Understanding the Borderline Brain: A Review of Neurobiological Findings in Borderline Personality Disorder (BPD)
Abstract
1. Introduction
1.1. Background
1.2. Current Limitations
1.3. Rationale for This Review
- Clarify the current understanding of BPD’s neurobiological mechanisms;
- Highlight translational gaps that hinder clinical application;
- Inform the development of personalized and multimodal treatment approaches.
1.4. Objectives
- To synthesize current evidence on the structural, functional, and neurochemical brain changes associated with BPD;
- To explore the role of genetic and epigenetic mechanisms, particularly in relation to early trauma;
- To discuss the cognitive and developmental implications of neurobiological alterations;
- To identify how neurobiological insights can inform clinical practice, including diagnosis, risk assessment, and treatment.
- To highlight future research directions, particularly those focused on biomarker validation, therapeutic innovation, and the development of integrated care models.
- (1)
- Which structural, functional, or neurochemical abnormalities remain significant after controlling for key comorbidities, such as depression, PTSD, and ADHD?
- (2)
- Which neuroimaging techniques demonstrate sufficient test–retest reliability (≥0.6) and replicability across studies?
- (3)
- To what extent do neurobiological markers differentiate BPD from other personality and affective disorders (i.e., transdiagnostic specificity)?
- (4)
- Which biomarkers show promise as clinically relevant predictors of treatment response or symptom trajectories?
2. Methodology
2.1. Review Type and Justification
2.2. Search Strategy
2.3. Search Terms and Syntax
2.4. Inclusion Criteria
- Studies published between 2000 and 2025.
- Articles focusing on individuals with a clinically diagnosed BPD based on DSM 5 or ICD-11 criteria.
- Studies must report empirical data (e.g., neuroimaging, genetics, epigenetics, neurochemistry, or cognition).
- Meta-analyses and systematic reviews relevant to the neurobiology of BPD.
- Studies examining comorbidity or transdiagnostic features were only included if they provided BPD-specific data.
2.5. Exclusion Criteria
- Non-empirical publications (e.g., theoretical articles, letters, commentaries and editorials).
- Case reports or single-subject studies lacking generalizability.
- Animal or preclinical studies not involving human subjects.
- Articles that were not available in full text or that were published in a language other than English.
- Studies where BPD was a secondary or incidental focus without subgroup analysis.
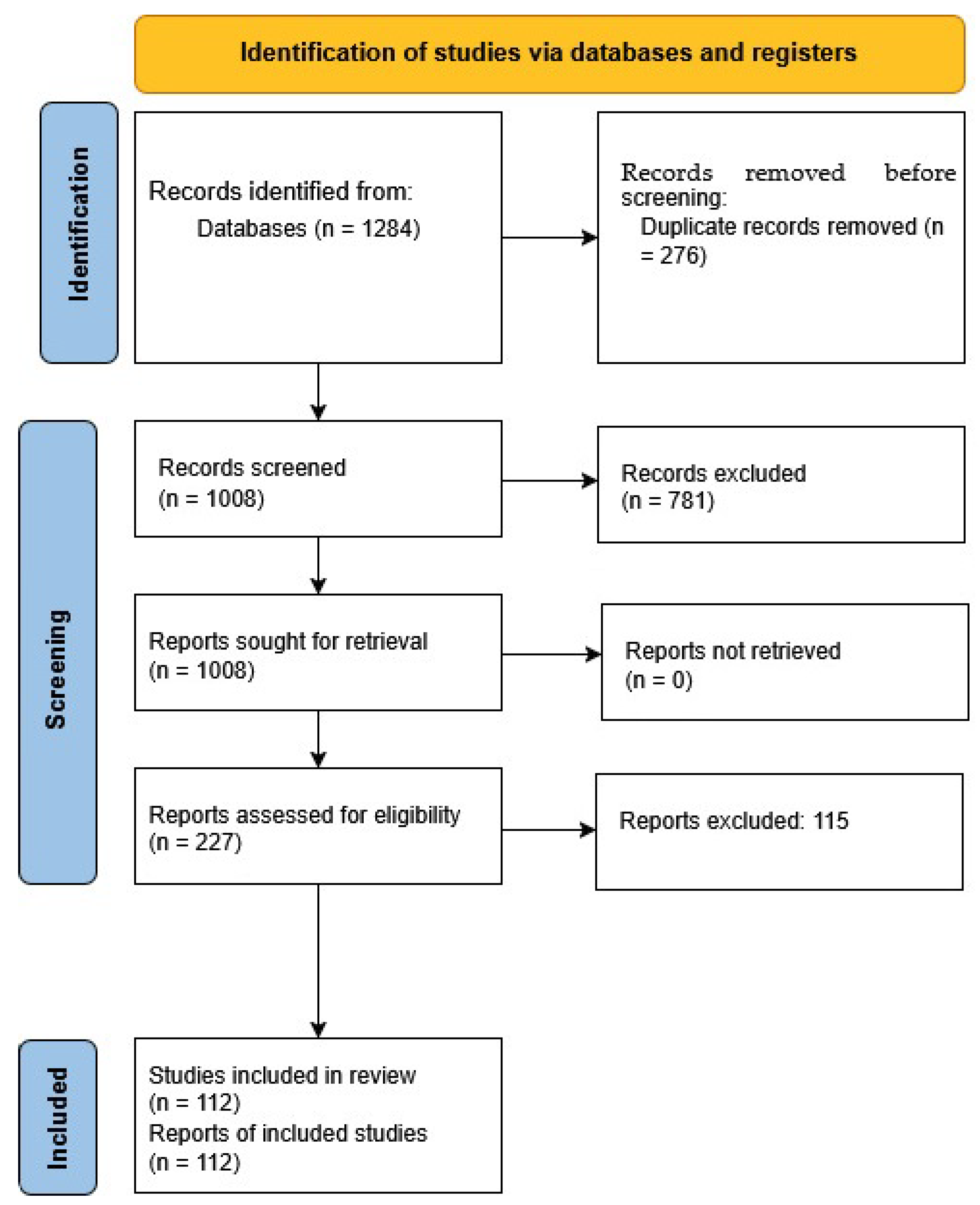
2.6. Database Filtering and Screening Process
2.7. Selection and Categorization
- Brain structure and function.
- Neurotransmitter systems.
- Genetic and epigenetic factors.
- Developmental contributions (e.g., childhood trauma).
- Cognitive and functional connectivity.
2.8. Data Synthesis
2.9. Quality Assessment and Statistical Considerations
3. Functional Connectivity Disruptions in Borderline Personality Disorder
3.1. Prefrontal–Amygdala Dysconnectivity
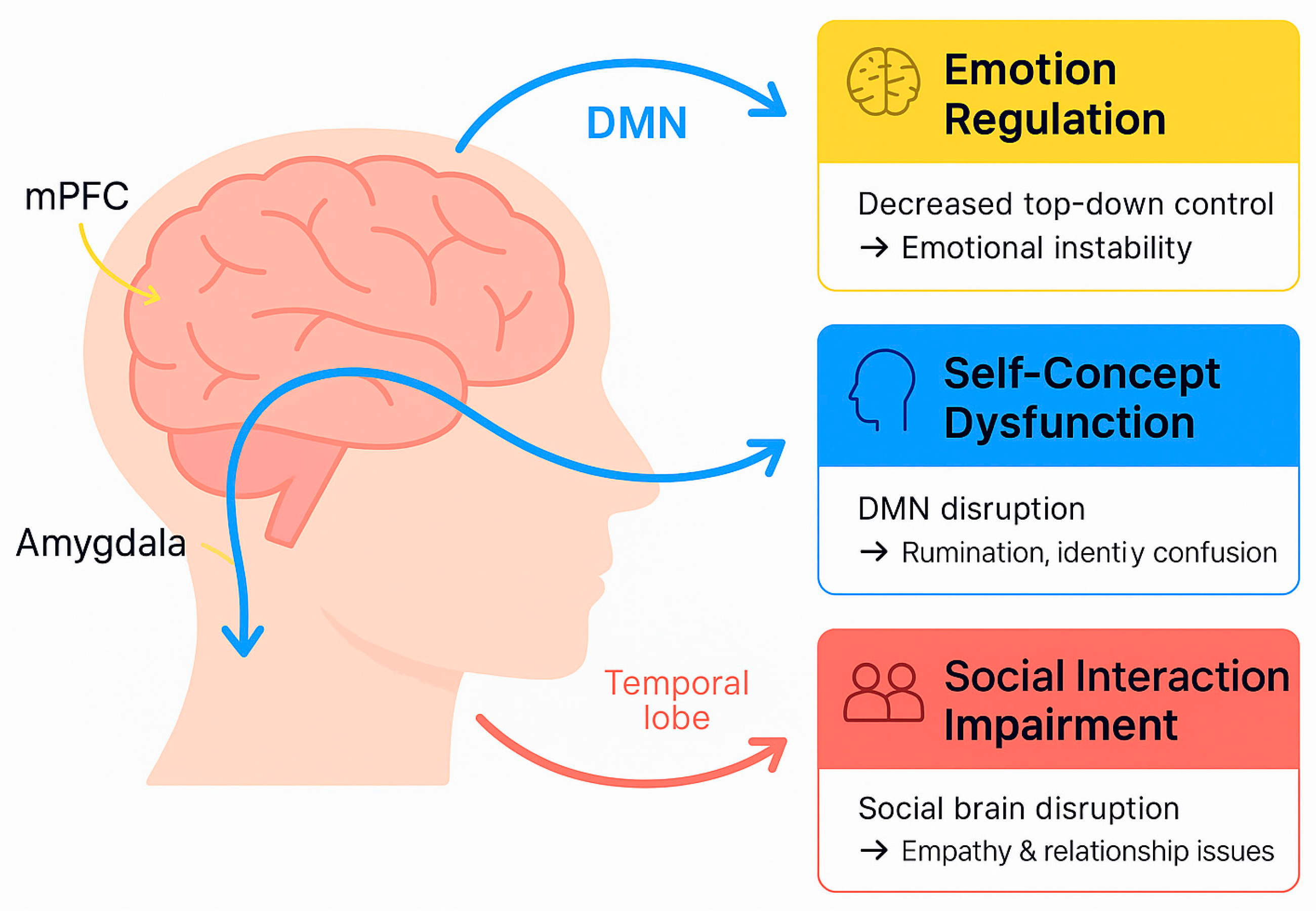
3.2. Default Mode Network Abnormalities
3.3. Social Brain and Mentalization Networks
3.4. Stress-Response System (HPA Axis) and Dynamic Reactivity
3.5. Mirror Neuron System and Interpersonal Misattunement
3.6. Machine Learning and Graph Theoretical Approaches
4. Neurostructural and Network-Level Correlates
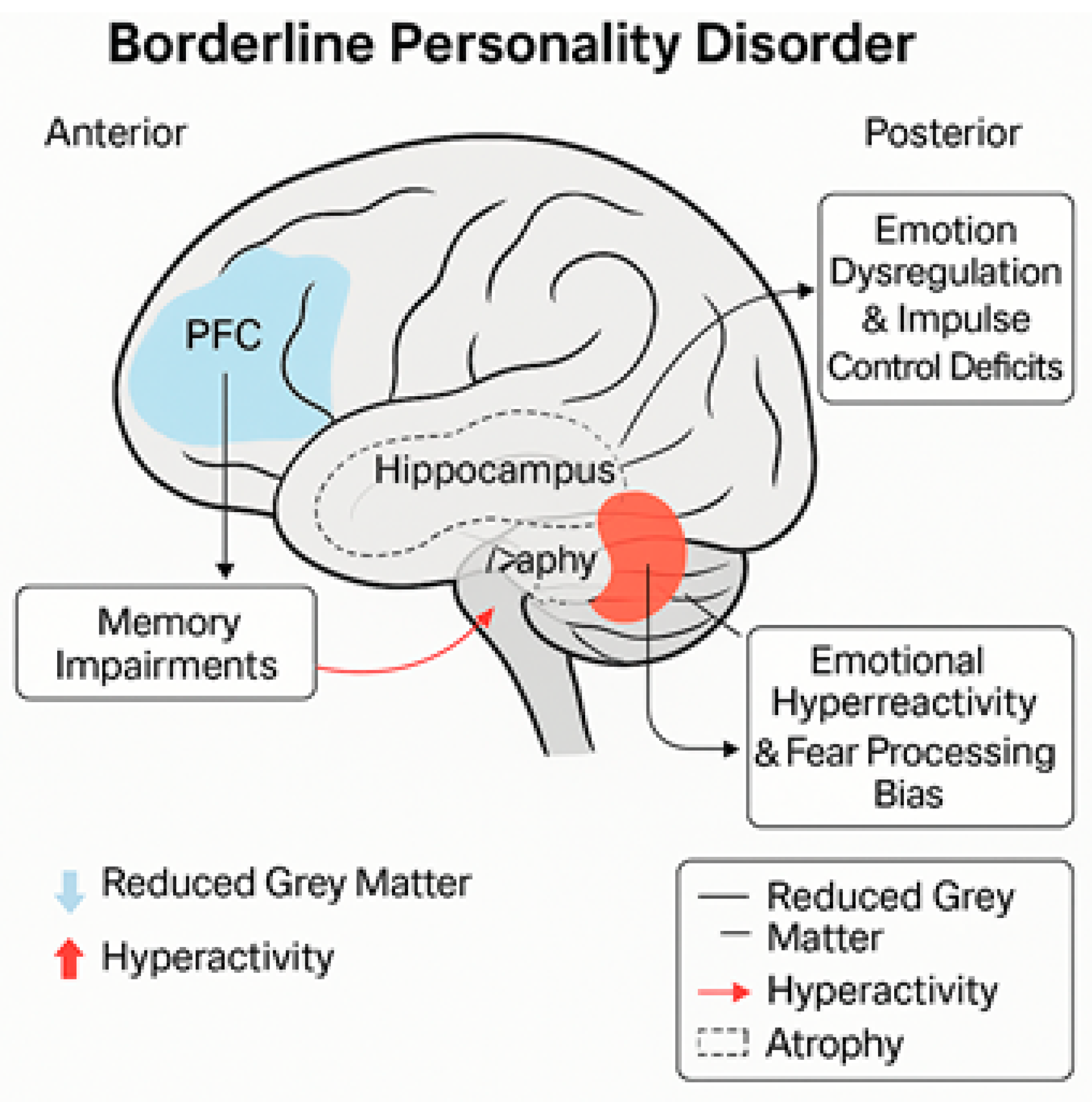
4.1. Prefrontal Cortex
4.2. Amygdala
4.3. Hippocampus
4.4. Parietal Cortex
4.5. Personality Dimensions and Network Efficiency
4.6. Meta-Analytic Findings
- -
- -
- -
5. Neurobiological Underpinnings
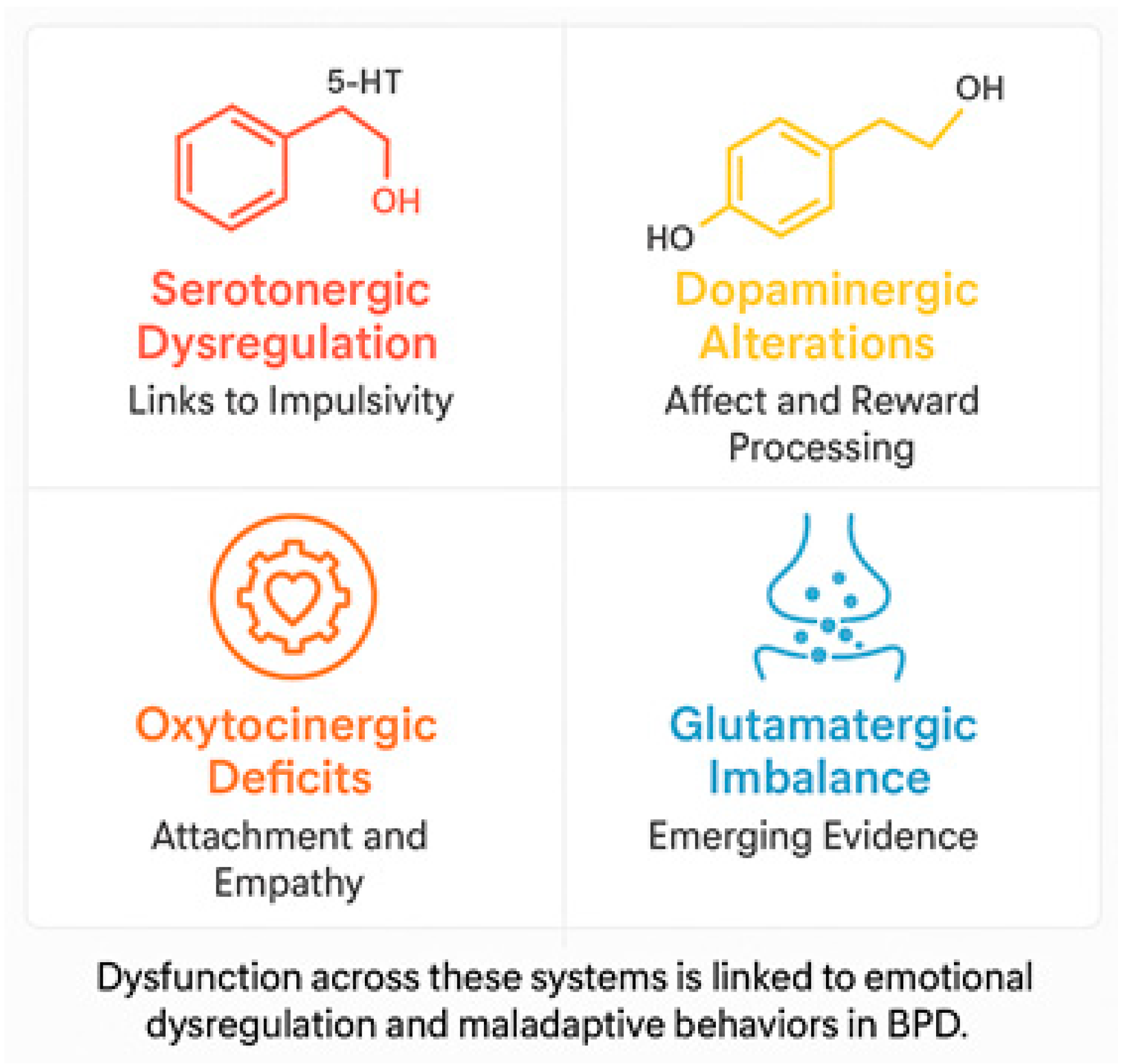
5.1. Neurotransmitter Dysfunctions
- Serotonin (5-HT): Dysregulation of the serotonergic system has been associated with the impulsive behavior, aggression, and mood swings that are typical of BPD. Reduced serotonin activity, particularly in the prefrontal cortex (PFC), is believed to be associated with difficulty controlling impulses and increased aggression [33,34]. Selective serotonin reuptake inhibitors (SSRIs) have demonstrated some therapeutic benefit in managing these symptoms, though the response may vary significantly between individuals [35].
- Dopamine (DA): Dysfunctions in the dopaminergic system have been associated with disturbances in reward processing, emotional regulation, and impulsivity. Neuroimaging studies have revealed alterations in mesolimbic circuitry and decreased dopamine receptor binding in the striatum, which are linked to heightened emotional sensitivity and impaired regulation of negative emotions [36,37].
- Glutamate: Recent evidence suggests that alterations in the glutamatergic system are associated with borderline personality disorder. Dysregulation of excitatory neurotransmission can exacerbate emotional instability and reinforce impulsive decision-making [11].
- Oxytocin and the opioid system: Disturbance of oxytocin, a neuropeptide that plays an essential role in attachment mechanisms and social cognition, is associated with the relational difficulties observed in BPD. Decreased oxytocin levels may impair the processing of emotional information, thereby exacerbating interpersonal relationship problems [32,38]. Furthermore, dysregulation in the endogenous opioid system is linked with chronic dysphoria and self-injurious behavior, which temporarily alleviates emotional distress by increasing opioid levels [31].
5.2. Functional Connectivity in Borderline Personality Disorder
5.3. Key Connectivity Disruptions
- Prefrontal Cortex (PFC) and Amygdala Connectivity:
- Default mode network (DMN) dysfunction:
- Social brain networks dysfunction:
5.4. Stress-Response System Dysregulation
5.5. Electrophysiological Biomarkers
6. Genetic and Epigenetic Factors
6.1. Genetic Contributions
6.2. Epigenetic Contributions
- Early-life trauma effect: Adverse events, including abuse and neglect, during early years can leave epigenetic marks that disrupt stress regulation mechanisms. For example, hypermethylation of the NR3C1 gene, which encodes the glucocorticoid receptor, has been observed in patients with borderline personality disorder, suggesting a potential disruption in the hypothalamic–pituitary–adrenal (HPA) axis [15,16]. This dysregulation increases the vulnerability to emotional dysregulation and hyperactive stress responses.
- The interplay between genetic predisposition and environmental stress conditions is a fundamental component to BPD pathogenesis. For instance, individuals with certain polymorphisms of the 5-HTTLPR gene are more susceptible to traumatic events during their early years and exhibit heightened emotional and behavioral responses [46,47].
6.3. Genetic and Epigenetic Studies’ Limitations
- Lack of specificity: Although candidate genetic and epigenetic markers have been suggested, no gene or epigenetic alteration has been reliably linked to BPD. The disorder’s heterogeneity and high comorbidity levels further complicate the identification of definitive markers.
- Limited sample sizes: Many genetic and epigenetic BPD-specific investigations have small sample sizes, which makes their findings less generalizable and credible [9].
- Transdiagnostic factors: The majority of the genetic and epigenetic changes that were observed are not specific to BPD, but appear in various other psychiatric disorders, including PTSD, depression, and anxiety, and are, therefore, not viable as specific biomarkers [48].
7. Neurodevelopmental Contributions
7.1. Effect of Childhood Adversity
- Structural and functional consequences: Repeated exposure to trauma during critical periods of neurodevelopment can interfere with the normal maturation of key brain structures, such as the hippocampus, amygdala, and prefrontal cortex [21,46]. These structures play a significant role in regulating emotions, consolidating memories, and adapting to stress.
- HPA axis dysregulation: Early-life adversity is associated with hyperactivation of the hypothalamic–pituitary–adrenal (HPA) axis, resulting in increased cortisol levels and heightened stress sensitivity [15,16] dysregulation is responsible for the emotional dysregulation and impulsive behavior exhibited in BPD.
7.2. Attachment Disruptions
- Neurodevelopment: Disruptions to early attachment relationships can adversely affect the limbic and cortical systems, emotional regulation, and social cognition. The amygdala becomes hyperactive, and the prefrontal cortex less connected, thereby enhancing interpersonal hypersensitivity and problems with emotional regulation [32,51].
- Impairments in mentalization: Insecure attachment disrupts the development of mentalization, or the ability to understand others’ mental states. Impaired mentalization is a central feature of BPD, leading to misunderstandings of emotional cues and hypersensitivity to rejection [52].
7.3. Developmental Timing and Vulnerability
- Critical periods: Early windows of development are crucial in forming neural pathways related to the stress response and emotional regulation. Disruption during these periods can result in permanent deficits in self-regulation, attachment, and cognitive processing [46].
- Social contexts: Chronic social stressors, such as bullying, social deprivation, or re-victimization, also contribute to developmental vulnerabilities, perpetuating the cycle of emotional dysregulation and interpersonal conflict in BPD [54].
7.4. Neurodevelopmental Mechanisms
- Neuroplasticity: Although adverse experiences in early life can have a negative effect on brain development, the concept of neuroplasticity offers a positive outlook on the potential for intervention. Some therapeutic interventions, such as dialectical behavior therapy (DBT) and mentalization-based therapy (MBT), can restructure maladaptive neural circuits and achieve functional adaptation [53].
- Epigenetics: Epigenetic alterations provide a mechanistic link between early experience and subsequent neurodevelopmental outcomes. For example, methylation of the glucocorticoid receptor gene (NR3C1), which is linked to trauma, affects the functioning of HPA, resulting in heightened emotional reactivity and stress sensitivity [15].
8. Cognitive Deficits
8.1. Executive Functioning
- Deficits: Meta-analyses suggest that subdomain of executive functioning most affected by BPD is inhibition. This is characterized by an inability to inhibit impulsive responses and manage emotions [22]. These deficits in inhibition subsequently hinder decision-making processes and flexibility in complex situations.
8.2. Memory Impairments
- Spatial vs. verbal memory: Long-term spatial memory is severely impaired, whereas long-term verbal memory remains largely intact. This may be due to differential levels of hippocampal activation, with the hippocampus being more active during the processing of spatial information [22].
- Emotional memory: Affect dysregulation in BPD can exacerbate memory distortions, particularly for emotionally significant or traumatic experiences. There is impaired memory for neutral events, but increased memory for negative emotional stimuli [57].
8.3. Attention and Visuospatial Skills
- Attention: Deficits in sustained and selective attention are a hallmark of BPD, causing difficulties in work, school, and interpersonal relationships [58].
- Visuospatial skills: Deficits in spatial planning and organization, linked to parietal cortex pathology, disrupt the ability to navigate through complex tasks involving constructional and visual processing [22].
8.4. Functional Connectivity and Cognitive Deficits
9. Clinical Implications
9.1. Improved Diagnostic Accuracy
- Biomarkers: Neuroimaging techniques have revealed changes in the morphology of key brain regions, particularly the prefrontal cortex (PFC), amygdala, and hippocampus, which play a crucial role in emotion regulation and impulsive behavior.
- Heterogeneity and comorbidities: Distinguishing BPD from other psychiatric disorders, including PTSD, bipolar disorder, and major depression, will be important in clarifying BPD heterogeneity. Combining neurobiological data with clinical evaluation could clarify the characteristics that are shared versus those that are specific to each disorder [9].
9.2. Therapeutic Interventions
- Novel approaches to tailoring pharmacotherapy to genetic polymorphisms, e.g., serotonin transporter and dopamine receptor gene polymorphisms, to realize optimal therapeutic gains [65].
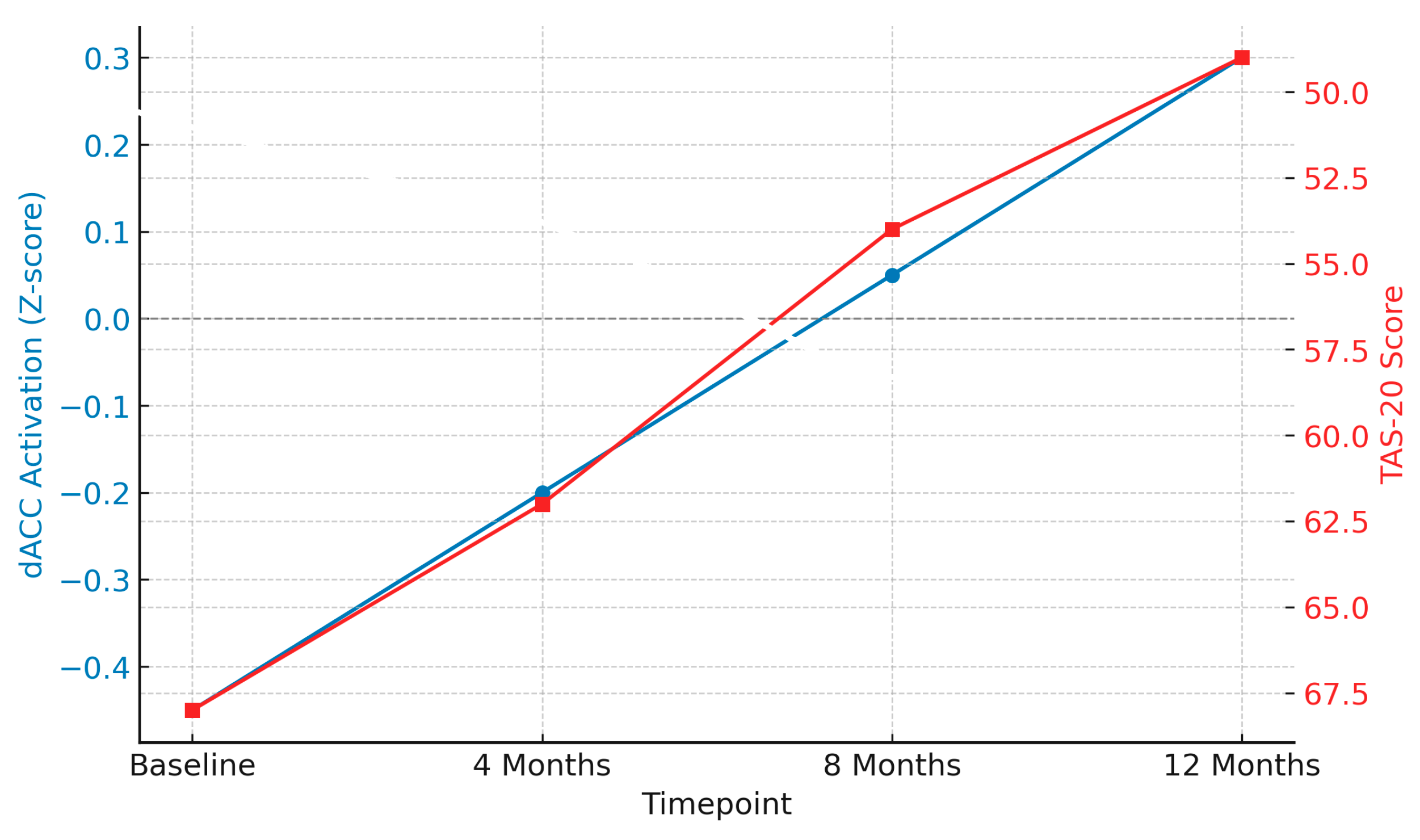
- Neuromodulation: Approaches, such as transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS), offer promising non-invasive interventions for BPD. TMS uses magnetic pulses to stimulate specific brain regions, most commonly the dorsolateral prefrontal cortex (DLPFC), which is involved in executive functioning and emotion regulation. TMS has been shown to increase prefrontal activity and reduce limbic hyperreactivity, which could improve emotional control and impulsivity in patients with BPD [66,67].
- Longitudinal effects of psychodynamic psychotherapy on neural activity and emotional functioning:
9.3. Preventive Interventions
- Adolescent interventions: The MOBY (Making Our BPD Youth) trial is a landmark randomized controlled trial evaluating early intervention strategies for adolescents and young adults presenting with borderline personality features. Conducted in Australia, the trial involved 139 participants aged 15–25, comparing 3 conditions: integrated cognitive analytic therapy (ICAT), specialized early intervention using adaptive DBT principles, and a control group receiving general clinical management [53].
9.4. Psychoeducation and Empathy Development
- The development of reliable biomarkers to improve early diagnosis and predict treatment outcomes.
- Refining neuromodulation techniques to target particular brain networks implicated in BPD.
- Longitudinal research to determine the association between neurobiological changes and symptom relapse and remission.
- Treatment efficacy: combining biology and psychology for individualized treatment.
10. Limitations and Challenges
10.1. Methodological Limitations in Neuroimaging Research
- Small sample sizes: Most neuroimaging research on BPD features small sample sizes, which compromises both the statistical power and external validity of the results. These small samples do not fully capture the heterogeneity of the disorder completely and could lead to inflated effect sizes or the identification of false positives [28].
- Imaging protocol variation: Inconsistencies in neuroimaging protocols, including differences in fMRI conditions and preprocessing procedures, hinder the comparison of results across different studies. Without standardized protocols, it is impossible to integrate findings into unifying models that describe the neurobiological underpinnings of BPD [73].
- Limitations of longitudinal data: Many studies rely heavily on cross-sectional methodology, which restricts our knowledge of the progression of neural alterations in BPD over time. Longitudinal studies are necessary to adequately explain the timing of neurobiological alterations as well as in relation to treatments [74].
10.2. Heterogeneity and Co-Morbidities
- Symptom heterogeneity: BPD is highly heterogeneous, with significant variability in symptom manifestation among subjects. This variability poses challenges for identifying specific neural correlates and may require subgroup analyses to better refine neurobiological models [75].
- Comorbid psychiatric disorders: The high prevalence of comorbid disorders, such as depression, PTSD, anxiety, and substance use disorders, makes it difficult to determine BPD-specific neural abnormalities [76]. Neurobiological findings could represent transdiagnostic effects rather than BPD-specific effects.
10.3. Ethical Considerations in Research
- Participant vulnerability: Given the high level of trauma and emotional instability characteristic of BPD samples, ethical issues can take on an even greater importance. Researchers must be sensitive to avoid exacerbating psychological distress or retraumatizing participants [77].
- Risk of stigmatization: Research on the neurobiological basis of BPD has the potential to contribute to stigmatization. The findings can be misinterpreted as indicating inherent deficits, thereby reinforcing negative stereotypes about individuals with BPD. Therefore, care should be taken when reporting research findings to enhance understanding and reduce stigma [9].
10.4. Challenges in Genetic and Epigenetic Research
- Lack of specificity: Despite research into genetic and epigenetic processes identifying some potential markers, no single marker has been regularly shown to be associated with borderline personality disorder [48]. The interaction between genetic risk factors and other psychiatric disorders makes it difficult to identify clear-cut biomarkers.
- Underpowered and small studies: Most genetic and epigenetic studies are underpowered and small since they include low sample sizes in order to detect subtle yet significant associations [9]. Large well-powered studies are the only ones that can generate proof of validation for promising epigenetic and genetic markers.
10.5. Technological and Analytical Limitations
- Complexity of neural networks: The intricacy of brain connectivity, along with how it is modulated by environmental influences, poses significant challenges to current neuroimaging techniques. Advanced methodologies, such as network analysis and machine learning, are required to elucidate the complex neural interactions observed in BPD [78].
- Limited practical application: Translating neurobiological findings into clinical practice remains difficult. Although neuroimaging and genetic findings offer the prospect of personalized medicine, their everyday use is also restricted by costs, access, and the need for specialized expertise [66].
- These challenges can be addressed using the following approaches:
- Large multi-site studies with standardized procedures can enhance the reliability and relevance of neurobiological findings.
- Longitudinal prospective studies to track neural and psychological changes over time, and their relationship to clinical recovery.
- Ethical standards to maintain participant well-being and reduce stigma.
- Cutting-edge analytical methods for investigating complex brain–behavior relationships in BPD.
10.6. Addressing Research Challenges
11. Discussion
11.1. Comparison with Prior Reviews
11.2. Functional and Clinical Interpretation
11.3. Points of Divergence and Methodological Gaps
11.4. Integrative Advances: From Network Disruption to Predictive Biomarkers
11.4.1. Functional Connectivity and Network Efficiency
11.4.2. Machine Learning and Diagnostic Precision
11.4.3. Predictive Modeling of Treatment Response:
12. Critical Synthesis and Future Directions
12.1. Transdiagnostic Comparisons
12.2. Heterogeneity Within BPD
12.3. Clinical Implications of Classifier Accuracy
12.4. Limitations and Future Directions
- Large, multicenter studies with harmonized protocols;
- Longitudinal and prospective study designs;
- Stratification by clinical subtypes and comorbidities;
- Greater use of effective and dynamic connectivity analyses;
- Adoption of risk-of-bias assessment frameworks in systematic reviews.
13. Conclusions
- (1)
- The construction of multimodal biomarker panels integrating functional connectivity profiles with epigenetic markers (e.g., FKBP5 methylation).
- (2)
- Randomized neuromodulation trials (e.g., TMS or tDCS) stratified by network phenotype (e.g., DMN vs. salience-dominant dysconnectivity).
- (3)
- Implementation studies testing the clinical utility of predictive models with ≥70% accuracy for monitoring early treatment response in weeks 6–8.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013. [Google Scholar]
- Paris, J. Chronic suicidality among patients with BPD. Psychiatry Serv. 2002, 53, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Grant, B.F.; Chou, S.P.; Goldstein, R.B.; Huang, B.; Stinson, F.S.; Saha, T.D.; Smith, S.M.; Dawson, D.A.; Pulay, A.J.; Pickering, R.P.; et al. Prevalence, correlates, disability, and comorbidity of DSM-IV borderline personality disorder: Results from the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. J. Clin. Psychiatry 2008, 69, 533–545. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trull, T.J.; Jahng, S.; Tomko, R.L.; Wood, P.K.; Sher, K.J. Revised NESARC personality disorder diagnoses: Gender, prevalence, and comorbidity with substance dependence disorders. J. Pers. Disord. 2010, 24, 412–426. [Google Scholar] [CrossRef] [PubMed]
- Ansell, E.B.; Sanislow, C.A.; McGlashan, T.H.; Grilo, C.M. Psychosocial impairment and treatment utilization by patients with borderline personality disorder. Psychiatry Serv. 2007, 58, 821–827. [Google Scholar]
- Zimmerman, M.; Rothschild, L.; Chelminski, I. The prevalence of DSM-IV personality disorders in psychiatric outpatients. Am. J. Psychiatry 2005, 162, 1911–1918. [Google Scholar] [CrossRef] [PubMed]
- Gunderson, J.G.; Stout, R.L.; McGlashan, T.H.; Shea, M.T.; Morey, L.C.; Grilo, C.M.; Zanarini, M.C.; Yen, S.; Markowitz, J.C.; Sanislow, C.; et al. Ten-year course of borderline personality disorder: Psychopathology and function from the Collaborative Longitudinal Personality Disorders Study. Arch. Gen. Psychiatry 2011, 68, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Kernberg, O.F. Borderline personality organization. J. Am. Psychoanal. Assoc. 1967, 15, 641–685. [Google Scholar] [CrossRef] [PubMed]
- Leichsenring, F.; Fonagy, P.; Heim, N.; Kernberg, O.F.; Leweke, F.; Luyten, P.; Steinert, C. BPD: A comprehensive review of diagnosis and clinical presentation, etiology, treatment, and current controversies. World Psychiatry 2024, 23, 4–25. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Krause-Utz, A.; Winter, D.; Niedtfeld, I.; Schmahl, C. The latest neuroimaging findings in borderline personality disorder. Curr. Psychiatry Rep. 2014, 16, 438. [Google Scholar] [CrossRef] [PubMed]
- Noor, L.; Hoffmann, J.; Meller, T.; Gaser, C.; Nenadić, I. Amygdala functional connectivity in borderline personality disorder. Psychiatry Res. Neuroimaging 2024, 340, 111808. [Google Scholar] [CrossRef] [PubMed]
- Choe, J.; Lin, S.; Zhang, W.; Liu, Q.; Wang, L.; Ramirez-Moya, J.; Du, P.; Kim, W.; Tang, S.; Sliz, P.; et al. mRNA circularization by METTL3-eIF3h enhances translation and promotes oncogenesis. Nature 2018, 561, 556–560. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schmidt, M.F.; Gan, Z.Y.; Komander, D.; Dewson, G. Ubiquitin signalling in neurodegeneration: Mechanisms and therapeutic opportunities. Cell Death Differ. 2021, 28, 570–590. [Google Scholar] [CrossRef] [PubMed]
- Perroud, N.; Paoloni-Giacobino, A.; Prada, P.; Olié, E.; Salzmann, A.; Nicastro, R.; Guillaume, S.; Mouthon, D.; Stouder, C.; Dieben, K.; et al. Increased methylation of glucocorticoid receptor gene (NR3C1) in adults with a history of childhood maltreatment: A link with the severity and type of trauma. Transl. Psychiatry 2011, 1, e59. [Google Scholar] [CrossRef] [PubMed]
- Drews, E.; Fertuck, E.A.; Koenig, J.; Kaess, M.; Arntz, A. Hypothalamic-pituitary-adrenal axis functioning in BPD: A meta-analysis. Neurosci. Biobehav. Rev. 2019, 96, 316–334. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, R.M. Towards precision forensic psychiatry: An advanced machine learning EEG model for high-accuracy borderline personality disorder diagnosis. Preprints 2025, 2025020527. [Google Scholar] [CrossRef]
- Schulze, L.; Schmahl, C.; Niedtfeld, I. Neural Correlates of Disturbed Emotion Processing in Borderline Personality Disorder: A Multimodal Meta-Analysis. Biol. Psychiatry 2016, 79, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, A.C.; Rodrigo, A.; Zakzanis, K.K. Amygdala and hippocampal volume reductions as candidate endophenotypes for borderline personality disorder: A meta-analysis of magnetic resonance imaging studies. J. Psychiatry Res. 2012, 46, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Nysæter, T.E.; Nordahl, H.M.; Havik, O.E. A preliminary study of the naturalistic course of non-manualized psychotherapy for outpatients with borderline personality disorder. Nord. J. Psychiatry 2010, 64, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Schmahl, C.; Vermetten, E.; Elzinga, B.; Bremner, J.D. Magnetic resonance imaging of hippocampal and amygdala volume in women with childhood abuse and borderline personality disorder. Psychiatry Res. Neuroimaging 2003, 122, 193–198. [Google Scholar] [CrossRef] [PubMed]
- D’Iorio, A.; Di Benedetto, G.L.; Santangelo, G. A meta-analysis on the neuropsychological correlates of BPD: An update. Neurosci. Biobehav. Rev. 2024, 165, 105860. [Google Scholar] [CrossRef] [PubMed]
- Schulze, R.J.; Schott, M.B.; Casey, C.A.; Tuma, P.L.; McNiven, M.A. The cell biology of the hepatocyte: A membrane trafficking machine. J. Cell Biol. 2019, 218, 2096–2112. [Google Scholar] [CrossRef] [PubMed]
- Schurz, H.; Naranbhai, V.; Yates, T.A.; Gilchrist, J.J.; Parks, T.; Dodd, P.J.; Möller, M.; Hoal, E.G.; Morris, A.P.; Hill, A.V.S.; et al. Multi-ancestry meta-analysis of host genetic susceptibility to tuberculosis identifies shared genetic architecture. eLife 2024, 13, e84394. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, A.; Medaglia, J.; Ayaz, H.; Chute, D. Abnormal prefrontal cortex response during affective processing in borderline personality disorder. Psychiatry Res. 2010, 182, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Michel, C.A.; Schneck, N.; Mann, J.J.; Ochsner, K.N.; Brodsky, B.S.; Stanley, B. Prefrontal cortex engagement during emotion regulation as a potential predictor of treatment response in BPD. J. Affect. Disord. 2024, 364, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Wingenfeld, K.; Spitzer, C.; Mensebach, C.; Grabe, H.J.; Hill, A.; Gast, U.; Schlosser, N.; Höpp, H.; Beblo, T.; Driessen, M. Die deutsche Version des Childhood Trauma Questionnaire (CTQ): Erste Befunde zu den psychometrischen Kennwerten [The German version of the Childhood Trauma Questionnaire (CTQ): Preliminary psychometric properties]. Psychother. Psychosom. Med. Psychol. 2010, 60, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Minzenberg, M.J.; Fan, J.; New, A.S.; Tang, C.Y.; Siever, L.J. Frontolimbic dysfunction in response to facial emotion in BPD: An event-related fMRI study. Psychiatry Res. 2007, 155, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Giap, T.T.; Lee, M.; Jeong, H.; Jeong, M.; Go, Y. Patient- and family-centered care interventions for improving the quality of health care: A review of systematic reviews. Int. J. Nurs. Stud. 2018, 87, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Bandelow, B.; Schmahl, C.; Falkai, P.; Wedekind, D. BPD: A dysregulation of the endogenous opioid system? Psychol. Rev. 2010, 117, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Herpertz, S.C.; Bertsch, K. A new perspective on the pathophysiology of BPD: A model of the role of oxytocin. Am. J. Psychiatry 2015, 172, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Martín-Blanco, A.; Ferrer, M.; Soler, J.; Salvà, J.; Alvarez, E.; Pascual, J.C. Association between methylation of the glucocorticoid receptor gene, childhood maltreatment and clinical severity in borderline personality disorder. J. Psychiatr. Res. 2014, 57, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Coccaro, E.F.; Siever, L.J.; Klar, H.M.; Maurer, G.; Cochrane, K.; Cooper, T.B.; Mohs, R.C.; Davis, K.L. Serotonergic studies in patients with affective and personality disorders. Correlates with suicidal and impulsive aggressive behavior. Arch. Gen. Psychiatry 1989, 46, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, J.C.; Weissman, M.M. Interpersonal psychotherapy: Principles and applications. World Psychiatry 2004, 3, 136. [Google Scholar] [PubMed]
- Soloff, P.H.; Abraham, K.; Diwadkar, V.A. SSRI fluvoxamine reduces rapid mood shifts but has variable effects on impulsivity and aggression in female BPD patients: A 6-week randomized controlled trial. Biol. Psychiatry 2002, 52, 800–807. [Google Scholar]
- Leyton, M.; Okazawa, H.; Diksic, M.; Paris, J.; Rosa, P.; Mzengeza, S.; Young, S.N.; Blier, P.; Benkelfat, C. Brain Regional alpha-[11C]methyl-L-tryptophan trapping in impulsive subjects with borderline personality disorder. Am. J. Psychiatry 2001, 158, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Friedel, R.O. Dopamine dysfunction in borderline personality disorder: A hypothesis. Neuropsychopharmacology 2004, 29, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- di Giacomo, E.; Andreini, E.; Santambrogio, J.; Arcara, A.; Clerici, M. The interplay between BPD and oxytocin: A systematic narrative review on possible contribution and treatment options. Front. Psychiatry 2024, 15, 1439615. [Google Scholar] [CrossRef] [PubMed]
- Schuermann, B.; Kathmann, N.; Stiglmayr, C.; Renneberg, B.; Endrass, T. Impaired decision making and feedback evaluation in BPD. Psychol. Med. 2011, 41, 1917–1927. [Google Scholar] [CrossRef] [PubMed]
- Salas, F.; Nvo-Fernández, M.; Leiva-Bianchi, M.; Sáez, D.A.; Páeza, G.S.; García, M.V.; Villacura-Herrera, C. Components of event-related potentials and BPD: A meta-analysis. Eur. J. Psychotraumatol. 2024, 15, 2297641. [Google Scholar] [CrossRef] [PubMed]
- White, C.; Gunderson, J.; Zanarini, M.; Hudson, J. Family Studies of Borderline Personality Disorder: A Review. Harv. Rev. Psychiatry 2003, 11, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Skoglund, C.; Tiger, A.; Rück, C.; Petrovic, P.; Asherson, P.; Hellner, C.; Mataix-Cols, D.; Kuja-Halkola, R. Familial risk and heritability of diagnosed borderline personality disorder: A register study of the Swedish population. Mol. Psychiatry 2021, 26, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Gelernter, J.; Kranzler, H.; Coccaro, E.; Siever, L.; New, A.; Mulgrew, C.L. D4 dopamine-receptor (DRD4) alleles and novelty seeking in substance-dependent, personality-disorder, and control subjects. Am. J. Hum. Genet. 1997, 61, 1144–1152. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beauchaine, T.P.; Klein, D.N.; Crowell, S.E.; Derbidge, C.; Gatzke-Kopp, L. Multifinality in the development of personality disorders: A biology × sex × environment interaction model of antisocial and borderline traits. Dev. Psychopathol. 2009, 21, 735–770. [Google Scholar] [CrossRef] [PubMed]
- Witt, K.; Spittal, M.J.; Carter, G.; Pirkis, J.; Hetrick, S.; Currier, D.; Robinson, J.; Milner, A. Effectiveness of online and mobile telephone applications (‘apps’) for the self-management of suicidal ideation and self-harm: A systematic review and meta-analysis. BMC Psychiatry 2017, 17, 297. [Google Scholar] [CrossRef] [PubMed]
- Teicher, M.H.; Andersen, S.L.; Polcari, A.; Anderson, C.M.; Navalta, C.P.; Kim, D.M. The neurobiological consequences of early stress and childhood maltreatment. Neurosci. Biobehav. Rev. 2003, 27, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.; Palmier-Claus, J.; Branitsky, A.; Mansell, W.; Warwick, H.; Varese, F. Childhood adversity and borderline personality disorder: A meta-analysis. Acta Psychiatr. Scand. 2020, 141, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Fox, D.J. Antisocial, Narcissistic, and Borderline Personality Disorders: A New Conceptualization of Development, Reinforcement, Expression, and Treatment; Routledge: Oxfordshire, UK, 2020. [Google Scholar]
- Zanarini, M.C.; Frankenburg, F.R.; Hennen, J.; Reich, D.B.; Silk, K.R. Prediction of the 10-year course of borderline personality disorder. Am. J. Psychiatry. 2006, 163, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Kaurin, A.; Beeney, J.E.; Stepp, S.D.; Scott, L.N.; Woods, W.C.; Pilkonis, P.A.; Wright, A.G.C. Attachment and Borderline Personality Disorder: Differential Effects on Situational Socio-Affective Processes. Affect. Sci. 2020, 1, 117–127. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schore, A.N. Effects of a secure attachment relationship on right brain development, affect regulation, and infant mental health. Infant. Ment. Health J. 2001, 22, 7–66. [Google Scholar] [CrossRef]
- Kabadayan, T. Borderline Personality Disorder and Treatment Methods for More Effective Interpersonal Relationships; Azusa Pacific University: Azusa, CA, USA, 2022. [Google Scholar]
- Chanen, A.M.; Betts, J.K.; Jackson, H.; Cotton, S.M.; Gleeson, J.; Davey, C.G.; Thompson, K.; Perera, S.; Rayner, V.; Andrewes, H.; et al. Effect of 3 Forms of Early Intervention for Young People With Borderline Personality Disorder: The MOBY Randomized Clinical Trial. JAMA Psychiatry 2022, 79, 109–119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vanwoerden, S.; Greiner, I.; Ensink, K.; Sharp, C. The relations between self-and caregiver-focused reflective function and theory of mind in the context of borderline pathology in adolescence. Psychiatry Res. 2019, 273, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Hazlett, E.A.; New, A.S.; Newmark, R.; Haznedar, M.M.; Lo, J.N.; Speiser, L.J.; Chen, A.D.; Mitropoulou, V.; Minzenberg, M.; Siever, L.J.; et al. Reduced anterior and posterior cingulate gray matter in borderline personality disorder. Biol. Psychiatry 2005, 58, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Friedman, N.P.; Robbins, T.W. The role of prefrontal cortex in cognitive control and executive function. Neuropsychopharmacology 2022, 47, 72–89. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Soloff, P.H.; Chowdury, A.; Diwadkar, V.A. Affective interference in borderline personality disorder: The lethality of suicidal behavior predicts functional brain profiles. J. Affect. Disord. 2019, 252, 253–262. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ruocco, A. The neuropsychology of borderline personality disorder: A meta-analysis and review. Psychiatry Res. 2006, 137, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Visintin, E.; De Panfilis, C.; Amore, M.; Balestrieri, M.; Wolf, R.C.; Sambataro, F. Mapping the brain correlates of borderline personality disorder: A functional neuroimaging meta-analysis of resting state studies. J. Affect. Disord. 2016, 204, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Schnell, K.; Herpertz, S.C. Effects of psychotherapy on brain activity in BPD. J. Psychiatr. Res. 2007, 41, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Fonagy, P.; Luyten, P. A developmental, mentalization-based approach to the understanding and treatment of borderline personality disorder. Dev. Psychopathol. 2009, 21, 1355–1381. [Google Scholar] [CrossRef] [PubMed]
- Labbé, I.Y.Q. Mentalization and Epigenetic Changes in Psycotherapy of Adolescents Diagnosed with Borderline Personality Disorder; Pontificia Universidad Catolica de Chile (Chile): Santiago, Chile, 2021. [Google Scholar]
- Wykes, T.; Reeder, C. Cognitive Remediation Therapy for Schizophrenia: Theory and Practice; Routledge: Oxfordshire, UK, 2005. [Google Scholar]
- Tang, Y.-Y.; Hölzel, B.K.; Posner, M.I. The neuroscience of mindfulness meditation. Nat. Rev. Neurosci. 2015, 16, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Amad, A.; Ramoz, N.; Thomas, P.; Jardri, R.; Gorwood, P. Genetics of borderline personality disorder: Systematic review and proposal of an integrative model. Neurosci. Biobehav. Rev. 2014, 40, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Yahya, A.S.; Khawaja, S.; Williams, P.S.; Naguib, M. Neuromodulation approaches for borderline personality disorder. Prog. Neurol. Psychiatry 2022, 26, 38–43. [Google Scholar] [CrossRef]
- Mansour, M.E.M.; Alsaadany, K.R.; Ahmed, M.A.E.; Elmetwalli, A.E.; Serag, I. Non-invasive brain stimulation for borderline personality disorder: A systematic review and network meta-analysis. Ann. Gen. Psychiatry 2025, 24, 24. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moffa, A.H.; Brunoni, A.R.; Nikolin, S.; Loo, C.K. Transcranial Direct Current Stimulation in Psychiatric Disorders: A Comprehensive Review. Psychiatr. Clin. North. Am. 2018, 41, 447–463. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Daini, S.; Calcagni, M.L.; Bruno, I.; De Risio, S. Neural correlates of psychodynamic psychotherapy in borderline disorders--a pilot investigation. Psychother. Psychosom. 2007, 76, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Uscinska, M.; Mattiot, A.P.; Bellino, S. Treatment-induced brain plasticity in psychiatric disorders. In Behavioral Neuroscience; Palermo, S., Morese, R., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Chanen, A.M.; Sharp, C.; Hoffman, P.; Schmeck, K. Prevention and early intervention for BPD: A novel public health priority. World Psychiatry 2017, 16, 215–216. [Google Scholar] [CrossRef] [PubMed]
- Fonagy, P.; Luyten, P. A multilevel perspective on the development of borderline personality disorder. In Developmental Psychopathology: Maladaptation and Psychopathology, 3rd ed.; Cicchetti, D., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 726–792. [Google Scholar] [CrossRef]
- Degasperi, G.; Cristea, I.A.; Di Rosa, E.; Costa, C.; Gentili, C. Parsing variability in borderline personality disorder: A meta-analysis of neuroimaging studies. Transl. Psychiatry 2021, 11, 314. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aleva, A.; de Boois, G.; Hessels, C.J.; Laceulle, O.M. The Cross-Sectional and Longitudinal Associations between Household Chaos, Perceived Stress, and Borderline Personality Disorder Features in Outpatient Youth. Youth 2024, 4, 1469–1480. [Google Scholar] [CrossRef]
- Hallquist, M.N.; Pilkonis, P.A. Refining the phenotype of borderline personality disorder: Diagnostic criteria and beyond. Personal. Disord. 2012, 3, 228–244. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Zanarini, M.C. Comorbidity of borderline personality disorder: Current status and future directions. Psychiatr. Clin. 2018, 41, 583–593. [Google Scholar]
- Gordon, B.G. Vulnerability in Research: Basic Ethical Concepts and General Approach to Review. Ochsner J. 2020, 20, 34–38. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, T.; Cullen, K.R.; Mueller, B.; Schreiner, M.W.; Lim, K.O.; Schulz, S.C.; Parhi, K.K. Network analysis of functional brain connectivity in borderline personality disorder using resting-state fMRI. Neuroimage Clin. 2016, 11, 302–315. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lahnakoski, J.M.; Nolte, T.; Solway, A.; Vilares, I.; Hula, A.; Feigenbaum, J.; Lohrenz, T.; King-Casas, B.; Fonagy, P.; Montague, P.R.; et al. A machine-learning approach for differentiating borderline personality disorder from community participants with brain-wide functional connectivity. J. Affect. Disord. 2024, 360, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Wolf, R.C.; Mass, R.; Beblo, T. Altered default mode network connectivity in borderline personality disorder during self-relevance evaluation. J. Psychiatry Neurosci. 2011, 36, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Nunes, P.M.; Wenzel, A.; Tavares Borges, K.; Ribeiro Porto, C.; Maiato Caminha, R.; Reis de Oliveira, I. Volumes of the hippocampus and amygdala in patients with borderline personality disorder: A meta-analysis. J. Pers. Disord. 2009, 23, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Silvers, J.A.; Hubbard, A.D.; Biggs, E.L.; Shu, J.; Fertuck, E.A.; Chaudhury, S.; Grunebaum, M.F.; Weber, J.; Kober, H.; Chesin, M.; et al. Affective lability and difficulties with regulation are differentially associated with amygdala and prefrontal response in women with borderline personality disorder. Psychiatry Res. Neuroimaging 2016, 254, 74–82. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brüne, M. On the role of oxytocin in borderline personality disorder. Br. J. Clin. Psychol. 2016, 55, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Stepp, S.D.; Lazarus, S.A.; Byrd, A.L. A systematic review of risk factors prospectively associated with borderline personality disorder: Taking stock and moving forward. Personal. Disord. 2016, 7, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, S.; Angeles-Valdez, D.; Rodríguez-Delgado, A.; Fresán, A.; Miranda, E.; Alcalá-Lozano, R.; Duque-Alarcón, X.; Arango de Montis, I.; Garza-Villarreal, E.A. Machine learning detects predictors of symptom severity and impulsivity after dialectical behavior therapy skills training group in borderline personality disorder. J. Psychiatr. Res. 2022, 151, 42–49. [Google Scholar] [CrossRef] [PubMed]
| Population Type | Prevalence (%) | Reference |
|---|---|---|
| General population (lifetime) | 1.6 | [3] |
| General population (12-month) | 0.7 | [4] |
| Primary care patients | 6.4 | [5] |
| Outpatient psychiatric samples | 10.0 | [6] |
| Inpatient psychiatric samples | 20.0 | [7] |
| Domain | Key Brain Regions/Systems | Associated Symptoms in BPD | Representative Studies |
|---|---|---|---|
| Structural | PFC, amygdala, hippocampus, parietal cortex | Emotional instability, impulsivity, memory impairments | [18,28] |
| Functional connectivity | PFC–amygdala, DMN, mentalizing networks | Poor emotion regulation, self-disturbance, social cognition deficits | [11,29] |
| Neurochemistry | Serotonin, dopamine, glutamate, oxytocin | Impulsivity, dysphoria, interpersonal sensitivity | [30,31] |
| Genetics/epigenetics | 5-HTTLPR, DRD4, NR3C1 | Increased vulnerability to trauma, emotional reactivity | [15,32] |
| Cognitive | Executive function, memory, attention | Planning deficits, dissociation, hypersensitivity | [22,25] |
| Neurobiological Feature | Clinical Manifestation | Therapeutic Implication |
|---|---|---|
| PFC–amygdala disconnect | Affective lability, reactive aggression | Targeted psychotherapy (DBT), neuromodulation (TMS) |
| Hippocampal atrophy | Dissociative symptoms, trauma sensitivity | Trauma-informed care, EMDR |
| Low serotonin/dopamine | Impulsivity, suicidality | SSRIs, pharmacogenetic approaches |
| Oxytocin dysregulation | Interpersonal dysfunction | Social cognition training, oxytocin-based trials |
| Epigenetic trauma imprinting | Stress sensitivity | Early intervention, resilience-building programs |
| Condition | DMN Hyperconnectivity | Hyper-Connected Precuneus States | Salience Network Disruption | Emotional Dysregulation | Reversibility with Treatment |
|---|---|---|---|---|---|
| Borderline personality disorder (BPD) | Yes | Stable, dominant | Yes | Severe | Partial (e.g., DBT, MBT) |
| Post-traumatic stress disorder (PTSD) | Yes | Contextual, trauma-linked | Yes | High | Moderate (e.g., trauma therapy) |
| Cocaine use disorder (CUD) | Yes | Less consistent | Moderate | Moderate | Limited |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannoulis, E.; Nousis, C.; Sula, I.-J.; Georgitsi, M.-E.; Malogiannis, I. Understanding the Borderline Brain: A Review of Neurobiological Findings in Borderline Personality Disorder (BPD). Biomedicines 2025, 13, 1783. https://doi.org/10.3390/biomedicines13071783
Giannoulis E, Nousis C, Sula I-J, Georgitsi M-E, Malogiannis I. Understanding the Borderline Brain: A Review of Neurobiological Findings in Borderline Personality Disorder (BPD). Biomedicines. 2025; 13(7):1783. https://doi.org/10.3390/biomedicines13071783
Chicago/Turabian StyleGiannoulis, Eleni, Christos Nousis, Ioanna-Jonida Sula, Maria-Evangelia Georgitsi, and Ioannis Malogiannis. 2025. "Understanding the Borderline Brain: A Review of Neurobiological Findings in Borderline Personality Disorder (BPD)" Biomedicines 13, no. 7: 1783. https://doi.org/10.3390/biomedicines13071783
APA StyleGiannoulis, E., Nousis, C., Sula, I.-J., Georgitsi, M.-E., & Malogiannis, I. (2025). Understanding the Borderline Brain: A Review of Neurobiological Findings in Borderline Personality Disorder (BPD). Biomedicines, 13(7), 1783. https://doi.org/10.3390/biomedicines13071783






