Causal Relationship Between Serum Uric Acid and Atherosclerotic Disease: A Mendelian Randomization and Transcriptomic Analysis
Abstract
1. Introduction
2. Materials and Methods
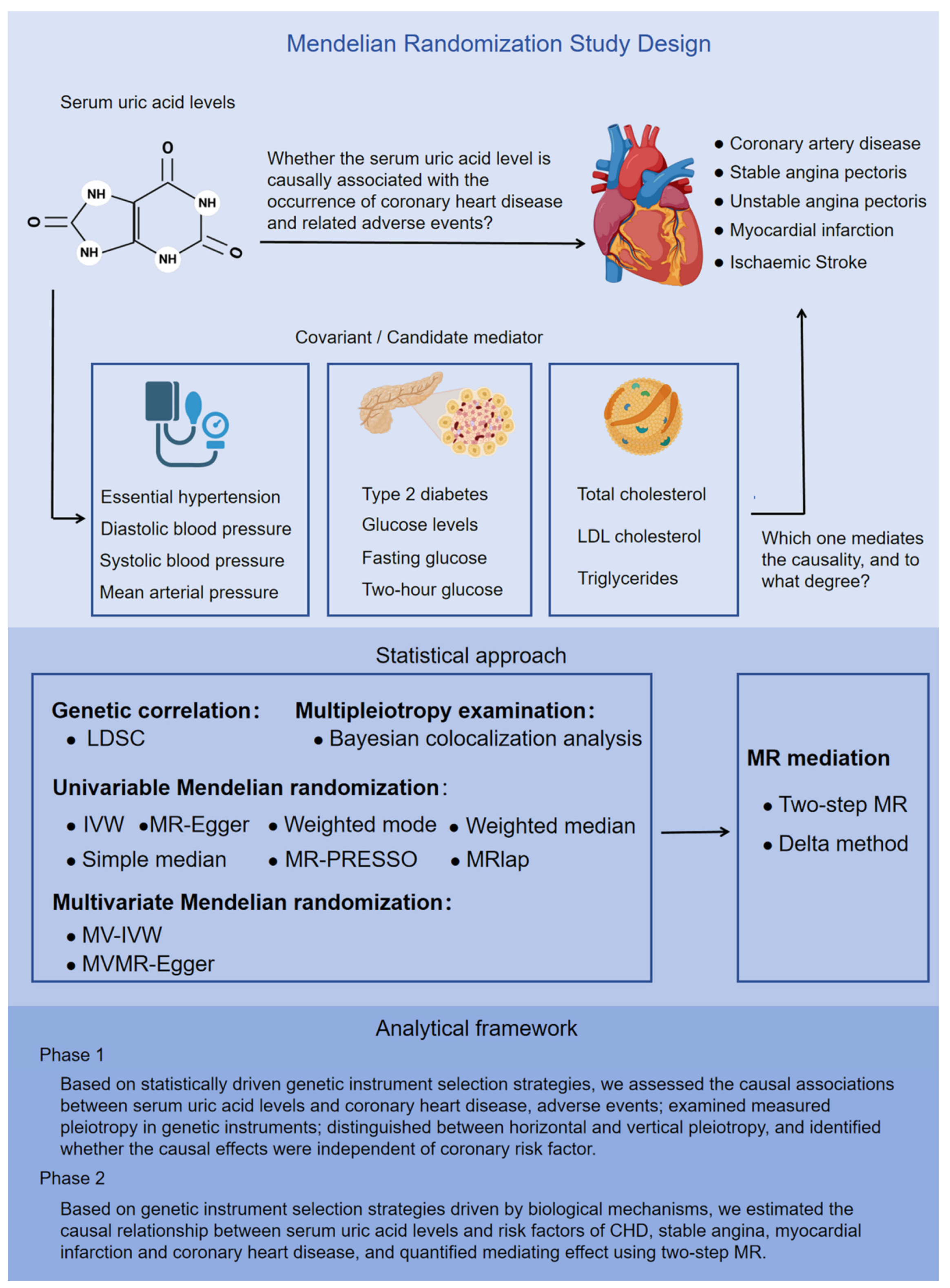
2.1. Study Design
2.2. Data Resources
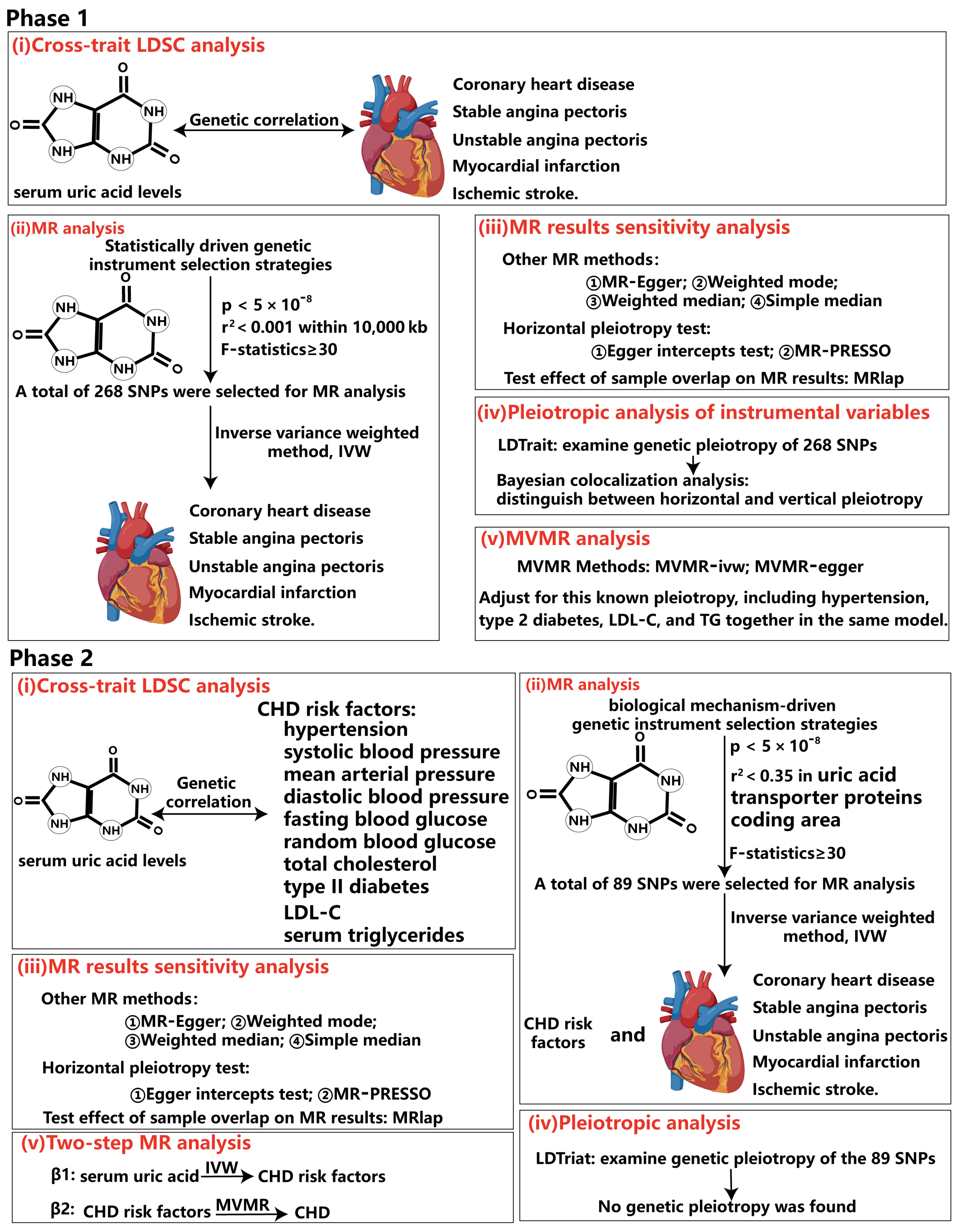
2.3. Genetic Instrument Selection
2.4. Statistical Analysis
2.4.1. Cross-Trait Linkage Disequilibrium Score Regression Analysis (LDSC)
2.4.2. UVMR and MVMR Analysis
2.4.3. Bayesian Colocalization Analysis
2.5. LDtrait
2.6. LDmatrix
2.7. Transcriptome Difference Analysis and Enrichment Analysis
2.8. Analysis of Mouse scRNA-Seq Data
2.9. High-Dimensional Weighted Gene Co-Expression Network Analysis (hdWGCNA)
2.10. Code Availability
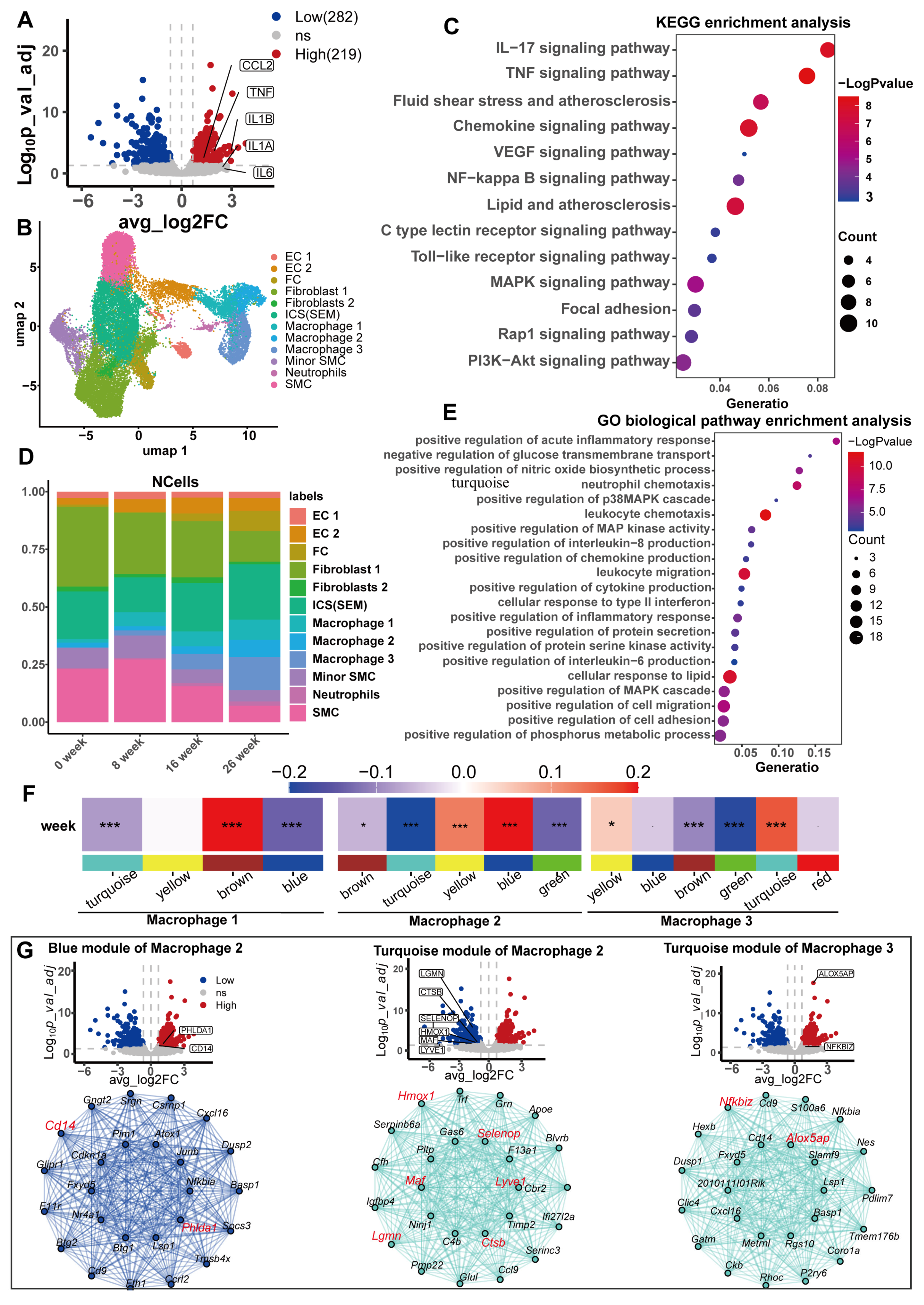
3. Results
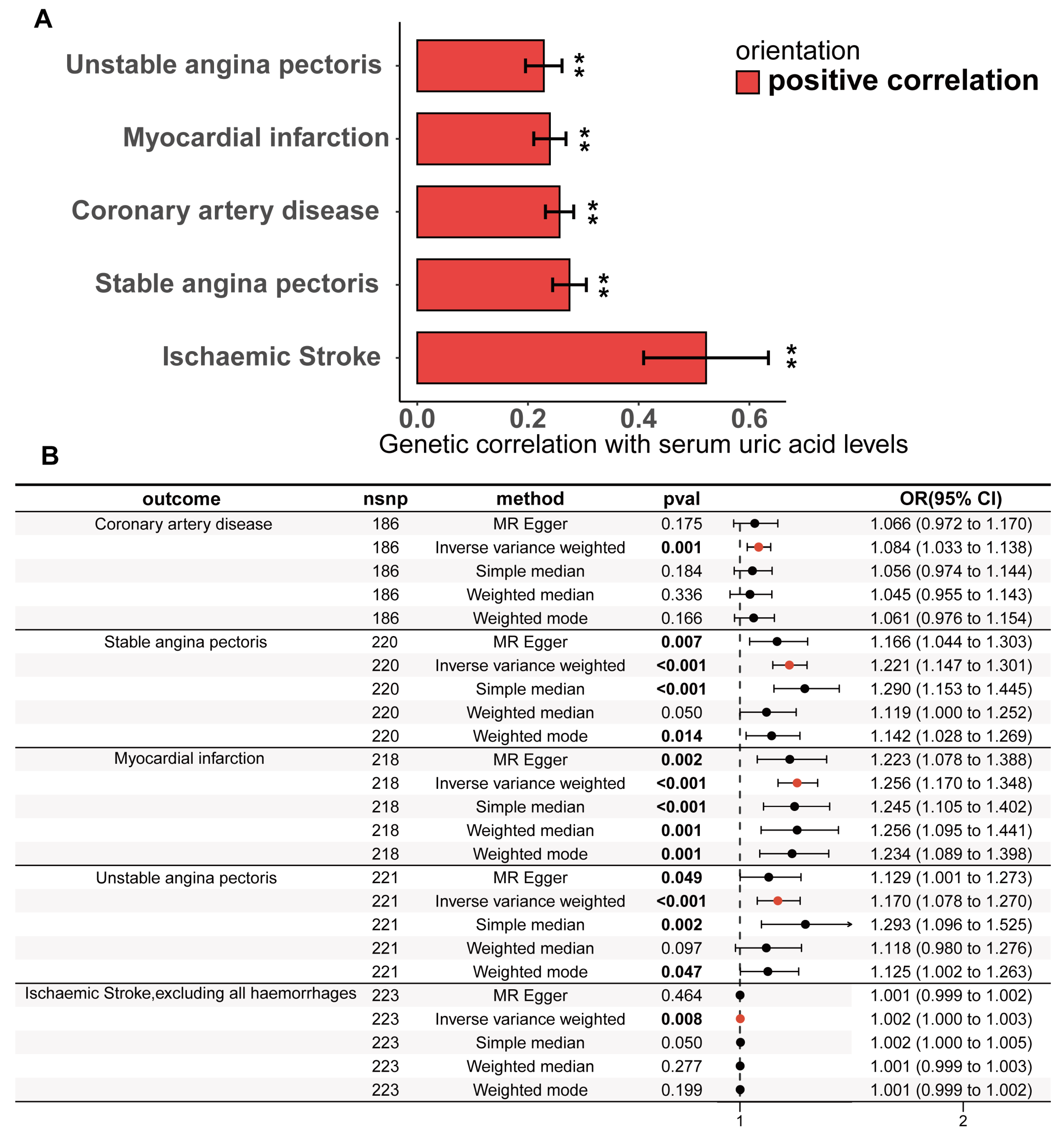
3.1. The Causality Between Serum Urate Concentrations and CHD
3.2. Pleiotropic Analysis of Instrumental Variables
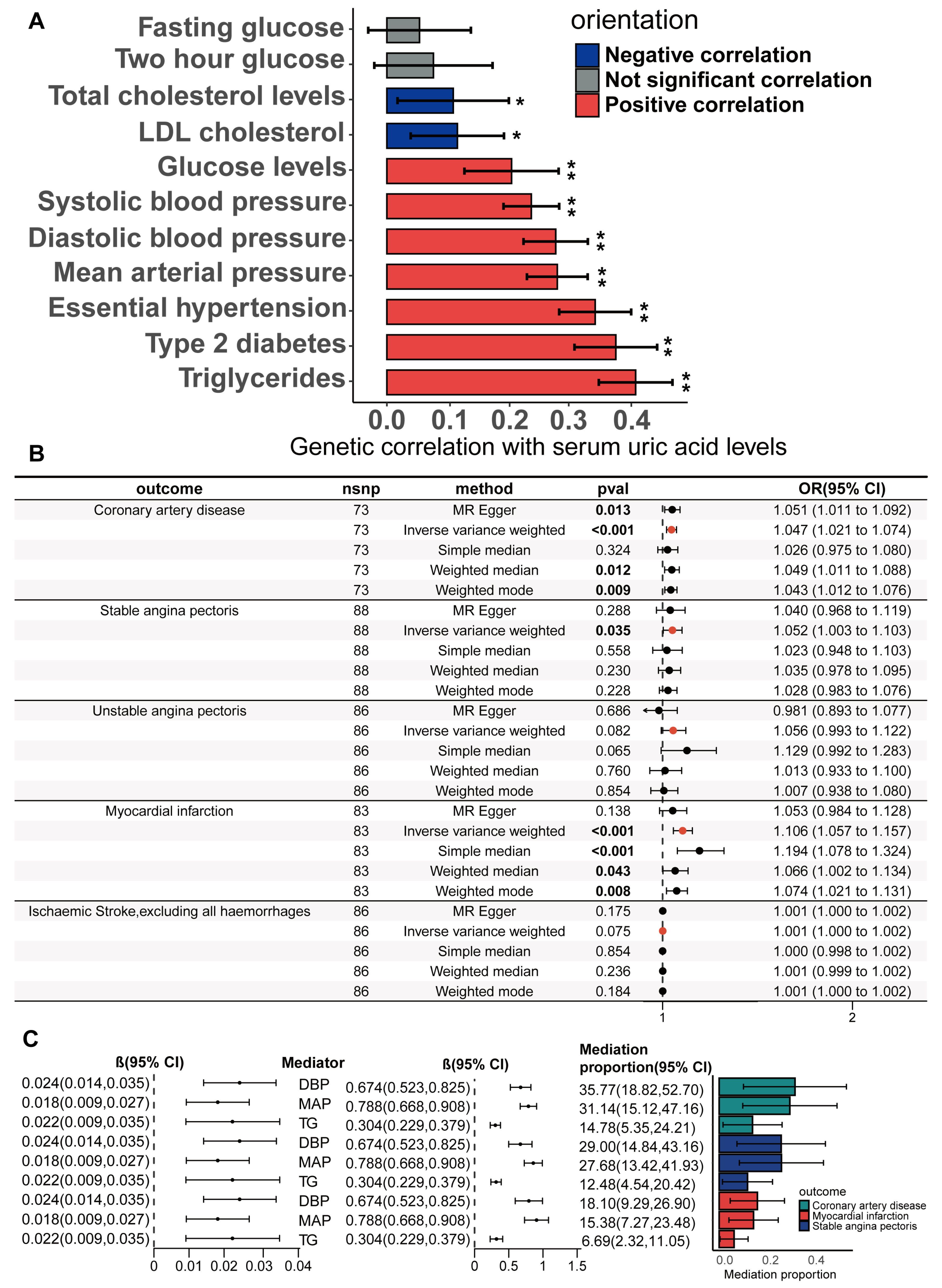
3.3. Causality Between Serum Urate Concentrations and CHD Risk Factors Based on the Biologically Driven Genetic Instrument Selection Strategy
3.4. Mediators Between the Serum Urate Concentrations and CHD, SAP and MI
3.5. Potential Mechanisms Mediating the Causality Between Serum Urate and CHD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CHD | coronary heart disease |
| SAP | unstable angina pectoris |
| MI | myocardial infarction |
| SNPs | single nucleotide polymorphisms |
| IVs | instrumental variables |
| MR | Mendelian randomization |
| MR-PRESSO | Mendelian randomization pleiotropy RESidual sum and outlier |
| MRlap | Mendelian randomization with Lasso penalty |
| GWAS | genome-wide association study |
| IVW | inverse variance weighted |
| OR | odds ratios |
| LD | linkage disequilibrium |
| LDL | low density lipoprotein |
| PC | principal components |
| PPH3 | posterior probability for hypothesis 3 |
| PPH4 | posterior probability for hypothesis 4 |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| GO | gene ontology |
| scRNA | single-cell RNA sequencing |
| DEGs | differentially expressed genes |
| TNF | tumor necrosis facto |
| CCL2 | C-C motif chemokine ligand 2 |
| CD14 | cluster of differentiation 14 |
| PHLDA1 | pleckstrin homology like domain family A member 1 |
| HMOX-1 | heme oxygenase 1 |
| LYVE1 | lymphatic vessel endothelial hyaluronan receptor 1 |
| SELENOP | selenoprotein P |
| MAF | MAF bZIP transcription factor |
| CTSB | cathepsin B |
| LGMN | legumain |
| NFKBIZ | nuclear factor kappa B inhibitor zeta |
| ALOX5AP | arachidonate 5-lipoxygenase activating protein |
| CARDIoGRAMplusC4D | plus C4D The Coronary ARtery DIsease Genome wide Replication and Meta-analysis plus The Coronary Artery Disease Genetics |
| WD | Western diet |
| EC | vascular endothelial cell |
| FC | fibroblast |
| SMC | smooth muscle cell |
| ICS | intermediate cell state |
| SEM | SMC-derived intermediate cells |
References
- Chen-Xu, M.; Yokose, C.; Rai, S.K.; Pillinger, M.H.; Choi, H.K. Contemporary Prevalence of Gout and Hyperuricemia in the United States and Decadal Trends: The National Health and Nutrition Examination Survey, 2007–2016. Arthritis Rheumatol. 2019, 71, 991–999. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, X.; Wu, J.; Huang, Z.; Zhao, Z.; Zhang, X.; Xue, Y.; Wan, W.; Li, C.; Zhang, W.; et al. Prevalence of Hyperuricemia Among Chinese Adults: Findings From Two Nationally Representative Cross-Sectional Surveys in 2015–16 and 2018–19. Front. Immunol. 2021, 12, 791983. [Google Scholar] [CrossRef]
- Li, K.; Li, K.; Yao, Q.; Shui, X.; Zheng, J.; He, Y.; Lei, W. The potential relationship of coronary artery disease and hyperuricemia: A cardiometabolic risk factor. Heliyon 2023, 9, e16097. [Google Scholar] [CrossRef]
- Yu, W.; Cheng, J.D. Uric Acid and Cardiovascular Disease: An Update From Molecular Mechanism to Clinical Perspective. Front. Pharmacol. 2020, 11, 582680. [Google Scholar] [CrossRef]
- Li, B.; Chen, L.; Hu, X.; Tan, T.; Yang, J.; Bao, W.; Rong, S. Association of Serum Uric Acid With All-Cause and Cardiovascular Mortality in Diabetes. Diabetes Care 2023, 46, 425–433. [Google Scholar] [CrossRef]
- Morikawa, N.; Chen, L.; Hu, X.; Tan, T.; Yang, J.; Bao, W.; Rong, S. Serum Urate Trajectory in Young Adulthood and Incident Cardiovascular Disease Events by Middle Age: CARDIA Study. Hypertension 2021, 78, 1211–1218. [Google Scholar] [CrossRef]
- Chu, X.; Lu, Y.; Mei, M.; Peng, P.; Zhao, Y.; Fu, G.; Qiu, F.; Jin, C. Correlation Between Serum Uric Acid Levels and Coronary Plaque Characteristics on Optical Coherence Tomography. Int. Heart J. 2022, 63, 806–813. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Qi, Y.; Zhang, S.; Shi, K.; Lin, H.; Grossfeld, P.; Wang, W.; Wu, T.; Qu, X.; et al. High Level of Serum Uric Acid induced Monocyte Inflammation is Related to Coronary Calcium Deposition in the Middle-Aged and Elder Population of China: A five-year Prospective Cohort Study. J. Inflamm. Res. 2022, 15, 1859–1872. [Google Scholar] [CrossRef]
- Ekici, B.; Kütük, U.; Alhan, A.; Töre, H.F. The relationship between serum uric acid levels and angiographic severity of coronary heart disease. Kardiol. Pol. 2015, 73, 533–538. [Google Scholar] [CrossRef]
- Yu, J.; Han, J.; Mao, J.; Guo, L.; Gao, W. Association between serum uric acid level and the severity of coronary artery disease in patients with obstructive coronary artery disease. Chin. Med. J. 2014, 127, 1039–1045. [Google Scholar] [CrossRef]
- Lan, M.; Liu, B.; He, Q. Evaluation of the association between hyperuricemia and coronary artery disease: A STROBE-compliant article. Medicine 2018, 97, e12926. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Song, B.; Yu, H.; Zhang, X.; Shao, Y. Impact of serum uric acid levels on the clinical prognosis and severity of coronary artery disease in patients with acute coronary syndrome and hypertension after percutaneous coronary intervention: A prospective cohort study. BMJ Open 2022, 12, e052031. [Google Scholar] [CrossRef]
- Liu, R.; Xu, F.; Zhou, Y.; Liu, T. The characteristics of risk factors in Chinese young women with acute coronary syndrome. BMC Cardiovasc. Disord. 2020, 20, 290. [Google Scholar] [CrossRef]
- Strasak, A.; Ruttmann, E.; Brant, L.; Kelleher, C.; Klenk, J.; Concin, H.; Diem, G.; Pfeiffer, K.; Ulmer, H. Serum uric acid and risk of cardiovascular mortality: A prospective long-term study of 83,683 Austrian men. Clin. Chem. 2008, 54, 273–284. [Google Scholar] [CrossRef]
- de Abajo, F.J.; Gil, M.J.; Rodríguez, A.; García-Poza, P.; Álvarez, A.; Bryant, V.; García-Rodríguez, L.A. Allopurinol use and risk of non-fatal acute myocardial infarction. Heart 2015, 101, 679–685. [Google Scholar] [CrossRef]
- Bredemeier, M.; Lopes, L.M.; Eisenreich, M.A.; Hickmann, S.; Bongiorno, G.K.; d’Avila, R.; Morsch, A.L.B.; da Silva Stein, F.; Campos, G.G.D. Xanthine oxidase inhibitors for prevention of cardiovascular events: A systematic review and meta-analysis of randomized controlled trials. BMC Cardiovasc. Disord. 2018, 18, 24. [Google Scholar] [CrossRef]
- Søltoft Larsen, K.; Pottegård, A.; Lindegaard, H.M.; Hallas, J. Impact of Urate Level on Cardiovascular Risk in Allopurinol Treated Patients. A Nested Case-Control Study. PLoS ONE 2016, 11, e0146172. [Google Scholar] [CrossRef]
- Maloberti, A.; Bossi, I.; Tassistro, E.; Rebora, P.; Racioppi, A.; Nava, S.; Soriano, F.; Piccaluga, E.; Piccalò, G.; Oreglia, J.; et al. Uric acid in chronic coronary syndromes: Relationship with coronary artery disease severity and left ventricular diastolic parameter. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1501–1508. [Google Scholar] [CrossRef]
- Yang, F.; Lu, Y.; Chen, S.; Wang, K.; Hu, T.; Cui, H. Sex-specific effect of serum urate levels on coronary heart disease and myocardial infarction prevention: A Mendelian randomization study. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 1266–1274. [Google Scholar] [CrossRef]
- Vallée, A. Association between serum uric acid and arterial stiffness in a large-aged 40-70 years old population. J. Clin. Hypertens. 2022, 24, 885–897. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, L.; Li, Z.; Ni, W.; Wang, L.; Mei, W.; Ruan, G.; Shi, Z.; Dai, C. Association Between Serum Uric Acid Levels and Traditional Cardiovascular Risk Factors in Xiamen Residents of China: A Real-World Study. Front. Cardiovasc. Med. 2022, 9, 913437. [Google Scholar] [CrossRef]
- Han, Y.; Han, K.; Han, X.; Yin, Y.; Di, H.; Wu, J.; Zhang, Y.; Zeng, X. Serum Uric Acid Might Be Positively Associated With Hypertension in Chinese Adults: An Analysis of the China Health and Nutrition Survey. Front. Med. 2021, 8, 755509. [Google Scholar] [CrossRef]
- Tian, X.; Chen, S.; Wang, P.; Xu, Q.; Zhang, Y.; Zhang, X.; Wu, S.; Luo, Y.; Wang, A. Temporal relationship between hyperuricemia and hypertension and its impact on future risk of cardiovascular disease. Eur. J. Intern. Med. 2023, 111, 82–89. [Google Scholar] [CrossRef]
- Galvão, A.I.R.; Beleigoli, A.M.R.; Vidigal, P.G.; Duncan, B.B.; Schmidt, M.I.; Appleton, S.L.; Barreto, S.M.; Diniz, M. The positive association between serum uric acid, impaired fasting glucose, impaired glucose tolerance, and diabetes mellitus in the ELSA-Brasil study. Cad. Saude Publica 2021, 37, e00255920. [Google Scholar] [CrossRef]
- Tan, M.Y.; Mo, C.Y.; Li, F.; Zhao, Q. The association between serum uric acid and hypertriglyceridemia: Evidence from the national health and nutrition examination survey (2007–2018). Front. Endocrinol. 2023, 14, 1215521. [Google Scholar] [CrossRef]
- Han, R.; Zhang, Y.; Jiang, X. Relationship Between Four Non-Insulin-Based Indexes of Insulin Resistance and Serum Uric Acid in Patients with Type 2 Diabetes: A Cross-Sectional Study. Diabetes. Metab. Syndr. Obes. 2022, 15, 1461–1471. [Google Scholar] [CrossRef]
- Yu, T.Y.; Jee, J.H.; Bae, J.C.; Jin, S.M.; Baek, J.H.; Lee, M.K.; Kim, J.H. Serum uric acid: A strong and independent predictor of metabolic syndrome after adjusting for body composition. Metabolism 2016, 65, 432–440. [Google Scholar] [CrossRef]
- Gill, D.; Cameron, A.C.; Burgess, S.; Li, X.; Doherty, D.J.; Karhunen, V.; Abdul-Rahim, A.H.; Taylor-Rowan, M.; Zuber, V.; Tsao, P.S.; et al. Urate, Blood Pressure, and Cardiovascular Disease: Evidence From Mendelian Randomization and Meta-Analysis of Clinical Trials. Hypertension 2021, 77, 383–392. [Google Scholar] [CrossRef]
- Lawlor, D.A.; Harbord, R.M.; Sterne, J.A.; Timpson, N.; Davey Smith, G. Mendelian randomization: Using genes as instruments for making causal inferences in epidemiology. Stat. Med. 2008, 27, 1133–1163. [Google Scholar] [CrossRef]
- Hemani, G.; Zheng, J.; Elsworth, B.; Wade, K.H.; Haberland, V.; Baird, D.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R.; et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife 2018, 7, e34408. [Google Scholar] [CrossRef]
- Elsworth, B.; Lyon, M.; Alexander, T.; Liu, Y.; Matthews, P.; Hallett, J.; Bates, P.; Palmer, T.; Haberland, V.; Smith, G.D.; et al. The MRC IEU OpenGWAS data infrastructure. bioRxiv 2008. bioRxiv:2020.2008.2010.244293. [Google Scholar] [CrossRef]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Yarmolinsky, J.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Higgins, J.P.T.; Timpson, N.J.; Dimou, N.; et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA 2021, 326, 1614–1621. [Google Scholar] [CrossRef]
- Sakaue, S.; Kanai, M.; Tanigawa, Y.; Karjalainen, J.; Kurki, M.; Koshiba, S.; Narita, A.; Konuma, T.; Yamamoto, K.; Akiyama, M.; et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat. Genet. 2021, 53, 1415–1424. [Google Scholar] [CrossRef]
- Mi, H.; Muruganujan, A.; Thomas, P.D. PANTHER in 2013: Modeling the evolution of gene function, and other gene attributes, in the context of phylogenetic trees. Nucleic Acids Res. 2013, 41, D377–D386. [Google Scholar] [CrossRef]
- Burgess, S.; Zuber, V.; Valdes-Marquez, E.; Sun, B.B.; Hopewell, J.C. Mendelian randomization with fine-mapped genetic data: Choosing from large numbers of correlated instrumental variables. Genet. Epidemiol. 2017, 41, 714–725. [Google Scholar] [CrossRef]
- Woolf, B.; Rajasundaram, S.; Cronjé, H.T.; Yarmolinsky, J.; Burgess, S.; Gill, D. A drug target for erectile dysfunction to help improve fertility, sexual activity, and wellbeing: Mendelian randomisation study. BMJ 2023, 383, e076197. [Google Scholar] [CrossRef]
- Teslovich, T.M.; Musunuru, K.; Smith, A.V.; Edmondson, A.C.; Stylianou, I.M.; Koseki, M.; Pirruccello, J.P.; Ripatti, S.; Chasman, D.I.; Willer, C.J.; et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature 2010, 466, 707–713. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G. Avoiding bias from weak instruments in Mendelian randomization studies. Int. J. Epidemiol. 2011, 40, 755–764. [Google Scholar] [CrossRef]
- Bulik-Sullivan, B.; Finucane, H.K.; Anttila, V.; Gusev, A.; Day, F.R.; Loh, P.R.; Duncan, L.; Perry, J.R.; Patterson, N.; Robinson, E.B.; et al. An atlas of genetic correlations across human diseases and traits. Nat. Genet. 2015, 47, 1236–1241. [Google Scholar] [CrossRef]
- Bulik-Sullivan, B.; KLoh, P.R.; Finucane, H.K.; Ripke, S.; Yang, J.; Patterson, N.; Daly, M.J.; Price, A.L.; Neale, B.M. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 2015, 47, 291–295. [Google Scholar] [CrossRef]
- Glickman, M.E.; Rao, S.R.; Schultz, M.R. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J. Clin. Epidemiol. 2014, 67, 850–857. [Google Scholar] [CrossRef]
- Giambartolomei, C.; Vukcevic, D.; Schadt, E.E.; Franke, L.; Hingorani, A.D.; Wallace, C.; Plagnol, V. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet. 2014, 10, e1004383. [Google Scholar] [CrossRef]
- Lin, S.H.; Brown, D.W.; Machiela, M.J. LDtrait: An Online Tool for Identifying Published Phenotype Associations in Linkage Disequilibrium. Cancer Res. 2020, 80, 3443–3446. [Google Scholar] [CrossRef]
- Breeze, C.E.; Haugen, E.; Gutierrez-Arcelus, M.; Yao, X.; Teschendorff, A.; Beck, S.; Dunham, I.; Stamatoyannopoulos, J.; Franceschini, N.; Machiela, M.J.; et al. FORGEdb: A tool for identifying candidate functional variants and uncovering target genes and mechanisms for complex diseases. Genome Biol. 2024, 25, 3. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Franzén, O.; Gan, L.M.; Björkegren, J.L.M. PanglaoDB: A web server for exploration of mouse and human single-cell RNA sequencing data. Database 2019, 2019, baz046. [Google Scholar] [CrossRef]
- Morabito, S.; Reese, F.; Rahimzadeh, N.; Miyoshi, E.; Swarup, V. hdWGCNA identifies co-expression networks in high-dimensional transcriptomics data. Cell Rep. Methods 2023, 3, 100498. [Google Scholar] [CrossRef]
- Ross, R. Atherosclerosis--an inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef]
- Robbins, C.S.; Chudnovskiy, A.; Rauch, P.J.; Figueiredo, J.L.; Iwamoto, Y.; Gorbatov, R.; Etzrodt, M.; Weber, G.F.; Ueno, T.; van Rooijen, N.; et al. Extramedullary hematopoiesis generates Ly-6C(high) monocytes that infiltrate atherosclerotic lesions. Circulation 2012, 125, 364–374. [Google Scholar] [CrossRef]
- Geng, S.; Chen, K.; Yuan, R.; Peng, L.; Maitra, U.; Diao, N.; Chen, C.; Zhang, Y.; Hu, Y.; Qi, C.F.; et al. The persistence of low-grade inflammatory monocytes contributes to aggravated atherosclerosis. Nat. Commun. 2016, 7, 13436. [Google Scholar] [CrossRef]
- Tian, X.; Chen, S.; Zhang, Y.; Zhang, X.; Xu, Q.; Wang, P.; Wu, S.; Luo, Y.; Wang, A. Serum uric acid variation and the risk of cardiovascular disease: A prospective cohort study. Eur. J. Intern. Med. 2023, 112, 37–44. [Google Scholar] [CrossRef]
- Crişan, T.O.; Cleophas, M.C.P.; Novakovic, B.; Erler, K.; van de Veerdonk, F.L.; Stunnenberg, H.G.; Netea, M.G.; Dinarello, C.A.; Joosten, L.A.B. Uric acid priming in human monocytes is driven by the AKT-PRAS40 autophagy pathway. Proc. Natl. Acad. Sci. USA 2017, 114, 5485–5490. [Google Scholar] [CrossRef]
- Martínez-Reyes, C.P.; Manjarrez-Reyna, A.N.; Méndez-García, L.A.; Aguayo-Guerrero, J.A.; Aguirre-Sierra, B.; Villalobos-Molina, R.; López-Vidal, Y.; Bobadilla, K.; Escobedo, G. Uric Acid Has Direct Proinflammatory Effects on Human Macrophages by Increasing Proinflammatory Mediators and Bacterial Phagocytosis Probably via URAT1. Biomolecules 2020, 10, 576. [Google Scholar] [CrossRef]
- Barbosa, K.B.; Volp, A.C.; Hermsdorff, H.H.; Navarro-Blasco, I.; Zulet, M.; Martínez, J.A.; Bressan, J. Relationship of oxidized low density lipoprotein with lipid profile and oxidative stress markers in healthy young adults: A translational study. Lipids Health Dis. 2011, 10, 61. [Google Scholar] [CrossRef]
- Weng, H.; Li, H.; Zhang, Z.; Zhang, Y.; Xi, L.; Zhang, D.; Deng, C.; Wang, D.; Chen, R.; Chen, G.; et al. Association between uric acid and risk of venous thromboembolism in East Asian populations: A cohort and Mendelian randomization study. Lancet Reg. Health West. Pac. 2023, 39, 100848. [Google Scholar] [CrossRef]
- Li, P.; Zhang, L.; Zhang, M.; Zhou, C.; Lin, N. Uric acid enhances PKC-dependent eNOS phosphorylation and mediates cellular ER stress: A mechanism for uric acid-induced endothelial dysfunction. Int. J. Mol. Med. 2016, 37, 989–997. [Google Scholar] [CrossRef]
- Liang, W.Y.; Zhu, X.Y.; Zhang, J.W.; Feng, X.R.; Wang, Y.C.; Liu, M.L. Uric acid promotes chemokine and adhesion molecule production in vascular endothelium via nuclear factor-kappa B signaling. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 187–194. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhao, Y.; Zhang, Y.; Shi, W.; Li, X.; Shyy, J.Y.; He, M.; Wang, L. Gout-induced endothelial impairment: The role of SREBP2 transactivation of YAP. FASEB J. 2021, 35, e21613. [Google Scholar] [CrossRef]
- Mazzali, M.; Hughes, J.; Kim, Y.G.; Jefferson, J.A.; Kang, D.H.; Gordon, K.L.; Lan, H.Y.; Kivlighn, S.; Johnson, R.J. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension 2001, 38, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Lozada, L.G.; Tapia, E.; Bautista-García, P.; Soto, V.; Avila-Casado, C.; Vega-Campos, I.P.; Nakagawa, T.; Zhao, L.; Franco, M.; Johnson, R.J. Effects of febuxostat on metabolic and renal alterations in rats with fructose-induced metabolic syndrome. Am. J. Physiol. Ren. Physiol. 2008, 294, F710–F718. [Google Scholar] [CrossRef] [PubMed]
- García-Arroyo, F.E.; Monroy-Sánchez, F.; Muñoz-Jiménez, I.; Gonzaga, G.; Andrés-Hernando, A.; Zazueta, C.; Juárez-Rojas, J.G.; Lanaspa, M.A.; Johnson, R.J.; Sánchez-Lozada, L.G. Allopurinol Prevents the Lipogenic Response Induced by an Acute Oral Fructose Challenge in Short-Term Fructose Fed Rats. Biomolecules 2019, 9, 601. [Google Scholar] [CrossRef] [PubMed]
- Ziga-Smajic, N.; Skrbo, S.; Muratovic, S.; Pehlivanovic, B.; Lagumdzija, D.; Omerovic, N. Comparison of the Effects of Allopurinol and Febuxostat on the Values of Triglycerides in Hyperuricemic Patients. Med. Arch. 2020, 74, 172–176. [Google Scholar] [CrossRef]
- Choi, Y.J.; Shin, H.S.; Choi, H.S.; Park, J.W.; Jo, I.; Oh, E.S.; Lee, K.Y.; Lee, B.H.; Johnson, R.J.; Kang, D.H. Uric acid induces fat accumulation via generation of endoplasmic reticulum stress and SREBP-1c activation in hepatocytes. Lab. Investig. 2014, 94, 1114–1125. [Google Scholar] [CrossRef]
- Coto, E.; Reguero, J.R.; Avanzas, P.; Pascual, I.; Martín, M.; Hevia, S.; Morís, C.; Díaz-Molina, B.; Lambert, J.L.; Alonso, B.; et al. Gene variants in the NF-KB pathway (NFKB1, NFKBIA, NFKBIZ) and risk for early-onset coronary artery disease. Immunol. Lett. 2019, 208, 39–43. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Zhang, X.; Yan, C.; Kang, J.; Liang, Z.; Liu, S.; Feng, X.; Han, Y. Association of ALOX5AP haplotypes with susceptibility to coronary artery disease in a Chinese Han population. Eur. J. Intern. Med. 2012, 23, e119–e123. [Google Scholar] [CrossRef]
- Spanbroek, R.; Grabner, R.; Lotzer, K.; Hildner, M.; Urbach, A.; Ruhling, K.; Moos, M.P.; Kaiser, B.; Cohnert, T.U.; Wahlers, T.; et al. Expanding expression of the 5-lipoxygenase pathway within the arterial wall during human atherogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 1238–1243. [Google Scholar] [CrossRef]
- Ayer, A.; Zarjou, A.; Agarwal, A.; Stocker, R. Heme Oxygenases in Cardiovascular Health and Disease. Physiol. Rev. 2016, 96, 1449–1508. [Google Scholar] [CrossRef]
- Handy, D.E.; Joseph, J.; Loscalzo, J. Selenium, a Micronutrient That Modulates Cardiovascular Health via Redox Enzymology. Nutrients 2021, 13, 3238. [Google Scholar] [CrossRef]
- Liu, H.; Xu, H.; Huang, K. Selenium in the prevention of atherosclerosis and its underlying mechanisms. Metallomics 2017, 9, 21–37. [Google Scholar] [CrossRef] [PubMed]
- von Scheidt, M.; Zhao, Y.; de Aguiar Vallim, T.Q.; Che, N.; Wierer, M.; Seldin, M.M.; Franzén, O.; Kurt, Z.; Pang, S.; Bongiovanni, D.; et al. Transcription Factor MAFF (MAF Basic Leucine Zipper Transcription Factor F) Regulates an Atherosclerosis Relevant Network Connecting Inflammation and Cholesterol Metabolism. Circulation 2021, 143, 1809–1823. [Google Scholar] [CrossRef] [PubMed]
- Takaoka, M.; Zhao, X.; Lim, H.Y.; Magnussen, C.G.; Ang, O.; Suffee, N.; Schrank, P.R.; Ong, W.S.; Tsiantoulas, D.; Sommer, F.; et al. Early intermittent hyperlipidaemia alters tissue macrophages to fuel atherosclerosis. Nature 2024, 634, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Davey Smith, G.; Davies, N.M.; Dudbridge, F.; Gill, D.; Glymour, M.M.; Hartwig, F.P.; Kutalik, Z.; Holmes, M.V.; Minelli, C.; et al. Guidelines for performing Mendelian randomization investigations: Update for summer 2023. Wellcome Open Res 2019, 4, 186. [Google Scholar] [CrossRef]
- Bowden, J.; Del Greco, M.F.; Minelli, C.; Davey Smith, G.; Sheehan, N.; Thompson, J. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat. Med. 2017, 36, 1783–1802. [Google Scholar] [CrossRef]
- Mounier, N.; Kutalik, Z. Bias correction for inverse variance weighting Mendelian randomization. Genet. Epidemiol. 2023, 47, 314–331. [Google Scholar] [CrossRef]
- Grant, A.J.; Burgess, S. Pleiotropy robust methods for multivariable Mendelian randomization. Stat. Med. 2021, 40, 5813–5830. [Google Scholar] [CrossRef]
- Rees, J.M.B.; Wood, A.M.; Burgess, S. Extending the MR-Egger method for multivariable Mendelian randomization to correct for both measured and unmeasured pleiotropy. Stat. Med. 2017, 36, 4705–4718. [Google Scholar] [CrossRef]
- Carter, A.R.; Sanderson, E.; Hammerton, G.; Richmond, R.C.; Davey Smith, G.; Heron, J.; Taylor, A.E.; Davies, N.M.; Howe, L.D. Mendelian randomisation for mediation analysis: Current methods and challenges for implementation. Eur. J. Epidemiol. 2021, 36, 465–478. [Google Scholar] [CrossRef]
- MacKinnon, D.P.; Lockwood, C.M.; Hoffman, J.M.; West, S.G.; Sheets, V. A comparison of methods to test mediation and other intervening variable effects. Psychol. Methods 2002, 7, 83–104. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef]
- Hartwig, F.P.; Davey Smith, G.; Bowden, J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int. J. Epidemiol. 2017, 46, 1985–1998. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef]
- Verbanck, M.; Chen, C.Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef]

| SNP_UA | Position (GRCh37) | GWAS Trait | PMID | SNP_Other_Trait | Position (GRCh37) | r2 | p-Value | PP.H3.abf | PP.H4.abf |
|---|---|---|---|---|---|---|---|---|---|
| rs77924615 | 16:20392332 | Diastolic blood pressure | 38689001 | rs77924615 | chr16:20392332 | 1 | 2.00 × 10−39 | 1.06 × 10−6 | 0.99999893 |
| rs72681698 | 14:51207741 | Diastolic blood pressure | 35762941 | rs72677850 | chr14:50849397 | 1 | 3.00 × 10−10 | 1.78 × 10−5 | 0.99998222 |
| rs2219647 | 16:51733405 | Diastolic blood pressure | 38689001 | rs9932220 | chr16:51758116 | 0.89426289 | 2.00 × 10−13 | 0.22711367 | 0.77288099 |
| rs2219647 | 16:51733405 | Essential hypertension | 32589924 | rs9932220 | chr16:51758116 | 0.89426289 | 2.00 × 10−8 | 0.00491713 | 0.99464745 |
| rs963837 | 11:30749090 | Systolic blood pressure | 33230300 | rs3925584 | chr11:30760335 | 0.955974843 | 3.00 × 10−9 | 0.11073903 | 0.88785727 |
| rs77924615 | 16:20392332 | Systolic blood pressure | 38689001 | rs77924615 | chr16:20392332 | 1 | 2.00 × 10−21 | 1.94 × 10−6 | 0.99999805 |
| rs72681698 | 14:51207741 | Systolic blood pressure | 30578418 | rs72677850 | chr14:50849397 | 1 | 2.00 × 10−16 | 0.18104193 | 0.81895644 |
| rs2219647 | 16:51733405 | Systolic blood pressure | 38689001 | rs9932220 | chr16:51758116 | 0.89426289 | 1.00 × 10−10 | 0.23499299 | 0.76500700 |
| rs2823139 | 21:16576783 | Systolic blood pressure | 30578418 | rs2823139 | chr21:16576783 | 1 | 9.00 × 10−12 | 0.22394859 | 0.77605130 |
| rs11128603 | 3:12385828 | Triglyceride levels | 34887591 | rs1801282 | chr3:12393125 | 1 | 5.00 × 10−26 | 0.00035804 | 0.99964193 |
| rs2060824 | 2:61484556 | Triglyceride levels | 34887591 | rs766448 | chr2:61735446 | 0.915200932 | 4.00 × 10−11 | 1.49 × 10−8 | 0.99999998 |
| rs146787580 | 2:203412513 | Triglyceride levels | 32203549 | rs3731696 | chr2:203431804 | 0.916666667 | 6.00 × 10−13 | 5.21 × 10−9 | 0.99999999 |
| rs2012385 | 2:242422405 | Triglyceride levels | 32203549 | rs4675812 | chr2:242395674 | 0.711111111 | 1.00 × 10−12 | 4.32 × 10−9 | 0.99999999 |
| rs2943645 | 2:227099180 | Triglycerides | 34594039 | rs56256300 | chr2:227098186 | 1 | 7.00 × 10−67 | 4.66 × 10−9 | 0.99999999 |
| rs1260326 | 2:27730940 | Triglycerides | 34594039 | rs1260326 | chr2:27730940 | 1 | 7.00 × 10−102 | 3.50 × 10−8 | 0.99999996 |
| rs114165349 | 1:27021913 | Triglycerides | 34594039 | rs114165349 | chr1:27021913 | 1 | 4.00 × 10−29 | 9.05 × 10−8 | 0.99999991 |
| rs2229357 | 12:57843711 | Triglycerides | 34594039 | rs2122982 | chr12:57781893 | 1 | 3.00 × 10−26 | 0.00026704 | 0.99973295 |
| rs10211562 | 2:111930796 | Triglycerides | 34594039 | rs10211562 | chr2:111930796 | 1 | 1.00 × 10−10 | 9.34 × 10−9 | 0.99999999 |
| rs12096443 | 1:50984962 | Triglycerides | 30275531 | rs1278530 | chr1:50889255 | 0.854051968 | 8.00 × 10−12 | 1.60 × 10−6 | 0.99999839 |
| rs1260326 | 2:27730940 | Two-hour glucose | 34059833 | rs1260326 | chr2:27730940 | 1 | 6.00 × 10−12 | 6.31 × 10−5 | 0.99958036 |
| rs1260326 | 2:27730940 | Type 2 diabetes | 34594039 | rs1260326 | chr2:27730940 | 1 | 6.00 × 10−29 | 3.71 × 10−13 | 1 |
| rs11128603 | 3:12385828 | Type 2 diabetes | 30595370 | rs17036160 | chr3:12329783 | 1 | 1.00 × 10−11 | 0.00098573 | 0.99901423 |
| rs76895963 | 12:4384844 | Type 2 diabetes | 30595370 | rs76895963 | chr12:4384844 | 1 | 2.00 × 10−31 | 4.38 × 10−7 | 0.99999956 |
| rs62106258 | 2:417167 | Type 2 diabetes | 30297969 | rs62107261 | chr2:422144 | 1 | 4.00 × 10−12 | 0.01668743 | 0.92439756 |
| rs10899125 | 11:75517332 | Type 2 diabetes | 32541925 | rs11236524 | chr11:75464344 | 1 | 1.00 × 10−8 | 5.45 × 10−7 | 0.99999945 |
| rs10211562 | 2:111930796 | Type 2 diabetes | 30054458 | rs10169613 | chr2:111934977 | 0.936140351 | 4.00 × 10−8 | 9.85 × 10−7 | 0.99999901 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Mei, S.; Ma, X.; Wuyun, Q.; Zhou, L.; Luo, Q.; Cai, Z.; Yan, J. Causal Relationship Between Serum Uric Acid and Atherosclerotic Disease: A Mendelian Randomization and Transcriptomic Analysis. Biomedicines 2025, 13, 1838. https://doi.org/10.3390/biomedicines13081838
Wang S, Mei S, Ma X, Wuyun Q, Zhou L, Luo Q, Cai Z, Yan J. Causal Relationship Between Serum Uric Acid and Atherosclerotic Disease: A Mendelian Randomization and Transcriptomic Analysis. Biomedicines. 2025; 13(8):1838. https://doi.org/10.3390/biomedicines13081838
Chicago/Turabian StyleWang, Shitao, Shuai Mei, Xiaozhu Ma, Qidamugai Wuyun, Li Zhou, Qiushi Luo, Ziyang Cai, and Jiangtao Yan. 2025. "Causal Relationship Between Serum Uric Acid and Atherosclerotic Disease: A Mendelian Randomization and Transcriptomic Analysis" Biomedicines 13, no. 8: 1838. https://doi.org/10.3390/biomedicines13081838
APA StyleWang, S., Mei, S., Ma, X., Wuyun, Q., Zhou, L., Luo, Q., Cai, Z., & Yan, J. (2025). Causal Relationship Between Serum Uric Acid and Atherosclerotic Disease: A Mendelian Randomization and Transcriptomic Analysis. Biomedicines, 13(8), 1838. https://doi.org/10.3390/biomedicines13081838








