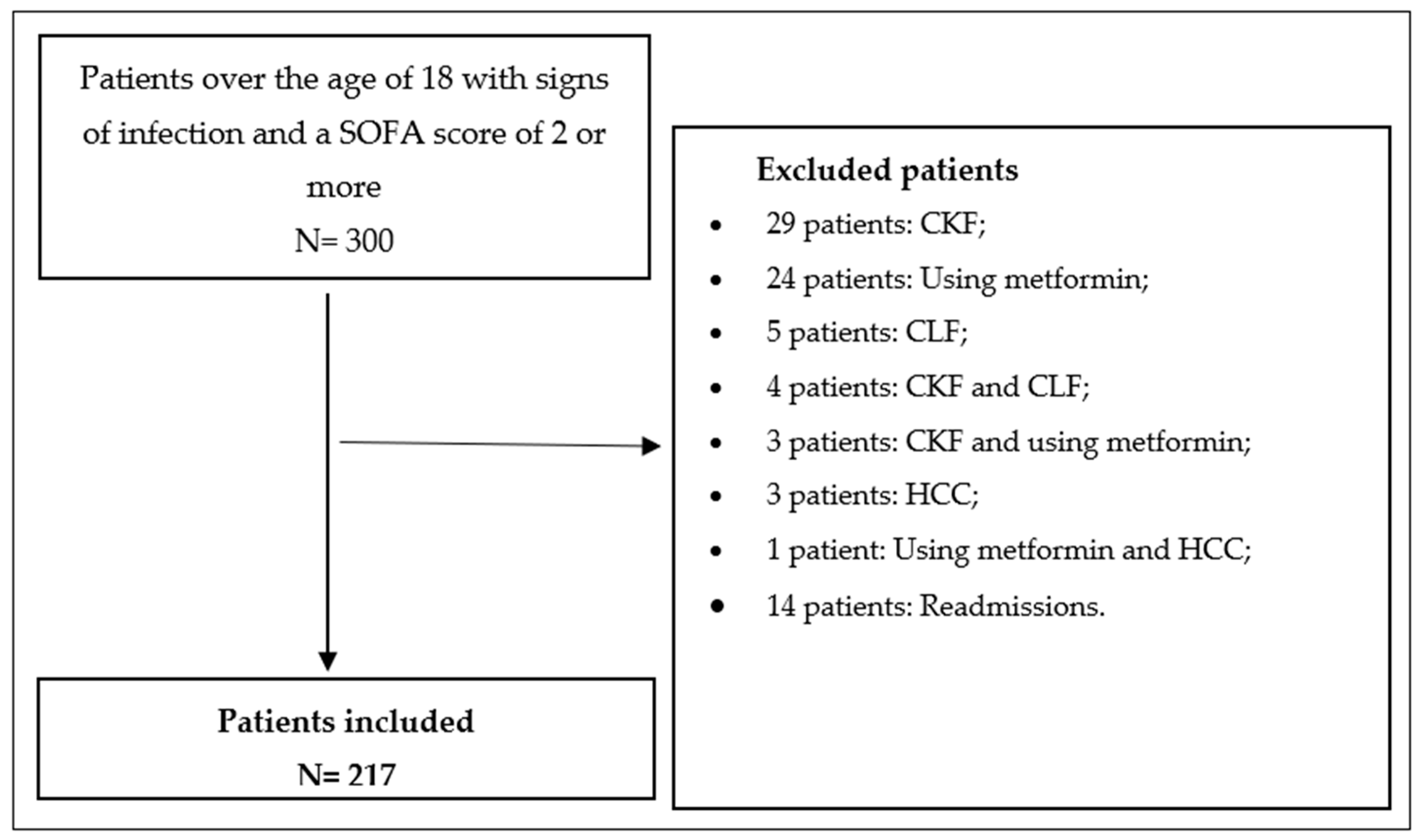
- Lactate, BE, or albumin follow-ups were missing for any reason;
- Insufficient information about the outcome could not be found;
- Type 2 lactatemia reasons (using metformin, chronic liver disease, etc.);
- Having chronic kidney disease;
- Antibiotics not started;
- Having acute gastrointestinal bleeding;
- Pregnant women;
- Trauma patients;
- Acute myocardial infarction patients;
- Repeated admissions of the same patient.
2.2. Stastistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABE | Alactic base excess |
| AUC | Area under the curve |
| BE | Base excess |
| COPD | Chronic obstructive pulmonary disease |
| ED | Emergency department |
| FIO2 | Fraction of inspired oxygen |
| HCC | Hepatocellular carcinoma |
| ICU | Intensive care unit |
| LC | Lactate clearance |
| MAP | Mean arterial pressure |
| n | Number |
| OR | Odds ratio |
| PaO2 | Partial pressure of oxygen |
| ROC | Receiver operating characteristic |
| SOFA | Sepsis-related (sequential) organ failure assessment |
| Std | Standard |
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, R.; Winters, M.E.; Vilke, G.M.; Wardi, G. Does Early and Appropriate Antibiotic Administration Improve Mortality in Emergency Department Patients with Severe Sepsis or Septic Shock? J. Emerg. Med. 2017, 53, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Lambden, S.; Laterre, P.F.; Levy, M.M.; Francois, B. The SOFA score-development, utility and challenges of accurate assessment in clinical trials. Crit. Care 2019, 23, 374. [Google Scholar] [CrossRef]
- Lee, C.C.; Chen, S.Y.; Tsai, C.L.; Wu, S.-C.; Chiang, W.-C.; Wang, J.-L.; Sun, H.-Y.; Chen, S.-C.; Chen, W.-J.; Hsueh, P.-R. Prognostic value of mortality in emergency department sepsis score, procalcitonin, and C-reactive protein in patients with sepsis at the emergency department. Shock 2008, 29, 322–327. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, C.; Jia, Y. Evaluation of the Mortality in Emergency Department Sepsis score combined with procalcitonin in septic patients. Am. J. Emerg. Med. 2013, 31, 1086–1091. [Google Scholar] [CrossRef]
- Seo, M.H.; Choa, M.; You, J.S.; Lee, H.S.; Hong, J.H.; Park, Y.S.; Chung, S.P.; Park, I. Hypoalbuminemia, Low Base Excess Values, and Tachypnea Predict 28-Day Mortality in Severe Sepsis and Septic Shock Patients in the Emergency Department. Yonsei Med. J. 2016, 57, 1361–1369. [Google Scholar] [CrossRef]
- Pierrakos, C.; Vincent, J.L. Sepsis biomarkers: A review. Crit. Care 2010, 14, R15. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Vasques, F.; Camporota, L.; Meessen, J.; Romitti, F.; Pasticci, I.; Duscio, E.; Vassalli, F.; Forni, L.G.; Payen, D.; et al. Understanding Lactatemia in Human Sepsis. Potential Impact for Early Management. Am. J. Respir. Crit. Care Med. 2019, 200, 582–589. [Google Scholar] [CrossRef]
- Alhindawi, M.H.; Hamadneh, K.N.A.; Adamat, M.A.; Alnajada, E.M. Acute Dropping in Albumin Level as an Early Predictive Sign of Septic Shock Mahmoud Hifith Alhindawi; MD, Khaled Nor ALdeen Hamadneh; MD, Mohammad Awwad. IAR J. Anaesthesiol. Crit. Care 2021, 2. [Google Scholar] [CrossRef]
- Lichtenauer, M.; Wernly, B.; Ohnewein, B.; Franz, M.; Kabisch, B.; Muessig, J.; Masyuk, M.; Lauten, A.; Schulze, P.C.; Hoppe, U.C.; et al. The Lactate/Albumin Ratio: A Valuable Tool for Risk Stratification in Septic Patients Admitted to ICU. Int. J. Mol. Sci. 2017, 18, 1893. [Google Scholar] [CrossRef]
- Siggaard-Andersen, O. An acid-base chart for arterial blood with normal and pathophysiological reference areas. Scand. J. Clin. Lab. Investig. 1971, 27, 239–245. [Google Scholar] [CrossRef]
- Takahashi, N.; Nakada, T.A.; Walley, K.R.; Russell, J.A. Significance of lactate clearance in septic shock patients with high bilirubin levels. Sci. Rep. 2021, 11, 6313. [Google Scholar] [CrossRef]
- Hajian-Tilaki, K. Sample size estimation in diagnostic test studies of biomedical informatics. J. Biomed. Inform. 2014, 48, 193–204. [Google Scholar] [CrossRef]
- Rannikko, J.; Syrjänen, J.; Seiskari, T.; Aittoniemi, J.; Huttunen, R. Sepsis-related mortality in 497 cases with blood culture-positive sepsis in an emergency department. Int. J. Infect. Dis. 2017, 58, 52–57. [Google Scholar] [CrossRef]
- Morr, M.; Lukasz, A.; Rübig, E.; Pavenstädt, H.; Kümpers, P. Sepsis recognition in the emergency department—impact on quality of care and outcome? BMC Emerg. Med. 2017, 17, 11. [Google Scholar] [CrossRef] [PubMed]
- Gunes Ozaydin, M.; Guneysel, O.; Saridogan, F.; Ozaydin, V. Are scoring systems sufficient for predicting mortality due to sepsis in the emergency department? Turk. J. Emerg. Med. 2016, 17, 25–28. [Google Scholar] [CrossRef]
- Park, M.; Azevedo, L.C.; Maciel, A.T.; Pizzo, V.R.; Noritomi, D.T.; da Cruz Neto, L.M. Evolutive standard base excess and serum lactate level in severe sepsis and septic shock patients resuscitated with early goal-directed therapy: Still outcome markers? Clinics 2006, 61, 47–52. [Google Scholar] [CrossRef]
- Javed, A.; Guirgis, F.W.; Sterling, S.A.; Puskarich, M.A.; Bowman, J.; Robinson, T.; Jones, A.E. Clinical predictors of early death from sepsis. J. Crit. Care 2017, 42, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, A.; Novack, V.; Bichovsky, Y.; Klein, M.; Dreiher, J. Serum Albumin Levels as a Predictor of Mortality in Patients with Sepsis: A Multicenter Study. Isr. Med. Assoc. J. 2022, 24, 454–459. [Google Scholar]
- Yuan, J.; Liu, X.; Liu, Y.; Li, W.; Chen, X.; Chen, Q.; Xiao, C.; Wan, Y.; Li, S.; Li, Q.; et al. Association between base excess and 28-day mortality in sepsis patients: A secondary analysis based on the MIMIC- IV database. Heliyon 2023, 9, e15990. [Google Scholar] [CrossRef] [PubMed]
- Marty, P.; Roquilly, A.; Vallée, F.; Luzi, A.; Ferré, F.; Fourcade, O.; Asehnoune, K.; Minville, V. Lactate clearance for death prediction in severe sepsis or septic shock patients during the first 24 hours in Intensive Care Unit: An observational study. Ann. Intensive Care 2013, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, Y.; Yang, B.; Zhao, J.; Tong, Q.; Yuan, Y.; Kang, Y.; Ren, T. Association between alactic base excess on mortality in sepsis patients: A retrospective observational study. J. Intensive Care 2025, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Freund, Y.; Lemachatti, N.; Krastinova, E.; Van Laer, M.; Claessens, Y.-E.; Avondo, A.; Occelli, C.; Feral-Pierssens, A.-L.; Truchot, J.; Ortega, M.; et al. Prognostic Accuracy of Sepsis-3 Criteria for In-Hospital Mortality Among Patients With Suspected Infection Presenting to the Emergency Department. JAMA 2017, 317, 301–308. [Google Scholar] [CrossRef] [PubMed]
| Score | |||||
|---|---|---|---|---|---|
| System | 0 | 1 | 2 | 3 | 4 |
| Respiration | |||||
| PaO2/FIO2, mmHg | ≥400 (53.3) | <400 (53.3) | <300 (40) | <200 (26.7) with respiratory support | <100 (13.3) with respiratory support |
| Coagulation | |||||
| Platelets, ×103/μL | ≥150 | <150 | <100 | <50 | <20 |
| Liver | |||||
| Bilirubin, mg/dL (μmol/L) | <1.2 (20) | 1.2–1.9 (20–32) | 2.0–5.9 (33–101) | 6.0–11.9 (102–204) | >12.0 (204) |
| Cardiovascular | MAP ≥ 70 mmHg | MAP < 70 mmHg | Dopamine < 5 or any dose dobutamine * | Dopamine 5.1–15 or epinephrine ≤ 0.1 or norepinephrine ≤ 0.1 * | Dopamine > 15 or epinephrine > 0.1 or norepinephrine > 0.1 * |
| Central Nervus System | |||||
| Glasgow Coma Scale score ** | 15 | 13–14 | 10–12 | 6–9 | <6 |
| Renal | |||||
| Creatine, mg/dL (μmol/L) | <1.2 (110) | 1.2–1.9 (110–170) | 2.0–3.4 (171–299) | 3.5–4.9 (300–440) | >5.0 (440) |
| Urine output, mL/d | <500 | <200 | |||
| Characteristics | n = 217 |
|---|---|
| Age yr | 67.5 ± 16.5 |
| Sex no. (%) | |
| Male | 131 (60.4) |
| Female | 86 (39.6) |
| Comorbidities no. (%) | |
| No disease | 2 (0.9) |
| Malignite | 122 (56.2) |
| Hypertension | 87 (40.1) |
| Coronary Artery Disease | 51 (23.5) |
| Asthma/COPD * | 46 (21.7) |
| Diabetes Mellitus | 40 (18.4) |
| Konjestif Kalp Yetmezliği | 31 (14.3) |
| Alzheimer’s/Parkinson’s/Demans | 31 (14.3) |
| Arrhythmia | 24 (11.1) |
| Cerebrovascular Disease | 23 (10.7) |
| Benign Prostatic Hypertrophy | 14 (6.5) |
| Rheumatological Disease | 13 (6) |
| Hypothyroidism/Hyperthyroidism | 10 (4.6) |
| Epilepsy | 8 (3.7) |
| Others ** | 48 (22.1) |
| Sistolic Blood Pressure mmHg | 106 ± 44 |
| Mean Arterial Pressure mmHg | 79 ± 28 |
| SOFA Scores no. (%) | |
| 2–6 | 170 (78.3) |
| 7–9 | 37 (17.1) |
| 10–14 | 10 (4.6) |
| Site of Infections no. (%) | |
| Pulmonary | 134 (61.8) |
| Urinary | 36 (16.6) |
| Bacteremia | 25 (11.5) |
| Intra-abdominal | 13 (6) |
| Soft Tissue | 8 (3.7) |
| Central Nervous System | 1 (0.5) |
| Mean (Std. Deviation) | |
|---|---|
| 0 h lactate * | 2.40 (1.9) |
| 0 h albumin * | 3 (0.7) |
| 0 h BE | −1.47 (6.57) |
| 24 h lactate * | 1.7 (1.3) |
| 24 h albumin | 2.65 (0.53) |
| 24 h BE | −1.44 (6.04) |
| 48 h lactate * | 1.5 (1) |
| 48 h albumin | 2.57 (0.51) |
| 48 h BE | −1.3 (6.1) |
| 24 h LC * | 26.7 (54.5) |
| 48 h LC * | 24 (50) |
| 0 h ABE | 1.39 (6.25) |
| 24 h ABE * | 0.2 (7.7) |
| 48 h ABE | 0.72 (5.56) |
| Sepsis (n = 196) | Septic Shock (n = 21) | |||
|---|---|---|---|---|
| Survival | Nonsurvival | Survival | Nonsurvival | |
| 0 h lactate | 2.1 (1.5–3.1) | 2.65 (1.6–4.5) | 2.92 (1.13) | 4.04 (1.98) |
| p value | 0.056 * | 0.14 † | ||
| 0 h albumin | 3.07 (0.49) | 2.68 (0.63) | 2.81 (0.57) | 2.65 (0.69) |
| p value | 0.000 † | 0.56 † | ||
| 0 h BE | −1.43 (6.1) | −1.01 (7.48) | −1.94 (7.46) | −5.7 (4.95) |
| p value | 0.576 † | 0.156 † | ||
| 24 h lactate | 1.5 (1.2–2.1) | 2 (1.5–3) | 2.2 (1.5–2.9) | 2.6 (2.15–4.5) |
| p value | 0.001 * | 0.268 * | ||
| 24 h albumin | 2.76 (0.48) | 2.49 (0.61) | 2.51 (0.45) | 2.3 (0.35) |
| p value | 0.004 † | 0.27 † | ||
| 24 h BE | −0.94 (5.35) | −1.29 (6.95) | −4.41 (7.01) | −7.45 (5.29) |
| p value | 0.279 † | 0.138 † | ||
| 48 h lactate | 1.5 (1.1–2) | 1.6 (1.2–3) | 2.05 (1.5–2.55) | 1.4 (1.2–2.2) |
| p value | 0.058 * | 0.151 * | ||
| 48 h albumin | 2.68 (0.48) | 2.37 (0.52) | 2.49 (0.47) | 2.21 (0.57) |
| p value | 0.000 † | 0.286 † | ||
| 48 h BE | −0.4 (5.13) | −2.03 (6.69) | −3.85 (7.39) | −8.13 (9.44) |
| p value | 0.075 † | 0.305 † | ||
| 0 h ABE | 1.32 (5.71) | 1.69 (6.92) | 1.31 (7.68) | −1.83 (2.79) |
| p value | 0.696 † | 0.285 † | ||
| 24 h ABE | 0.93 (5.24) | 0.94 (6.49) | −1.22 (7.63) | −4.73 (3.94) |
| p value | 0.990 † | 0.247 † | ||
| 48-h ABE | 1.23 (5.11) | 0.37 (6.24) | −1.21 (6.54) | −3.07 (5.12) |
| p value | 0.330 † | 0.553 † | ||
| 24 h LC | 32.04 (50) | 18.75 (56.67) | 25.53 (50.37) | 27.62 (78.74) |
| p value | 0.037 * | 0.664 * | ||
| 48 h LC | 24.57 (54.27) | 23.08 (48.05) | 21.35 (57.19) | 37.88 (142.86) |
| p value | 0.430 * | 0.640 * | ||
| SOFA Score | Sepsis (N = 196) n(%) | Septic Shock (N = 21) n(%) | ||
|---|---|---|---|---|
| Survival | Nonsurvival | Survival | Nonsurvival | |
| 2–6 | 115 (71.9) | 45 (28.1) | 5 (50) | 5 (50) |
| 7–9 | 16 (55.2) | 13 (44.8) | 7 (87.5) | 1 (12.5) |
| 10–14 | 3 (43.3) | 4 (56.7) | 1 (33.3) | 2 (66.7) |
| p value | 0.045 | 0.188 | ||
| Model-1 | Model-2 | Model-3 | ||||
|---|---|---|---|---|---|---|
| AUC | OR | AUC | OR | AUC | OR | |
| 0 h lactate | 0.517 | 1.143 | - | - | - | 1.116 |
| p value | 0.707 | 0.038 | - | - | 0.103 | |
| 0 h albumin | - | - | 0.604 | 0.267 | - | 0.281 |
| p value | - | - | 0.020 | 0.000 | 0.103 | |
| 0 h lactate + albumin | - | - | - | - | 0.604 | - |
| p value | - | - | - | - | 0.019 | - |
| 24 h lactate | 0.570 | 1.635 | - | - | - | 1.580 |
| p value | 0.125 | 0.001 | - | - | 0.001 | |
| 24 h albumin | - | - | 0.540 | 0.366 | - | 0.409 |
| p value | - | - | 0.373 | 0.002 | 0.008 | |
| 24 h lactate + albumin | - | - | - | - | 0.580 | - |
| p value | - | - | - | - | 0.078 | - |
| 48 h lactate | 0.588 | 1.610 | - | - | - | 1.516 |
| p value | 0.060 | 0.001 | - | - | 0.005 | |
| 48 h albumin | - | - | 0.540 | 0.270 | - | 0.318 |
| p value | - | - | 0.395 | 0.000 | 0.002 | |
| 48 h lactate + albumin | - | - | - | - | 0.617 | - |
| p value | - | - | - | - | 0.013 | - |
| Hospital Mortality | ||
|---|---|---|
| AUC | p Value | |
| 0 h BE | 0.500 | 0.995 |
| 24 h BE | 0.479 | 0.639 |
| 48 h BE | 0.426 | 0.095 |
| 0 h ABE | 0.510 | 0.837 |
| 24 h ABE | 0.495 | 0.915 |
| 48 h ABE | 0.506 | 0.674 |
| 24 h LC | 0.412 | 0.047 |
| 48 h LC | 0.475 | 0.580 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gur, A.; Ozturk Ince, E.; Metin Aksu, N. Co-Evaluation of Lactate, Base Excess, and Albumin as Predictors of Mortality in Sepsis by Excluding the Factors That Affect Their Levels: An Observational Study. Biomedicines 2025, 13, 1932. https://doi.org/10.3390/biomedicines13081932
Gur A, Ozturk Ince E, Metin Aksu N. Co-Evaluation of Lactate, Base Excess, and Albumin as Predictors of Mortality in Sepsis by Excluding the Factors That Affect Their Levels: An Observational Study. Biomedicines. 2025; 13(8):1932. https://doi.org/10.3390/biomedicines13081932
Chicago/Turabian StyleGur, Aysenur, Elif Ozturk Ince, and Nalan Metin Aksu. 2025. "Co-Evaluation of Lactate, Base Excess, and Albumin as Predictors of Mortality in Sepsis by Excluding the Factors That Affect Their Levels: An Observational Study" Biomedicines 13, no. 8: 1932. https://doi.org/10.3390/biomedicines13081932
APA StyleGur, A., Ozturk Ince, E., & Metin Aksu, N. (2025). Co-Evaluation of Lactate, Base Excess, and Albumin as Predictors of Mortality in Sepsis by Excluding the Factors That Affect Their Levels: An Observational Study. Biomedicines, 13(8), 1932. https://doi.org/10.3390/biomedicines13081932






