Mechanically Induced Pulpitis: A Rat Model That Preserves Animal Well-Being
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Procedures
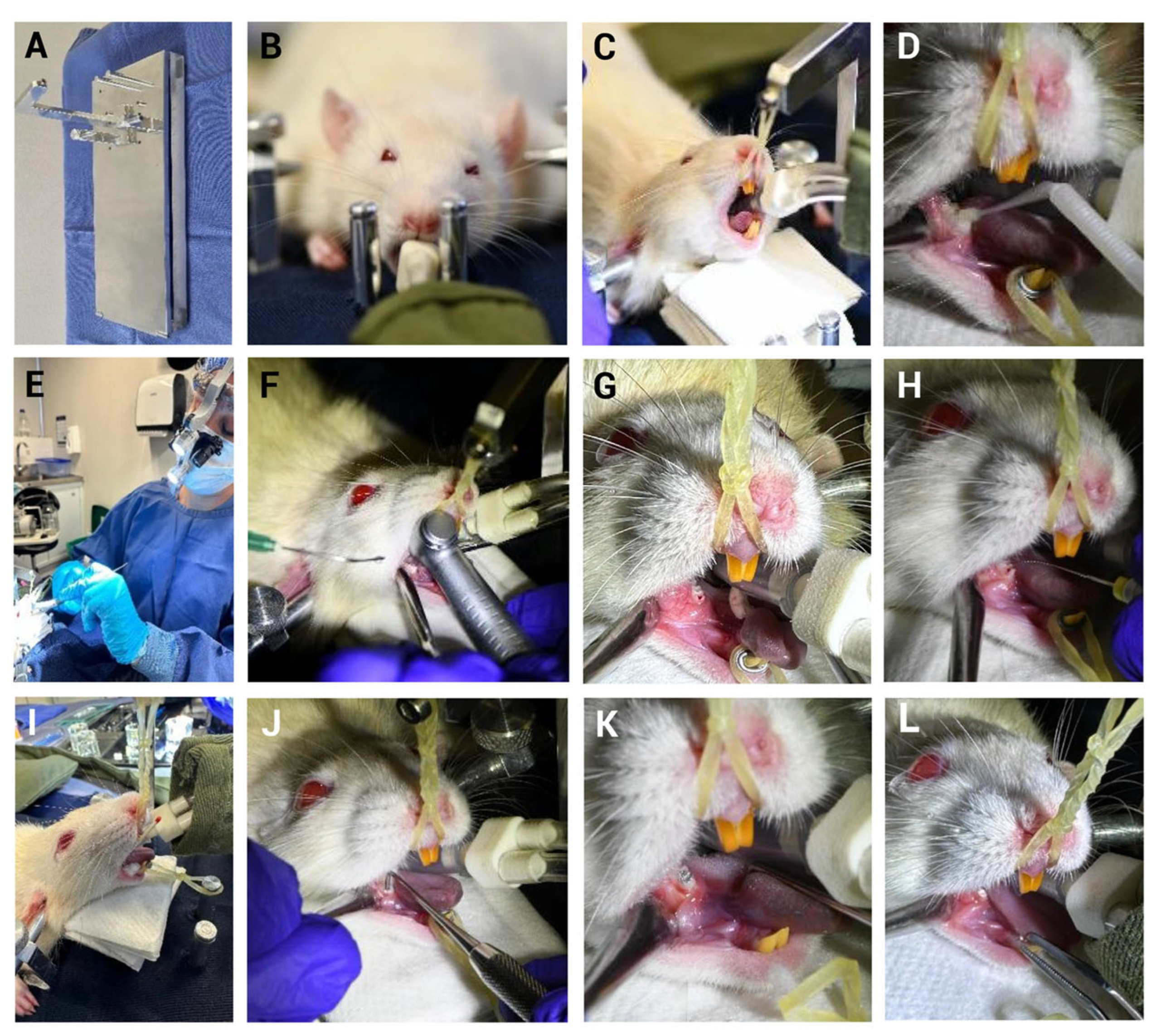
2.2.1. Endotracheal Intubation
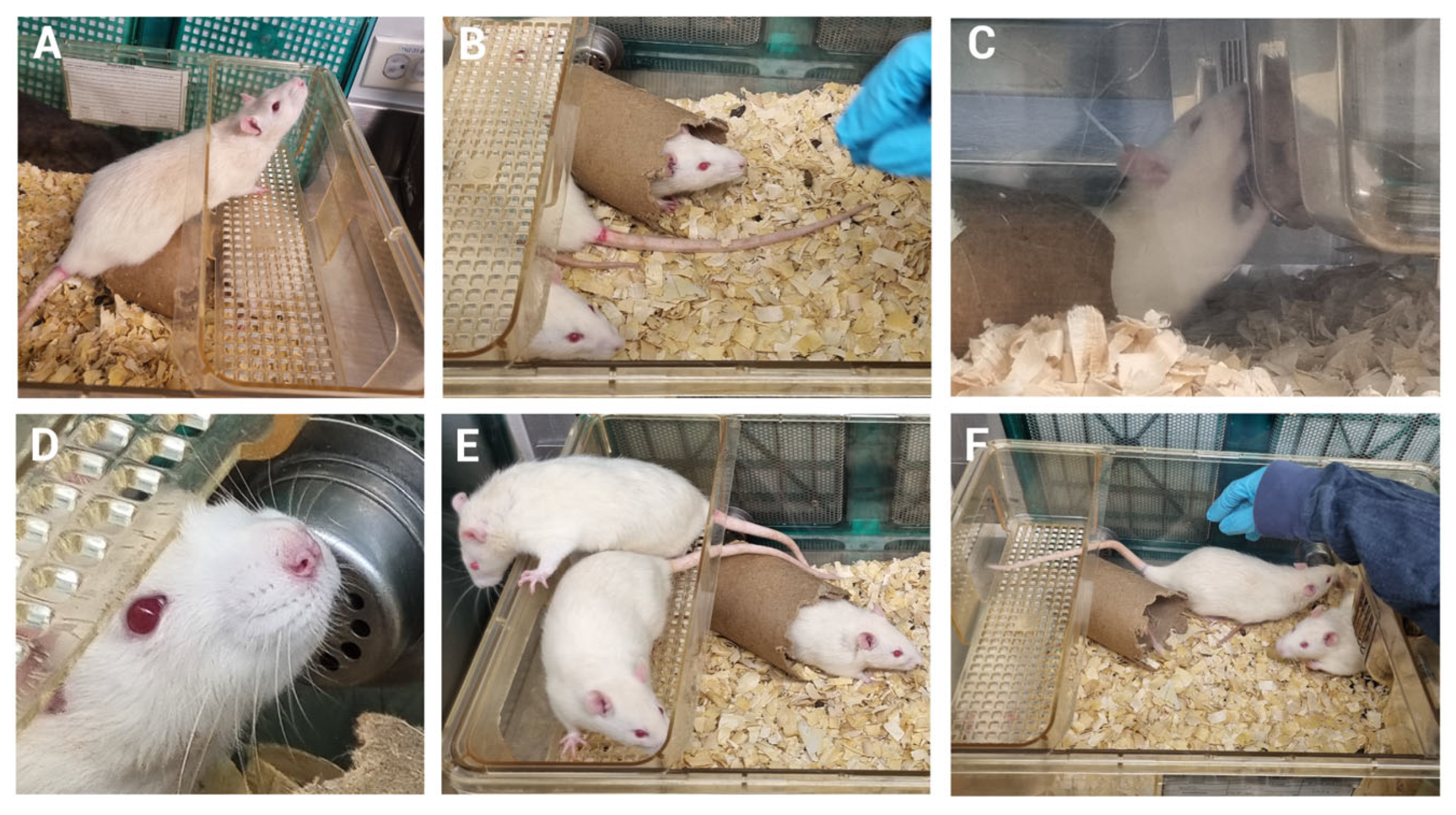
2.2.2. Mechanical Induction of Pulpitis (MIP)
2.3. Well-Being Assessment
2.4. Statistical Analysis
3. Results
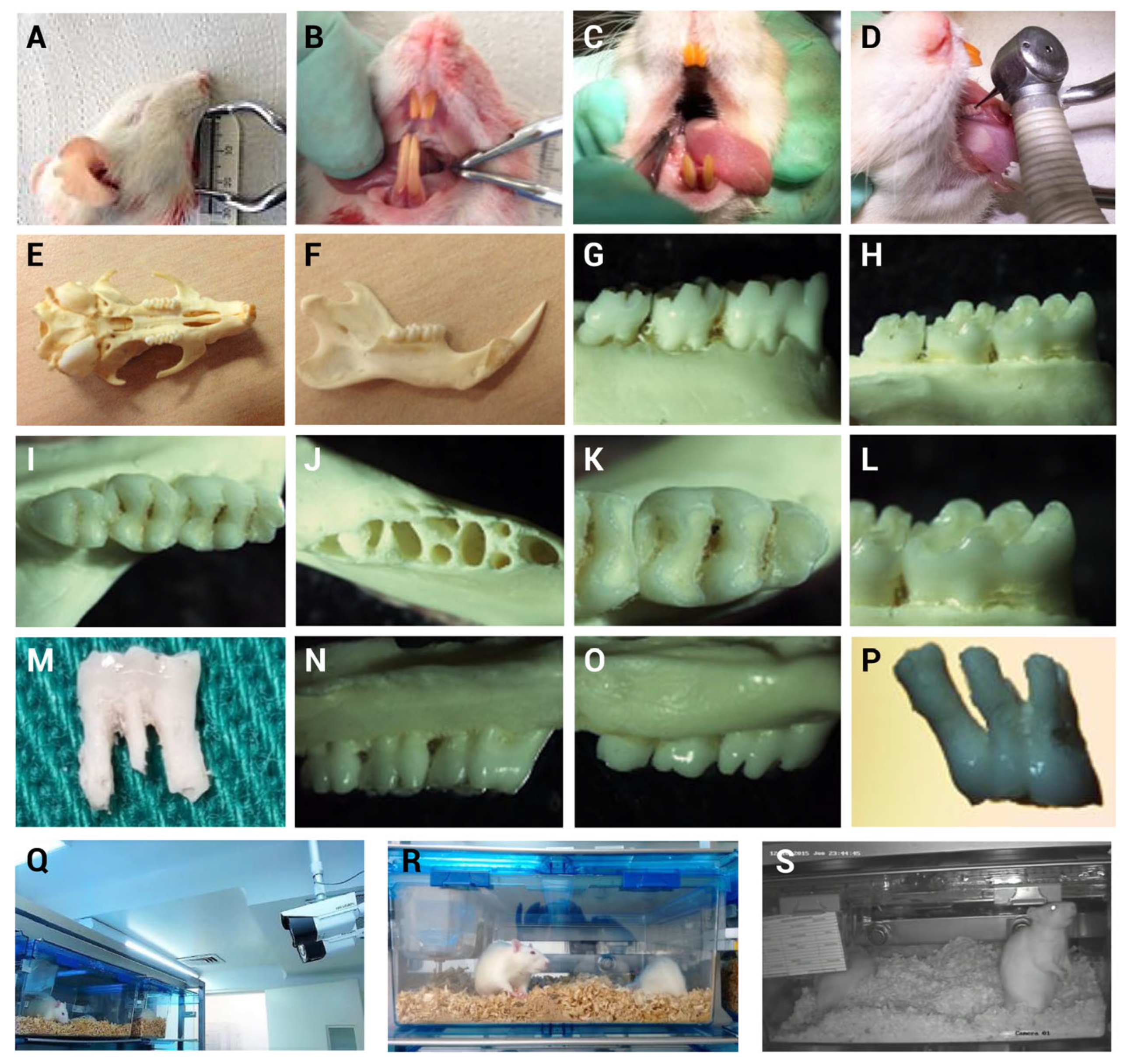
3.1. Oral Opening and Dentition Characteristics
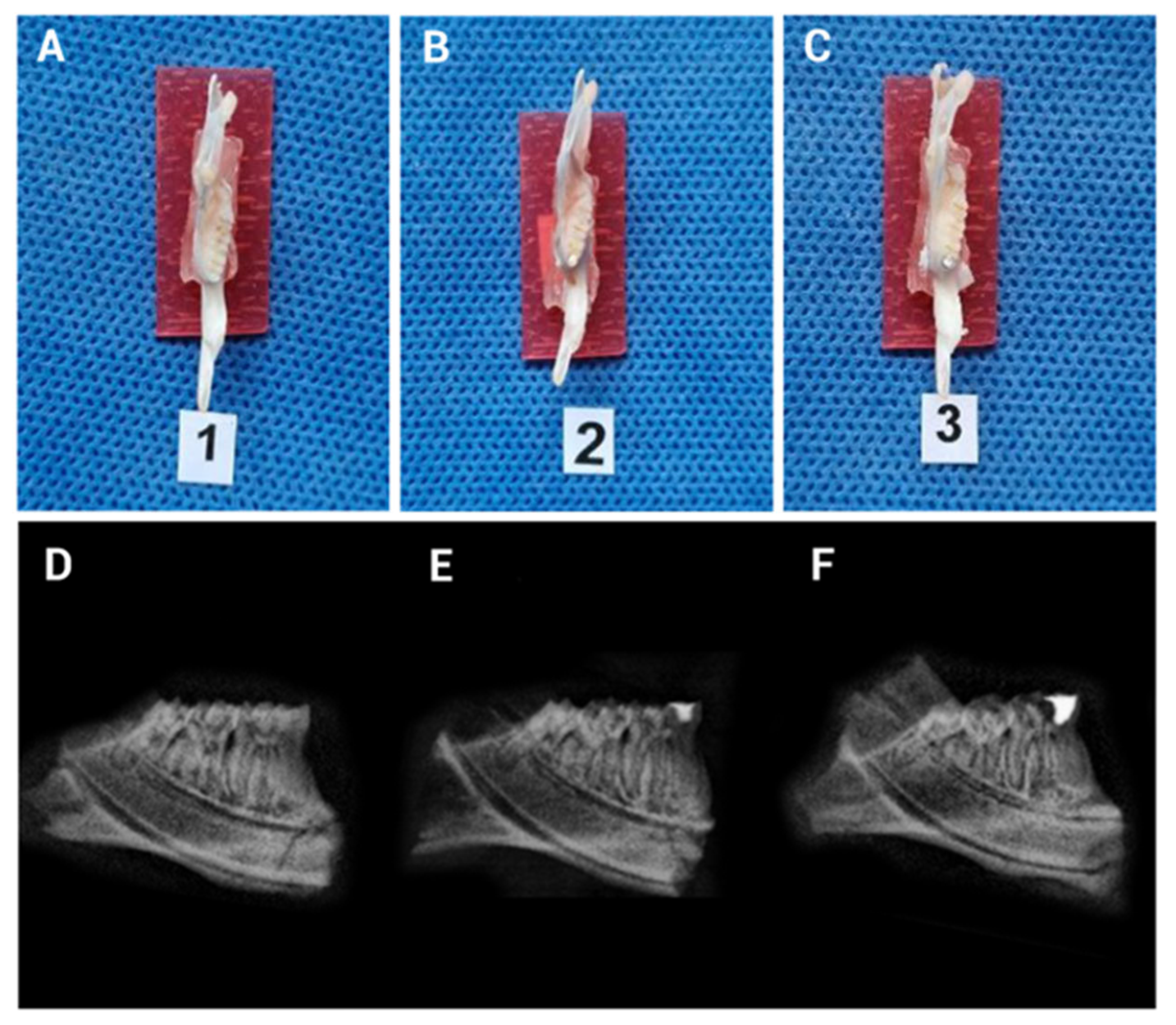
3.2. Implementation of the Mechanical Induction Model of Pulpitis
3.3. MIP Does Not Alter Animal Well-Being in the Early Stages
3.4. Animal Well-Being Is Preserved Following Five Days of MIP Induction
3.5. The Behavior of the Animals Is Unchanged During Monitoring
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| CGRP | Calcitonin gene-related peptide |
| NKA | Neurokinin A |
| NPY | Neuropeptide Y |
| VIP | Vasoactive Intestinal Peptide |
| CFA | Complete Freund’s Adjuvant |
| SPF | Specific pathogen-free |
| CIEFOPUJ | Research and Ethics Committee of the Faculty of Dentistry at Pontificia Universidad Javeriana. |
| IACUC | Institutional Animal Care and Use Committee |
| ARRIVE | Animal Research: Reporting of In Vivo Experiments |
| FELASA | Federation of European Laboratory Animal Science Associations |
| MIP | Mechanical Induction of Pulpitis |
| NC | Negative Control |
| MIPA | Mechanical Induction of Pulpitis sealed with amalgam |
| MIPZ | Mechanical Induction of Pulpitis sealed with zinc phosphate cement |
| CP | Capsaicin |
| H&E | Hematoxylin and Eosin |
| ANOVA | Analysis of variance |
| HR | Heart Rate |
| RR | Respiratory Rate |
| SpO2 | Peripheral capillary oxygen saturation |
| CV | Coefficients of Variation |
| GPL | General Public License |
| HUSI | Hospital Universitario San Ignacio |
References
- Li, A.; Li, Z.; Chiu, W.; Xiong, C.; Chen, Q.; Chen, J.; Lai, X.; Li, W.; Ke, Q.; Liu, J.; et al. Efficient Treatment of Pulpitis via Transplantation of Human Pluripotent Stem Cell-Derived Pericytes Partially through LTBP1-Mediated T Cell Suppression. Biomedicines 2023, 11, 3199. [Google Scholar] [CrossRef]
- Gaudin, A.; Renard, E.; Hill, M.; Bouchet-Delbos, L.; Bienvenu-Louvet, G.; Farges, J.C.; Cuturi, M.C.; Alliot-Licht, B. Phenotypic analysis of immunocompetent cells in healthy human dental pulp. J. Endod. 2015, 41, 621–627. [Google Scholar] [CrossRef]
- Caviedes-Bucheli, J.; Munoz, H.R.; Azuero-Holguin, M.M.; Ulate, E. Neuropeptides in dental pulp: The silent protagonists. J. Endod. 2008, 34, 773–788. [Google Scholar] [CrossRef]
- Sacerdote, P.; Levrini, L. Peripheral mechanisms of dental pain: The role of substance P. Mediat. Inflamm. 2012, 2012, 951920. [Google Scholar] [CrossRef]
- Richert, R.; Ducret, M.; Alliot-Licht, B.; Bekhouche, M.; Gobert, S.; Farges, J.C. A critical analysis of research methods and experimental models to study pulpitis. Int. Endod. J. 2022, 55 (Suppl. 1), 14–36. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.; Njeh, A.; Uzunoglu, E. Is Pulp Inflammation a Prerequisite for Pulp Healing and Regeneration? Mediat. Inflamm. 2015, 2015, 347649. [Google Scholar] [CrossRef]
- Karim, I.A.; Cooper, P.R.; About, I.; Tomson, P.L.; Lundy, F.T.; Duncan, H.F. Deciphering Reparative Processes in the Inflamed Dental Pulp. Front. Dent. Med. 2021, 2, 651219. [Google Scholar] [CrossRef]
- Schmalz, G.; Widbiller, M.; Galler, K.M. Clinical Perspectives of Pulp Regeneration. J. Endod. 2020, 46, S161–S174. [Google Scholar] [CrossRef]
- Schmalz, G.; Widbiller, M.; Galler, K.M. Erratum to Clinical Perspectives of Pulp Regeneration [J Endodon 46 (2020) S161–S174]. J. Endod. 2021, 47, 335. [Google Scholar] [CrossRef] [PubMed]
- Zaky, S.H.; Shehabeldin, M.; Ray, H.; Sfeir, C. The role of inflammation modulation in dental pulp regeneration. Eur. Cell Mater. 2021, 41, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Aubeux, D.; Renard, E.; Pérez, F.; Tessier, S.; Geoffroy, V.; Gaudin, A. Review of Animal Models to Study Pulp Inflammation. Front. Dent. Med. 2021, 2, 673552. [Google Scholar] [CrossRef]
- Dammaschke, T. Rat molar teeth as a study model for direct pulp capping research in dentistry. Lab. Anim. 2010, 44, 1–6. [Google Scholar] [CrossRef]
- Moghadam, A.; Moghadam, N.; Doremami, V.; Pishghadam, S.; Mafi, A. A New Experimental Technique for Complete Extraction of Mandibular First Molar Teeth in Rats. J. Vet. Dent. 2024, 41, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Cooper, P.R.; Holder, M.J.; Smith, A.J. Inflammation and regeneration in the dentin-pulp complex: A double-edged sword. J. Endod. 2014, 40 (Suppl. 4), S46–S51. [Google Scholar] [CrossRef] [PubMed]
- Minic, S.; Florimond, M.; Sadoine, J.; Valot-Salengro, A.; Chaussain, C.; Renard, E.; Boukpessi, T. Evaluation of Pulp Repair after BiodentineTM Full Pulpotomy in a Rat Molar Model of Pulpitis. Biomedicines 2021, 9, 784. [Google Scholar] [CrossRef] [PubMed]
- Renard, E.; Gaudin, A.; Bienvenu, G.; Amiaud, J.; Farges, J.C.; Cuturi, M.C.; Moreau, A.; Alliot-Licht, B. Immune Cells and Molecular Networks in Experimentally Induced Pulpitis. J. Dent. Res. 2016, 95, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Kawagishi, E.; Nakakura-Ohshima, K.; Nomura, S.; Ohshima, H. Pulpal responses to cavity preparation in aged rat molars. Cell Tissue Res. 2006, 326, 111–122. [Google Scholar] [CrossRef]
- He, Y.; Gan, Y.; Lu, J.; Feng, Q.; Wang, H.; Guan, H.; Jiang, Q. Pulpal Tissue Inflammatory Reactions after Experimental Pulpal Exposure in Mice. J. Endod. 2017, 43, 90–95. [Google Scholar] [CrossRef]
- Shi, X.; Li, Z.; He, Y.; Jiang, Q.; Yang, X. Effect of different dental burs for experimental induction of pulpitis in mice. Arch. Oral Biol. 2017, 83, 252–257. [Google Scholar] [CrossRef]
- Filippini, H.F.; Scalzilli, P.A.; Costa, K.M.; Freitas, R.D.S.; Campos, M.M. Activation of trigeminal ganglion satellite glial cells in CFA-induced tooth pulp pain in rats. PLoS ONE 2018, 13, e0207411. [Google Scholar] [CrossRef]
- Rossi, H.L.; See, L.P.; Foster, W.; Pitake, S.; Gibbs, J.; Schmidt, B.; Mitchell, C.H.; Abdus-Saboor, I. Evoked and spontaneous pain assessment during tooth pulp injury. Sci. Rep. 2020, 10, 2759. [Google Scholar] [CrossRef]
- Diaz, L.; Zambrano, E.; Flores, M.E.; Contreras, M.; Crispin, J.C.; Aleman, G.; Bravo, C.; Armenta, A.; Valdes, V.J.; Tovar, A.; et al. Ethical Considerations in Animal Research: The Principle of 3R’s. Rev. Investig. Clin. 2020, 73, 199–209. [Google Scholar] [CrossRef]
- Bronstad, A.; Newcomer, C.E.; Decelle, T.; Everitt, J.I.; Guillen, J.; Laber, K. Current concepts of Harm-Benefit Analysis of Animal Experiments—Report from the AALAS-FELASA Working Group on Harm-Benefit Analysis—Part 1. Lab. Anim. 2016, 50 (Suppl. 1), 1–20. [Google Scholar] [CrossRef]
- Chandwani, N.D.; Pawar, M.G.; Tupkari, J.V.; Yuwanati, M. Histological evaluation to study the effects of dental amalgam and composite restoration on human dental pulp: An in vivo study. Med. Princ. Pract. 2014, 23, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Snuggs, H.M.; Cox, C.F.; Powell, C.S.; White, K.C. Pulpal healing and dentinal bridge formation in an acidic environment. Quintessence Int. 1993, 24, 501–510. [Google Scholar] [PubMed]
- Kanjevac, T.; Taso, E.; Stefanovic, V.; Petkovic-Curcin, A.; Supic, G.; Markovic, D.; Djukic, M.; Djuran, B.; Vojvodic, D.; Sculean, A.; et al. Estimating the Effects of Dental Caries and Its Restorative Treatment on Periodontal Inflammatory and Oxidative Status: A Short Controlled Longitudinal Study. Front. Immunol. 2021, 12, 716359. [Google Scholar] [CrossRef]
- Hill, E.E.; Lott, J. A clinically focused discussion of luting materials. Aust. Dent. J. 2011, 56 (Suppl. 1), 67–76. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.J.; Shih, A.; Miletic, G.; Miletic, V. Continual systemic infusion of lidocaine provides analgesia in an animal model of neuropathic pain. Pain 2002, 97, 267–273. [Google Scholar] [CrossRef]
- MacDougall, L.M.; Hethey, J.A.; Livingston, A.; Clark, C.; Shmon, C.L.; Duke-Novakovski, T. Antinociceptive, cardiopulmonary, and sedative effects of five intravenous infusion rates of lidocaine in conscious dogs. Vet. Anaesth. Analg. 2009, 36, 512–522. [Google Scholar] [CrossRef]
- Basith, S.; Cui, M.; Hong, S.; Choi, S. Harnessing the Therapeutic Potential of Capsaicin and Its Analogues in Pain and Other Diseases. Molecules 2016, 21, 966. [Google Scholar] [CrossRef]
- Mora-Boga, R. Use of topical capsaicin for pain management: Review and evidence-based indications. Semergen 2025, 51, 102312. [Google Scholar] [CrossRef]
- Rifai, K.; Chidiac, J.J.; Hawwa, N.; Baliki, M.; Jabbur, S.J.; Saade, N.E. Occlusion of dentinal tubules and selective block of pulp innervation prevent the nociceptive behaviour induced in rats by intradental application of irritants. Arch. Oral Biol. 2004, 49, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Sotocinal, S.G.; Sorge, R.E.; Zaloum, A.; Tuttle, A.H.; Martin, L.J.; Wieskopf, J.S.; Mapplebeck, J.C.; Wei, P.; Zhan, S.; Zhang, S.; et al. The Rat Grimace Scale: A partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol. Pain. 2011, 7, 55. [Google Scholar] [CrossRef]
- Roughan, J.V.; Flecknell, P.A. Behavioural effects of laparotomy and analgesic effects of ketoprofen and carprofen in rats. Pain 2001, 90, 65–74. [Google Scholar] [CrossRef]
- Pain and Distress in Daboratory Rodents and Lagomorphs. Report of the Federation of European Laboratory Animal Science Associations (FELASA) Working Group on Pain and Distress accepted by the FELASA Board of Management November 1992. Lab. Anim. 1994, 28, 97–112. [Google Scholar] [CrossRef]
- Guillen, J. FELASA guidelines and recommendations. J. Am. Assoc. Lab. Anim. Sci. 2012, 51, 311–321. [Google Scholar]
- Foley, P.L.; Kendall, L.V.; Turner, P.V. Clinical Management of Pain in Rodents. Comp. Med. 2019, 69, 468–489. [Google Scholar] [CrossRef]
- Lumb, A.B. Just a little oxygen to breathe as you go off to sleep…is it always a good idea? Br. J. Anaesth. 2007, 99, 769–771. [Google Scholar] [CrossRef]
- Bodrumlu, E. Biocompatibility of retrograde root filling materials: A review. Aust. Endod. J. 2008, 34, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, Y.N.; Wang, Y.H.; Chen, Y.; Hou, J.L.; Wang, D.Y.; Shi, L.; Shen, J. Animal models of oral infectious diseases. Front. Oral Health 2025, 6, 1571492. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.; van den Broek, F.A.; Martorell, J.C.; Hackbarth, H.; Ruksenas, O.; Zeller, W. Principles and practice in ethical review of animal experiments across Europe: Summary of the report of a FELASA working group on ethical evaluation of animal experiments. Lab. Anim. 2007, 41, 143–160. [Google Scholar] [CrossRef] [PubMed]
- Gomez de Segura, I.A.; Seeldrayers, S.; Flecknell, P. Current practices of pain assessment and analgesic use in laboratory mice: A 2022 FELASA Working Group survey. Lab. Anim. 2025, 59, 396–412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, B.; Pawluski, J.; Steinbusch, H.W.M.; Kirthana Kunikullaya, U.; Song, C. The effect of chronic stress on behaviors, inflammation and lymphocyte subtypes in male and female rats. Behav. Brain Res. 2023, 439, 114220. [Google Scholar] [CrossRef]
- Ma, W.; Zheng, W.H.; Kar, S.; Quirion, R. Morphine treatment induced calcitonin gene-related peptide and substance P increases in cultured dorsal root ganglion neurons. Neuroscience 2000, 99, 529–539. [Google Scholar] [CrossRef]
- Powell, K.J.; Ma, W.; Sutak, M.; Doods, H.; Quirion, R.; Jhamandas, K. Blockade and reversal of spinal morphine tolerance by peptide and non-peptide calcitonin gene-related peptide receptor antagonists. Br. J. Pharmacol. 2000, 131, 875–884. [Google Scholar] [CrossRef]
- Ferreira, S.H. Peripheral analgesia: Mechanism of the analgesic action of aspirin-like drugs and opiate-antagonists. Br. J. Clin. Pharmacol. 1980, 10 (Suppl. 2), 237S–245S. [Google Scholar] [CrossRef]
- Koponen, M.E.; Forget, P. Pharmacological Interventions for Opioid-Induced Hyperalgesia: A Scoping Review of Preclinical Trials. J. Clin. Med. 2022, 11, 7060. [Google Scholar] [CrossRef]
- Rabben, T. Effects of the NMDA receptor antagonist ketamine in electrically induced A delta-fiber pain. Methods Find. Exp. Clin. Pharmacol. 2000, 22, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Hillhouse, T.M.; Negus, S.S. Effects of the noncompetitive N-methyl-d-aspartate receptor antagonists ketamine and MK-801 on pain-stimulated and pain-depressed behaviour in rats. Eur. J. Pain 2016, 20, 1229–1240. [Google Scholar] [CrossRef]
- Brookshire, H.L.; English, R.V.; Nadelstein, B.; Weigt, A.K.; Gift, B.W.; Gilger, B.C. Efficacy of COX-2 inhibitors in controlling inflammation and capsular opacification after phacoemulsification cataract removal. Vet. Ophthalmol. 2015, 18, 175–185. [Google Scholar] [CrossRef]
- Stokes, E.L.; Flecknell, P.A.; Richardson, C.A. Reported analgesic and anaesthetic administration to rodents undergoing experimental surgical procedures. Lab. Anim. 2009, 43, 149–154. [Google Scholar] [CrossRef] [PubMed]
- King, H.; Reiber, M.; Philippi, V.; Stirling, H.; Aulehner, K.; Bankstahl, M.; Bleich, A.; Buchecker, V.; Glasenapp, A.; Jirkof, P.; et al. Anesthesia and analgesia for experimental craniotomy in mice and rats: A systematic scoping review comparing the years 2009 and 2019. Front. Neurosci. 2023, 17, 1143109. [Google Scholar] [CrossRef]
- Pan, Y.; Cohen, S. Reporting practices of anesthetic and analgesic use in rodent orthopedic research. Sci. Rep. 2024, 14, 26225. [Google Scholar] [CrossRef]
- Pogatzki-Zahn, E.; Segelcke, D.; Zahn, P. Mechanisms of acute and chronic pain after surgery: Update from findings in experimental animal models. Curr. Opin. Anaesthesiol. 2018, 31, 575–585. [Google Scholar] [CrossRef]
- Munk, A.; Philippi, V.; Buchecker, V.; Bankstahl, M.; Glasenapp, A.; Blutke, A.; Michelakaki, E.; Talbot, S.R.; Huwyler, J.; Jirkof, P.; et al. Refining pain management in mice by comparing multimodal analgesia and NSAID monotherapy for neurosurgical procedures. Sci. Rep. 2024, 14, 18691. [Google Scholar] [CrossRef]
- Smith, D.; Anderson, D.; Degryse, A.D.; Bol, C.; Criado, A.; Ferrara, A.; Franco, N.H.; Gyertyan, I.; Orellana, J.M.; Ostergaard, G.; et al. Classification and reporting of severity experienced by animals used in scientific procedures: FELASA/ECLAM/ESLAV Working Group report. Lab. Anim. 2018, 52 (Suppl. 1), 5–57. [Google Scholar] [CrossRef] [PubMed]
- Otto, K.; Thaden, A.K. Anaesthesia, Analgesia and Euthanasia. Lab. Mouse 2012, 739–759. [Google Scholar] [CrossRef]
- Korczeniewska, O.A.; Khan, J.; Tao, Y.; Eliav, E.; Benoliel, R. Effects of Sex and Stress on Trigeminal Neuropathic Pain-Like Behavior in Rats. J. Oral Facial Pain Headache 2017, 31, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.M.; Hallikas, O.; Das Roy, R.; Vaananen, V.; Stenberg, O.E.; Hakkinen, T.J.; Francois, J.C.; Asher, R.J.; Klein, O.D.; Holzenberger, M.; et al. The developmental basis for scaling of mammalian tooth size. Proc. Natl. Acad. Sci. USA 2023, 120, e2300374120. [Google Scholar] [CrossRef]
- Kermeoglu, F.; Sayiner, S.; Sehirli, A.O.; Savtekin, G.; Aksoy, U. Does alpha-lipoic acid therapeutically effective against experimentally induced-acute pulpitis in rats? Aust. Endod. J. 2023, 49, 87–91. [Google Scholar] [CrossRef]
- Kobayashi, K.; Uchida, T.; Kuroda, Y.; Yamashita, A.; Ohba, E.; Ochiai, T.; Sadahiro, M. Aortobifemoral Bypass Grafting with Reversed L-Shaped Technique for Endograft Infection. Ann. Thorac. Cardiovasc. Surg. 2020, 26, 369–372. [Google Scholar] [CrossRef]
- Tomasello, G.; Damiani, F.; Cassata, G.; Palumbo, V.D.; Sinagra, E.; Damiani, P.; Bruno, A.; Cicero, L.; Cupido, F.; Carini, F.; et al. Simple and fast orotracheal intubation procedure in rats. Acta Biomed. 2016, 87, 13–15. [Google Scholar] [PubMed]
- Jahshan, F.; Ertracht, O.; Abu Ammar, A.; Ronen, O.; Srouji, S.; Apel-Sarid, L.; Eisenbach, N.; Atar, S.; Sela, E.; Gruber, M. A novel rat model for assessment of laryngotracheal injury following transoral intubation. Int. J. Pediatr. Otorhinolaryngol. 2018, 113, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Marschner, L.; Wogensen, E.; Mogensen, J.; Abelson, K. Implementation of a Functional Observation Battery for the Assessment of Postoperative Well-being in Rats Subjected to Fimbria-Fornix Transection. In Vivo 2016, 30, 77–82. [Google Scholar] [PubMed]
- Martin, L.J.; Hathaway, G.; Isbester, K.; Mirali, S.; Acland, E.L.; Niederstrasser, N.; Slepian, P.M.; Trost, Z.; Bartz, J.A.; Sapolsky, R.M.; et al. Reducing social stress elicits emotional contagion of pain in mouse and human strangers. Curr. Biol. 2015, 25, 326–332. [Google Scholar] [CrossRef]
- Okamoto, M.; Takahashi, Y.; Komichi, S.; Ali, M.; Yoneda, N.; Ishimoto, T.; Nakano, T.; Hayashi, M. Novel evaluation method of dentin repair by direct pulp capping using high-resolution micro-computed tomography. Clin. Oral Investig. 2018, 22, 2879–2887. [Google Scholar] [CrossRef]
- Okamoto, M.; Takahashi, Y.; Komichi, S.; Ali, M.; Watanabe, M.; Hayashi, M. Effect of tissue inhibitor of metalloprotease 1 on human pulp cells in vitro and rat pulp tissue in vivo. Int. Endod. J. 2019, 52, 1051–1062. [Google Scholar] [CrossRef]
- Maeda, H.; Hashiguchi, I.; Nakamuta, H.; Toriya, Y.; Wada, N.; Akamine, A. Histological study of periapical tissue healing in the rat molar after retrofilling with various materials. J. Endod. 1999, 25, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Glickman, G.N. AAE Consensus Conference on Diagnostic Terminology: Background and perspectives. J. Endod. 2009, 35, 1619–1620. [Google Scholar] [CrossRef] [PubMed]
- Eba, H.; Murasawa, Y.; Iohara, K.; Isogai, Z.; Nakamura, H.; Nakamura, H.; Nakashima, M. The anti-inflammatory effects of matrix metalloproteinase-3 on irreversible pulpitis of mature erupted teeth. PLoS ONE 2012, 7, e52523. [Google Scholar] [CrossRef]
- Lin, J.J.; Du, Y.; Cai, W.K.; Kuang, R.; Chang, T.; Zhang, Z.; Yang, Y.X.; Sun, C.; Li, Z.Y.; Kuang, F. Toll-like receptor 4 signaling in neurons of trigeminal ganglion contributes to nociception induced by acute pulpitis in rats. Sci. Rep. 2015, 5, 12549. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, J.; Qi, F.; Kang, Y.; Zhang, T.; Wang, L. Acute pulpitis promotes purinergic signaling to induce pain in rats via P38MAPK/NF-kappaB signaling pathway. Mol. Pain 2024, 20, 17448069241234451. [Google Scholar] [CrossRef] [PubMed]
- Mogil, J.S. The translatability of pain across species. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20190286. [Google Scholar] [CrossRef] [PubMed]
- Berman, L.H.; Hargreaves, K.M. Cohen Pathways of the Pulp, 12th ed.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Okiji, T.; Kosaka, T.; Kamal, A.M.; Kawashima, N.; Suda, H. Age-related changes in the immunoreactivity of the monocyte/macrophage system in rat molar pulp. Arch. Oral Biol. 1996, 41, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Ye, J.R.; Asiri, N.M.; Chae, Y.K.; Choi, S.C.; Nam, O.H. Biocompatibility and Bioactivity of a Dual-Cured Resin-Based Calcium Silicate Cement: In Vitro and in vivo Evaluation. J. Endod. 2024, 50, 235–242. [Google Scholar] [CrossRef]
- Sukarawan, W.; Rattanawarawipa, P.; Yaemkleebbua, K.; Nowwarote, N.; Pavasant, P.; Limjeerajarus, C.N.; Osathanon, T. Wnt3a promotes odonto/osteogenic differentiation in vitro and tertiary dentin formation in a rat model. Int. Endod. J. 2023, 56, 514–529. [Google Scholar] [CrossRef]
- Jia, P.; Li, J.; Wu, J.; Li, X.; Mao, S.; Wang, S.; Dong, Y. Alkaline Treatment Enhances the Anti-Inflammatory and Reparative Potential of Dentin Matrix Proteins in Inflamed Pulp. J. Endod. 2025, in press. [Google Scholar] [CrossRef]



| 3 h | 5 Days | |||||
|---|---|---|---|---|---|---|
| Parameter | NC (n = 2) | MIPZ (n = 10) | MIPA (n = 10) | NC (n = 2) | MIPZ (n = 10) | MIPA (n = 10) |
| % Coronal area affected by inflammatory infiltrate | 1 = 100% | 2 = 40% | 1 = 20% | 1 = 100% | 2 = 40% | 2 = 40% |
| 3 = 60% | 2 = 80% | 3 = 60% | 3 = 60% | |||
| % Necrosis | 1 = 100% | 1 = 20% | 1 = 80% | 1 = 100% | 2 = 60% | 1 = 20% |
| 2 = 40% | 2 = 40% | |||||
| 3 = 20% | 3 = 20% | 3 = 40% | 3 = 40% | |||
| 4 = 20% | ||||||
| Treatments | |||
|---|---|---|---|
| Parameter | MIPA | MIPZ | NC |
| n | (n = 10) | (n = 10) | (n = 2) |
| Weight [g] | 382.5 (33.56) | 394.1 (32.8) | 316 (7.36) |
| Food consumption [g] | 9.9 (5.37) | 10.4 (5.7) | 9.97 (5.75) |
| Water consumption [39] | 9.6 (7.04) | 9.6 (6.7) | 8.5 (6.6) |
| Treatment | Parameters | Wi (g) | W1 (g) | W2 (g) | FCi-1 | FC 1-2 | WCi-1 | WC 1-2 |
|---|---|---|---|---|---|---|---|---|
| MIPZ | μ | 393.9 | 394.6 | 393.9 | 15.6 | 5.2 | 15.9 | 3.2 |
| σ | 34.1 | 33.9 | 34.0 | 2.1 | 1.8 | 1.8 | 0.6 | |
| MIPA | μ | 382 | 383.2 | 382.3 | 14.8 | 5.0 | 16.3 | 2.9 |
| σ | 35.6 | 34.6 | 34.1 | 2.2 | 1.7 | 2.2 | 0.4 | |
| NC | μ | 312.6 | 315.5 | 316.0 | 14.8 | 5.2 | 14.2 | 2.8 |
| σ | 11.5 | 9.9 | 9.9 | 2.6 | 1.0 | 1.2 | 0.4 |
| Parameter | Treatments | ||
|---|---|---|---|
| MIPA | MIPZ | NC | |
| Weight [g] | (n = 10) | (n = 10) | (n = 2) |
| μ (σ) | 373 (24.7) | 376.9 (23.4) | 363.2 (30.7) |
| Mean confidence interval | [33] | [370.2–383.5] | [34] |
| Coefficient of variation | 6.60% | 6.20% | 8.50% |
| Food consumption [g] | (n = 10) | (n = 10) | (n = 2) |
| μ (σ) | 18.6 (3.23) | 17.3 (2.95) | 19.4 (2.78) |
| Mean confidence interval | [17.6–19.6] | [16.3–18.3] | [17.1–21.8] |
| Coefficient of variation | 17.40% | 17% | 14.30% |
| Water consumption [39] | (n = 10) | (n = 10) | (n = 2) |
| μ (σ) | 21.4 (7.3) | 16.9 (3.8) | 19 (4.7) |
| Mean confidence interval | [19–23.7] | [15.7–18.2] | [15.1–23] |
| Coefficient of variation | 34.1% | 22.5% | 24.7% |
| Descriptive Statistics | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIPZ | Wi | W1 | W2 | W3 | W6 | FCi-1 | FC1-2 | FC2-3 | FC3-6 | WCi-1 | WC1-2 | WC2-3 | WC3-6 |
| μ | 376.8 | 377.3 | 375.4 | 375.5 | 379.5 | 16.6 | 19.9 | 15.7 | 17.0 | 17.9 | 13.0 | 18.5 | 18.3 |
| σ | 24.0 | 24.1 | 23.2 | 25.3 | 25.3 | 1.1 | 4.4 | 1.4 | 2.0 | 3.6 | 2.3 | 2.0 | 4.2 |
| MIPA | Wi | W1 | W2 | W3 | W6 | FCi-1 | FC1-2 | FC2-3 | FC3-6 | WCi-1 | WC1-2 | WC2-3 | WC3-6 |
| μ | 371.4 | 374.2 | 371.8 | 371.9 | 375.8 | 17.3 | 21.6 | 16.7 | 18.8 | 23.3 | 20.3 | 21.8 | 20.1 |
| σ | 25.9 | 25.3 | 25.6 | 24.9 | 27.0 | 1.6 | 3.8 | 1.6 | 3.1 | 8.2 | 9.0 | 7.9 | 3.6 |
| NC | Wi | W1 | W2 | W3 | W6 | FCi-1 | FC1-2 | FC2-3 | FC3-6 | WCi-1 | WC1-2 | WC2-3 | WC3-6 |
| μ | 361.8 | 362.6 | 363.2 | 363.7 | 364.8 | 18.3 | 22.9 | 19.2 | 17.6 | 14.2 | 22.5 | 20.2 | 19.3 |
| σ | 41.6 | 40.9 | 41.3 | 40.9 | 41.1 | 1.3 | 1.6 | 4.0 | 0.7 | 5.9 | 3.5 | 4.5 | 3.8 |
| T3a Weight | |||
|---|---|---|---|
| Total percentage of gain or loss in each period () | MIPZ % (g) | MIPA % (g) | NC % (g) |
| Overall growth () | 4.1 (15.4) | 8.7 (31.5) | 0.5 (1.7) |
| Overall growth () | 0.85 (2.8) | 0 (0) | 0.32 (1.2) |
| Overall growth () | 4.11 (16) | 3.14 (11.4) | 0.29 (1.0) |
| Overall growth () | 10.6 (39.7) | 10.1 (38.7) | 0.57 (2.1) |
| Overall decrease () | 2.8 (10.6) | 1.1 (3.9) | 0 (0) |
| Overall decrease () | 5.6 (21.3) | 6.4 (23.9) | 0 (0) |
| Overall decrease ) | 4.2 (15.5) | 2.6 (9.8) | 0 (0) |
| Overall decrease () | 0 (0) | 0 (0) | 0 (0) |
| T3b Food consumption | MIPZ % (g) | MIPA % (g) | NC % (g) |
| Overall growth () | 212.7 (36.2) | 262.5 (44) | 50.4 (9.2) |
| Overall growth () | 16.7 (2.5) | 0 (0) | 0 (0) |
| Overall growth () | 107.9 (16.4) | 141.6 (24) | 11.04 (1.8) |
| Overall decrease () | 14.3 (2.5) | 9.3 (1.8) | 0 (0) |
| Overall decrease () | 189.4 (44.4) | 202.9 (49) | 33.2 (7.4) |
| Overall decrease ) | 22.2 (3.4) | 15.4 (2.2) | 22.3 (4.9) |
| T3c Water consumption | MIPZ % (mL) | MIPA % (mL) | NC % (mL) |
| Overall growth () | 0 (0) | 298 (49.8) | 159.3 (16.7) |
| Overall growth () | 485.8 (55.3) | 436 (64.2) | 16.5 (3.3) |
| Overall growth () | 163.6 (26) | 140.2 (22.1) | 29.4 (5) |
| Overall decrease () | 264.7 (49) | 270 (86) | 0 (0) |
| Overall decrease () | 0 (0) | 149.5 (49.8) | 32 (8) |
| Overall decrease ) | 138.5 (27.7) | 125.2 (38.4) | 29.5 (6.7) |
| ∆ Overall GPW0 | ∆ Overall PPW0 | Index GPW0 | Index PPW0 | |
|---|---|---|---|---|
| MIPZ | 31 | 4.5 | 6.2 | 0.9 |
| MIPA | 49.2 | 5.2 | 9.84 | 1.04 |
| NC | 6 | 0 | 1.2 | 0 |
| Total | 86.2 | 9.7 | 17.24 | 1.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bedoya, M.A.; Moreno, G.C.; Durán, C.; Camacho, A.; Pirela, A.E.; Rojas Lozano, S.; Mejía, M.; Herrera, E.; Camacho, L.-S.R.; Jaramillo, L.; et al. Mechanically Induced Pulpitis: A Rat Model That Preserves Animal Well-Being. Biomedicines 2025, 13, 1925. https://doi.org/10.3390/biomedicines13081925
Bedoya MA, Moreno GC, Durán C, Camacho A, Pirela AE, Rojas Lozano S, Mejía M, Herrera E, Camacho L-SR, Jaramillo L, et al. Mechanically Induced Pulpitis: A Rat Model That Preserves Animal Well-Being. Biomedicines. 2025; 13(8):1925. https://doi.org/10.3390/biomedicines13081925
Chicago/Turabian StyleBedoya, María Alexandra, Gloria Cristina Moreno, Camilo Durán, Adriana Camacho, Angel Eduardo Pirela, Stefany Rojas Lozano, Maddy Mejía, Eddy Herrera, Luz-Stella Rodríguez Camacho, Lorenza Jaramillo, and et al. 2025. "Mechanically Induced Pulpitis: A Rat Model That Preserves Animal Well-Being" Biomedicines 13, no. 8: 1925. https://doi.org/10.3390/biomedicines13081925
APA StyleBedoya, M. A., Moreno, G. C., Durán, C., Camacho, A., Pirela, A. E., Rojas Lozano, S., Mejía, M., Herrera, E., Camacho, L.-S. R., Jaramillo, L., & Roa, N. S. (2025). Mechanically Induced Pulpitis: A Rat Model That Preserves Animal Well-Being. Biomedicines, 13(8), 1925. https://doi.org/10.3390/biomedicines13081925






