The Cannabinoid Pharmacology of Bone Healing: Developments in Fusion Medicine
Abstract
1. Introduction
2. Epidemiology
3. Current Clinical Evidence in Spine Surgery
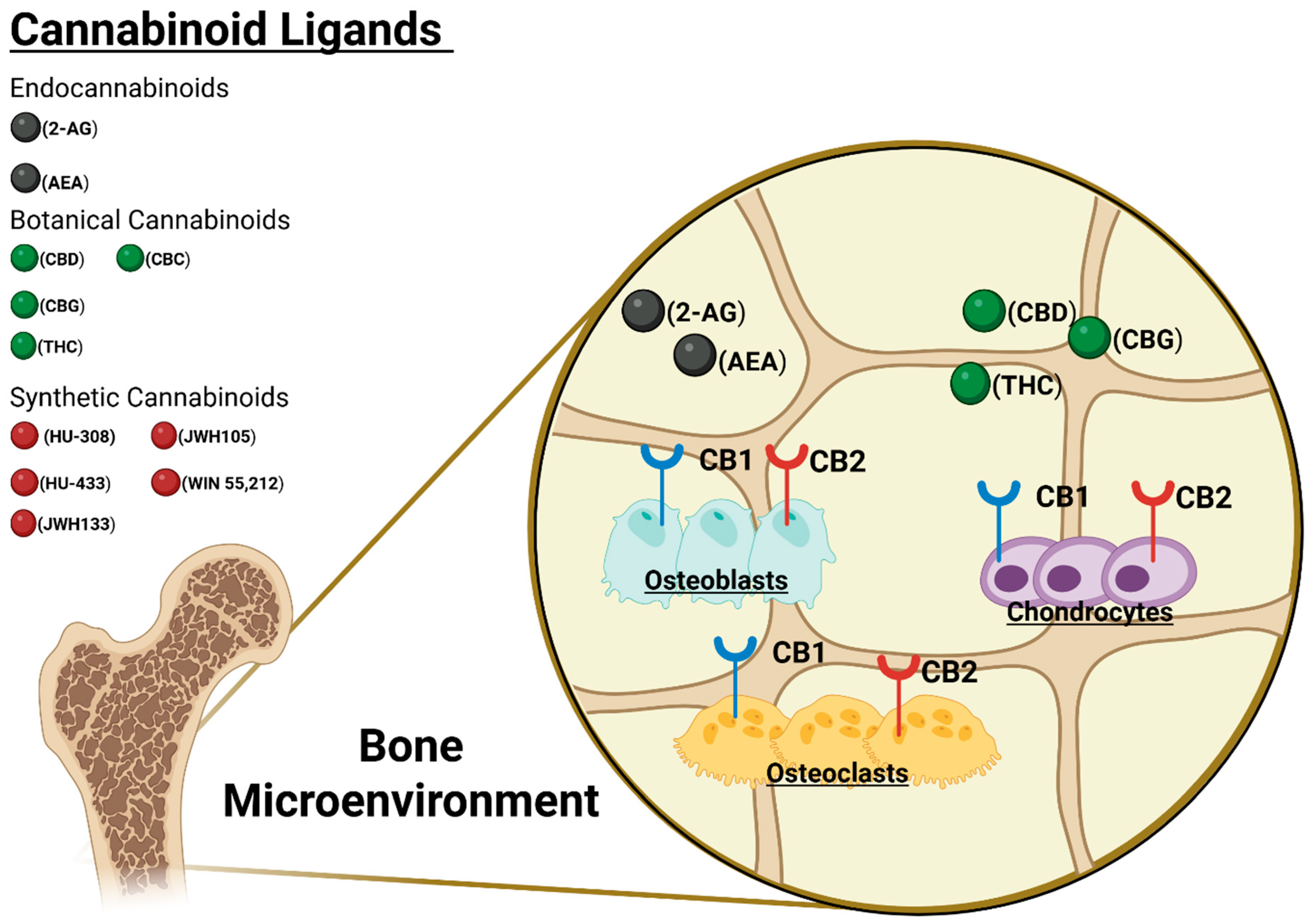
4. Endocannabinoid Physiology and Bony Healing/Fusion
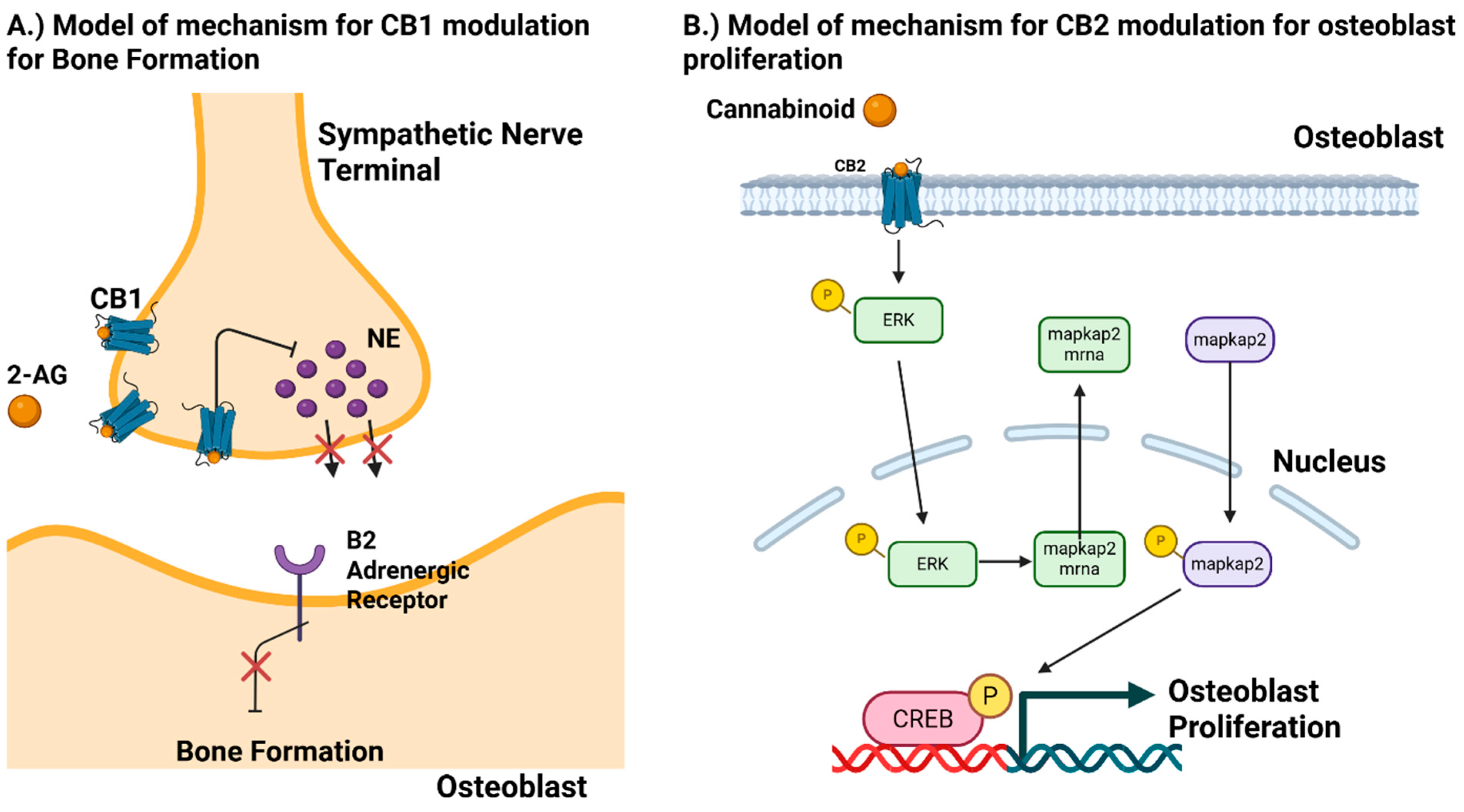
5. CB1 Receptor
| Genotype | Phenotype/Effect | Murine Strain | Sex | Age | Citations |
|---|---|---|---|---|---|
| CB1 Related Knock-Outs | |||||
| CB1−/− | Elevated trabecular & cortical bone mass (young adults) | C57BL/6J | M/F | 8–11 weeks | Idris 2005 [52] |
| CB1−/− | Age-related osteoporosis with low bone formation and fatty marrow | C57BL/6J | M/F | 12 months | Idris 2009 [46] |
| CB1−/− | Suppressed bone formation via heightened sympathetic tone | C57BL/6J | M/F | 3 months | Tam 2008 [47] |
| CB1−/− | Elongated femur & tibia from growth-plate expansion | C57BL/6J | M/F | 6 weeks | Wasserman 2015 [53] |
| CB2 Related Knock-Outs | |||||
| CB2−/− | Accelerated trabecular bone loss | C57BL/6J | M/F | 8–11 weeks or 51 weeks | Ofek 2006 [54] |
| CB2−/− | Accelerated Cortical Expansion | C57BL/6J | M/F | 8–11 weeks or 51 weeks | Ofek 2006 [54] |
| CB2−/− | Increased activity of Trabecular Osteoblasts | C57BL/6J | M/F | 8–11 weeks or 51 weeks | Ofek 2006 [54] |
| CB2−/− | Increased Activity of Osteoclasts | C57BL/6J | M/F | 8–11 weeks or 51 weeks | Ofek 2006 [54] |
| CB2−/− | Decreased Diaphyseal Osteoblast precursors | C57BL/6J | M/F | 8–11 weeks or 51 weeks | Ofek 2006 [54] |
| CB2−/− | Increased Cortical Bone | C57BL/6 | M/F | 3 months | Khalid 2015 [55] |
| CB2−/− | Reduced capacity to form bone & no protective effects from CB2 agonists | C57BL/6 | F | 8 weeks | Sophocleous 2011 [56] |
| CB2−/− | Low Bone Turnover & High Trabecular Bone Mass–In young females | CD1 | M/F | 12 Months | Sophocleous 2014 [57] |
| P62 KO | Osteoanabolic Effect | Pg62 Mice | M/F | 3 months | Keller 2022 [44] |
| P62 KO | Increased number of Osteoblasts and Osteoclasts | Pg62 Mice | M/F | 3 months | Keller 2022 [44] |
| CB2−/− | Young female mice had higher trabecular bone mass but lost more with age. | CD1 Mice | M/F | 3 months | Sophocleous 2014 [57] |
| CB2−/− | Similar bone volume decrease compared to wild-type & bone mass reduced with age. | C57BL/6 Mice | M/F | 3 months | Sophocleous 2014 [57] |
| Study Design | Strain | Sex | Age | Receptor | Ligand | Agonist or Antagonist | Phenotype/Effect | Citation |
|---|---|---|---|---|---|---|---|---|
| CB1 Related In Vivo Models | ||||||||
| Ovariectomy-induced bone loss (global KO ± rimonabant) | C57BL/6J | F | Adult | CB1 | Rimonabant | Antagonist | Bone mass preserved; osteoclast suppression | Idris 2005 [52] |
| Mild TBI remote bone-gain model | C57BL/6J | M | 12 weeks | CB1 | 2-AG | Agonist | CB1-dependent bone formation; absent in KO | Tam 2008 [47] |
| Glucocorticoid osteoporosis (methyl-pred ± rimonabant) | Sprague-Dawley | M | 3–4 mo. & 12–14 mo. | CB1 | Rimonabant | Antagonist | Antagonist prevents loss in young; worsens loss in old | Samir 2014 [58] |
| Calvarial TBI bone-gain model ± rimonabant | ICR & C57BL/6J | M | 6 & 12 weeks | CB1 | Rimonabant | Antagonist | Skull bone gain CB1-dependent; Antagonist blocks | Eger 2019 [59] |
| Diabetic bone loss, renal-tubule CB1 KO | C57BL/6J | M | Adult | CB1 | CB1 antag | Antagonist | KO/antagonist prevent loss via increased EPO and formation | Baraghithy 2021 [60] |
| CB2 Related In Vivo Models | ||||||||
| Ovariectomy-Induced Bone Loss | C57BL/J | M/F | 3 vs. 6 months | CB2 | OGP | Agonist & Allosteric Modulator | Age-Related Decline in Bone Mass | Rapheal-Mizrahi 2022 [45] |
| Glucocorticoid induced Osteonecrosis | Sprague Dawley | M | 10 weeks | CB2 | JWH133 | Agonist | Alleviation of Glucocorticoid induced Osteonecrosis | Sun 2021 [61] |
| Glucocorticoid induced Osteonecrosis | Sprague Dawley | M | 10 weeks | CB2 | JWH133 | Agonist | Osteogenic protection via GSK/3B/B-Catenin signaling pathway | Sun 2021 [61] |
| Glucocorticoid induced Osteonecrosis | Sprague Dawley Rat | M | 10 weeks | CB2 | JWH133 | Agonist | Stimulation of Angiogenesis | Sun 2021 [61] |
| Ovariectomy-Induced Bone Loss | C57BL/6J Mice | M/F | 10 weeks | CB2 | HU-308 | Agonist | Protective of Bone Loss | Smoum 2015 [62] |
| Ovariectomy-Induced Bone Loss | C57BL/6J Mice | M/F | 10 weeks | CB2 | HU- 433 | Agonist | Protective of Bone Loss (3–4 order of magnitude more potent) | Smoum 2015 [62] |
| Ovariectomy-Induced Bone Loss | Mice | F | 10 weeks | CB2 | 2-AG | Agonist | Up-regulated Notch 1 Expression and promoted differentiation of hbMSCSs | Tian 2021 [63] |
| Ovariectomy-Induced Bone Loss | C3H Mice | F | 8 or 51 weeks | CB2 | HU-308 | Agonist | Attenuates Bone loss, stimulated cortical thickness, suppresses osteoclast number and stimulates endocortical bone formation. Suppressed Osteoclast genesis. By reducing the availability of RANKL. | Ofek 2006 [54] |
| Cell Line | Cell-Type | Receptor | Ligand | Agonist or Antagonist | Effect | Citation |
|---|---|---|---|---|---|---|
| PDLSCs | MSCs (periodontal) | CB1 | R(+)-Methanandamide | Agonist | Increased osteogenic differentiation (TNF/IFN-resistant) | Yan 2019 [49] |
| hBMSCs | MSCs (bone marrow) | CB1 | CB1 over-expression | Genetic OE | Increased mineralization; rescued TNF/IFN inhibition | Yan 2022 [50] |
| Mouse BMSCs | MSCs (bone marrow) | CB1 | SR141716A (Rimonabant) | Antagonist | Induced apoptosis; decreased mineralized matrix | Gowran 2013 [51] |
| CD14⁺-derived OCs | Osteoclasts | CB1 | Anandamide (AEA) | Agonist | Increased osteoclast formation + resorption | |
| BMSCs | MSCs (bone marrow) | CB2 | JWH133 | Agonist | Osteogenic protection Via GSK/3B/B-Catenin signaling pathway | Sun 2021 [61] |
| BMSCs | MSCs (bone marrow) | CB2 | JWH133 | Agonist | Stimulation of Angiogenesis Via endothelial cell migration & VEGF | Sun 2021 [61] |
| C57BL/6 Mice | BMSCs | CB2 | CBD | Agonist | Osteogenic Differentiation Via p38 MAPK signaling pathway | Li 2022 [64] |
| Human Bone Marrow | Osteoblasts | CB2 | JWH-133 | Agonist | Improves Osteogenesis | Rossi 2015 [65] |
| Mouse Calvarial cells | Osteoblasts | CB2 | HU-308/HU-433 | Agonist | Protection of Bone Loss | Smoum 2015 [62] |
| MC3T3 E1 | Osteoblasts | CB2 | HU-308 | Agonist | Osteoblast Proliferation Via Erk1/2 phosphorylation and Map-kapk2 protein synthesis in G protein cyclin D1 mitogenic axis | Ofek 2011 [66] |
| BMSCs & RAW264.7 cell line | Osteoblasts & Osteoclasts | CB2 | Desmethoxyyangoni, Flavokawain A, Echinatin, Mangiferin, 11-keto-B-boswelic acid | Agonist | Promote Osteogeneis & Inhibit Osteoclast Differentiation | Hu 2022 [67] |
| Human Osteoblasts | Osteoblasts | CB2 | OGP | Agonist | Proliferation of Osteoblasts | Raphael-Mizrahi 2022 [45] |
| Mouse BDMS | BDMS | CB2 | OGP | Agonist | Attenuation of Osteoclast Differentiation | Raphael-Mizrahi 2022 [45] |
| Mouse BDMS | BDMS | CB2 | OGP | Agonist | Anti-Inflammatory Activity in Macrophages | Raphael-Mizrahi 2022 [45] |
| Mouse BMDS | BDMS & Osteoblasts | CB2 | HU-308 | Agonist | Direct stimulation of stromal cells/osteoblasts and inhibition of monocytes/osteoclasts by direct inhibition of RANKL expression | Ofek 2006 [54] |
| Human Bone Marrow | Osteoblasts | TRPV1 | Resiniferatoxin (RTX) | Agonist | Improves Osteogenesis | Rossi 2015 [68] |
6. CB2 Receptor
6.1. Cannabidiol (CBD)
6.2. Cannabigerol (CBG)
6.3. Tetrahydrocannabinol (THC)
7. Conclusions
Funding
Conflicts of Interest
References
- Touw, M. The Religious and Medicinal Uses of Cannabis in China, India and Tibet. J. Psychoact. Drugs 1981, 13, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L. An Archaeological and Historical Account of Cannabis in China. Econ Bot. 1974, 28, 437–448. [Google Scholar] [CrossRef]
- Zuardi, A.W. History of cannabis as a medicine: A review. Braz. J. Psychiatry 2006, 28, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Russo, E. Cannabis in India: Ancient lore and modern medicine. In Cannabinoids as Therapeutics; Mechoulam, R., Ed.; Milestones in Drug Therapy MDT; Birkhäuser-Verlag: Basel, Switzerland, 2005; pp. 1–22. [Google Scholar] [CrossRef]
- O’Shaughnessy, W.B. On the Preparations of the Indian Hemp, or Gunjah. Prov. Med. J. Retrosp. Med. Sci. 1843, 5, 363–369. [Google Scholar]
- Quintero, J.-M.; Diaz, L.-E.; Galve-Roperh, I.; Bustos, R.-H.; Leon, M.-X.; Beltran, S.; Dodd, S. The endocannabinoid system as a therapeutic target in neuropathic pain: A review. Expert. Opin. Ther. Targets 2024, 28, 739–755. [Google Scholar] [CrossRef] [PubMed]
- Copeland, J.; Swift, W. Cannabis use disorder: Epidemiology and management. Int. Rev. Psychiatry 2009, 21, 96–103. [Google Scholar] [CrossRef]
- Mechoulam, R.; Shvo, Y. Hashish—I: The structure of Cannabidiol. Tetrahedron 1963, 19, 2073–2078. [Google Scholar] [CrossRef]
- Gaoni, Y.; Mechoulam, R. Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish. J. Am. Chem. Soc. 1964, 86, 1646–1647. [Google Scholar] [CrossRef]
- Crocq, M.A. History of cannabis and the endocannabinoid system. Dialogues Clin. Neurosci. 2020, 22, 223–228. [Google Scholar] [CrossRef]
- Kogan, N.M.; Melamed, E.; Wasserman, E.; Raphael, B.; Breuer, A.; Stok, K.S.; Sondergaard, R.; Escudero, A.V.V.; Baraghithy, S.; Attar-Namdar, M.; et al. Cannabidiol, a Major Non-Psychotropic Cannabis Constituent Enhances Fracture Healing and Stimulates Lysyl Hydroxylase Activity in Osteoblasts. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2015, 30, 1905–1913. [Google Scholar] [CrossRef]
- Delling, F.N.; Vittinghoff, E.; Dewland, T.A.; Pletcher, M.J.; Olgin, J.E.; Nah, G.; Aschbacher, K.; Fang, C.D.; Lee, E.S.; Fan, S.M.; et al. Does cannabis legalisation change healthcare utilisation? A population-based study using the healthcare cost and utilisation project in Colorado, USA. BMJ Open 2019, 9, e027432. [Google Scholar] [CrossRef] [PubMed]
- Belatti, D.A.; Phisitkul, P. Trends in orthopedics: An analysis of Medicare claims, 2000–2010. Orthopedics 2013, 36, e366–e372. [Google Scholar] [CrossRef]
- Rajaee, S.S.; Bae, H.W.; Kanim, L.E.A.; Delamarter, R.B. Spinal fusion in the United States: Analysis of trends from 1998 to 2008. Spine 2012, 37, 67–76. [Google Scholar] [CrossRef] [PubMed]
- World Drug Report 2024. United Nations: Office on Drugs and Crime. Available online: https://www.unodc.org/unodc/en/data-and-analysis/world-drug-report-2024.html (accessed on 29 May 2025).
- 2022 NSDUH Annual National Report | CBHSQ Data. Available online: https://www.samhsa.gov/data/report/2022-nsduh-annual-national-report (accessed on 29 May 2025).
- Bhaskar, A.; Bell, A.; Boivin, M.; Briques, W.; Brown, M.; Clarke, H.; Cyr, C.; Eisenberg, E.; De Oliveira Silva, R.F.; Frohlich, E.; et al. Consensus recommendations on dosing and administration of medical cannabis to treat chronic pain: Results of a modified Delphi process. J. Cannabis Res. 2021, 3, 22. [Google Scholar] [CrossRef]
- Khan, S.P.; Pickens, T.A.; Berlau, D.J. Perspectives on Cannabis as a Substitute for Opioid Analgesics. Pain Manag. 2019, 9, 191–203. [Google Scholar] [CrossRef]
- Kayani, B.; Howard, L.C.; Neufeld, M.E.; Garbuz, D.S.; Masri, B.A. Cannabis and Pain Control After Total Hip and Knee Arthroplasty. Orthop. Clin. 2023, 54, 407–415. [Google Scholar] [CrossRef]
- Kleeman-Forsthuber, L.T.; Dennis, D.A.; Jennings, J.M. Medicinal Cannabis in Orthopaedic Practice. JAAOS—J. Am. Acad. Orthop. Surg. 2020, 28, 268. [Google Scholar] [CrossRef]
- Price, R.L.; Charlot, K.V.; Frieler, S.; Dettori, J.R.; Oskouian, R.; Chapman, J.R. The Efficacy of Cannabis in Reducing Back Pain: A Systematic Review. Glob. Spine J. 2022, 12, 343–352. [Google Scholar] [CrossRef]
- Pinsger, M.; Schimetta, W.; Volc, D.; Hiermann, E.; Riederer, F.; Pölz, W. Nutzen einer Add-On-Therapie mit dem synthetischen Cannabinomimetikum Nabilone bei Patienten mit chronischen Schmerzzuständen—Eine randomisierte kontrollierte Studie. Wien. Klin. Wochenschr. 2006, 118, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Moon, A.S.; LeRoy, T.E.; Yacoubian, V.; Gedman, M.; Aidlen, J.P.; Rogerson, A. Cannabis Use Is Associated With Increased Use of Prescription Opioids Following Posterior Lumbar Spinal Fusion Surgery. Glob. Spine J. 2024, 14, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Brenne, J.; Burney, E.; Mauer, K.; Orina, J.; Philipp, T.; Yoo, J. Risks associated with chronic cannabis use on opioid use, length of stay, and revision rate for patients undergoing posterior lumbar interbody fusion. Spine J. 2024, 24, 851–857. [Google Scholar] [CrossRef]
- Barkay, G.; Solomito, M.J.; Kostyun, R.O.; Esmende, S.; Makanji, H. The effect of cannabis use on postoperative complications in patients undergoing spine surgery: A national database study. N. Am. Spine Soc. J. NASSJ 2023, 16, 100265. [Google Scholar] [CrossRef]
- Chaliparambil, R.K.; Mittal, M.; Gibson, W.; Ahuja, C.; Dahdaleh, N.S.; El Tecle, N. Association Between Preoperative Cannabis Use and Increased Rate of Revision Surgery Following Spinal Fusion: A Systematic Review and Meta-Analysis. Cureus 2024, 16, e61828. [Google Scholar] [CrossRef]
- Tao, X.; Matur, A.V.; Khalid, S.; Shukla, G.; Vorster, P.; Childress, K.; Garner, R.; Gibson, J.; Cass, D.; Mejia Munne, J.C.; et al. Cannabis Use is Associated With Higher Rates of Pseudarthrosis Following TLIF: A Multi-Institutional Matched-Cohort Study. Spine 2024, 49, 412. [Google Scholar] [CrossRef]
- Lambrechts, M.J.; D’Antonio, N.D.; Toci, G.R.; Karamian, B.A.; Farronato, D.; Pezzulo, J.; Breyer, G.; Canseco, J.A.; Woods, B.; Hilibrand, A.S.; et al. Marijuana Use and its Effect on Clinical Outcomes and Revision Rates in Patients Undergoing Anterior Cervical Discectomy and Fusion. Spine 2022, 47, 1558. [Google Scholar] [CrossRef]
- Kulpa, J.; Harrison, A.; Rudolph, L.; Eglit, G.M.L.; Turcotte, C.; Bonn-Miller, M.O.; Peters, E.N. Oral Cannabidiol Treatment in Two Postmenopausal Women with Osteopenia: A Case Series. Cannabis Cannabinoid Res. 2023, 8, S83–S89. [Google Scholar] [CrossRef]
- Kulpa, J.; Eglit, G.; Hill, M.L.; MacNair, L.; Yardley, H.; Ware, M.A.; Bonn-Miller, M.O.; Peters, E.N. Serum Markers of Bone Turnover Following Controlled Administration of Two Medical Cannabis Products in Healthy Adults. Cannabis Cannabinoid Res. 2024, 9, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Keating, G.M. Delta-9-Tetrahydrocannabinol/Cannabidiol Oromucosal Spray (Sativex®): A Review in Multiple Sclerosis-Related Spasticity. Drugs 2017, 77, 563–574. [Google Scholar] [CrossRef]
- Di Marzo, V.; Bifulco, M.; De Petrocellis, L. The endocannabinoid system and its therapeutic exploitation. Nat. Rev. Drug Discov. 2004, 3, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Raphael-Mizrahi, B.; Gabet, Y. The Cannabinoids Effect on Bone Formation and Bone Healing. Curr. Osteoporos. Rep. 2020, 18, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Alger, B.E. Endocannabinoid Signaling in Neural Plasticity. In Behavioral Neurobiology of the Endocannabinoid System; Kendall, D., Alexander, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 141–172. [Google Scholar] [CrossRef]
- Castillo, P.E.; Younts, T.J.; Chávez, A.E.; Hashimotodani, Y. Endocannabinoid Signaling and Synaptic Function. Neuron 2012, 76, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Haspula, D.; Clark, M.A. Cannabinoid Receptors: An Update on Cell Signaling, Pathophysiological Roles and Therapeutic Opportunities in Neurological, Cardiovascular, and Inflammatory Diseases. Int. J. Mol. Sci. 2020, 21, 7693. [Google Scholar] [CrossRef] [PubMed]
- Cuddihey, H.; MacNaughton, W.K.; Sharkey, K.A. Role of the Endocannabinoid System in the Regulation of Intestinal Homeostasis. Cell. Mol. Gastroenterol. Hepatol. 2022, 14, 947–963. [Google Scholar] [CrossRef] [PubMed]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Turcotte, C.; Blanchet, M.-R.; Laviolette, M.; Flamand, N. The CB2 receptor and its role as a regulator of inflammation. Cell. Mol. Life Sci. 2016, 73, 4449–4470. [Google Scholar] [CrossRef]
- Turcotte, C.; Chouinard, F.; Lefebvre, J.S.; Flamand, N. Regulation of inflammation by cannabinoids, the endocannabinoids 2-arachidonoyl-glycerol and arachidonoyl-ethanolamide, and their metabolites. J. Leukoc. Biol. 2015, 97, 1049–1070. [Google Scholar] [CrossRef]
- Wilson, R.I.; Nicoll, R.A. Endocannabinoid Signaling in the Brain. Science 2002, 296, 678–682. [Google Scholar] [CrossRef]
- Ehrenkranz, J.; Levine, M.A. Bones and Joints: The Effects of Cannabinoids on the Skeleton. J. Clin. Endocrinol. Metab. 2019, 104, 4683–4694. [Google Scholar] [CrossRef]
- Silver, R.J. The Endocannabinoid System of Animals. Animals 2019, 9, 686. [Google Scholar] [CrossRef]
- Keller, C.; Yorgan, T.A.; Rading, S.; Schinke, T.; Karsak, M. Impact of the Endocannabinoid System on Bone Formation and Remodeling in p62 KO Mice. Front. Pharmacol. 2022, 13, 858215. [Google Scholar] [CrossRef]
- Raphael-Mizrahi, B.; Attar-Namdar, M.; Chourasia, M.; Cascio, M.G.; Shurki, A.; Tam, J.; Neuman, M.; Rimmerman, N.; Vogel, Z.; Shteyer, A.; et al. Osteogenic growth peptide is a potent anti-inflammatory and bone preserving hormone via cannabinoid receptor type 2. eLife 2022, 11, e65834. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.I.; Sophocleous, A.; Landao-Bassonga, E.; Canals, M.; Milligan, G.; Baker, D.; van’t Hof, R.J.; Ralston, S.H. Cannabinoid Receptor Type 1 Protects against Age- Related Osteoporosis by Regulating Osteoblast and Adipocyte Differentiation in Marrow Stromal Cells. Cell Metab. 2009, 10, 139–147. [Google Scholar] [CrossRef]
- Tam, J.; Trembovler, V.; Di Marzo, V.; Petrosino, S.; Leo, G.; Alexandrovich, A.; Regev, E.; Casap, N.; Shteyer, A.; Ledent, C.; et al. The cannabinoid CB1 receptor regulates bone formation by modulating adrenergic signaling. FASEB J. 2008, 22, 285–294. [Google Scholar] [CrossRef]
- Tam, J.; Ofek, O.; Fride, E.; Ledent, C.; Gabet, Y.; Müller, R.; Zimmer, A.; Mackie, K.; Mechoulam, R.; Shohami, E.; et al. Involvement of Neuronal Cannabinoid Receptor CB1 in Regulation of Bone Mass and Bone Remodeling. Mol. Pharmacol. 2006, 70, 786–792. [Google Scholar] [CrossRef]
- Yan, W.; Cao, Y.; Yang, H.; Han, N.; Zhu, X.; Fan, Z.; Du, J.; Zhang, F. CB1 enhanced the osteo/dentinogenic differentiation ability of periodontal ligament stem cells via p38 MAPK and JNK in an inflammatory environment. Cell Prolif. 2019, 52, e12691. [Google Scholar] [CrossRef]
- Yan, W.; Li, L.; Ge, L.; Zhang, F.; Fan, Z.; Hu, L. The cannabinoid receptor I (CB1) enhanced the osteogenic differentiation of BMSCs by rescue impaired mitochondrial metabolism function under inflammatory condition. Stem Cell Res. Ther. 2022, 13, 22. [Google Scholar] [CrossRef]
- Gowran, A.; McKayed, K.; Campbell, V.A. The Cannabinoid Receptor Type 1 Is Essential for Mesenchymal Stem Cell Survival and Differentiation: Implications for Bone Health. Stem Cells Int. 2013, 2013, 796715. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.I.; van ’t Hof, R.J.; Greig, I.R.; Ridge, S.A.; Baker, D.; Ross, R.A.; Ralston, S.H. Regulation of bone mass, bone loss and osteoclast activity by cannabinoid receptors. Nat. Med. 2005, 11, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, E.; Tam, J.; Mechoulam, R.; Zimmer, A.; Maor, G.; Bab, I. CB1 cannabinoid receptors mediate endochondral skeletal growth attenuation by Δ9-tetrahydrocannabinol. Ann. N. Y. Acad. Sci. 2015, 1335, 110–119. [Google Scholar] [CrossRef]
- Ofek, O.; Karsak, M.; Leclerc, N.; Fogel, M.; Frenkel, B.; Wright, K.; Tam, J.; Attar-Namdar, M.; Kram, V.; Shohami, E.; et al. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc. Natl. Acad. Sci. USA 2006, 103, 696–701. [Google Scholar] [CrossRef]
- Khalid, A.B.; Goodyear, S.R.; Ross, R.A.; Aspden, R.M.; Jin, Z. Effects of deleting cannabinoid receptor-2 on mechanical and material properties of cortical and trabecular bone. Cogent Eng. 2015, 2. [Google Scholar] [CrossRef]
- Sophocleous, A.; Landao-Bassonga, E.; van‘t Hof, R.J.; Idris, A.I.; Ralston, S.H. The Type 2 Cannabinoid Receptor Regulates Bone Mass and Ovariectomy-Induced Bone Loss by Affecting Osteoblast Differentiation and Bone Formation. Endocrinology 2011, 152, 2141–2149. [Google Scholar] [CrossRef]
- Sophocleous, A.; Idris, A.I.; Ralston, S.H. Genetic Background Modifies the Effects of Type 2 Cannabinoid Receptor Deficiency on Bone Mass and Bone Turnover. Calcif. Tissue Int. 2014, 94, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Samir, S.M.; Malek, H.A. Effect of cannabinoid receptors 1 modulation on osteoporosis in a rat model of different ages. J Physiol Pharmacol. 2014, 5, 687–694. [Google Scholar]
- Eger, M.; Bader, M.; Bree, D.; Hadar, R.; Nemirovski, A.; Tam, J.; Levy, D.; Pick, C.G.; Gabet, Y. Bone anabolic response in the calvaria following mild traumatic brain injury is mediated by the cannabinoid-1 receptor. Sci. Rep. 2019, 9, 16196. [Google Scholar] [CrossRef] [PubMed]
- Baraghithy, S.; Soae, Y.; Assaf, D.; Hinden, L.; Udi, S.; Drori, A.; Gabet, Y.; Tam, J. Renal proximal tubule cell cannabinoid-1 receptor regulates bone remodeling and mass via a kidney-to-bone axis. Cells 2021, 10, 414. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, W.; Yang, N.; Xue, Y.; Wang, T.; Wang, H.; Zheng, K.; Wang, Y.; Zhu, F.; Yang, H.; et al. Activation of cannabinoid receptor 2 alleviates glucocorticoid-induced osteonecrosis of femoral head with osteogenesis and maintenance of blood supply. Cell Death Dis. 2021, 12, 1035. [Google Scholar] [CrossRef]
- Smoum, R.; Baraghithy, S.; Chourasia, M.; Breuer, A.; Mussai, N.; Attar-Namdar, M.; Kogan, N.M.; Raphael, B.; Bolognini, D.; Cascio, M.G.; et al. CB2 cannabinoid receptor agonist enantiomers HU-433 and HU-308: An inverse relationship between binding affinity and biological potency. Proc. Natl. Acad. Sci. USA 2015, 112, 8774–8779. [Google Scholar] [CrossRef]
- Tian, F.; Yang, H.T.; Huang, T.; Chen, F.F.; Xiong, F.J. Involvement of CB2 signalling pathway in the development of osteoporosis by regulating the proliferation and differentiation of hBMSCs. J. Cell. Mol. Med. 2021, 25, 2426–2435. [Google Scholar] [CrossRef]
- Li, L.; Feng, J.; Sun, L.; Xuan, Y.; Wen, L.; Li, Y.; Yang, S.; Zhu, B.; Tian, X.; Li, S.; et al. Cannabidiol Promotes Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells in the Inflammatory Microenvironment via the CB2-dependent p38 MAPK Signaling Pathway. Int. J. Stem Cells 2022, 15, 405–414. [Google Scholar] [CrossRef]
- Rossi, F.; Bellini, G.; Tortora, C.; Bernardo, M.E.; Luongo, L.; Conforti, A.; Starc, N.; Manzo, I.; Nobili, B.; Locatelli, F.; et al. CB2 and TRPV1 receptors oppositely modulate in vitro human osteoblast activity. Pharmacol. Res. 2015, 99, 194–201. [Google Scholar] [CrossRef]
- Ofek, O.; Attar-Namdar, M.; Kram, V.; Dvir-Ginzberg, M.; Mechoulam, R.; Zimmer, A.; Frenkel, B.; Shohami, E.; Bab, I. CB2 cannabinoid receptor targets mitogenic Gi protein–cyclin D1 axis in osteoblasts. J. Bone Miner. Res. 2011, 26, 308–316. [Google Scholar] [CrossRef]
- Hu, S.-J.; Cheng, G.; Zhou, H.; Zhang, Q.; Zhang, Q.-L.; Wang, Y.; Shen, Y.; Lian, C.-X.; Ma, X.-Q.; Zhang, Q.-Y.; et al. Identification of Novel Cannabinoid CB2 Receptor Agonists from Botanical Compounds and Preliminary Evaluation of Their Anti-Osteoporotic Effects. Molecules 2022, 27, 702. [Google Scholar] [CrossRef]
- Rossi, F.; Bellini, G.; Tortora, C.; Bernardo, M.E.; Luongo, L.; Conforti, A.; Starc, N.; Manzo, I.; Nobili, B.; Locatelli, F.; et al. CB2 and TRPV1 receptors oppositely modulate in vitro human osteoblast activity. Pharmacol. Res. 2015, 99, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Raphael, B.; Gabet, Y. The skeletal endocannabinoid system: Clinical and experimental insights. J. Basic Clin. Physiol. Pharmacol. 2016, 27, 237–245. [Google Scholar] [CrossRef]
- Li, D.; Lin, Z.; Meng, Q.; Wang, K.; Wu, J.; Yan, H. Cannabidiol administration reduces sublesional cancellous bone loss in rats with severe spinal cord injury. Eur. J. Pharmacol. 2017, 809, 13–19. [Google Scholar] [CrossRef]
- Whyte, L.; Ford, L.; Ridge, S.; Cameron, G.; Rogers, M.; Ross, R. Cannabinoids and bone: Endocannabinoids modulate human osteoclast function in vitro. Br. J. Pharmacol. 2012, 165, 2584–2597. [Google Scholar] [CrossRef] [PubMed]
- Apostu, D.; Lucaciu, O.; Mester, A.; Benea, H.; Oltean-Dan, D.; Onisor, F.; Baciut, M.; Bran, S. Cannabinoids and bone regeneration. Drug Metab. Rev. 2019, 51, 65–75. [Google Scholar] [CrossRef]
- Fogel, H.; Yeritsyan, D.; Momenzadeh, K.; Kheir, N.; Yeung, C.M.; Abbasian, M.; Lozano, E.M.; Nazarian, R.M.; Nazarian, A. The effect of cannabinoids on single-level lumbar arthrodesis outcomes in a rat model. Spine J. 2024, 24, 1759–1772. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef]
- Kamali, A.; Oryan, A.; Hosseini, S.; Ghanian, M.H.; Alizadeh, M.; Baghaban Eslaminejad, M.; Baharvand, H. Cannabidiol-loaded microspheres incorporated into osteoconductive scaffold enhance mesenchymal stem cell recruitment and regeneration of critical-sized bone defects. Mater. Sci. Eng. C 2019, 101, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Khajuria, D.K.; Karuppagounder, V.; Nowak, I.; Sepulveda, D.E.; Lewis, G.S.; Norbury, C.C.; Raup-Konsavage, W.M.; Vrana, K.E.; Kamal, F.; Elbarbary, R.A. Cannabidiol and Cannabigerol, Nonpsychotropic Cannabinoids, as Analgesics that Effectively Manage Bone Fracture Pain and Promote Healing in Mice. J. Bone Miner. Res. 2023, 38, 1560–1576. [Google Scholar] [CrossRef] [PubMed]
- Ihejirika-Lomedico, R.; Patel, K.; Buchalter, D.B.; Kirby, D.J.; Mehta, D.; Dankert, J.F.; Muiños-López, E.; Ihejirika, Y.; Leucht, P. Non-psychoactive Cannabidiol Prevents Osteoporosis in an Animal Model and Increases Cell Viability, Proliferation, and Osteogenic Gene Expression in Human Skeletal Stem and Progenitor Cells. Calcif. Tissue Int. 2023, 112, 716–726. [Google Scholar] [CrossRef]
- Nielsen, S.S.R.; Pedersen, J.A.Z.; Sharma, N.; Wasehuus, P.K.; Hansen, M.S.; Møller, A.M.J.; Borggaard, X.G.; Rauch, A.; Frost, M.; Sondergaard, T.E.; et al. Human osteoclasts in vitro are dose dependently both inhibited and stimulated by cannabidiol (CBD) and Δ9-tetrahydrocannabinol (THC). Bone 2024, 181, 117035. [Google Scholar] [CrossRef]
- Gabet, Y. Therapeutic Potential of Cannabidiol and Cannabigerol in Fracture Healing. J. Bone Miner. Res. 2023, 38, 1547–1548. [Google Scholar] [CrossRef] [PubMed]
- Navarro, G.; Varani, K.; Reyes-Resina, I.; Sánchez de Medina, V.; Rivas-Santisteban, R.; Sánchez-Carnerero Callado, C.; Vincenzi, F.; Casano, S.; Ferreiro-Vera, C.; Canela, E.I.; et al. Cannabigerol Action at Cannabinoid CB1 and CB2 Receptors and at CB1–CB2 Heteroreceptor Complexes. Front. Pharmacol. 2018, 9, 632. [Google Scholar] [CrossRef]
- Sepulveda, D.E.; Morris, D.P.; Raup-Konsavage, W.M.; Sun, D.; Vrana, K.E.; Graziane, N.M. Cannabigerol (CBG) attenuates mechanical hypersensitivity elicited by chemotherapy-induced peripheral neuropathy. Eur. J. Pain 2022, 26, 1950–1966. [Google Scholar] [CrossRef]
- Amstutz, K.; Schwark, W.S.; Zakharov, A.; Gomez, B.; Lyubimov, A.; Ellis, K.; Venator, K.P.; Wakshlag, J.J. Single dose and chronic oral administration of cannabigerol and cannabigerolic acid-rich hemp extract in fed and fasted dogs: Physiological effect and pharmacokinetic evaluation. J. Vet. Pharmacol. Ther. 2022, 45, 245–254. [Google Scholar] [CrossRef]
- Charavarty, I.; Sengupta, D.; Bhattacharyya, P.; Ghosh, J.J. Effect of treatment with cannabis extract on the water and glycogen contents of the uterus in normal and estradiol-treated prepubertal rats. Toxicol. Appl. Pharmacol. 1975, 34, 513–516. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urreola, G.; Le, M.; Harris, A.; Castillo, J.A., Jr.; Saiz, A.M.; Shahzad, H.; Martin, A.R.; Kim, K.D.; Khan, S.; Price, R. The Cannabinoid Pharmacology of Bone Healing: Developments in Fusion Medicine. Biomedicines 2025, 13, 1891. https://doi.org/10.3390/biomedicines13081891
Urreola G, Le M, Harris A, Castillo JA Jr., Saiz AM, Shahzad H, Martin AR, Kim KD, Khan S, Price R. The Cannabinoid Pharmacology of Bone Healing: Developments in Fusion Medicine. Biomedicines. 2025; 13(8):1891. https://doi.org/10.3390/biomedicines13081891
Chicago/Turabian StyleUrreola, Gabriel, Michael Le, Alan Harris, Jose A. Castillo, Jr., Augustine M. Saiz, Hania Shahzad, Allan R. Martin, Kee D. Kim, Safdar Khan, and Richard Price. 2025. "The Cannabinoid Pharmacology of Bone Healing: Developments in Fusion Medicine" Biomedicines 13, no. 8: 1891. https://doi.org/10.3390/biomedicines13081891
APA StyleUrreola, G., Le, M., Harris, A., Castillo, J. A., Jr., Saiz, A. M., Shahzad, H., Martin, A. R., Kim, K. D., Khan, S., & Price, R. (2025). The Cannabinoid Pharmacology of Bone Healing: Developments in Fusion Medicine. Biomedicines, 13(8), 1891. https://doi.org/10.3390/biomedicines13081891






