Photobiomodulation Therapy Reduces Oxidative Stress and Inflammation to Alleviate the Cardiotoxic Effects of Doxorubicin in Human Stem Cell-Derived Ventricular Cardiomyocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. Photobiomodulation (PBM) Protocol
2.3. Doxorubicin Exposure
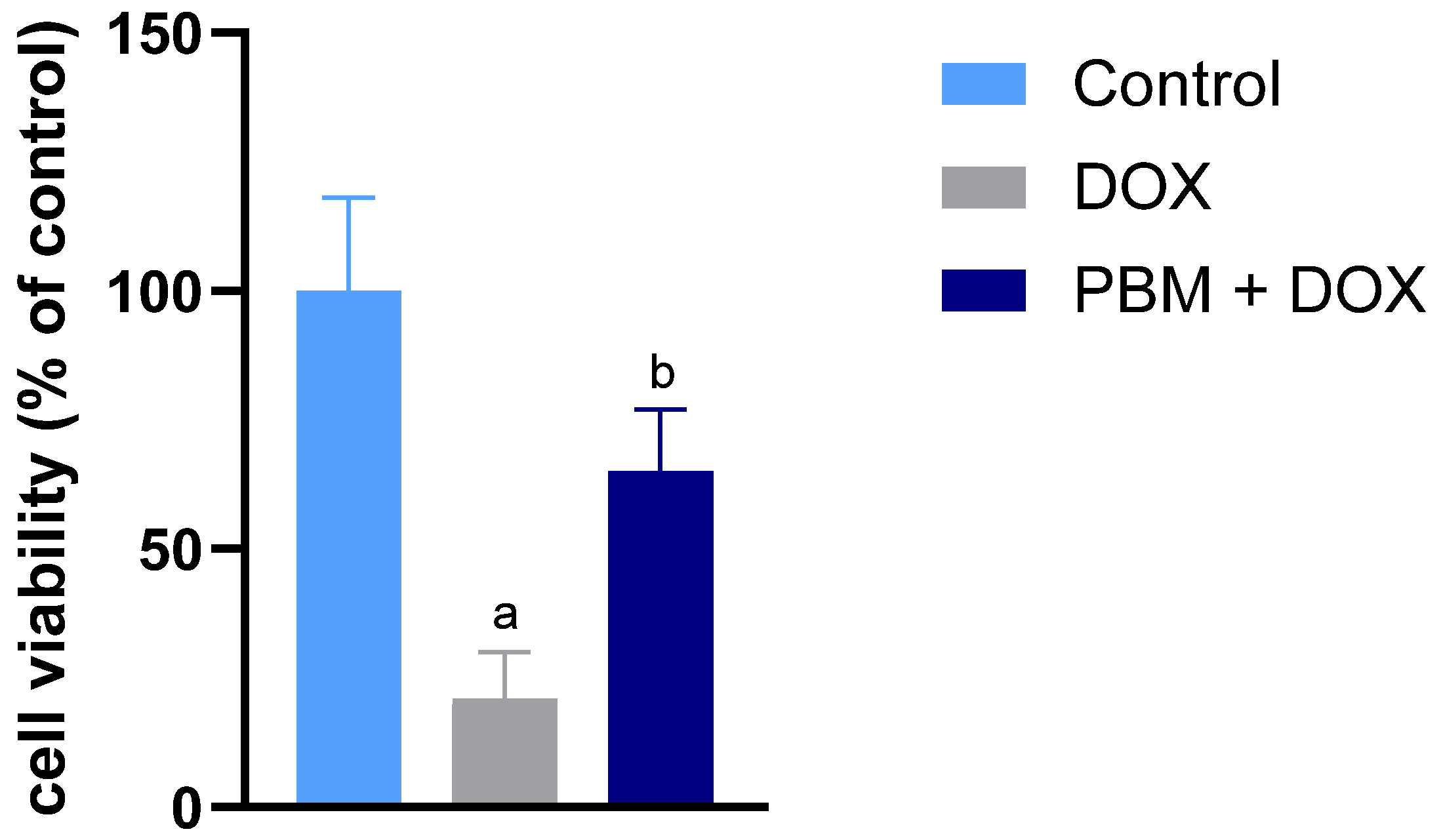
2.4. Cell Viability Assessment
2.5. Quantitative PCR for Gene Expression
2.5.1. RNA Extraction and Reverse Transcription
2.5.2. Quantitative Polymerase Chain Reaction (PCR)–qPCR
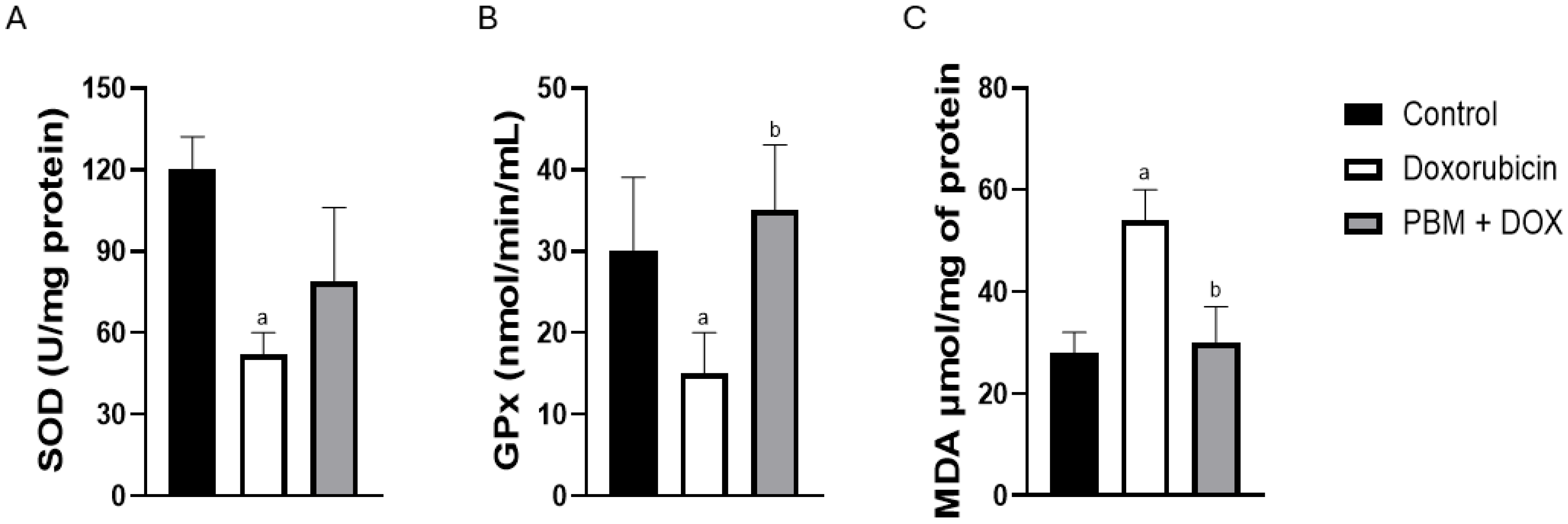
2.6. Analysis of Oxidative Stress-Related Enzymes
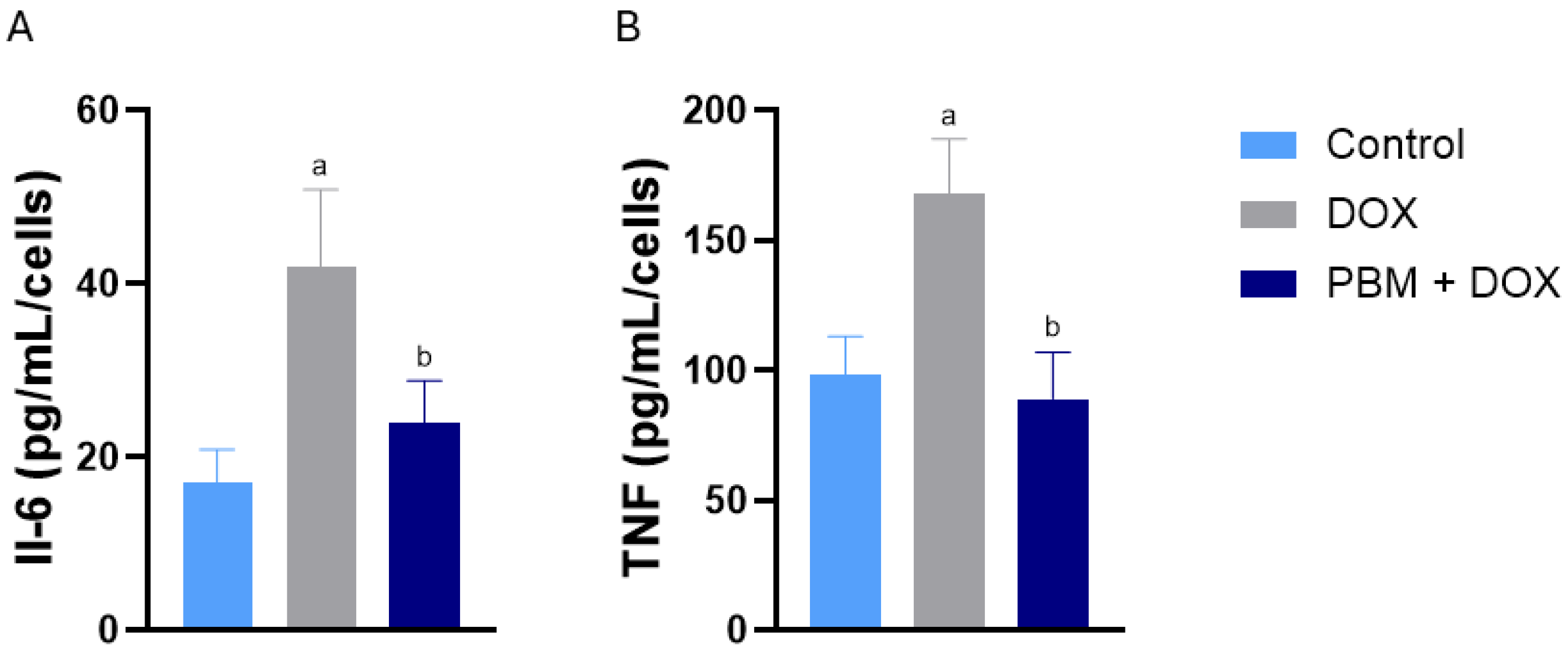
2.7. ELISA Assays
2.8. Statistical Analysis
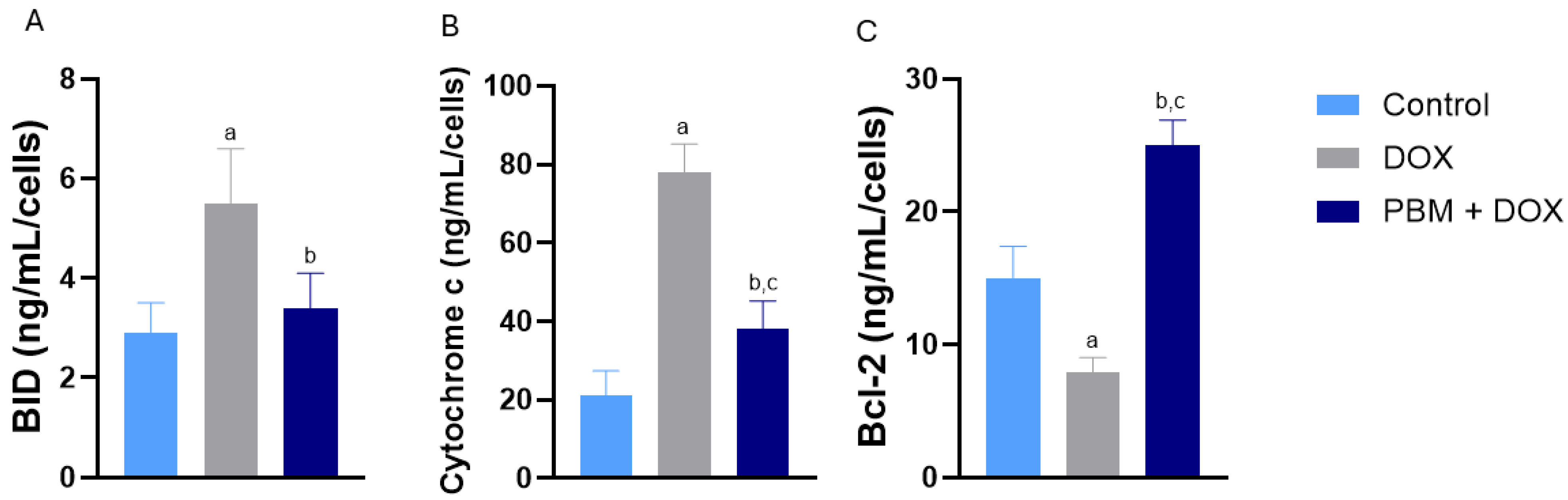
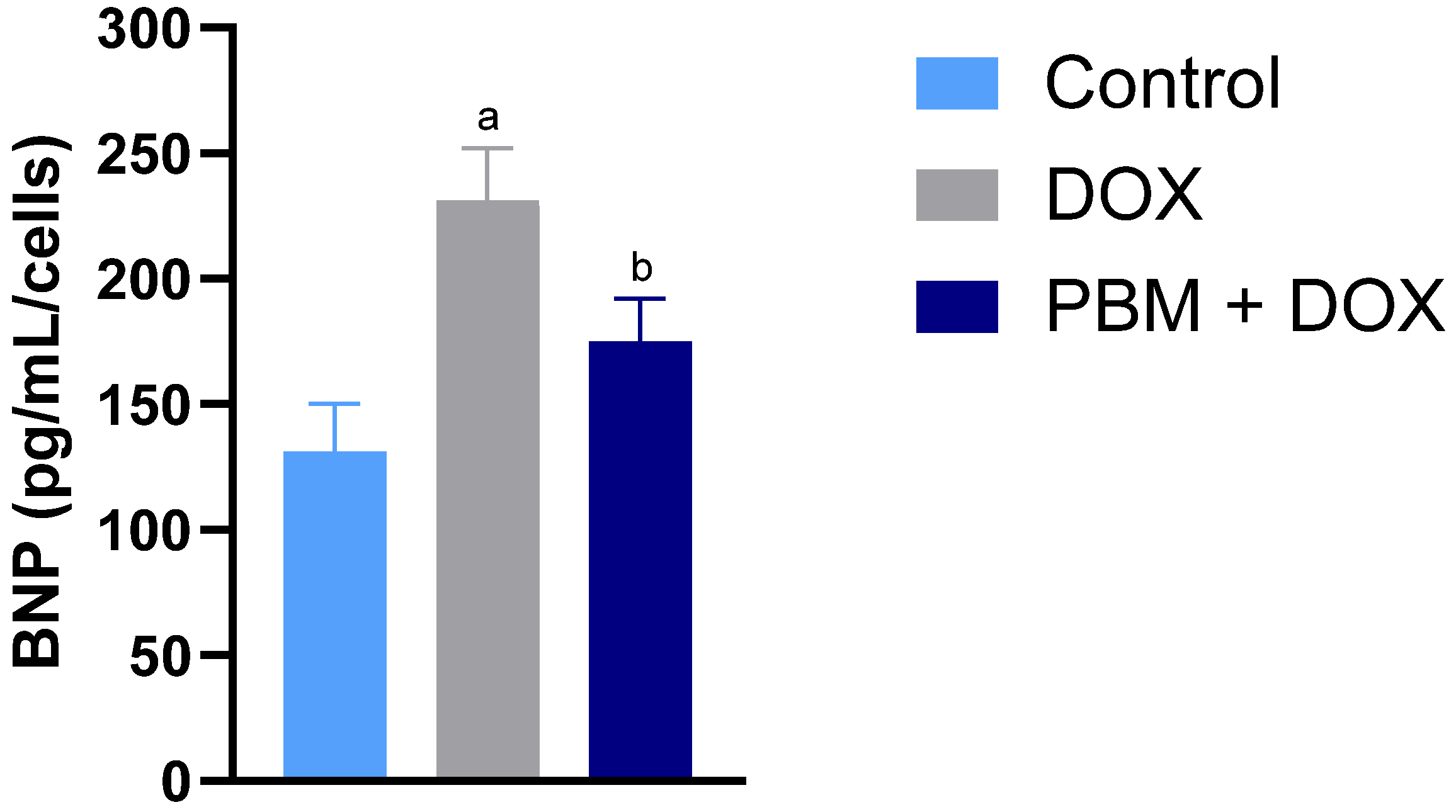
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DOX | Doxorubicin |
| PBM | Photobiomodulation |
| mRNA | Messenger ribonucleic acid |
| hiPSC-vCMs | Human-induced pluripotent stem cell-derived ventricular cardiomyocytes |
References
- Bisht, A.; Avinash, D.; Sahu, K.K.; Patel, P.; Das Gupta, G.; Das Kurmi, B. A comprehensive review on doxorubicin: Mechanisms, toxicity, clinical trials, combination therapies and nanoformulations in breast cancer. Drug Deliv. Transl. Res. 2024, 15, 102–133. [Google Scholar] [CrossRef]
- Al Khafaji, A.T.; Barakat, A.M.; Shayyal, A.J.; Taan, A.A.; Al-Aouadi, R.F.A. Managing Doxorubicin Cardiotoxicity: Insights Into Molecular Mechanisms and Protective Strategies. J. Biochem. Mol. Toxicol. 2025, 39, e70155. [Google Scholar] [CrossRef]
- Curigliano, G.; Cardinale, D.; Dent, S.; Criscitiello, C.; Aseyev, O.; Lenihan, D.; Cipolla, C.M. Cardiotoxicity of anticancer treatments: Epidemiology, detection, and management. CA Cancer J. Clin. 2016, 66, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Herman, E.; Young, R.; Krop, S. Doxorubicin-induced hypotension in the beagle dog. Inflamm. Res. 1978, 8, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhan, H. Dexrazoxane makes doxorubicin-induced heart failure a rare event in sarcoma patients receiving high cumulative doses. Cardio-Oncology 2025, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Cipolla, C.M. Chemotherapy-induced cardiotoxicity: Importance of early detection. Expert Rev. Cardiovasc. Ther. 2016, 14, 1297–1299. [Google Scholar] [CrossRef]
- Kong, C.-Y.; Guo, Z.; Song, P.; Zhang, X.; Yuan, Y.-P.; Teng, T.; Yan, L.; Tang, Q.-Z. Underlying the Mechanisms of Doxorubicin-Induced Acute Cardiotoxicity: Oxidative Stress and Cell Death. Int. J. Biol. Sci. 2022, 18, 760–770. [Google Scholar] [CrossRef]
- Dempke, W.C.M.; Zielinski, R.; Winkler, C.; Silberman, S.; Reuther, S.; Priebe, W. Anthracycline-induced cardiotoxicity—Are we about to clear this hurdle? Eur. J. Cancer. 2023, 185, 94–104. [Google Scholar] [CrossRef]
- Cardinale, D.; Biasillo, G.; Cipolla, C.M. Curing Cancer, Saving the Heart: A Challenge That Cardioncology Should Not Miss. Curr. Cardiol. Rep. 2016, 18, 51. [Google Scholar] [CrossRef]
- Talkhabi, M.; Aghdami, N.; Baharvand, H. Human cardiomyocyte generation from pluripotent stem cells: A state-of-art. Life Sci. 2016, 145, 98–113. [Google Scholar] [CrossRef]
- Rikhtegar, R.; Pezeshkian, M.; Dolati, S.; Safaie, N.; Rad, A.A.; Mahdipour, M.; Nouri, M.; Jodati, A.R.; Yousefi, M. Stem cells as therapy for heart disease: iPSCs, ESCs, CSCs, and skeletal myoblasts. Biomed. Pharmacother. 2019, 109, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Atum, A.L.B.; da Silva, J.A.A.; Marques, D.; Prates, R.A.; Consolim-Colombo, F.M.; Irigoyen, M.C.C.; Dalboni, M.A.; Chavantes, M.C.; Silva, J.A. Photobiomodulation therapy preconditioning modifies nitric oxide pathway and oxidative stress in human-induced pluripotent stem cell-derived ventricular cardiomyocytes treated with doxorubicin. Lasers Med. Sci. 2021, 37, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Malavazzi, T.C.; Fernandes, K.P.S.; Lopez, T.C.C.; Rodrigues, M.F.S.D.; Horliana, A.C.R.T.; Bussadori, S.K.; Mesquita-Ferrari, R.A. Effects of the invasive and non-invasive systemic photobiomodulation using low-level laser in experimental models: A systematic review. Lasers Med. Sci. 2023, 38, 137. [Google Scholar] [CrossRef] [PubMed]
- Ramanishankar, A.; S, A.S.; Begum, R.F.; Jayasankar, N.; Nayeem, A.; Prajapati, B.G.; Nirenjen, S. Unleashing light’s healing power: An overview of photobiomodulation for Alzheimer’s treatment. Future Sci. OA 2024, 10, FSO922. [Google Scholar] [CrossRef]
- Austin, E.; Geisler, A.N.; Nguyen, J.; Kohli, I.; Hamzavi, I.; Lim, H.W.; Jagdeo, J. Visible light. Part I: Properties and cutaneous effects of visible light. J. Am. Acad. Dermatol. 2021, 84, 1219–1231. [Google Scholar] [CrossRef]
- Malavazzi, T.C.d.S.; Andreo, L.; Martinelli, A.; Rodrigues, M.F.S.D.; Horliana, A.C.R.T.; Bussadori, S.K.; Fernandes, K.P.S.; Nunes, F.D.; Mesquita-Ferrari, R.A. Preventive and therapeutic vascular photobiomodulation decreases the inflammatory markers and enhances the muscle repair process in an animal model. J. Photochem. Photobiol. B Biol. 2024, 256, 112921. [Google Scholar] [CrossRef]
- Maulik, A.; Davidson, S.M.; Piotrowska, I.; Walker, M.; Yellon, D.M. Ischaemic Preconditioning Protects Cardiomyocytes from Anthracycline-Induced Toxicity via the PI3K Pathway. Cardiovasc. Drugs Ther. 2018, 32, 245–253. [Google Scholar] [CrossRef]
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular Advances and Pharmacologic Developments in Antitumor Activity and Cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef]
- Uche, N.; Dai, Q.; Lai, S.; Kolander, K.; Thao, M.; Schibly, E.; Sendaydiego, X.; Zielonka, J.; Benjamin, I.J. Carvedilol Phenocopies PGC-1α Overexpression to Alleviate Oxidative Stress, Mitochondrial Dysfunction and Prevent Doxorubicin-Induced Toxicity in Human iPSC-Derived Cardiomyocytes. Antioxidants 2023, 12, 1585. [Google Scholar] [CrossRef]
- Lewis, J.; Yaseen, B.; Wu, H.; Saraf, A. Novel 2D/3D Hybrid Organoid System for High-Throughput Drug Screening in iPSC Cardiomyocytes. Therapeutics 2025, 2, 11. [Google Scholar] [CrossRef]
- Chen, X.; Lu, N.; Huang, S.; Zhang, Y.; Liu, Z.; Wang, X. Assessment of doxorubicin toxicity using human cardiac organoids: A novel model for evaluating drug cardiotoxicity. Chem. Interact. 2023, 386, 110777. [Google Scholar] [CrossRef]
- Yang, J.; Lei, W.; Xiao, Y.; Tan, S.; Yang, J.; Lin, Y.; Yang, Z.; Zhao, D.; Zhang, C.; Shen, Z.; et al. Generation of human vascularized and chambered cardiac organoids for cardiac disease modelling and drug evaluation. Cell Prolif. 2024, 57, e13631. [Google Scholar] [CrossRef] [PubMed]
- Arzt, M.; Gao, B.; Mozneb, M.; Pohlman, S.; Cejas, R.B.; Liu, Q.; Huang, F.; Yu, C.; Zhang, Y.; Fan, X.; et al. Protein-encapsulated doxorubicin reduces cardiotoxicity in hiPSC-cardiomyocytes and cardiac spheroids while maintaining anticancer efficacy. Stem Cell Rep. 2023, 18, 1913–1924. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xiao, L.; Chen, L.; Wang, X. Doxorubicin-Induced Cardiac Remodeling: Mechanisms and Mitigation Strategies. Cardiovasc. Drugs Ther. 2025, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Christidi, E.; Brunham, L.R. Regulated cell death pathways in doxorubicin-induced cardiotoxicity. Cell Death Dis. 2021, 12, 339. [Google Scholar] [CrossRef]
- Hong, Y.; Li, X.; Li, J.; He, Q.; Huang, M.; Tang, Y.; Chen, X.; Chen, J.; Tang, K.-J.; Wei, C. H3K27ac acts as a molecular switch for doxorubicin-induced activation of cardiotoxic genes. Clin. Epigenet. 2024, 16, 91. [Google Scholar] [CrossRef]
- da Silva, P.A.V.; Dos Anjos, L.M.J.; Abduch, T.F.; Pereira, R.; Fonseca, A.d.S.d.; de Paoli, F. Photobiomodulation can alter mRNA levels cell death-related. Lasers Med. Sci. 2019, 34, 1373–1380. [Google Scholar] [CrossRef]
- Bender, A.; Opel, D.; Naumann, I.; Kappler, R.; Friedman, L.; von Schweinitz, D.; Debatin, K.-M.; Fulda, S. PI3K inhibitors prime neuroblastoma cells for chemotherapy by shifting the balance towards pro-apoptotic Bcl-2 proteins and enhanced mitochondrial apoptosis. Oncogene 2010, 30, 494–503. [Google Scholar] [CrossRef]
- Lauterwasser, J.; Fimm-Todt, F.; Oelgeklaus, A.; Schreiner, A.; Funk, K.; Falquez-Medina, H.; Klesse, R.; Jahreis, G.; Zerbes, R.M.; O’NEill, K.; et al. Hexokinases inhibit death receptor–dependent apoptosis on the mitochondria. Proc. Natl. Acad. Sci. USA 2021, 118, e2021175118. [Google Scholar] [CrossRef]
- Koleini, N.; Kardami, E. Autophagy and mitophagy in the context of doxorubicin-induced cardiotoxicity. Oncotarget 2017, 8, 46663–46680. [Google Scholar] [CrossRef]
- Abbas, M.M.; Kandil, Y.İ.; Abbas, M.A. R-(-)-carvone Attenuated Doxorubicin Induced Cardiotoxicity In Vivo and Potentiated Its Anticancer Toxicity In Vitro. Balk. Med. J. 2020, 37, 98–103. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, Q.; Xie, Y. TCFL5 knockdown sensitizes DLBCL to doxorubicin treatment via regulation of GPX4. Cell. Signal. 2023, 110, 110831. [Google Scholar] [CrossRef]
- Nascimento, J.J.A.C.; Machado, A.S.D.; Della-Santa, G.M.L.; Fernandes, D.C.; Ferreira, M.C.; Machado, G.A.P.; Chaves, B.C.G.; Costa, K.B.; Rocha-Vieira, E.; Oliveira, M.X.; et al. Effects of photobiomodulation therapy on functional recovery, angiogenesis and redox status in denervated muscle of rats. Einstein 2021, 19, eAO6001. [Google Scholar] [CrossRef]
- Tonetto, L.d.S.; da Silva, C.C.F.; Gonzatti, N.; Guex, C.G.; Hartmann, D.D.; Boschi, E.S.; Lago, P.D.; Trevisan, M.E.; Bauermann, L.d.F.; Jaenisch, R.B. Effects of photobiomodulation on oxidative stress in rats with type 2 diabetes mellitus. Lasers Med. Sci. 2023, 38, 90. [Google Scholar] [CrossRef]
- Evangelista, A.N.; dos Santos, F.F.; Martins, L.P.d.O.; Gaiad, T.P.; Machado, A.S.D.; Rocha-Vieira, E.; Costa, K.B.; Santos, A.P.; Oliveira, M.X. Photobiomodulation therapy on expression of HSP70 protein and tissue repair in experimental acute Achilles tendinitis. Lasers Med. Sci. 2020, 36, 1201–1208. [Google Scholar] [CrossRef]
- Assis, L.; Moretti, A.I.; Abrahão, T.B.; Cury, V.; Souza, H.P.; Hamblin, M.R.; Parizotto, N.A. Low-level laser therapy (808 nm) reduces inflammatory response and oxidative stress in rat tibialis anterior muscle after cryolesion. Lasers Surg. Med. 2012, 44, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Grandinetti, V.; Carlos, F.P.; Antonio, E.L.; de Oliveira, H.A.; dos Santos, L.F.N.; Yoshizaki, A.; Mansano, B.S.D.M.; Silva, F.A.; Porte, L.A.; Albuquerque-Pontes, G.M.; et al. Photobiomodulation therapy combined with carvedilol attenuates post-infarction heart failure by suppressing excessive inflammation and oxidative stress in rats. Sci. Rep. 2019, 9, 9425. [Google Scholar] [CrossRef] [PubMed]
- Hanna, A.D.; Lam, A.; Tham, S.; Dulhunty, A.F.; Beard, N.A. Adverse Effects of Doxorubicin and Its Metabolic Product on Cardiac RyR2 and SERCA2A. Mol. Pharmacol. 2014, 86, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Olson, R.D.; Gambliel, H.A.; Vestal, R.E.; Shadle, S.E.; Charlier, H.A.; Cusack, B.J. Doxorubicin Cardiac Dysfunction: Effects on Calcium Regulatory Proteins, Sarcoplasmic Reticulum, and Triiodothyronine. Cardiovasc. Toxicol. 2005, 5, 269–284. [Google Scholar] [CrossRef]
- Ho, C.Y.; Day, S.M.; Ashley, E.A.; Michels, M.; Pereira, A.C.; Jacoby, D.; Lakdawala, N.K.; Ware, J.S.; Helms, A.S.; Colan, S.D.; et al. Response by Ho et al to Letter Regarding Article, “Genotype and Lifetime Burden of Disease in Hypertrophic Cardiomyopathy: Insights From the Sarcomeric Human Cardiomyopathy Registry (SHaRe)”. Circulation 2019, 139, 1559–1560. [Google Scholar] [CrossRef]
- Podyacheva, E.; Danilchuk, M.; Toropova, Y. Molecular mechanisms of endothelial remodeling under doxorubicin treatment. Biomed. Pharmacother. 2023, 162, 114576. [Google Scholar] [CrossRef]
- Yin, Z.; Zhao, Y.; Li, H.; Yan, M.; Zhou, L.; Chen, C.; Wang, D.W. miR-320a mediates doxorubicin-induced cardiotoxicity by targeting VEGF signal pathway. Aging 2016, 8, 192–207. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, Y.; Chen, C.; Han, C.; Xue, L.; Xing, D.; Huang, O.; Tao, M. BNP as a marker for early prediction of anthracycline-induced cardiotoxicity in patients with breast cancer. Oncol. Lett. 2019, 18, 4992–5001. [Google Scholar] [CrossRef] [PubMed]
- Di Somma, S.; Magrini, L.; Pittoni, V.; Marino, R.; Mastrantuono, A.; Ferri, E.; Ballarino, P.; Semplicini, A.; Bertazzoni, G.; Carpinteri, G.; et al. In-hospital percentage BNP reduction is highly predictive for adverse events in patients admitted for acute heart failure: The Italian RED Study. Crit. Care 2010, 14, R116. [Google Scholar] [CrossRef] [PubMed]
- Marcelino, G.; Hiane, P.A.; Freitas, K.d.C.; Santana, L.F.; Pott, A.; Donadon, J.R.; Guimarães, R.d.C.A. Effects of Olive Oil and Its Minor Components on Cardiovascular Diseases, Inflammation, and Gut Microbiota. Nutrients 2019, 11, 1826. [Google Scholar] [CrossRef] [PubMed]
- Reis-Mendes, A.; Ferreira, M.; Padrão, A.I.; Duarte, J.A.; Duarte-Araújo, M.; Remião, F.; Carvalho, F.; Sousa, E.; Bastos, M.L.; Costa, V.M. The Role of Nrf2 and Inflammation on the Dissimilar Cardiotoxicity of Doxorubicin in Two-Time Points: A Cardio-Oncology In Vivo Study Through Time. Inflammation 2023, 47, 264–284. [Google Scholar] [CrossRef]
- Silva, L.M.G.; Gouveia, V.A.; Campos, G.R.S.; Dale, C.S.; da Palma, R.K.; de Oliveira, A.P.L.; Marcos, R.L.; Duran, C.C.G.; Cogo, J.C.; Junior, J.A.S.; et al. Photobiomodulation mitigates Bothrops jararacussu venom-induced damage in myoblast cells by enhancing myogenic factors and reducing cytokine production. PLOS Negl. Trop. Dis. 2024, 18, e0012227. [Google Scholar] [CrossRef]
- Imanaka-Yoshida, K. Tenascin-C in Heart Diseases—The Role of Inflammation. Int. J. Mol. Sci. 2021, 22, 5828. [Google Scholar] [CrossRef]
- Berthiaume, J.M.; Wallace, K.B. Persistent Alterations to the Gene Expression Profile of the Heart Subsequent to Chronic Doxorubicin Treatment. Cardiovasc. Toxicol. 2007, 7, 178–191. [Google Scholar] [CrossRef]
- Atum, A.L.B.; de Matos, L.P.; de Jesus, B.C.; Nasuk, G.R.; da Silva, G.A.; Gomes, C.P.; Pesquero, J.B.; Zamuner, S.R.; Silva Júnior, J.A. Impact of Prenatal Alcohol Exposure on the Development and Myocardium of Adult Mice: Morphometric Changes, Transcriptional Modu-lation of Genes Related to Cardiac Dysfunction, and Antioxidant Cardioprotection. Antioxidants 2023, 12, 256. [Google Scholar] [CrossRef]
- Putker, K.; Schneider, A.-F.; Van De Vijver, D.; Hildyard, J.; Aartsma-Rus, A.; van Putten, M.; Hitachi, K. Identification of suitable qPCR reference genes for the normalization of gene expression in the BL10-mdx and D2-mdx mouse models of Duchenne muscular dystrophy. PLoS ONE 2025, 20, e0318944. [Google Scholar] [CrossRef]
- Bahr, A.C.; Naasani, L.I.S.; de Gregório, E.; Wink, M.R.; Araujo, A.S.d.R.; Turck, P.; Lago, P.D. Photobiomodulation improves cell survival and death parameters in cardiomyocytes exposed to hypoxia/reoxygenation. J. Photochem. Photobiol. B Biol. 2024, 258, 112991. [Google Scholar] [CrossRef]
- Da Silva, D.; van Rensburg, M.J.; Crous, A.; Abrahamse, H. Photobiomodulation: A novel approach to promote trans-differentiation of adipose-derived stem cells into neuronal-like cells. Neural Regen. Res. 2024, 20, 598–608. [Google Scholar] [CrossRef]
- Moshksayan, K.; Harihara, A.; Mondal, S.; Hegarty, E.; Atherly, T.; Sahoo, D.K.; Jergens, A.E.; Mochel, J.P.; Allenspach, K.; Zoldan, J.; et al. OrganoidChip facilitates hydrogel-free immobilization for fast and blur-free imaging of organoids. Sci. Rep. 2023, 13, 11268. [Google Scholar] [CrossRef]
- Jang, J.; Jung, H.; Jeong, J.; Jeon, J.; Lee, K.; Jang, H.R.; Han, J.-W.; Lee, J. Modeling doxorubicin-induced-cardiotoxicity through breast cancer patient specific iPSC-derived heart organoid. Heliyon 2024, 10, e38714. [Google Scholar] [CrossRef]
| Signaling Pathway | Gene | DOX (Mean ± SE) | PBM+DOX (Mean ± SE) |
|---|---|---|---|
| Angiogenesis | VEGFA | 0.96 ± 0.12 | 1.67 ± 018 b,c |
| Apoptosis | BAX | 1.97 ± 0.28 a | 0.67 ± 0.06 b,c |
| FAS | 1.72 ± 0.33 a | 1.38 ± 0.51 | |
| TP53 | 3.34 ± 0.48 a | 1.76 ± 0.35 b,c | |
| Calcium kinetics | ATP2A2 | 0.47 ± 0.11 a | 0.94 ± 0.08 b |
| CASQ2 | 0.90 ± 0.12 | 0.76 ± 0.23 | |
| PLN | 1.12 ± 0.17 | 1.01 ± 0.28 | |
| RYR-2 | 0.49 ± 0.17 a | 0.84 ± 0.21 | |
| SLC8A1 | 0.63 ± 0.10 a | 0.56 ± 0.35 | |
| Oxidative stress | CAT | 0.24 ± 0.04 a | 1.64 ± 0.23 b,c |
| GPX4 | 0.60 ± 0.11 a | 1.89 ± 0.23 b,c | |
| HSPA1A/B | 2.19 ± 0.56 a | 1.21 ± 0.15 b | |
| SOD1 | 0.39 ± 0.09 a | 0.89 ± 0.12 b | |
| Cardiac hypertrophy | ACE | 0.43 ± 0.22 a | 0.93 ± 0.36 |
| ACE2 | 1.94 ± 0.30 a | 1.56 ± 0.41 | |
| AGTR1A | 1.80 ± 0.42 a | 1.55 ± 0.42 | |
| CABIN1 | 1.67 ± 0.21 a | 1.42 ± 0.64 | |
| CHP2 | 0.52 ± 0.16 a | 0.79 ± 0.42 | |
| EDN1 | 0.65 ± 0.15 a | 1.36 ± 0.17 b | |
| IGF1 | 0.44 ± 0.07 a | 0.79 ± 0.26 | |
| MYH6 | 0.65 ± 0.11 a | 1.73 ± 0.30 b | |
| MYH7 | 1.69 ± 0.19 a | 1.24 ± 0.37 | |
| NFATC3 | 1.24 ± 0.14 | 1.82 ± 0.21 b,c | |
| NPPA | 2.76 ± 0.38 a | 1.85 ± 0.27 b | |
| NPPB | 2.98 ± 0.70 a | 1.74 ± 0.25 b | |
| Inflammation | IL-6 | 3.14 ± 0.26 a | 1.34 ± 042 b |
| TNFRSF1a | 0.67 ± 0.17 a | 0.82 ± 0.25 | |
| TNF | 2.36 ± 0.24 a | 1.41 ± 0.21 b | |
| Extracellular matrix | COL1A1 | 0.46 ± 0.12 a | 0.86 ± 0.22 |
| COL3A1 | 1.35 ± 0.11 a | 1.87 ± 0.23 b,c | |
| MMP9 | 1.35 ± 0.14 a | 1.77 ± 0.14 b,c | |
| TGFB1 | 1.41 ± 018 a | 1.13 ± 0.12 | |
| TNC | 2.84 ± 0.48 a | 1.54 ± 0.35 b | |
| Cell metabolism | GAPDH | 0.89 ± 0.31 | 0.69 ± 0.44 |
| HK1 | 0.45 ± 0.22 a | 0.98 ± 0.31 | |
| NDUFA3 | 0.77 ± 0.25 | 0.83 ± 0.36 | |
| PFKM | 0.43 ± 0.27 a | 1.13 ± 0.21 b | |
| SLC2A1 | 1.56 ± 0.57 | 1.41 ± 0.49 | |
| TAZ | 1.24 ± 0.31 | 0.97 ± 0.34 | |
| UCP-2 | 0.33 ± 0.02 a | 1.94 ± 0.36 b,c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasuk, G.R.; de Matos, L.P.; Atum, A.L.B.; de Jesus, B.C.; Batista, J.G.C.; da Silva, G.A.; Martins, A.H.; Trivelin, M.L.A.; Duran, C.C.G.; de Oliveira, A.P.L.; et al. Photobiomodulation Therapy Reduces Oxidative Stress and Inflammation to Alleviate the Cardiotoxic Effects of Doxorubicin in Human Stem Cell-Derived Ventricular Cardiomyocytes. Biomedicines 2025, 13, 1781. https://doi.org/10.3390/biomedicines13071781
Nasuk GR, de Matos LP, Atum ALB, de Jesus BC, Batista JGC, da Silva GA, Martins AH, Trivelin MLA, Duran CCG, de Oliveira APL, et al. Photobiomodulation Therapy Reduces Oxidative Stress and Inflammation to Alleviate the Cardiotoxic Effects of Doxorubicin in Human Stem Cell-Derived Ventricular Cardiomyocytes. Biomedicines. 2025; 13(7):1781. https://doi.org/10.3390/biomedicines13071781
Chicago/Turabian StyleNasuk, Guilherme Rabelo, Leonardo Paroche de Matos, Allan Luís Barboza Atum, Bruna Calixto de Jesus, Julio Gustavo Cardoso Batista, Gabriel Almeida da Silva, Antonio Henrique Martins, Maria Laura Alchorne Trivelin, Cinthya Cosme Gutierrez Duran, Ana Paula Ligeiro de Oliveira, and et al. 2025. "Photobiomodulation Therapy Reduces Oxidative Stress and Inflammation to Alleviate the Cardiotoxic Effects of Doxorubicin in Human Stem Cell-Derived Ventricular Cardiomyocytes" Biomedicines 13, no. 7: 1781. https://doi.org/10.3390/biomedicines13071781
APA StyleNasuk, G. R., de Matos, L. P., Atum, A. L. B., de Jesus, B. C., Batista, J. G. C., da Silva, G. A., Martins, A. H., Trivelin, M. L. A., Duran, C. C. G., de Oliveira, A. P. L., Prates, R. d. A., Marcos, R. L., Zamuner, S. R., Baltatu, O. C., & Silva, J. A., Jr. (2025). Photobiomodulation Therapy Reduces Oxidative Stress and Inflammation to Alleviate the Cardiotoxic Effects of Doxorubicin in Human Stem Cell-Derived Ventricular Cardiomyocytes. Biomedicines, 13(7), 1781. https://doi.org/10.3390/biomedicines13071781










