Neuronal Pentraxin 2 as a Potential Biomarker for Nusinersen Therapy Response in Adults with Spinal Muscular Atrophy: A Pilot Study
Abstract
1. Introduction
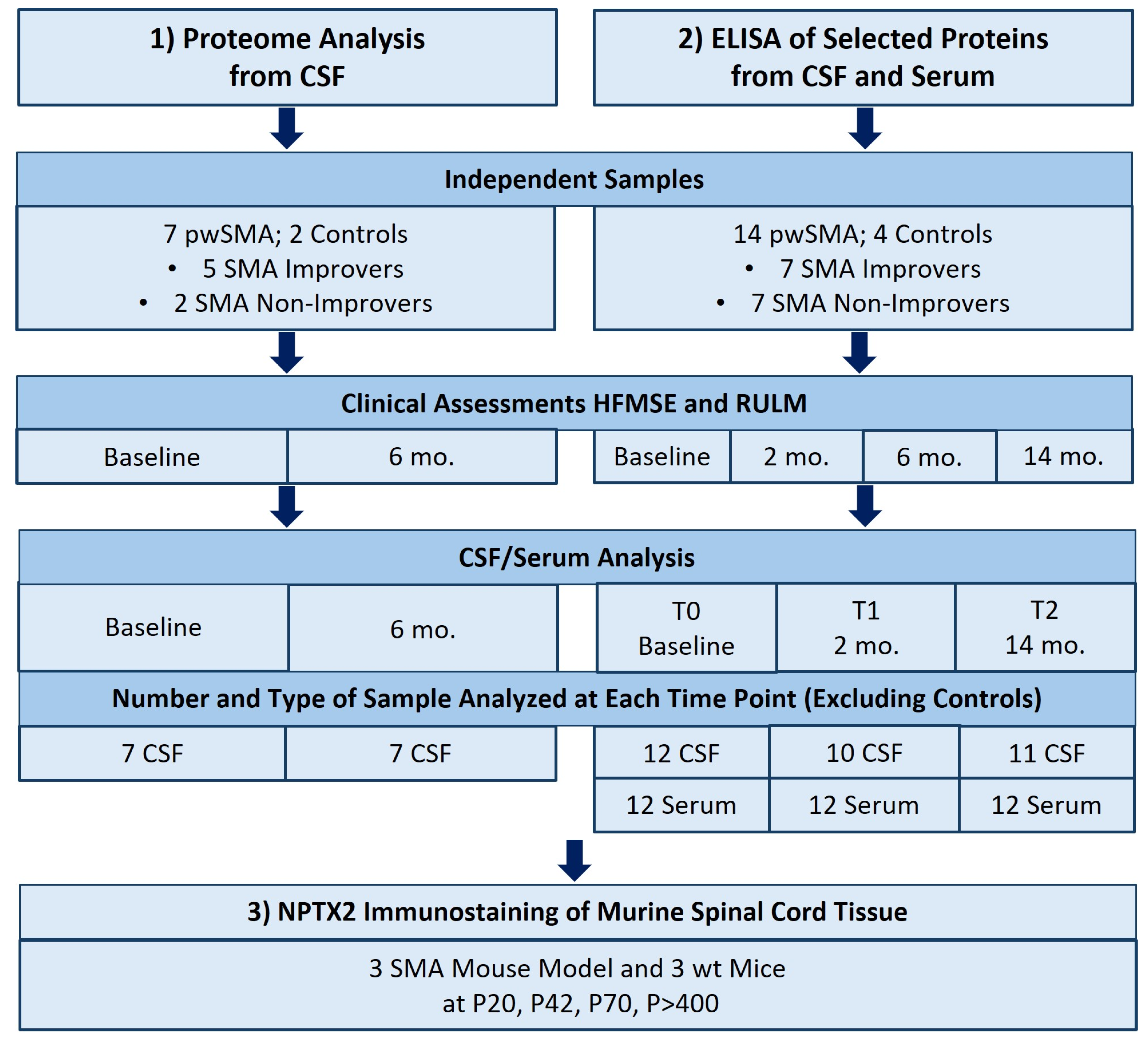
2. Materials and Methods
2.1. Study Design
2.2. Assessment of Motor Function
2.3. Proteome Analysis from CSF
2.4. Sample Selection for Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. ELISA from CSF and Serum
2.6. Animals
2.7. NPTX2 Immunostaining of Murine Spinal Cord Tissue Slices
2.8. Statistical Analysis
3. Results
3.1. Proteomic Profiling of CSF
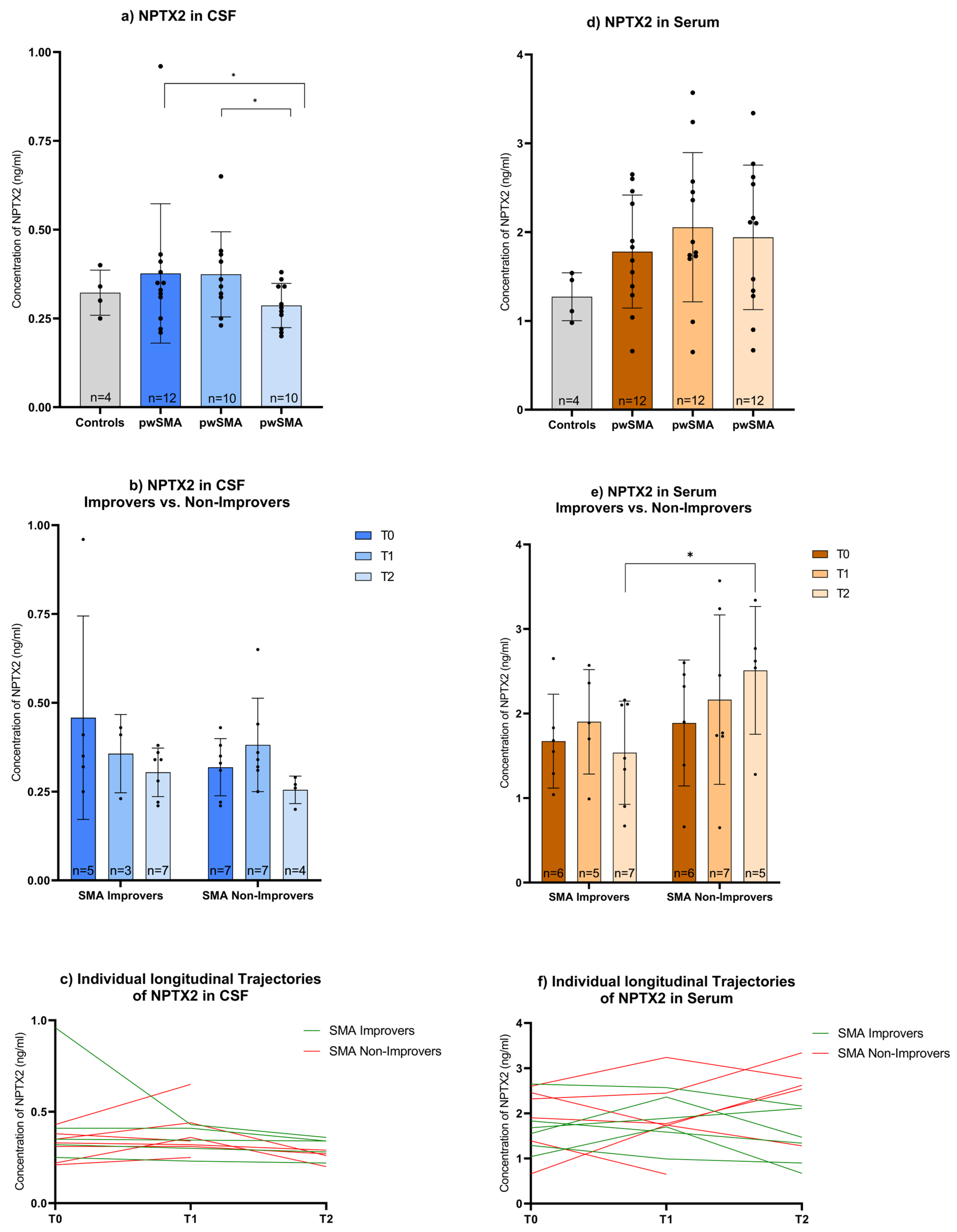
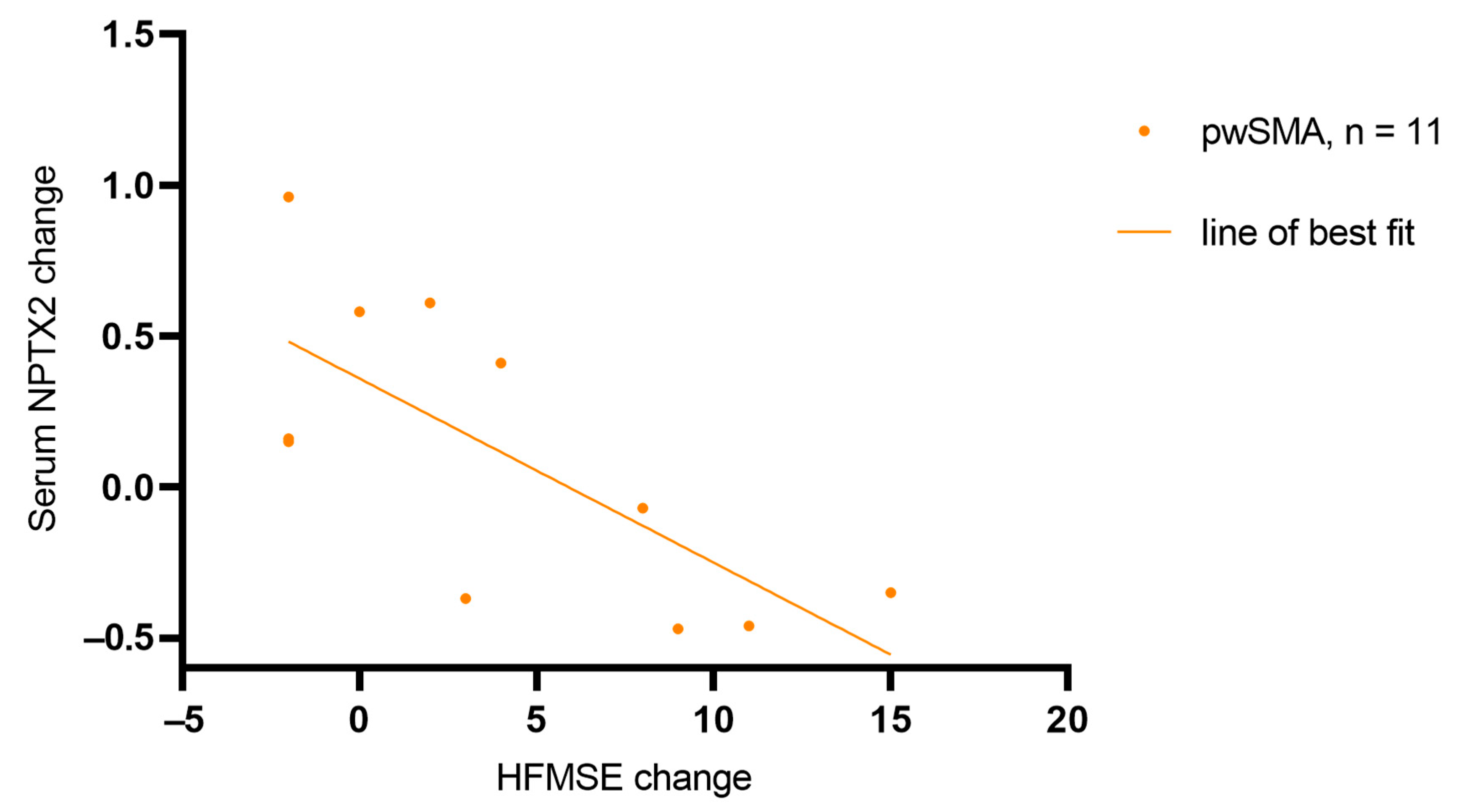
3.2. ELISA-Based Quantification of NPTX2, CNTN5, and ANXTR1 in CSF and Serum
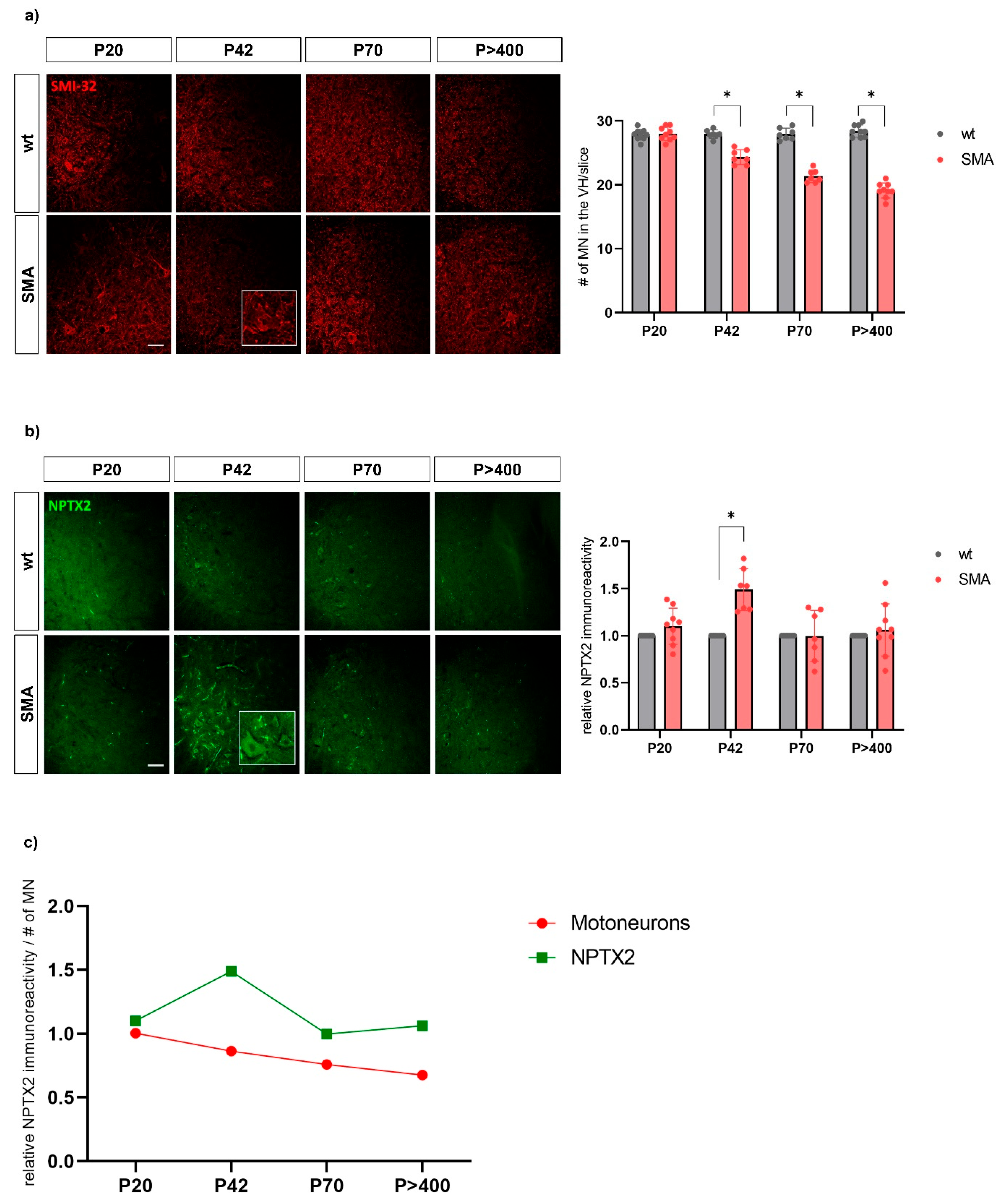
3.3. NPTX2 Immunostaining of Murine Spinal Cord Tissue Slices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lefebvre, S.; Bürglen, L.; Reboullet, S.; Clermont, O.; Burlet, P.; Viollet, L.; Benichou, B.; Cruaud, C.; Millasseau, P.; Zeviani, M.; et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell 1995, 80, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, S.; Burlet, P.; Liu, Q.; Bertrandy, S.; Clermont, O.; Munnich, A.; Dreyfuss, G.; Melki, J. Correlation between severity and SMN protein level in spinal muscular atrophy. Nat. Genet. 1997, 16, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Kolb, S.J.; Battle, D.J.; Dreyfuss, G. Molecular Functions of the SMN Complex. J. Child Neurol. 2007, 22, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Calucho, M.; Bernal, S.; Alías, L.; March, F.; Venceslá, A.; Rodríguez-Álvarez, F.J.; Aller, E.; Fernández, R.M.; Borrego, S.; Millán, J.M.; et al. Correlation between SMA type and SMN2 copy number revisited: An analysis of 625 unrelated Spanish patients and a compilation of 2834 reported cases. Neuromuscul. Disord. 2018, 28, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Wadman, R.I.; Wijngaarde, C.A.; Stam, M.; Bartels, B.; Otto, L.A.M.; Lemmink, H.H.; Schoenmakers, M.A.G.C.; Cuppen, I.; Van den Berg, L.H.; Van Der Pol, W.L. Muscle strength and motor function throughout life in a cross-sectional cohort of 180 patients with spinal muscular atrophy types 1c-4. Eur. J. Neurol. 2018, 25, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Hagenacker, T.; Wurster, C.D.; Günther, R.; Schreiber-Katz, O.; Osmanovic, A.; Petri, S.; Weiler, M.; Ziegler, A.; Kuttler, J.; Koch, J.C.; et al. Nusinersen in adults with 5q spinal muscular atrophy: A non-interventional, multicentre, observational cohort study. Lancet Neurol. 2020, 19, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Günther, R.; Wurster, C.D.; Brakemeier, S.; Osmanovic, A.; Schreiber-Katz, O.; Petri, S.; Uzelac, Z.; Hiebeler, M.; Thiele, S.; Walter, M.C.; et al. Long-term efficacy and safety of nusinersen in adults with 5q spinal muscular atrophy: A prospective European multinational observational study. Lancet Reg. Health—Eur. 2024, 39, 100862. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Deconinck, N.; Mazzone, E.S.; Nascimento, A.; Oskoui, M.; Saito, K.; Vuillerot, C.; Baranello, G.; Boespflug-Tanguy, O.; Goemans, N.; et al. Safety and efficacy of once-daily risdiplam in type 2 and non-ambulant type 3 spinal muscular atrophy (SUNFISH part 2): A phase 3, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2022, 21, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Brakemeier, S.; Lipka, J.; Schlag, M.; Kleinschnitz, C.; Hagenacker, T. Risdiplam improves subjective swallowing quality in non-ambulatory adult patients with 5q-spinal muscular atrophy despite advanced motor impairment. J. Neurol. 2024, 271, 2649–2657. [Google Scholar] [CrossRef] [PubMed]
- Maggi, L.; Bello, L.; Bonanno, S.; Govoni, A.; Caponnetto, C.; Passamano, L.; Grandis, M.; Trojsi, F.; Cerri, F.; Ferraro, M.; et al. Nusinersen safety and effects on motor function in adult spinal muscular atrophy type 2 and 3. J. Neurol. Neurosurg. Psychiatry 2020, 91, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Coratti, G.; Pera, M.C.; Montes, J.; Pasternak, A.; Scoto, M.; Baranello, G.; Messina, S.; Young, S.D.; Glanzman, A.M.; Duong, T.; et al. Different trajectories in upper limb and gross motor function in spinal muscular atrophy. Muscle Nerve 2021, 64, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Gaiani, A.; Martinelli, I.; Bello, L.; Querin, G.; Puthenparampil, M.; Ruggero, S.; Toffanin, E.; Cagnin, A.; Briani, C.; Pegoraro, E.; et al. Diagnostic and Prognostic Biomarkers in Amyotrophic Lateral Sclerosis. JAMA Neurol. 2017, 74, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.-J.; McGlasson, S.; Hunt, D.; Overell, J. Cerebrospinal fluid neurofilament light chain in multiple sclerosis and its subtypes: A meta-analysis of case–control studies. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Bayoumy, S.; Verberk, I.M.; Vermunt, L.; Willemse, E.; Dulk, B.D.; van der Ploeg, A.T.; Pajkrt, D.; Nitz, E.; Hout, J.M.v.D.; van der Post, J.; et al. Neurofilament light protein as a biomarker for spinal muscular atrophy: A review and reference ranges. Clin. Chem. Lab. Med. 2024, 62, 1252–1265. [Google Scholar] [CrossRef] [PubMed]
- Cordts, I.; Fuetterer, C.; Wachinger, A.; von Heynitz, R.; Kessler, T.; Freigang, M.; Quinten, A.L.; Bjelica, B.; Brakemeier, S.; Hobbiebrunken, E.; et al. Long-Term Dynamics of CSF and Serum Neurofilament Light Chain in Adult Patients With 5q Spinal Muscular Atrophy Treated With Nusinersen. Neurology 2025, 104, e213371. [Google Scholar] [CrossRef] [PubMed]
- Totzeck, A.; Stolte, B.; Kizina, K.; Bolz, S.; Schlag, M.; Thimm, A.; Kleinschnitz, C.; Hagenacker, T. Neurofilament Heavy Chain and Tau Protein Are Not Elevated in Cerebrospinal Fluid of Adult Patients with Spinal Muscular Atrophy during Loading with Nusinersen. Int. J. Mol. Sci. 2019, 20, 5397. [Google Scholar] [CrossRef] [PubMed]
- Birhanu, A.G. Mass spectrometry-based proteomics as an emerging tool in clinical laboratories. Clin. Proteom. 2023, 20, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Pera, M.C.; Coratti, G.; Forcina, N.; Mazzone, E.S.; Scoto, M.; Montes, J.; Pasternak, A.; Mayhew, A.; Messina, S.; Sframeli, M.; et al. Content validity and clinical meaningfulness of the HFMSE in spinal muscular atrophy. BMC Neurol. 2017, 17, 39. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, E.S.; Mayhew, A.; Montes, J.; Ramsey, D.; Fanelli, L.; Young, S.D.; Salazar, R.; De Sanctis, R.; Pasternak, A.; Glanzman, A.; et al. Revised upper limb module for spinal muscular atrophy: Development of a new module. Muscle Nerve 2017, 55, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.-F.; Xu, D.; Craig, M.T.; A Pelkey, K.; Chien, C.-C.; Shi, Y.; Zhang, J.; Resnick, S.; Pletnikova, O.; Salmon, D.; et al. NPTX2 and cognitive dysfunction in Alzheimer’s Disease. eLife 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Jang, Y.; Na, C.H. Discovery of Biomarkers for Amyotrophic Lateral Sclerosis from Human Cerebrospinal Fluid Using Mass-Spectrometry-Based Proteomics. Biomedicines 2023, 11, 1250. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, L.-I.; David, C.; Steffen, R.; Hezel, S.; Roos, A.; Schara-Schmidt, U.; Kleinschnitz, C.; Leo, M.; Hagenacker, T. Spinal astrocyte dysfunction drives motor neuron loss in late-onset spinal muscular atrophy. Acta Neuropathol. 2023, 145, 611–635. [Google Scholar] [CrossRef] [PubMed]
- Chapman, G.; Shanmugalingam, U.; Smith, P.D. The Role of Neuronal Pentraxin 2 (NP2) in Regulating Glutamatergic Signaling and Neuropathology. Front. Cell. Neurosci. 2020, 13, 575. [Google Scholar] [CrossRef] [PubMed]
- Bowerman, M.; Swoboda, K.J.; Michalski, J.; Wang, G.; Reeks, C.; Beauvais, A.; Murphy, K.; Woulfe, J.; Screaton, R.A.; Scott, F.W.; et al. Glucose metabolism and pancreatic defects in spinal muscular atrophy. Ann. Neurol. 2012, 72, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Reilly, A.; Beauvais, A.; Al-Aarg, M.; Yaworski, R.; Sutton, E.R.; Thebault, S.; Kothary, R. Peripheral defects precede neuromuscular pathology in the Smn2B/− mouse model of spinal muscular atrophy. J. Neuromuscul. Dis. 2024, 11, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Tsui, C.; Copeland, N.; Gilbert, D.; Jenkins, N.; Barnes, C.; Worley, P. Narp, a novel member of the pentraxin family, promotes neurite outgrowth and is dynamically regulated by neuronal activity. J. Neurosci. 1996, 16, 2463–2478. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C.; Park, J.M.; A Pelkey, K.; Grabenstatter, H.L.; Xu, D.; Linden, D.J.; Sutula, T.P.; McBain, C.J.; Worley, P.F. Narp regulates homeostatic scaling of excitatory synapses on parvalbumin-expressing interneurons. Nat. Neurosci. 2010, 13, 1090–1097. [Google Scholar] [CrossRef] [PubMed]
- O’bRien, R.J.; Xu, D.; Petralia, R.S.; Steward, O.; Huganir, R.L.; Worley, P. Synaptic Clustering of AMPA Receptors by the Extracellular Immediate-Early Gene Product Narp. Neuron 1999, 23, 309–323. [Google Scholar] [CrossRef] [PubMed]
- O'BRien, R.; Xu, D.; Mi, R.; Tang, X.; Hopf, C.; Worley, P. Synaptically Targeted Narp Plays an Essential Role in the Aggregation of AMPA Receptors at Excitatory Synapses in Cultured Spinal Neurons. J. Neurosci. 2002, 22, 4487–4498. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Wei, M.; Zhang, C.; Maxeiner, S.; Pak, C.; Botelho, S.C.; Trotter, J.; Sterky, F.H.; Südhof, T.C. Presynaptic Neuronal Pentraxin Receptor Organizes Excitatory and Inhibitory Synapses. J. Neurosci. 2016, 37, 1062–1080. [Google Scholar] [CrossRef] [PubMed]
- Cho, R.W.; Park, J.M.; Wolff, S.B.; Xu, D.; Hopf, C.; Kim, J.-A.; Reddy, R.C.; Petralia, R.S.; Perin, M.S.; Linden, D.J.; et al. mGluR1/5-Dependent Long-Term Depression Requires the Regulated Ectodomain Cleavage of Neuronal Pentraxin NPR by TACE. Neuron 2008, 57, 858–871. [Google Scholar] [CrossRef] [PubMed]
- A Schwarz, D.; Barry, G.; Mackay, K.B.; Manu, F.; Naeve, G.S.; Vana, A.M.; Verge, G.; Conlon, P.J.; Foster, A.C.; A Maki, R. Identification of differentially expressed genes induced by transient ischemic stroke. Mol. Brain Res. 2002, 101, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Leo, M.; Schmitt, L.-I.; Fleischer, M.; Steffen, R.; Osswald, C.; Kleinschnitz, C.; Hagenacker, T. Induction of Survival of Motor Neuron (SMN) Protein Deficiency in Spinal Astrocytes by Small Interfering RNA as an In Vitro Model of Spinal Muscular Atrophy. Cells 2022, 11, 558. [Google Scholar] [CrossRef] [PubMed]
- Welby, E.; Ebert, A.D. Diminished motor neuron activity driven by abnormal astrocytic EAAT1 glutamate transporter activity in spinal muscular atrophy is not fully restored after lentiviral SMN delivery. Glia 2023, 71, 1311–1332. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Costa, J.F.; Povedano, M.; Nascimiento-Osorio, A.E.; Escribano, A.M.; Garcia, S.K.; Dominguez, R.; Exposito, J.M.; González, L.; Marco, C.; Castillo, J.M.; et al. Validation of motor and functional scales for the evaluation of adult patients with 5q spinal muscular atrophy. Eur. J. Neurol. 2022, 29, 3666–3675. [Google Scholar] [CrossRef] [PubMed]
- Göverti, D.; Büyüklüoğlu, N.; Kaya, H.; Yüksel, R.N.; Yücel, Ç.; Göka, E. Neuronal pentraxin-2 (NPTX2) serum levels during an acute psychotic episode in patients with schizophrenia. Psychopharmacology 2022, 239, 2585–2591. [Google Scholar] [CrossRef] [PubMed]
- Shao, K.; Shan, S.; Ru, W.; Ma, C. Association between serum NPTX2 and cognitive function in patients with vascular dementia. Brain Behav. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Lunke, S.; Bouffler, S.E.; Patel, C.V.; Sandaradura, S.A.; Wilson, M.; Pinner, J.; Hunter, M.F.; Barnett, C.P.; Wallis, M.; Kamien, B.; et al. Integrated multi-omics for rapid rare disease diagnosis on a national scale. Nat. Med. 2023, 29, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Kessler, T.; Latzer, P.; Schmid, D.; Warnken, U.; Saffari, A.; Ziegler, A.; Kollmer, J.; Möhlenbruch, M.; Ulfert, C.; Herweh, C.; et al. Cerebrospinal fluid proteomic profiling in nusinersen-treated patients with spinal muscular atrophy. J. Neurochem. 2020, 153, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Panicucci, C.; Sahin, E.; Bartolucci, M.; Casalini, S.; Brolatti, N.; Pedemonte, M.; Baratto, S.; Pintus, S.; Principi, E.; D’aMico, A.; et al. Proteomics profiling and machine learning in nusinersen-treated patients with spinal muscular atrophy. Cell. Mol. Life Sci. 2024, 81, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Meneri, M.; Abati, E.; Gagliardi, D.; Faravelli, I.; Parente, V.; Ratti, A.; Verde, F.; Ticozzi, N.; Comi, G.P.; Ottoboni, L.; et al. Identification of Novel Biomarkers of Spinal Muscular Atrophy and Therapeutic Response by Proteomic and Metabolomic Profiling of Human Biological Fluid Samples. Biomedicines 2023, 11, 1254. [Google Scholar] [CrossRef] [PubMed]
- Zandl-Lang, M.; Züllig, T.; Holzer, M.; Eichmann, T.O.; Darnhofer, B.; Schwerin-Nagel, A.; Zobel, J.; Haidl, H.; Biebl, A.; Köfeler, H.; et al. Multi-omics profiling in spinal muscular atrophy (SMA): Investigating lipid and metabolic alterations through longitudinal CSF analysis of Nusinersen-treated patients. J. Neurol. 2025, 272, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wieske, L.; Martín-Aguilar, L.; Fehmi, J.; Lleixà, C.; Koel-Simmelink, M.J.; Chatterjee, M.; van Lierop, Z.; Killestein, J.; Verhamme, C.; Querol, L.; et al. Serum Contactin-1 in CIDP. Neurol.-Neuroimmunol. Neuroinflamm. 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.J.; Isensee, J.; Neel, D.V.; Quadros, A.U.; Zhang, H.-X.B.; Lauzadis, J.; Liu, S.M.; Shiers, S.; Belu, A.; Palan, S.; et al. Anthrax toxins regulate pain signaling and can deliver molecular cargoes into ANTXR2+ DRG sensory neurons. Nat. Neurosci. 2021, 25, 168–179. [Google Scholar] [CrossRef] [PubMed]

| Sex | Male (%) | Female (%) | |
|---|---|---|---|
| Proteome analysis | 5 (71) | 2 (29) | |
| ELISA Analysis | 10 (71) | 4 (29) | |
| SMA type | 2 | 3 | |
| Proteome analysis | 2 (29) | 5 (71) | |
| ELISA Analysis | 1 (7) | 13 (93) | |
| SMN2 copy number | 2 (%) | 3 (%) | 4 (%) |
| Proteome analysis | 0 (0) | 3 (43) | 4 (57) |
| ELISA Analysis | 1 (7) | 6 (43) | 7 (50) |
| Clinical classification | Non-Sitter | Sitter | Walker |
| Proteome analysis | 0 (0) | 4 (57) | 3 (43) |
| ELISA Analysis | 4 (29) | 1 (7) | 9 (64) |
| Spondylodesis | Yes (%) | No (%) | |
| Proteome analysis | 1 (14) | 6 (86) | |
| ELISA Analysis | 2 (14) | 12 (86) | |
| Non-invasive ventilation | Yes (%) | No (%) | |
| Proteome analysis | 2 (29) | 5 (71) | |
| ELISA Analysis | 3 (21) | 11 (79) | |
| Age | Mean (sd a) | Minimum | Maximum |
| Proteome analysis | 40.00 (14.28) | 26 | 65 |
| ELISA Analysis | 37.71 (12.86) | 22 | 62 |
| Disease duration in years | Mean (sd) | Minimum | Maximum |
| Proteome analysis | 35.01 (16.22) | 14 | 63 |
| ELISA Analysis | 29.08 (14.34) | 12 | 61 |
| Baseline HFMSE score | Mean (sd) | Minimum | Maximum |
| Proteome analysis | 28.00 (22.06) | 0 | 57 |
| ELISA Analysis | 32.50 (21.38) | 1 | 62 |
| Baseline RULM score | Mean (sd) | Minimum | Maximum |
| Proteome analysis | 25.00 (13.90) | 0 | 37 |
| ELISA Analysis | 29.64 (10.49) | 7 | 37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neuhoff, S.; Schmitt, L.-I.; Liebig, K.C.; Hezel, S.; Tilahun, N.I.; Kleinschnitz, C.; Leo, M.; Hagenacker, T. Neuronal Pentraxin 2 as a Potential Biomarker for Nusinersen Therapy Response in Adults with Spinal Muscular Atrophy: A Pilot Study. Biomedicines 2025, 13, 1821. https://doi.org/10.3390/biomedicines13081821
Neuhoff S, Schmitt L-I, Liebig KC, Hezel S, Tilahun NI, Kleinschnitz C, Leo M, Hagenacker T. Neuronal Pentraxin 2 as a Potential Biomarker for Nusinersen Therapy Response in Adults with Spinal Muscular Atrophy: A Pilot Study. Biomedicines. 2025; 13(8):1821. https://doi.org/10.3390/biomedicines13081821
Chicago/Turabian StyleNeuhoff, Svenja, Linda-Isabell Schmitt, Kai Christine Liebig, Stefanie Hezel, Nick Isana Tilahun, Christoph Kleinschnitz, Markus Leo, and Tim Hagenacker. 2025. "Neuronal Pentraxin 2 as a Potential Biomarker for Nusinersen Therapy Response in Adults with Spinal Muscular Atrophy: A Pilot Study" Biomedicines 13, no. 8: 1821. https://doi.org/10.3390/biomedicines13081821
APA StyleNeuhoff, S., Schmitt, L.-I., Liebig, K. C., Hezel, S., Tilahun, N. I., Kleinschnitz, C., Leo, M., & Hagenacker, T. (2025). Neuronal Pentraxin 2 as a Potential Biomarker for Nusinersen Therapy Response in Adults with Spinal Muscular Atrophy: A Pilot Study. Biomedicines, 13(8), 1821. https://doi.org/10.3390/biomedicines13081821







