Nicotine Therapy for Parkinson’s Disease: A Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Study Selection
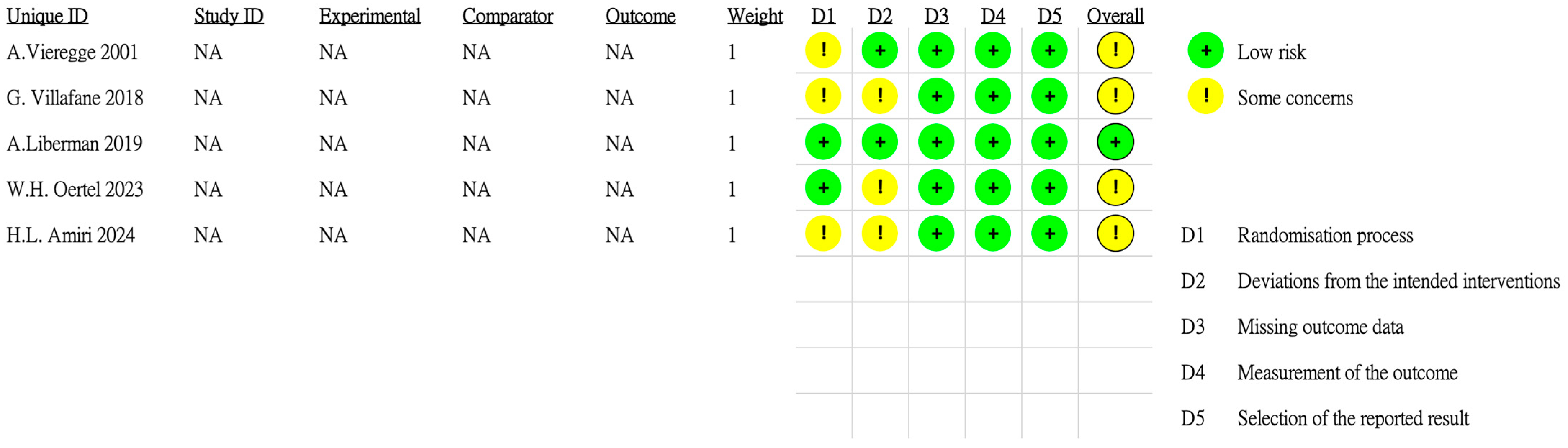
2.2. Data Extraction and Quality Assessment
2.3. Outcomes and Statistical Analysis
3. Results
3.1. Summary of Included Studies
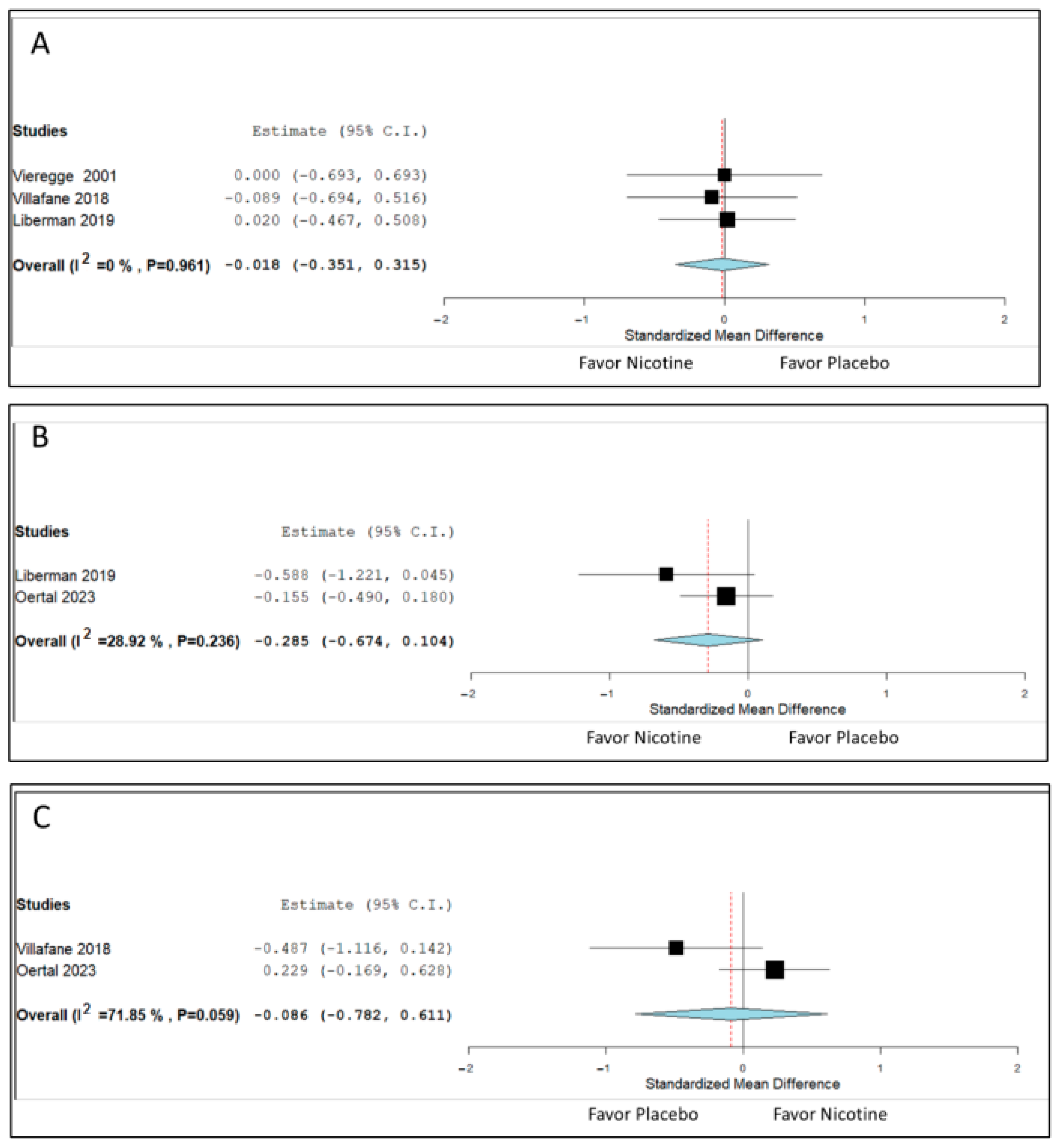
3.2. Changes in Motor Symptoms
3.3. Changes in ADLs, QoL, and Cognition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef] [PubMed]
- Vijiaratnam, N.; Simuni, T.; Bandmann, O.; Morris, H.R.; Foltynie, T. Progress towards therapies for disease modification in Parkinson’s disease. Lancet Neurol. 2021, 20, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Mari, Z.; Mestre, T.A. The Disease Modification Conundrum in Parkinson’s Disease: Failures and Hopes. Front. Aging Neurosci. 2022, 14, 810860. [Google Scholar] [CrossRef] [PubMed]
- Parkinson Study Group QE3 Investigators; Beal, M.F.; Oakes, D.; Shoulson, I.; Henchcliffe, C.; Galpern, W.R.; Haas, R.; Juncos, J.L.; Nutt, J.G.; Voss, T.S. A Randomized Clinical Trial of High-Dosage Coenzyme Q10 in Early Parkinson Disease: No Evidence of Benefit. JAMA Neurol. 2014, 71, 543–552. [Google Scholar] [CrossRef]
- Pioglitazone in early Parkinson’s disease: A phase 2, multicentre, double-blind, randomised trial. Lancet Neurol. 2015, 14, 795–803. [CrossRef]
- Vijiaratnam, N.; Girges, C.; Auld, G.; McComish, R.; King, A.; Skene, S.S.; Hibbert, S.; Wong, A.; Melander, S.; Gibson, R.; et al. Exenatide once a week versus placebo as a potential disease-modifying treatment for people with Parkinson’s disease in the UK: A phase 3, multicentre, double-blind, parallel-group, randomised, placebo-controlled trial. Lancet 2025, 405, 627–636. [Google Scholar] [CrossRef]
- Quik, M.; O’Leary, K.; Tanner, C.M. Nicotine and Parkinson’s disease: Implications for therapy. Mov. Disord. 2008, 23, 1641–1652. [Google Scholar] [CrossRef]
- Li, X.; Li, W.; Liu, G.; Shen, X.; Tang, Y. Association between cigarette smoking and Parkinson’s disease: A meta-analysis. Arch. Gerontol. Geriatr. 2015, 61, 510–516. [Google Scholar] [CrossRef]
- Mappin-Kasirer, B.; Pan, H.; Lewington, S.; Kizza, J.; Gray, R.; Clarke, R.; Peto, R. Tobacco smoking and the risk of Parkinson disease: A 65-year follow-up of 30,000 male British doctors. Neurology 2020, 94, e2132–e2138. [Google Scholar] [CrossRef]
- Lin, X.; Li, Q.; Pu, M.; Dong, H.; Zhang, Q. Significance of nicotine and nicotinic acetylcholine receptors in Parkinson’s disease. Front. Aging Neurosci. 2025, 17, 1535310. [Google Scholar] [CrossRef]
- Kelton, M.C.; Kahn, H.J.; Conrath, C.L.; Newhouse, P.A. The effects of nicotine on Parkinson’s disease. Brain Cogn. 2000, 43, 274–282. [Google Scholar]
- Lemay, S.; Chouinard, S.; Blanchet, P.; Masson, H.; Soland, V.; Beuter, A.; Bédard, M.A. Lack of efficacy of a nicotine transdermal treatment on motor and cognitive deficits in Parkinson’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 2004, 28, 31–39. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, A.; Lockhart, T.E.; Olson, M.C.; Smith Hussain, V.A.; Frames, C.W.; Sadreddin, A.; McCauley, M.; Ludington, E. Nicotine Bitartrate Reduces Falls and Freezing of Gait in Parkinson Disease: A Reanalysis. Front. Neurol. 2019, 10, 424. [Google Scholar] [CrossRef] [PubMed]
- Lorvand Amiri, H.; Hassan Javanbakht, M.; Mohammad Baghbanian, S.; Parsaeian, M. The effect of a nicotine-rich diet with/without redistribution of dietary protein on motor indices in patients with Parkinson’s disease: A randomized clinical trial. J. Clin. Neurosci. 2024, 129, 110845. [Google Scholar] [CrossRef] [PubMed]
- Oertel, W.H.; Müller, H.H.; Unger, M.M.; Schade-Brittinger, C.; Balthasar, K.; Articus, K.; Brinkman, M.; Venuto, C.S.; Tracik, F.; Eberling, J.; et al. Transdermal Nicotine Treatment and Progression of Early Parkinson’s Disease. NEJM Evid. 2023, 2, EVIDoa2200311. [Google Scholar] [CrossRef]
- Villafane, G.; Thiriez, C.; Audureau, E.; Straczek, C.; Kerschen, P.; Cormier-Dequaire, F.; Van Der Gucht, A.; Gurruchaga, J.M.; Quéré-Carne, M.; Evangelista, E.; et al. High-dose transdermal nicotine in Parkinson’s disease patients: A randomized, open-label, blinded-endpoint evaluation phase 2 study. Eur. J. Neurol. 2018, 25, 120–127. [Google Scholar] [CrossRef]
- Vieregge, A.; Sieberer, M.; Jacobs, H.; Hagenah, J.M.; Vieregge, P. Transdermal nicotine in PD: A randomized, double-blind, placebo-controlled study. Neurology 2001, 57, 1032–1035. [Google Scholar] [CrossRef]
- Fant, R.V.; Henningfield, J.E.; Shiffman, S.; Strahs, K.R.; Reitberg, D.P. A pharmacokinetic crossover study to compare the absorption characteristics of three transdermal nicotine patches. Pharmacol. Biochem. Behav. 2000, 67, 479–482. [Google Scholar] [CrossRef]
- Lane, J.D.; Westman, E.C.; Ripka, G.V.; Wu, J.; Chiang, C.-C.; Rose, J.E. Pharmacokinetics of a Transdermal Nicotine Patch Compared to Nicotine Gum. Drug Dev. Ind. Pharm. 1993, 19, 1999–2010. [Google Scholar] [CrossRef]
- Lindson, N.; Chepkin, S.C.; Ye, W.; Fanshawe, T.R.; Bullen, C.; Hartmann-Boyce, J. Different doses, durations and modes of delivery of nicotine replacement therapy for smoking cessation. Cochrane Database Syst. Rev. 2019, 2019, Cd013308. [Google Scholar] [CrossRef]
- Paulson, G.W.; Dadmehr, N. Is there a premorbid personality typical for Parkinson’s disease? Neurology 1991, 41, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Sieurin, J.; Gustavsson, P.; Weibull, C.E.; Feldman, A.L.; Petzinger, G.M.; Gatz, M.; Pedersen, N.L.; Wirdefeldt, K. Personality traits and the risk for Parkinson disease: A prospective study. Eur. J. Epidemiol. 2016, 31, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Noyce, A.J.; Lees, A.J.; Schrag, A.-E. The prediagnostic phase of Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2016, 87, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Bestwick, J.P.; Auger, S.D.; Simonet, C.; Rees, R.N.; Rack, D.; Jitlal, M.; Giovannoni, G.; Lees, A.J.; Cuzick, J.; Schrag, A.E.; et al. Improving estimation of Parkinson’s disease risk—The enhanced PREDICT-PD algorithm. npj Park. Dis. 2021, 7, 33. [Google Scholar] [CrossRef]
- Heinzel, S.; Berg, D.; Gasser, T.; Chen, H.; Yao, C.; Postuma, R.B.; the MDS Task Force on the Definition of Parkinson’s Disease. Update of the MDS research criteria for prodromal Parkinson’s disease. Mov. Disord. 2019, 34, 1464–1470. [Google Scholar] [CrossRef]


| Study (First Author, Publication Year) | Country/ Setting | Sample Size | Age (Years), Mean ± Standard Deviation | H and Y Stage | Intervention | Treatment Duration (Weeks) | Outcomes |
|---|---|---|---|---|---|---|---|
| Vieregge, 2001 [19] | Germany | 32 | I: 67 ± 6.8 C: 66 ± 7.5 | II or III | Transdermal patch, 35 mg/day | 3 | Motor function and ADL performance |
| Villafane, 2018 [18] | France | 42 | I: 58.0 ± 8.4 C: 56.9 ± 6.3 | II–IV | Transdermal patch, 90 mg/day | 39 | Motor function, ADL performance, and cognition |
| Lieberman, 2019 [15] | United States | 65 | I: 68.1 ± 8.3 C: 65.5 ± 7.2 | II–IV | Oral nicotine bitartrate, 24 mg/day | 10 | L-Dopa-induced dyskinesia, motor function, and ADL performance |
| Oertel, 2023 [17] | United States and Germany | 162 | I: 61.0 ± 9.5 C: 61.0 ± 10.3 | I or II | Transdermal patch, 28 mg/day | 52 | Motor function, cognition, depression, and quality of life |
| Amiri, 2024 [16] | Iran | 45 * | I: 61.53 ± 8.33 C: 60 ± 7.21 | Not reported | Nicotine-rich diet, 20 µg/day | 12 | Motor function and ADL performance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, C.-H.; Huang, T.-W.; Chiu, W.-T.; Chung, C.-C.; Hong, C.-T. Nicotine Therapy for Parkinson’s Disease: A Meta-Analysis of Randomized Controlled Trials. Biomedicines 2025, 13, 1814. https://doi.org/10.3390/biomedicines13081814
Liang C-H, Huang T-W, Chiu W-T, Chung C-C, Hong C-T. Nicotine Therapy for Parkinson’s Disease: A Meta-Analysis of Randomized Controlled Trials. Biomedicines. 2025; 13(8):1814. https://doi.org/10.3390/biomedicines13081814
Chicago/Turabian StyleLiang, Chih-Hung, Tsai-Wei Huang, Wei-Ting Chiu, Chen-Chih Chung, and Chien-Tai Hong. 2025. "Nicotine Therapy for Parkinson’s Disease: A Meta-Analysis of Randomized Controlled Trials" Biomedicines 13, no. 8: 1814. https://doi.org/10.3390/biomedicines13081814
APA StyleLiang, C.-H., Huang, T.-W., Chiu, W.-T., Chung, C.-C., & Hong, C.-T. (2025). Nicotine Therapy for Parkinson’s Disease: A Meta-Analysis of Randomized Controlled Trials. Biomedicines, 13(8), 1814. https://doi.org/10.3390/biomedicines13081814