Interspecies Interactions of Single- and Mixed-Species Biofilms of Candida albicans and Aggregatibacter actinomycetemcomitans
Abstract
1. Introduction
2. Materials and Methods
2.1. Microbial Strains and Culture Conditions
2.2. Antifungal and Antibacterial Drugs
2.3. Single- and Mixed-Microbial Biofilm Standardisation
2.4. Biofilm Viability Assay
2.5. Biofilm Biomass Quantification with Crystal Violet
2.6. Antimicrobial Susceptibility Testing (AST)
2.7. Examination of Spatial Arrangements of Biofilms Using PNA-FISH Combined with Confocal Laser Scanning Microscopy
2.8. Statistical Analysis
3. Results
3.1. Colony-Forming Unit (CFU) Analysis of Single- and Mixed-Species Biofilms of C. albicans and A. actinomycetemcomitans
3.2. Biomass Quantification with Crystal Violet
3.3. Antimicrobial Susceptibility Testing
3.4. Biofilm Structure Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atiencia-Carrera, M.B.; Cabezas-Mera, F.S.; Vizuete, K.; Debut, A.; Tejera, E.; Machado, A. Evaluation of the Biofilm Life Cycle between Candida albicans and Candida tropicalis. Front. Cell. Infect. Microbiol. 2022, 12, 953168. [Google Scholar] [CrossRef]
- Di Stefano, M.; Polizzi, A.; Santonocito, S.; Romano, A.; Lombardi, T.; Isola, G. Impact of Oral Microbiome in Periodontal Health and Periodontitis: A Critical Review on Prevention and Treatment. Int. J. Mol. Sci. 2022, 23, 5142. [Google Scholar] [CrossRef]
- Huang, F.; Song, Y.; Chen, W.; Liu, Q.; Wang, Q.; Liu, W.; Wang, X.; Wang, W. Effects of Candida albicans Infection on Defense Effector Secretion by Human Oral Mucosal Epithelial Cells. Arch. Oral Biol. 2019, 103, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Akrivopoulou, C.; Green, I.M.; Donos, N.; Nair, S.P.; Ready, D. Aggregatibacter actinomycetemcomitans Serotype Prevalence and Antibiotic Resistance in a UK Population with Periodontitis. J. Glob. Antimicrob. Resist. 2017, 10, 54–58. [Google Scholar] [CrossRef]
- Bachtiar, E.W.; Bachtiar, B.M.; Kusumaningrum, A.; Sunarto, H.; Soeroso, Y.; Sulijaya, B.; Apriyanti, E.; Theodorea, C.F.; Pratomo, I.P.; Efendi, D.; et al. ACE2 Expression in Saliva of Patients with COVID-19 and Its Association with Candida Albicans and Aggregatibacter actinomycetemcomitans. F1000Research 2022, 11, 557. [Google Scholar] [CrossRef]
- Bravo, E.; Arce, M.; Ribeiro-Vidal, H.; Herrera, D.; Sanz, M. The Impact of Candida albicans in the Development, Kinetics, Structure, and Cell Viability of Biofilms on Implant Surfaces—An In Vitro Study with a Validated Multispecies Biofilm Model. Int. J. Mol. Sci. 2024, 25, 3277. [Google Scholar] [CrossRef]
- Pawlowska, T.E. Symbioses between Fungi and Bacteria: From Mechanisms to Impacts on Biodiversity. Curr. Opin. Microbiol. 2024, 80, 102496. [Google Scholar] [CrossRef] [PubMed]
- Gulati, M.; Nobile, C.J. Candida Albicans Biofilms: Development, Regulation, and Molecular Mechanisms. Microbes Infect. 2016, 18, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Tristano, J.; Danforth, D.R.; Wargo, M.J.; Mintz, K.P. Regulation of Adhesin Synthesis in Aggregatibacter actinomycetemcomitans. Mol. Oral Microbiol. 2023, 38, 237–250. [Google Scholar] [CrossRef]
- Krueger, E.; Brown, A.C. Aggregatibacter actinomycetemcomitans Leukotoxin: From Mechanism to Targeted Anti-Toxin Therapeutics. Mol. Oral Microbiol. 2020, 35, 85–105. [Google Scholar] [CrossRef]
- Herbert, B.A.; Novince, C.M.; Kirkwood, K.L. Aggregatibacter actinomycetemcomitans, a Potent Immunoregulator of the Periodontal Host Defense System and Alveolar Bone Homeostasis. Mol. Oral Microbiol. 2015, 31, 207. [Google Scholar] [CrossRef]
- Shirtliff, M.E.; Peters, B.M.; Jabra-Rizk, M.A. Cross-Kingdom Interactions: Candida albicans and Bacteria. FEMS Microbiol. Lett. 2009, 299, 1–8. [Google Scholar] [CrossRef]
- Montelongo-Jauregui, D.; Srinivasan, A.; Ramasubramanian, A.K.; Lopez-Ribot, J.L. An In Vitro Model for Candida albicans–Streptococcus gordonii Biofilms on Titanium Surfaces. J. Fungi 2018, 4, 66. [Google Scholar] [CrossRef]
- Rodrigues, C.F.; Henriques, M. Liposomal and Deoxycholate Amphotericin B Formulations: Effectiveness against Biofilm Infections of Candida spp. Pathogens 2017, 6, 62. [Google Scholar] [CrossRef] [PubMed]
- Alsina, M.; Olle, E.; Frias, J. Improved, Low-Cost Selective Culture Medium for Actinobacillus actinomycetemcomitans. J. Clin. Microbiol. 2001, 39, 509–513. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lai, P.-C.; Walters, J.D. Azithromycin Kills Invasive Aggregatibacter Actinomycetemcomitans in Gingival Epithelial Cells. Antimicrob. Agents Chemother. 2013, 57, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_15.0_Breakpoint_Tables.pdf (accessed on 10 July 2025).
- Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Clinical_breakpoints/AFST_BP_v11.0.pdf (accessed on 18 April 2025).
- Rodrigues, C.F.; Alves, D.F.; Henriques, M. Combination of Posaconazole and Amphotericin B in the Treatment of Candida glabrata Biofilms. Microorganisms 2018, 6, 123. [Google Scholar] [CrossRef]
- Archive of EUCAST AST of Bacteria. Available online: https://www.eucast.org/ast_of_bacteria/previous_versions_of_documents (accessed on 1 May 2025).
- Mendes, L.; Rocha, R.; Azevedo, A.S.; Ferreira, C.; Henriques, M.; Pinto, M.G.; Azevedo, N.F. Novel Strategy to Detect and Locate Periodontal Pathogens: The PNA-FISH Technique. Microbiol. Res. 2016, 192, 185–191. [Google Scholar] [CrossRef]
- Shi, C.; Liu, J.; Li, W.; Zhao, Y.; Meng, L.; Xiang, M. Expression of Fluconazole Resistance-Associated Genes in Biofilm from 23 Clinical Isolates of Candida albicans. Braz. J. Microbiol. 2019, 50, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Wiernik, E.; Renuy, A.; Kab, S.; Steg, P.G.; Goldberg, M.; Zins, M.; Caligiuri, G.; Bouchard, P.; Carra, M.C. Prevalence of Self-Reported Severe Periodontitis: Data from the Population-Based CONSTANCES Cohort. J. Clin. Periodontol. 2024, 51, 884–894. [Google Scholar] [CrossRef]
- Liccardo, D.; Cannavo, A.; Spagnuolo, G.; Ferrara, N.; Cittadini, A.; Rengo, C.; Rengo, G. Periodontal Disease: A Risk Factor for Diabetes and Cardiovascular Disease. Int. J. Mol. Sci. 2019, 20, 1414. [Google Scholar] [CrossRef]
- Sanz, M.; Marco del Castillo, A.; Jepsen, S.; Gonzalez-Juanatey, J.R.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and Cardiovascular Diseases: Consensus Report. J. Clin. Periodontol. 2020, 47, 268–288. [Google Scholar] [CrossRef]
- Gasner, N.S.; Schure, R.S. Periodontal Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Van Dyke, T.E.; Bartold, P.M.; Reynolds, E.C. The Nexus Between Periodontal Inflammation and Dysbiosis. Front. Immunol. 2020, 11, 511. [Google Scholar] [CrossRef]
- Bayraktar, G.; Yılmaz Göler, A.M.; Aksu, B.; Öztürk Özener, H. Efficacy of Hypochlorous Acid as an Alternative Oral Antimicrobial Agent on Human Gingival Fibroblasts, Aggregatibacter actinomycetemcomitans, and Candida albicans Biofilms in Vitro. Biofouling 2023, 39, 980–989. [Google Scholar] [CrossRef]
- Saskianti, T.; Wardhani, K.K.; Fadhila, N.; Wahluyo, S.; Dewi, A.M.; Nugraha, A.P.; Ernawati, D.S.; Kanawa, M. Polymethylmethacrylate-Hydroxyapatite Antibacterial and Antifungal Activity against Oral Bacteria: An in Vitro Study. J. Taibah Univ. Med. Sci. 2023, 19, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Bachtiar, E.W.; Bachtiar, B.M.; Jarosz, L.M.; Amir, L.R.; Sunarto, H.; Ganin, H.; Meijler, M.M.; Krom, B.P. AI-2 of Aggregatibacter Actinomycetemcomitans Inhibits Candida Albicans Biofilm Formation. Front. Cell. Infect. Microbiol. 2014, 4, 94. [Google Scholar] [CrossRef] [PubMed]
- Plančak, D.; Musić, L.; Puhar, I. Quorum Sensing of Periodontal Pathogens. Acta Stomatol Croat 2015, 49, 234–241. [Google Scholar] [CrossRef]
- Elias, S.; Banin, E. Multi-Species Biofilms: Living with Friendly Neighbors. FEMS Microbiol. Rev. 2012, 36, 990–1004. [Google Scholar] [CrossRef]
- Olsen, I. New Principles in Ecological Regulation—Features from the Oral Cavity. Microb. Ecol. Health Dis. 2006, 18, 26–31. [Google Scholar] [CrossRef][Green Version]
- Velusamy, S.K.; Sampathkumar, V.; Ramasubbu, N.; Paster, B.J.; Fine, D.H. Aggregatibacter Actinomycetemcomitans Colonization and Persistence in a Primate Model. Proc. Natl. Acad. Sci. USA 2019, 116, 22307–22313. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Li, M.; Gregory, R.L. Bacterial Interactions in Dental Biofilm. Virulence 2011, 2, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Amarasinghe, J.J.; Scannapieco, F.A.; Haase, E.M. Transcriptional and Translational Analysis of Biofilm Determinants of Aggregatibacter actinomycetemcomitans in Response to Environmental Perturbation. Infect. Immun. 2009, 77, 2896–2907. [Google Scholar] [CrossRef]
- Nobile, C.J.; Johnson, A.D. Candida Albicans Biofilms and Human Disease. Annu. Rev. Microbiol. 2015, 69, 71–92. [Google Scholar] [CrossRef]
- Rodrigues, C.F.; Henriques, M. Portrait of Matrix Gene Expression in Candida glabrata Biofilms with Stress Induced by Different Drugs. Genes 2018, 9, 205. [Google Scholar] [CrossRef]
- Rodrigues, C.F.; Vilas Boas, D.; Haynes, K.; Henriques, M. The MNN2 Gene Knockout Modulates the Antifungal Resistance of Biofilms of Candida glabrata. Biomolecules 2018, 8, 130. [Google Scholar] [CrossRef]
- Alves, A.M.C.V.; Lopes, B.O.; Leite, A.C.R.d.M.; Cruz, G.S.; Brito, É.H.S.d.; Lima, L.F.d.; Černáková, L.; Azevedo, N.F.; Rodrigues, C.F. Characterization of Oral Candida spp. Biofilms in Children and Adults Carriers from Eastern Europe and South America. Antibiotics 2023, 12, 797. [Google Scholar] [CrossRef]
- Kong, E.F.; Tsui, C.; Kucharíková, S.; Andes, D.; Van Dijck, P.; Jabra-Rizk, M.A. Commensal Protection of Staphylococcus aureus against Antimicrobials by Candida albicans Biofilm Matrix. mBio 2016, 7. [Google Scholar] [CrossRef]
- Du, Q.; Ren, B.; Zhou, X.; Zhang, L.; Xu, X. Cross-Kingdom Interaction between Candida Albicans and Oral Bacteria. Front. Microbiol. 2022, 13, 911623. [Google Scholar] [CrossRef] [PubMed]
- Weigel, W.A.; Demuth, D.R. QseBC, a Two-component Bacterial Adrenergic Receptor and Global Regulator of Virulence in Enterobacteriaceae and Pasteurellaceae. Mol. Oral Microbiol. 2016, 31, 379–397. [Google Scholar] [CrossRef]
- Weigel, W.; Demuth, D.; Torres-Escobar, A.; Juárez-Rodríguez, M. Aggregatibacter Actinomycetemcomitans QseBC Is Activated by Catecholamines and Iron and Regulates Genes Encoding Proteins Associated with Anaerobic Respiration and Metabolism. Mol. Oral Microbiol. 2015, 30, 384–398. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.J.; Shelton, B.T.; Kruppa, M.D. Characterization of Genetic Determinants That Modulate Candida Albicans Filamentation in the Presence of Bacteria. PLoS ONE 2013, 8, e71939. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bao, K.; Bostanci, N.; Thurnheer, T.; Grossmann, J.; Wolski, W.E.; Thay, B.; Belibasakis, G.N.; Oscarsson, J. Aggregatibacter actinomycetemcomitans H-NS Promotes Biofilm Formation and Alters Protein Dynamics of Other Species within a Polymicrobial Oral Biofilm. npj Biofilms Microbiomes 2018, 4, 12. [Google Scholar] [CrossRef] [PubMed]
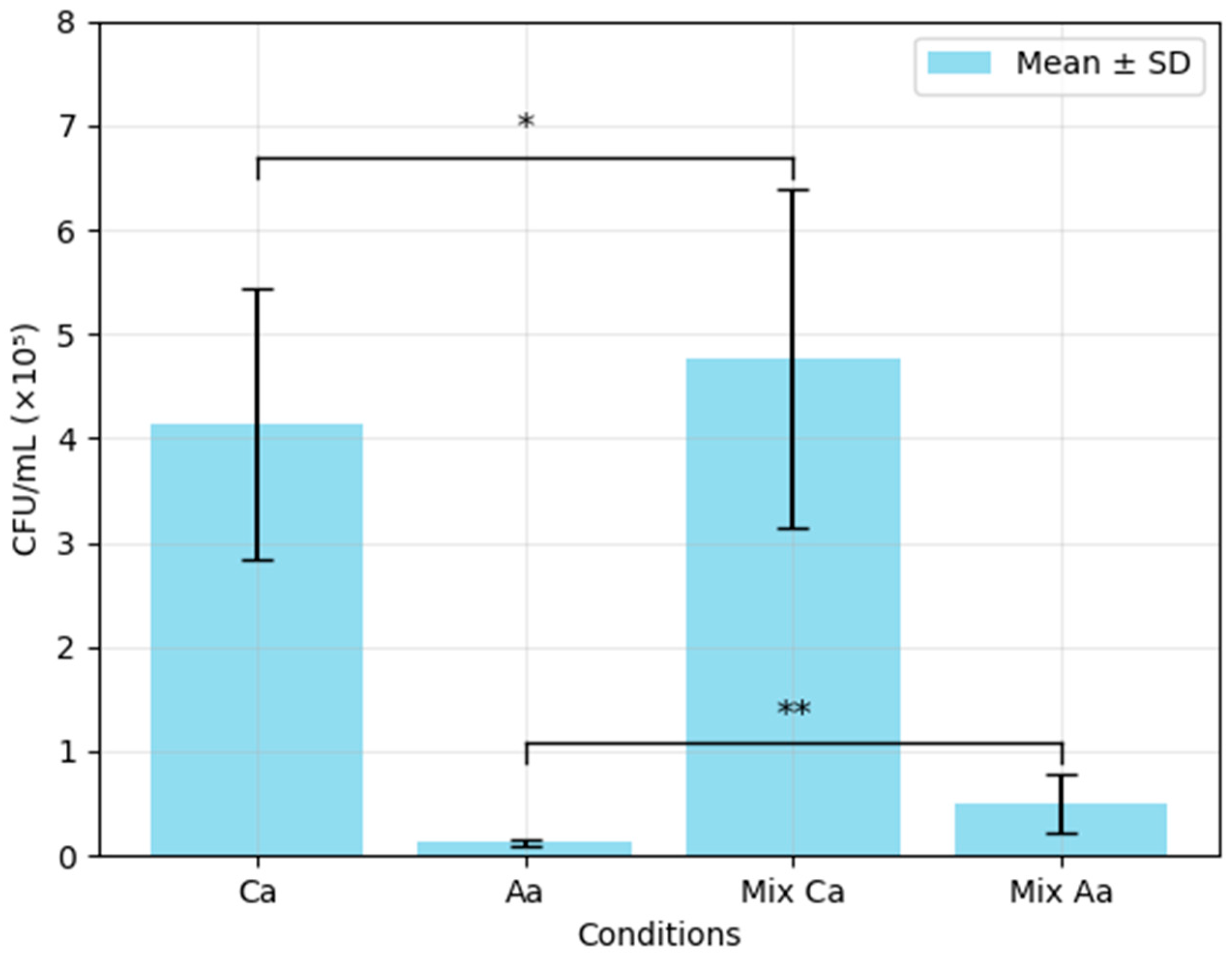
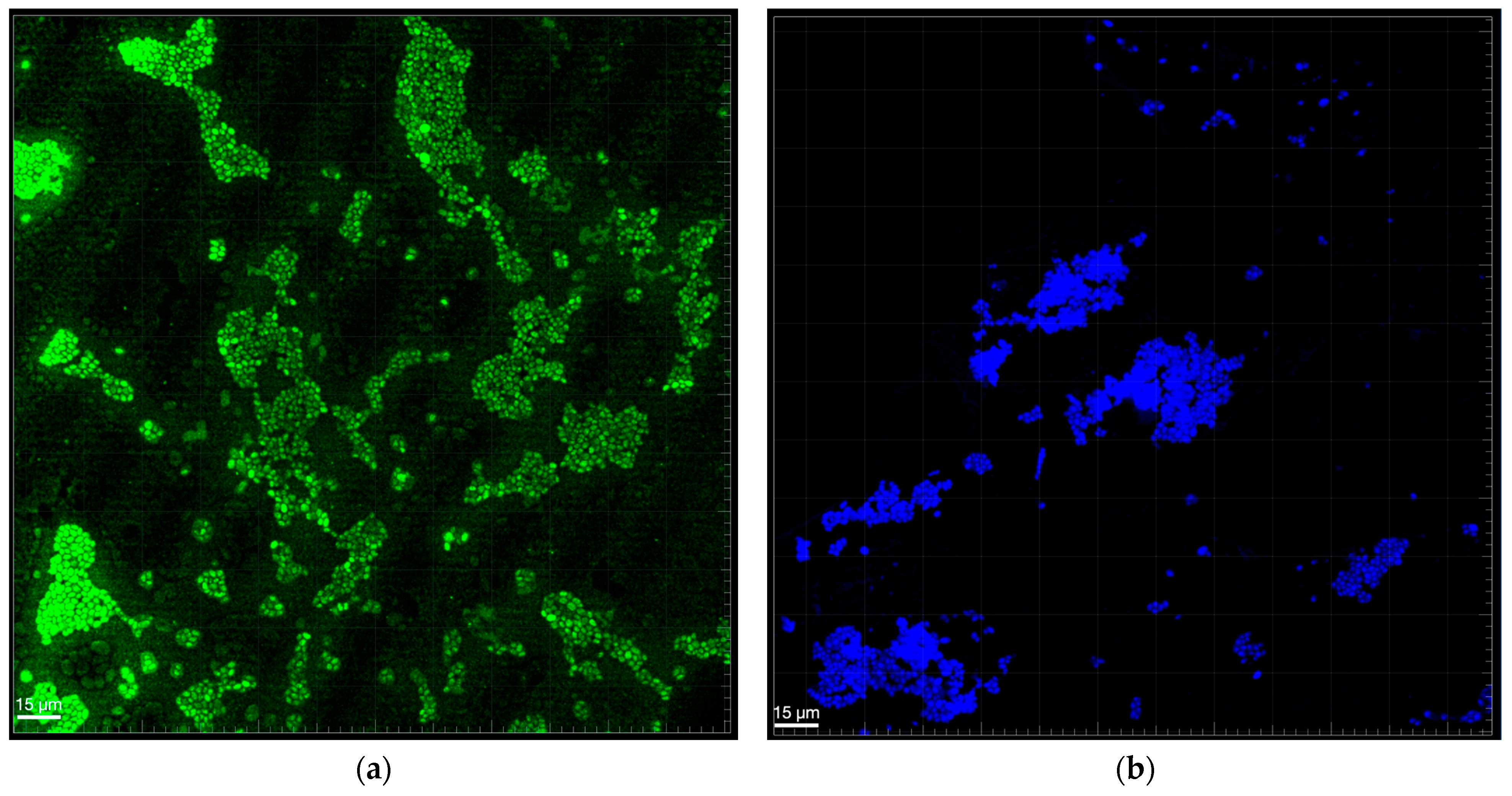

| Optical Density | % Reduction in Single-Species Biofilm Compared with Mixed-Species Biofilm | ||||
|---|---|---|---|---|---|
| Ca | Aa | Mixed | Species | ||
| 0.724 ± 0.17 | 0.397 ± 0.12 | 0.318 ± 0.06 | 56.07 ± 0.03 | p ≤ 0.001 | C. albicans |
| 19.16 ± 0.01 | p = 0.048 | A. actinomycetemcomitans | |||
| Species | Flu 2 mg/L | Flu 4 mg/L | Azm 0.125 mg/L | Azm 8 mg/L |
|---|---|---|---|---|
| C. albicans | Susceptible | Susceptible | - | - |
| A. actinomycetemcomitans | _ | _ | Resistant | Susceptible |
| Mixed biofilm | Intermediate | Intermediate | Resistant | Resistant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huc, A.; Azevedo, A.S.; Andrade, J.C.; Rodrigues, C.F. Interspecies Interactions of Single- and Mixed-Species Biofilms of Candida albicans and Aggregatibacter actinomycetemcomitans. Biomedicines 2025, 13, 1890. https://doi.org/10.3390/biomedicines13081890
Huc A, Azevedo AS, Andrade JC, Rodrigues CF. Interspecies Interactions of Single- and Mixed-Species Biofilms of Candida albicans and Aggregatibacter actinomycetemcomitans. Biomedicines. 2025; 13(8):1890. https://doi.org/10.3390/biomedicines13081890
Chicago/Turabian StyleHuc, Adèle, Andreia S. Azevedo, José Carlos Andrade, and Célia Fortuna Rodrigues. 2025. "Interspecies Interactions of Single- and Mixed-Species Biofilms of Candida albicans and Aggregatibacter actinomycetemcomitans" Biomedicines 13, no. 8: 1890. https://doi.org/10.3390/biomedicines13081890
APA StyleHuc, A., Azevedo, A. S., Andrade, J. C., & Rodrigues, C. F. (2025). Interspecies Interactions of Single- and Mixed-Species Biofilms of Candida albicans and Aggregatibacter actinomycetemcomitans. Biomedicines, 13(8), 1890. https://doi.org/10.3390/biomedicines13081890









