Amylin Receptor 1 Mutagenesis Revealed a Potential Role of Calcitonin Serine 29 in Receptor Interaction
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Line and Bacteria Cells Used
2.3. Expression Plasmids
2.4. Receptor Protein Purification
2.5. Synthetic Peptides
2.6. Fluorescence Polarization/Anisotropy (FP) Receptor–Ligand Binding Assay
2.7. Homology Model Structures
2.8. Statistical Analysis
3. Results
3.1. Rationale of Selecting Amylin Receptor Residues for Mutagenesis
3.2. Mutational Effects of Calcitonin Receptor D101 and N135 Residues on Peptide Probe Affinity
3.3. Mutational Effects of Calcitonin Receptor E123 and N124 Residues on Peptide Probe Affinity
3.4. Mutational Effects of the Calcitonin Receptor D97 Residue on Peptide Probe Affinity
4. Discussion
4.1. Effects of FITC Labeling to the Calcitonin Fragment on Receptor ECD Binding
4.2. Limitation of the Current Study in Interpreting Peptide Ligand Interaction
4.3. Alanine-Scanning Mutagenesis Studies on Peptide Interaction with Amylin Receptor 1
4.4. Future Studies and Closing Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hay, D.L.; Chen, S.; Lutz, T.A.; Parkes, D.G.; Roth, J.D. Amylin: Pharmacology, Physiology, and Clinical Potential. Pharmacol. Rev. 2015, 67, 564–600. [Google Scholar] [CrossRef] [PubMed]
- McQueen, J. Pramlintide acetate. Am. J. Health Syst. Pharm. 2005, 62, 2363–2372. [Google Scholar] [CrossRef] [PubMed]
- Kruse, T.; Hansen, J.L.; Dahl, K.; Schaffer, L.; Sensfuss, U.; Poulsen, C.; Schlein, M.; Hansen, A.M.; Jeppesen, C.B.; de la Cour, C.D.; et al. Development of Cagrilintide, a Long-Acting Amylin Analogue. J. Med. Chem. 2021, 64, 11183–11194. [Google Scholar] [CrossRef] [PubMed]
- ID NCT05567796, A Research Study to See How Well CagriSema Helps People with Excess Body Weight Lose Weight (REDEFINE 1). 2025. Available online: https://ClinicalTrials.gov (accessed on 2 June 2025).
- Enebo, L.B.; Berthelsen, K.K.; Kankam, M.; Lund, M.T.; Rubino, D.M.; Satylganova, A.; Lau, D.C. Safety, tolerability, pharmacokinetics, and pharmacodynamics of concomitant administration of multiple doses of cagrilintide with semaglutide 2.4 mg for weight management: A randomised, controlled, phase 1b trial. Lancet 2021, 397, 1736–1748. [Google Scholar] [CrossRef] [PubMed]
- Lau, D.C.; Erichsen, L.; Francisco, A.M.; Satylganova, A.; le Roux, C.W.; McGowan, B.; Pedersen, S.D.; Pietilainen, K.H.; Rubino, D.; Batterham, R.L. Once-weekly cagrilintide for weight management in people with overweight and obesity: A multicentre, randomised, double-blind, placebo-controlled and active-controlled, dose-finding phase 2 trial. Lancet 2021, 398, 2160–2172. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Belousoff, M.J.; Liang, Y.; Johnson, R.M.; Josephs, T.M.; Fletcher, M.M.; Christopoulos, A.; Hay, D.L.; Danev, R.; Wootten, D.; et al. A structural basis for amylin receptor phenotype. Science 2022, 375, eabm9609. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.L.; Khoshouei, M.; Radjainia, M.; Zhang, Y.; Glukhova, A.; Tarrasch, J.; Thal, D.M.; Furness, S.G.; Christopoulos, G.; Coudrat, T.; et al. Phase-plate cryo-EM structure of a class B GPCR-G-protein complex. Nature 2017, 546, 118–123. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sexton, P.M.; Findlay, D.M.; Martin, T.J. Calcitonin. Curr. Med. Chem. 1999, 6, 1067–1093. [Google Scholar] [CrossRef] [PubMed]
- Christopoulos, G.; Perry, K.J.; Morfis, M.; Tilakaratne, N.; Gao, Y.; Fraser, N.J.; Main, M.J.; Foord, S.M.; Sexton, P.M. Multiple amylin receptors arise from receptor activity-modifying protein interaction with the calcitonin receptor gene product. Mol. Pharmacol. 1999, 56, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Tilakaratne, N.; Christopoulos, G.; Zumpe, E.T.; Foord, S.M.; Sexton, P.M. Amylin receptor phenotypes derived from human calcitonin receptor/RAMP coexpression exhibit pharmacological differences dependent on receptor isoform and host cell environment. J. Pharmacol. Exp. Ther. 2000, 294, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Keov, P.; Christopoulos, G.; Hick, C.A.; Glendorf, T.; Ballarin-Gonzalez, B.; Wootten, D.; Sexton, P.M. Development of a Novel Assay for Direct Assessment of Selective Amylin Receptor Activation Reveals Novel Differences in Behavior of Selective and Nonselective Peptide Agonists. Mol. Pharmacol. 2024, 105, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Gostynska, S.E.; Karim, J.A.; Ford, B.E.; Gordon, P.H.; Babin, K.M.; Inoue, A.; Lambert, N.A.; Pioszak, A.A. Amylin receptor subunit interactions are modulated by agonists and determine signaling. BioRxiv 2024. BioRxiv:2024.10.09.617487. [Google Scholar]
- Roder, C.; Kupreichyk, T.; Gremer, L.; Schafer, L.U.; Pothula, K.R.; Ravelli, R.B.; Willbold, D.; Hoyer, W.; Schroder, G.F. Cryo-EM structure of islet amyloid polypeptide fibrils reveals similarities with amyloid-beta fibrils. Nat. Struct. Mol. Biol. 2020, 27, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Boyer, D.R.; Sawaya, M.R.; Ge, P.; Eisenberg, D.S. Cryo-EM structure and inhibitor design of human IAPP (amylin) fibrils. Nat. Struct. Mol. Biol. 2020, 27, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Shigihara, N.; Fukunaka, A.; Hara, A.; Komiya, K.; Honda, A.; Uchida, T.; Abe, H.; Toyofuku, Y.; Tamaki, M.; Ogihara, T.; et al. Human IAPP-induced pancreatic beta cell toxicity and its regulation by autophagy. J. Clin. Invest. 2014, 124, 3634–3644. [Google Scholar] [CrossRef] [PubMed]
- Green, J.; Goldsbury, C.; Mini, T.; Sunderji, S.; Frey, P.; Kistler, J.; Cooper, G.; Aebi, U. Full-length rat amylin forms fibrils following substitution of single residues from human amylin. J. Mol. Biol. 2003, 326, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Aricescu, A.R.; Lu, W.; Jones, E.Y. A time- and cost-efficient system for high-level protein production in mammalian cells. Acta Crystallogr. D Biol. Crystallogr. 2006, 62, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Lee, S. Development of High Affinity Calcitonin Analog Fragments Targeting Extracellular Domains of Calcitonin Family Receptors. Biomolecules 2021, 11, 1364. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Hay, D.L.; Pioszak, A.A. Calcitonin and Amylin Receptor Peptide Interaction Mechanisms: Insights into Peptide-Binding Modes and Allosteric Modulation of the Calcitonin Receptor by Receptor Activity-Modifying Proteins. J. Biol. Chem. 2016, 291, 8686–8700. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, S.; Booe, J.M.; Gingell, J.J.; Sjoelund, V.; Hay, D.L.; Pioszak, A.A. N-Glycosylation of Asparagine 130 in the Extracellular Domain of the Human Calcitonin Receptor Significantly Increases Peptide Hormone Affinity. Biochemistry 2017, 56, 3380–3393. [Google Scholar] [CrossRef] [PubMed]
- Roehrl, M.H.; Wang, J.Y.; Wagner, G. A general framework for development and data analysis of competitive high-throughput screens for small-molecule inhibitors of protein-protein interactions by fluorescence polarization. Biochemistry 2004, 43, 16056–16066. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Jeong, Y.; Simms, J.; Warner, M.L.; Poyner, D.R.; Chung, K.Y.; Pioszak, A.A. Calcitonin Receptor N-Glycosylation Enhances Peptide Hormone Affinity by Controlling Receptor Dynamics. J. Mol. Biol. 2020, 432, 1996–2014. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Belousoff, M.J.; Johnson, R.M.; Keov, P.; Mariam, Z.; Deganutti, G.; Christopoulos, G.; Hick, C.A.; Reedtz-Runge, S.; Glendorf, T.; et al. Structural and dynamic features of cagrilintide binding to calcitonin and amylin receptors. Nat. Commun. 2025, 16, 3389. [Google Scholar] [CrossRef] [PubMed]
- Lee, S. Development of the novel amylin and calcitonin receptor activators by peptide mutagenesis. Arch. Biochem. Biophys. 2024, 762, 110191. [Google Scholar] [CrossRef] [PubMed]
- Bower, R.L.; Yule, L.; Rees, T.A.; Deganutti, G.; Hendrikse, E.R.; Harris, P.W.; Kowalczyk, R.; Ridgway, Z.; Wong, A.G.; Swierkula, K.; et al. Molecular Signature for Receptor Engagement in the Metabolic Peptide Hormone Amylin. ACS Pharmacol. Transl. Sci. 2018, 1, 32–49. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Booe, J.M.; Walker, C.S.; Barwell, J.; Kuteyi, G.; Simms, J.; Jamaluddin, M.A.; Warner, M.L.; Bill, R.M.; Harris, P.W.; Brimble, M.A.; et al. Structural Basis for Receptor Activity-Modifying Protein-Dependent Selective Peptide Recognition by a G Protein-Coupled Receptor. Mol. Cell 2015, 58, 1040–1052. [Google Scholar] [CrossRef] [PubMed]
- Gingell, J.J.; Simms, J.; Barwell, J.; Poyner, D.R.; Watkins, H.A.; Pioszak, A.A.; Sexton, P.M.; Hay, D.L. An allosteric role for receptor activity-modifying proteins in defining GPCR pharmacology. Cell. Discov. 2016, 2, 16012. [Google Scholar] [CrossRef] [PubMed]
- Johansson, E.; Hansen, J.L.; Hansen, A.M.; Shaw, A.C.; Becker, P.; Schaffer, L.; Reedtz-Runge, S. Type II Turn of Receptor-bound Salmon Calcitonin Revealed by X-ray Crystallography. J. Biol. Chem. 2016, 291, 13689–13698. [Google Scholar] [CrossRef] [PubMed]
- Maso, E.D.; Glukhova, A.; Zhu, Y.; Garcia-Nafria, J.; Tate, C.G.; Atanasio, S.; Reynolds, C.A.; Ramirez-Aportela, E.; Carazo, J.; Hick, C.A.; et al. The Molecular Control of Calcitonin Receptor Signaling. ACS Pharmacol. Transl. Sci. 2019, 2, 31–51. [Google Scholar] [CrossRef] [PubMed]
- Maso, E.D.; Zhu, Y.; Pham, V.; Reynolds, C.A.; Deganutti, G.; Hick, C.A.; Yang, D.; Christopoulos, A.; Hay, D.L.; Wang, M.; et al. Extracellular loops 2 and 3 of the calcitonin receptor selectively modify agonist binding and efficacy. Biochem. Pharmacol. 2018, 150, 214–244. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.; Zhu, Y.; Maso, E.D.; Reynolds, C.A.; Deganutti, G.; Atanasio, S.; Hick, C.A.; Yang, D.; Christopoulos, A.; Hay, D.L.; et al. Deconvoluting the Molecular Control of Binding and Signaling at the Amylin 3 Receptor: RAMP3 Alters Signal Propagation through Extracellular Loops of the Calcitonin Receptor. ACS Pharmacol. Transl. Sci. 2019, 2, 183–197. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoare, S.R. Mechanisms of peptide and nonpeptide ligand binding to Class B G-protein-coupled receptors. Drug Discov. Today 2005, 10, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Parthier, C.; Reedtz-Runge, S.; Rudolph, R.; Stubbs, M.T. Passing the baton in class B GPCRs: Peptide hormone activation via helix induction? Trends Biochem. Sci. 2009, 34, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Booe, J.M.; Warner, M.L.; Pioszak, A.A. Picomolar Affinity Antagonist and Sustained Signaling Agonist Peptide Ligands for the Adrenomedullin and Calcitonin Gene-Related Peptide Receptors. ACS Pharmacol. Transl. Sci. 2020, 3, 759–772. [Google Scholar] [CrossRef] [PubMed]



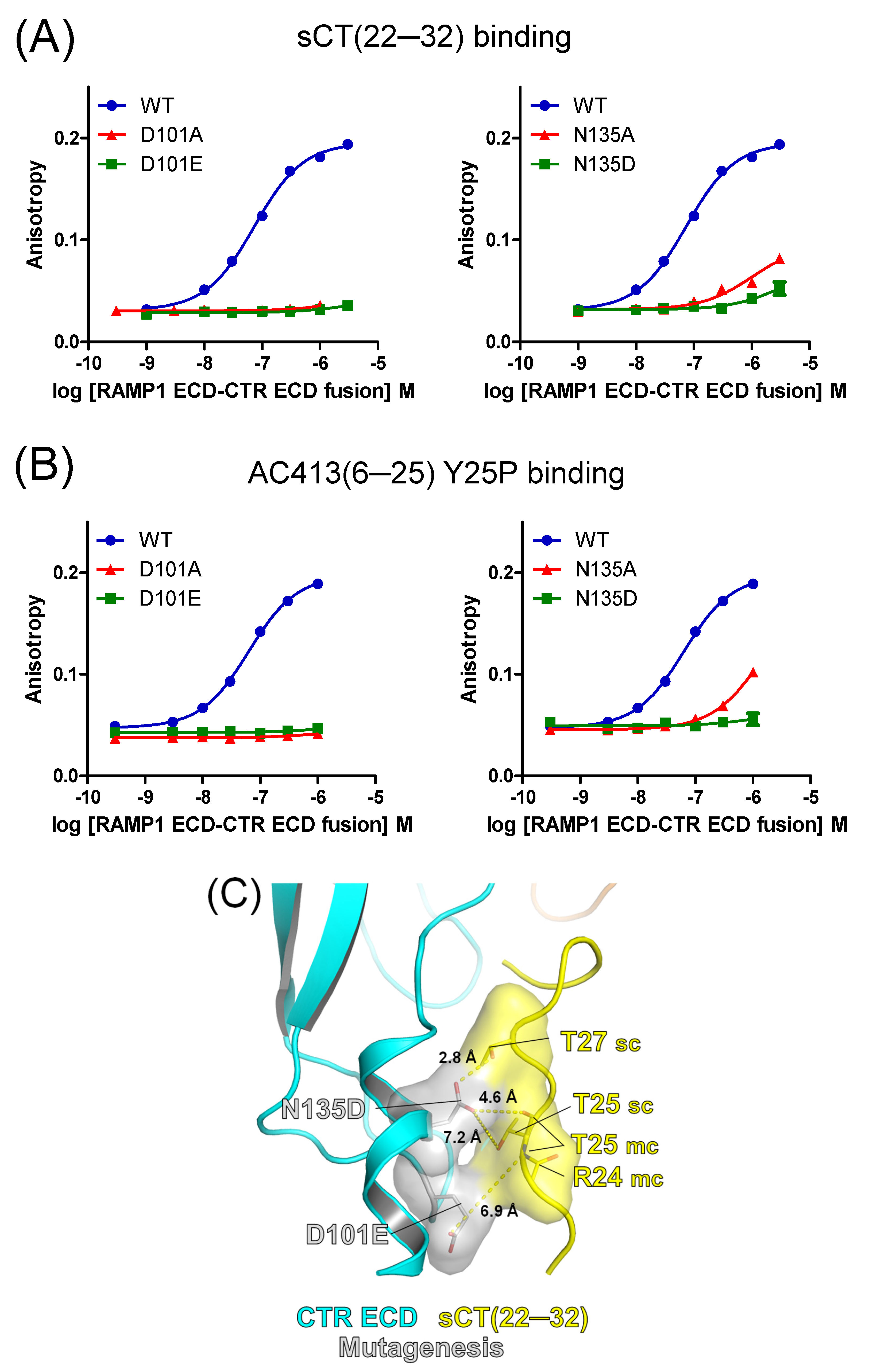



| Figure and Peptide Probe | Amylin Receptor 1 ECD | N | pKD Mean ± S.D. | Mean KD |
|---|---|---|---|---|
| FITC-sCT(22–32) | WT | 3 | 7.09 ± 0.13 | 81 nM |
| FITC-AC413(6–25) Y25P | WT | 4 | 7.24 ± 0.17 | 57 nM |
| Figure 3A FITC-sCT(22–32) | D101A | 3 | N.D. | N.D. |
| D101E | 3 | N.D. | N.D. | |
| N135A | 3 | N.D. | N.D. | |
| N135D | 3 | N.D. | N.D. | |
| Figure 3B FITC-AC413(6–25) Y25P | D101A | 3 | N.D. | N.D. |
| D101E | 3 | N.D. | N.D. | |
| N135A | 3 | N.D. | N.D. | |
| N135D | 3 | N.D. | N.D. | |
| Figure 4A FITC-sCT(22–32) | E123A | 3 | 6.44 ± 0.27 a | 365 nM |
| E123D | 3 | 7.28 ± 0.21 c | 53 nM | |
| N124A | 3 | N.D. | N.D. | |
| N124D | 3 | 7.29 ± 0.19 | 52 nM | |
| Figure 4B FITC-AC413(6–25) Y25P | E123A | 3 | 6.89 ± 0.17 b | 129 nM |
| E123D | 3 | 6.87 ± 0.20 b | 136 nM | |
| N124A | 3 | 6.33 ± 0.08 b | 467 nM | |
| N124D | 3 | 6.09 ± 0.10 b | 822 nM | |
| Figure 5A FITC-sCT(22–32) | D97A | 3 | 6.26 ± 0.23 a | 548 nM |
| D97E | 3 | 6.79 ± 0.16 d | 161 nM | |
| Figure 5B FITC-AC413(6–25) Y25P | D97A | 3 | 7.21 ± 0.10 | 62 nM |
| D97E | 3 | 7.09 ± 0.12 | 81 nM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, H.; Jang, J.; Park, M.; Yun, J.; Jin, J.; Lee, S. Amylin Receptor 1 Mutagenesis Revealed a Potential Role of Calcitonin Serine 29 in Receptor Interaction. Biomedicines 2025, 13, 1787. https://doi.org/10.3390/biomedicines13071787
Song H, Jang J, Park M, Yun J, Jin J, Lee S. Amylin Receptor 1 Mutagenesis Revealed a Potential Role of Calcitonin Serine 29 in Receptor Interaction. Biomedicines. 2025; 13(7):1787. https://doi.org/10.3390/biomedicines13071787
Chicago/Turabian StyleSong, Hyeseon, Jaehyeok Jang, Minjae Park, Junsu Yun, Jeongwoo Jin, and Sangmin Lee. 2025. "Amylin Receptor 1 Mutagenesis Revealed a Potential Role of Calcitonin Serine 29 in Receptor Interaction" Biomedicines 13, no. 7: 1787. https://doi.org/10.3390/biomedicines13071787
APA StyleSong, H., Jang, J., Park, M., Yun, J., Jin, J., & Lee, S. (2025). Amylin Receptor 1 Mutagenesis Revealed a Potential Role of Calcitonin Serine 29 in Receptor Interaction. Biomedicines, 13(7), 1787. https://doi.org/10.3390/biomedicines13071787








