1. Introduction
The mechanisms responsible for neuronal damage induced by stress remained partially unclear, prompting ongoing research efforts to elucidate these mechanisms. Stress-induced impairment, particularly chronic or severe stress, has been associated with an increased risk of various diseases and health conditions, including cardiovascular problems, gastrointestinal issues, immune system dysfunction, endocrine disorders, and neurological disorders [
1]. However, in the presented experiments and research, we focused on stress as a known risk factor for mental health conditions such as depression.
The hypothalamic–pituitary–adrenal (HPA) axis plays a central role in the pathophysiology of depression, a disorder traditionally managed with psychotherapy and pharmacological agents targeting monoaminergic systems [
2,
3,
4]. Despite the availability of newer monoamine reuptake inhibitors and receptor antagonists, current treatments are still based on drugs discovered in the 1960s and early 1970s, and remain limited by adverse effects such as sedation, gastrointestinal disturbances, sexual dysfunction, or weight gain.
The HPA axis regulates the physiological response to stress and modulates key processes including metabolism, immune function, digestion, mood, and sexual behavior. Upon stress exposure, glucocorticoids (GCs)—primarily cortisol in humans and corticosterone (CORT) in rodents—are secreted by the adrenal cortex. These hormones influence neurogenesis, synaptic plasticity, and neuronal survival, and are essential for restoring homeostasis. However, chronic stress can lead to HPA axis hyperactivity and dysregulation, resulting in sustained elevations of circulating GCs. Prolonged GC exposure has been implicated in immune suppression [
5], metabolic disturbances [
6], osteoporosis [
7], mood disorders [
8], hippocampal atrophy [
9], and cognitive impairment [
10,
11,
12].
Since we know that excessive GC levels may have a huge impact on neuron condition and lead to their damage in several regions of the brain, we proposed to search for a drug candidate with neuroprotective potency, using an in vitro model of neuronal damage caused by CORT. Using CORT in cell models offers a controlled and consistent method to study how stress affects cellular processes.
In trying to develop new antidepressant medicines, opioid peptides will be used as hypothetical therapeutic agents. Opioid receptors are widely expressed in the central and peripheral nervous system and the non-neuronal tissues [
13]. Ligands for these receptors are compounds of exogenous and endogenous origin, with different structures and functions; among them are opioid peptides (i.e., enkephalins, endorphins, and dynorphins). They remain essential for alleviating pain. However, in addition to their classic role in pain inhibition, opioid peptides are involved in physiological actions such as immune responses, feeding, drinking, locomotor activation, and the regulation of mood and memory [
14]. Moreover, they show diverse biological effects, including cytoprotective and neuroprotective effects [
15,
16]. A growing body of evidence suggests that the opioid system, as well as opioid peptides, promote the survival of neurons during the development of the nervous system, influencing their proliferation and migration (see more in [
16]). Moreover, our recent studies showed that two highly selective endogenous µ-opioid receptor (MOR) ligands, endomorphin-1 (EM-1, Tyr-Pro-Trp-Phe-NH
2) and endomorphin-2 (EM-2, Tyr-Pro-Phe-Phe-NH
2), significantly recovered the neurotoxicity induced by 6-hydroxydopamine (6-OHDA) in the human neuroblastoma cell line (SH-SY5Y) [
17]. They increased cell viability (7–10%), and EM-1 (at the dose of 1 μM) showed a significant decrease in mitochondrial membrane potential (∆Ψm) depolarization, while EM-2 (at the dose of 1–10 μM) diminished caspase-3 activity.
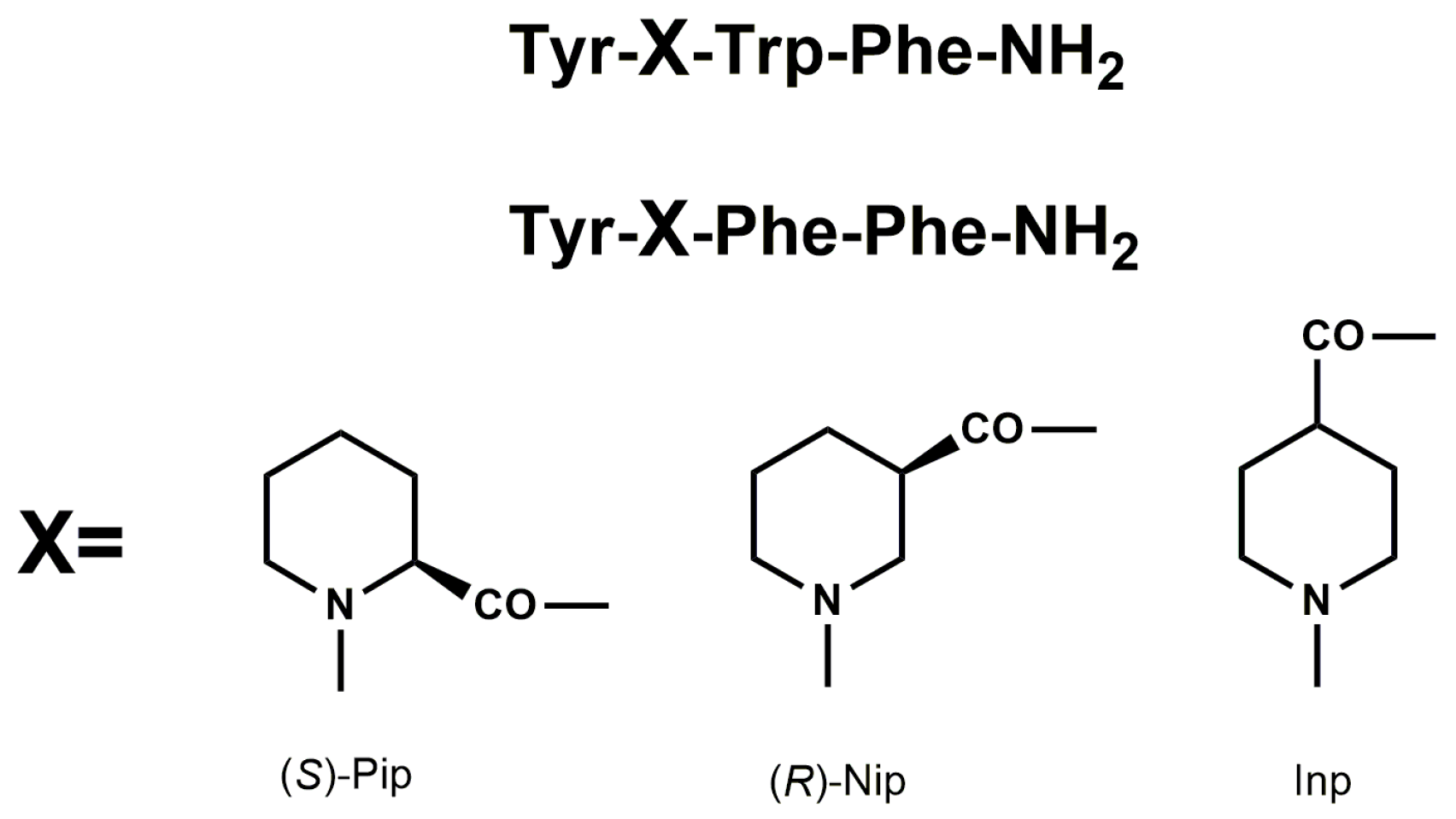
Endogenous and naturally occurring opioid peptides may serve as important leads for the design of peptide analogs with better pharmacodynamic and pharmacokinetic properties. Previously, we have described synthesis, together with biological evaluation of EM analogs, modified in position 2 by incorporation of unnatural amino acids with six-membered heterocyclic rings, such as piperidine-2-, 3-, and 4-carboxylic acids ((
S)-Pip, (
R)-Nip, and Inp, respectively) (
Figure 1) [
18,
19,
20].
Modified EM analogs showed enhanced μ-opioid receptor affinity, functional potency, metabolic stability, and in vivo antinociceptive effects after intracerebroventricular (icv) administration, which was completely reversed by β-funaltrexamine (β-FNA), a selective µ-opioid receptor antagonist, highlighting their potential for further development.
Taking into account the satisfactory biological activity of the obtained EM analogs, we decided to check their neuroprotective profile. To explore this effect, we used a CORT-induced in vitro model of depression using SH-SY5Y cells. Additionally, we proposed an anti-inflammatory study by assessing the ability to reduce nitric oxide (NO), tumor necrosis factor alpha (TNF-α), and interleukin 6 (IL-6) production in macrophage RAW 264.7 cells damaged by lipopolysaccharide (LPS) and interferon gamma (IFN-γ).
In this study, peptides 2, 3, 6, and 7 showed the best increase in cell survival in the CORT-injured cell model. However, further studies of the mechanism revealed that the greatest neuroprotective potential concerns analogs 3 and 7. These peptides most effectively suppressed the changes caused by CORT administration. They significantly reduced the release of lactate dehydrogenase (LDH) and inhibited reactive oxygen species (ROS) production and [Ca2+]i release. Peptides improved mitochondrial function and enhanced ATP production while reducing apoptosis and caspase-3 activity. Additionally, they modulated the balance of pro- and anti-apoptotic genes (Bax and Bcl-2, respectively) expression. We also suggested that the attenuation of the cytotoxic effect of CORT additionally resulted from the peptide’s ability to increase the mRNA and protein levels of brain-derived neurotrophic factor (BDNF). Moreover, in the LPS- and IFN-γ-induced damage model of RAW 264.7 cells, analogs 3 and 7 reduced the inflammatory response.
2. Materials and Methods
2.1. Peptide Synthesis
Peptides were synthesized by standard solid-phase procedures, as described before [
18,
19,
20], using techniques for 9-fluorenylmethoxycarbonyl (Fmoc)-protected amino acids on MBHA Rink-Amide peptide resin (100–200 mesh, 0.80 mM/g, Novabiochem) and 2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium tetrafluoroborate (TBTU) as a coupling agent. Crude peptides were purified by preparative reversed-phase HPLC on a Vydac C18 column (10 μm, 22 × 250 mm) equipped with a Vydac guard cartridge. For purification, a linear gradient of 0–100% acetonitrile containing 0.1% TFA over 15 min. at a flow rate of 15 mL/min. was used. The purity of the final peptides was verified by analytical HPLC employing a Vydac C18 column (5 μm, 4.6 × 250 mm) and the solvent system of 0.1% TFA in water (A) and 80% acetonitrile in water containing 0.1% TFA (B). A linear gradient of 0–100% solvent B over 25 min. at a flow rate of 1 mL/min. was used for the analysis. The absorbance was monitored at 214 nm. The final purity of all peptides was >98%. Calculated values for protonated molecular ions were in agreement with those determined by FAB mass spectrometry. The physicochemical data of tested peptides are presented in
Table 1. The combination of 2D 1H NMR measurements and molecular modeling calculations were published earlier [
18,
19,
20].
2.2. Cell Culture and Treatment
The human neuroblastoma cell line (SH-SY5Y) was purchased from the European Collection of Cell Cultures (ECACC) and cultivated at 37 °C in a humidified atmosphere of 5% CO
2 in the air with Dulbecco’s Modified Eagle Medium (DMEM): Nutrient Mixture F-12 (Ham’s) (1:1) (Gibco/Life Technologies, Carlsbad, CA, USA) containing 2 mM glutamine, antibiotics (100 U/mL penicillin and 100 µg/mL streptomycin) (Sigma-Aldrich, St. Louis, MO, USA), and 10% (
v/
v) heat-inactivated fetal bovine serum (FBS, Biological Industries, Beit-Haemek, Israel) according to the previously described protocol [
17,
21]. The mouse macrophage-like RAW 264.7 cells were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA) and maintained in DMEM (Gibco™, Grand Island, NY, USA) supplemented with 10% bovine calf serum (BCS, Gibco™, Grand Island, NY, USA) and 1% penicillin–streptomycin (Sigma-Aldrich, St. Louis, MO, USA) [
21]. The number of cells was assessed under a phase-contrast microscope based on the exclusion of trypan blue dye. The cultured cells were maintained for 3 days to allow for adhering to the plates. Subculture was performed using 0.25% trypsin/EDTSA (Gibco/Life Technologies, Carlsbad, CA, USA) after the cells reached confluence.
The tested peptides and CORT were dissolved in sterile DMSO and further diluted with a culture medium. The final concentration of DMSO in cell cultures was less than 0.1% (v/v). Controls without and with 0.1% DMSO were performed in each experiment. At the used concentration, DMSO did not affect the observed parameters.
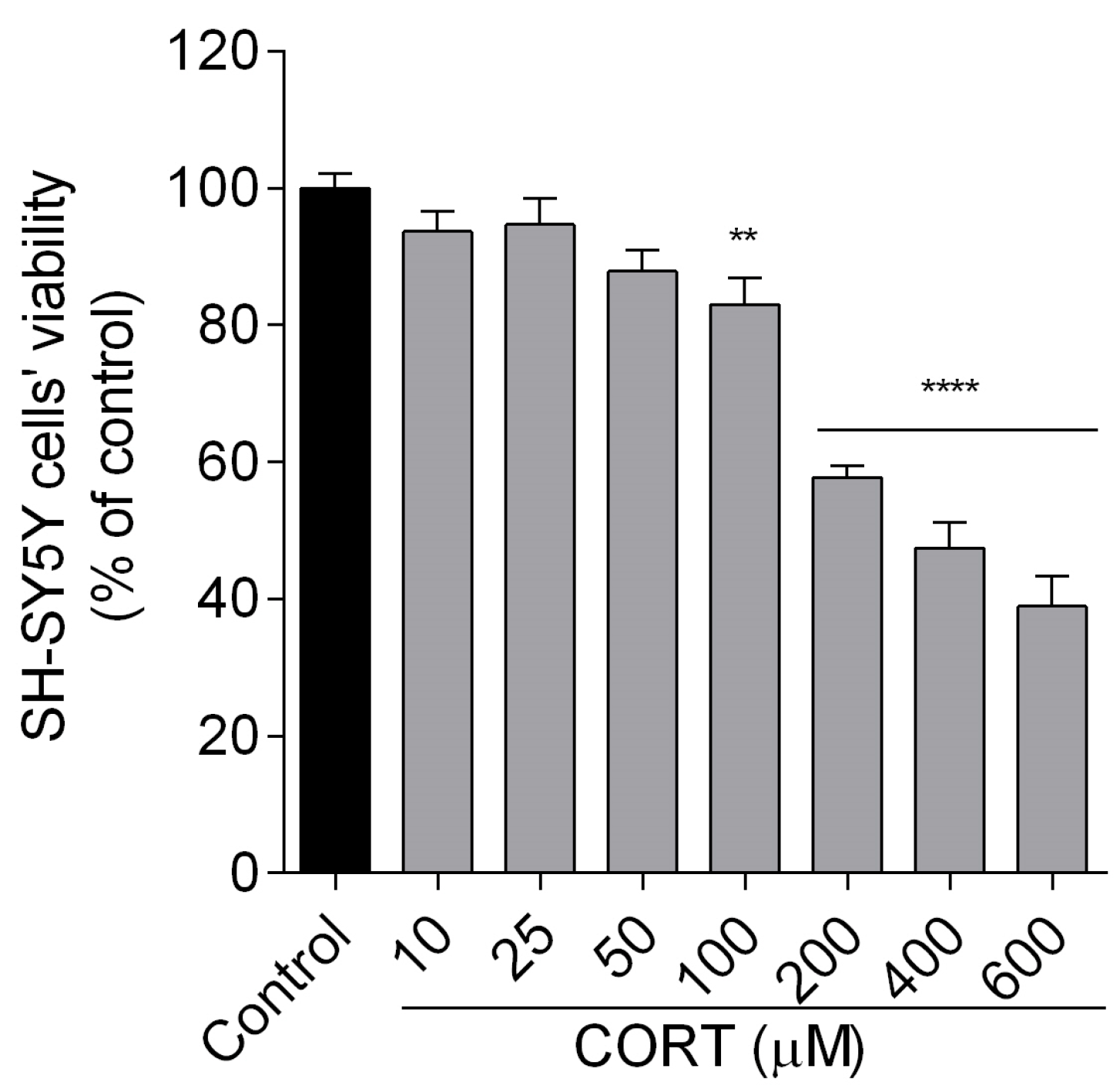
To measure the cytotoxic effect of the tested compounds, the SH-SY5Y cells were incubated for 24 h with different doses of peptides (0.01, 0.1, 1 µM) and CORT (10, 25, 50, 100, 200, 400, 600 µM) alone.
In a cell model imitating stress-induced damages, peptides and CORT were co-incubated for 24 h. To study the inflammatory effect, 24 h after RAW 264.7 seeding, LPS/IFN-γ and peptides were added for the next 24 h.
2.3. Cytotoxic and Neuroprotective Activities of Peptides
The MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay, which measures the activity of cellular dehydrogenases as an indicator of cell viability and cytotoxicity, was performed according to the Mosmann method [
22] with minor modifications. It is a quantitative method based on the reduction in tetrazolium yellow dye named MTT by metabolically active cells to purple formazan crystals. The concentration of formazan was measured calorimetrically. Exponentially growing cells were seeded into 96-well plates at a density of approximately 2 × 10
4 cells/well and left to grow for 24 h under controlled conditions (37 °C; 5% CO
2). Subsequently, SH-SY5Y cells were exposed for 24 h to peptides (0.01–1 µM) and CORT (200 µM). After 24 h of incubation, cells were incubated with 20 µL of MTT tetrazolium salt solution (5 mg/mL in phosphate-buffered saline, PBS) for 1.5 h under a controlled condition (37 °C; 5% CO
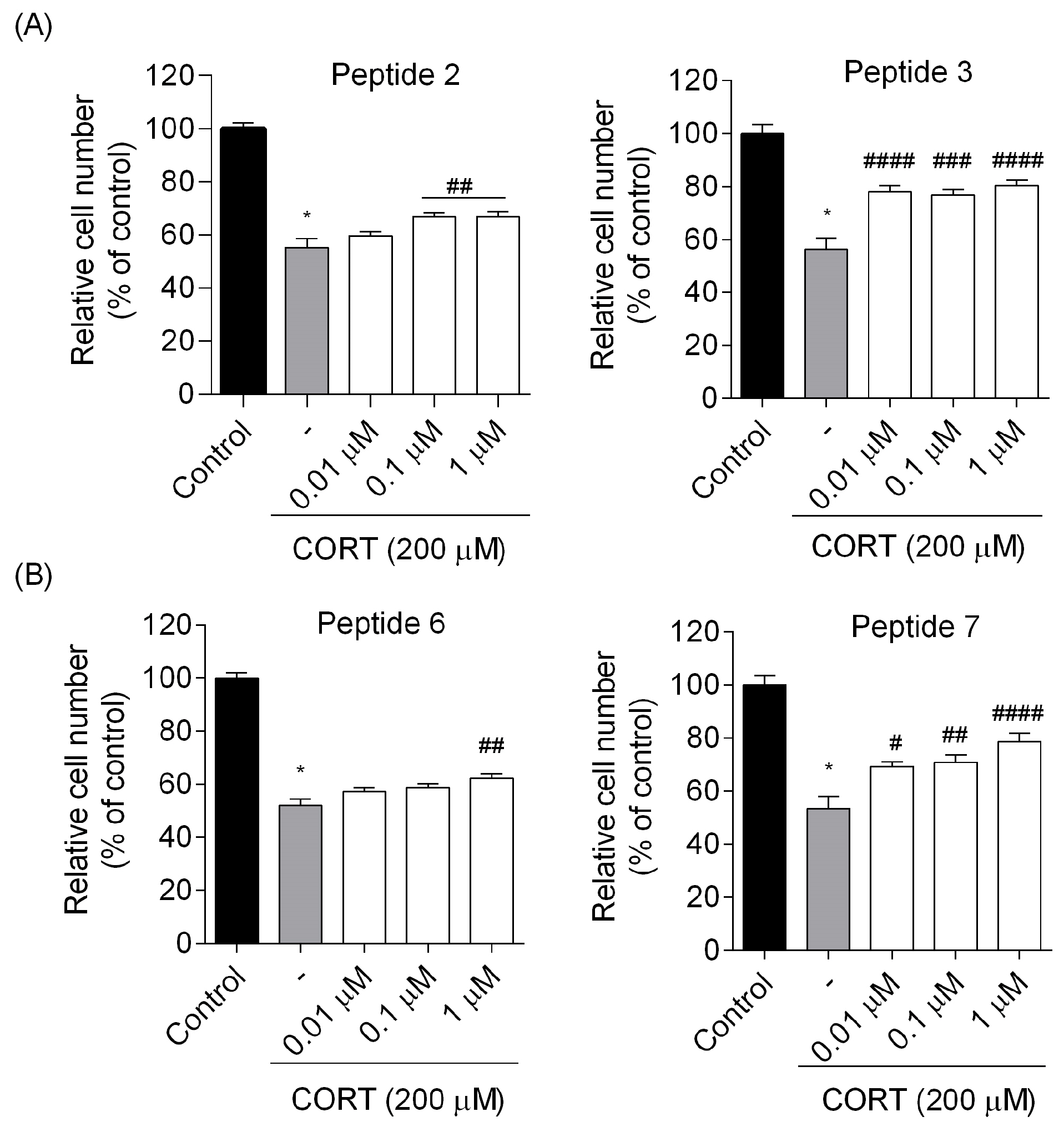
2). Following the incubation time, the medium was aspirated, and DMSO (100 µL) was added to each well to dissolve the crystals, whose absorbance was measured at 560 nm using FlexStation 3 Multi-Mode Microplate Reader (Molecular Devices, LLC, San Jose, CA, USA). The experimental design included vehicle controls (non-treated cells) and blanks (wells without cells). The cell viability rates were determined as a percentage relative to the viability of the control group (non-treated cells). The data were expressed as mean ± SEM of three to four independent experiments.
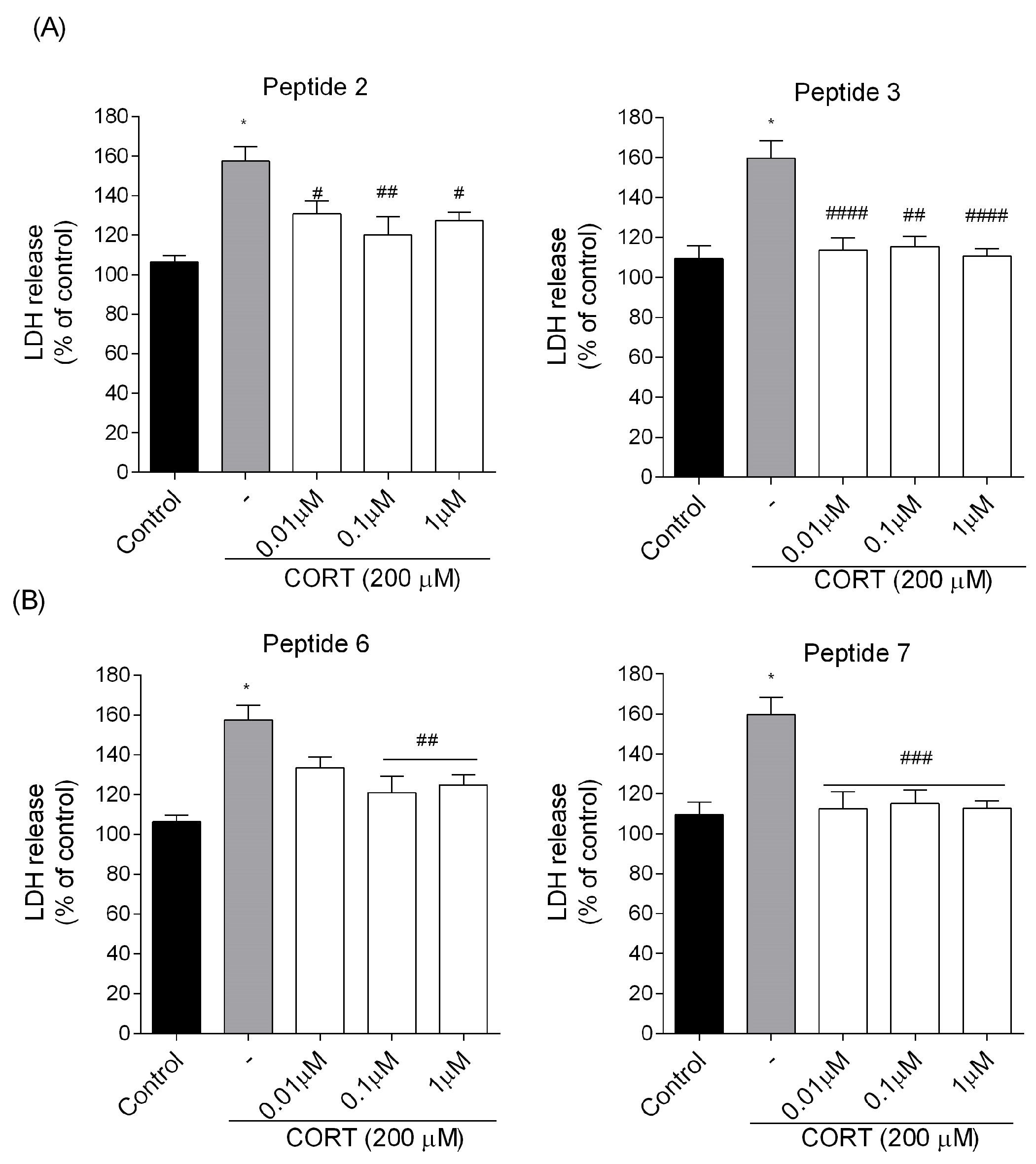
Additionally, to evaluate the release of lactate dehydrogenase (LDH) into the SH-SY5Y cell culture medium upon cell damage or lysis, we used a commercially available CytoTox 96® Non-Radioactive Cytotoxicity Assay (Promega Corporation, Madison, WI, USA) according to the manufacturer’s instructions. The assay measures LDH activity based on the conversion of a tetrazolium salt into a red formazan product. The color intensity of the red formazan is directly proportional to the amount of LDH released and hence the number of damaged/lysed cells. Briefly, SH-SY5Y cells were seeded into 96-well plates at a density of approximately 2 × 104 cells/well and left to grow for 24 h under controlled conditions (37 °C; 5% CO2). Then, they were exposed to peptides (0.01–1 µM) and CORT (200 µM) for 24 h. At the end of the incubation time, 50 µL of the sample was incubated with CytoTox 96® Reagent in the dark for 30 min at room temperature. Then, 50 μL of Stop Solution was added, and finally, absorbance was read at 490 nm at 37 °C using FlexStation 3 Multi-Mode Microplate Reader (Molecular Devices, LLC., San Jose, CA, USA). The experimental design included control group (non-treated cells) and blanks (wells without cells). The release of LDH was determined as a percentage relative to the LDH leakage of the control. The data were expressed as mean ± SEM of three independent experiments.
2.4. Trypan Blue Exclusion Assay
We further investigated the impact of co-incubated peptides and CORT on cell viability using the Trypan blue exclusion assay to validate the results. Trypan blue is a diazo dye that selectively enters only dead cells, binding to intracellular proteins and staining them blue. Conversely, viable cells with undamaged cellular membranes resist staining and do not take up the dye, allowing them to be distinguished from the dead cells. Briefly, SH-SY5Y cells were seeded at a density of 4 × 105 cells/well in 6-well plates and left to grow for 24 h under controlled conditions (37 °C; 5% CO2). After 24 h of incubation with various concentrations of the tested compounds, cells were detached by trypsinization, centrifuged, and the pellet was resuspended in a fresh growth medium. From the cell suspension, 15 µL was transferred to an Eppendorf tube, to which an equivalent volume of 0.4% trypan blue dye (Sigma-Aldrich, St Louis, MO, USA) was added and loaded onto the counting chambers. The experimental design included vehicle control (non-treated cells). The cell viability rates were determined as a percentage relative to the viability of the vehicle control (non-treated group). The data were expressed as mean ± SEM of three independent experiments. Cells were counted using an inverted microscope (magnification 40×, Motic Images Plus version 3.0).
2.5. Determination of Cell Morphology Using Wright–Giemsa Staining
The morphological changes in SH-SY5Y cells induced by CORT and peptides were analyzed by Wright–Giemsa (Merck KGaA, Darmstadt, Germany) staining according to the method described by Dlugosz-Pokorska et al. [
23]. The dye stains the cytoplasm an orange to pink color, while the nucleus is blue to purple, depending on the acidity of the cytoplasmic content. Briefly, SH-SY5Y cells were seeded at a density of 4 × 10
5 cells/well in 6-well plates and left to grow for 24 h under controlled conditions (37 °C; 5% CO
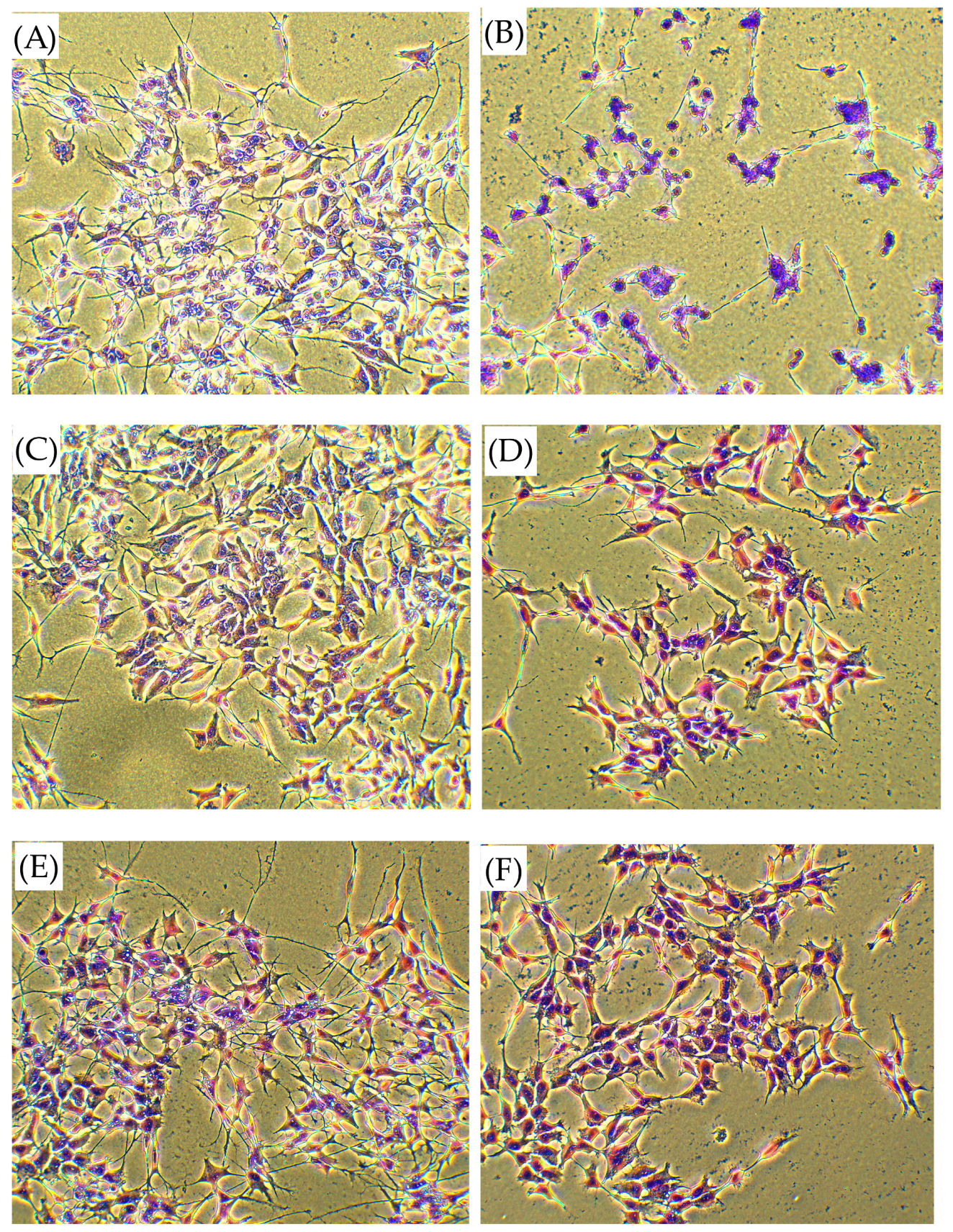
2). After 24 h of incubation with various concentrations of the tested compounds, the cell culture medium was removed, and the cells were rinsed twice with PBS. The cells were then fixed with cold methanol for 5 min and washed with fresh PBS again. A staining procedure was performed using a Wright–Giemsa solution for 30 min, followed by a rinse with water and subsequent drying. Morphological changes in the SH-SY5Y cells were examined using an inverted microscope (magnification 40×, Motic Images Plus version 3.0) with a built-in camera (Motic Moticam 2300, 3.0M Pixel USB2.0, Merazet, Poznań, Poland and photographed.
2.6. Determination of Intracellular Reactive Oxygen Species (ROS)
Intracellular ROS levels were analyzed using 5(6)-carboxy-2′,7′-dichlorofluorescein diacetate (Carboxy-H2DCFDA, Sigma-Aldrich, St. Louis, MO, USA) according to the method previously described by Silva et al. [
24] with minor changes. Carboxy-H2DCFDA is a cell-permeant and non-fluorescent indicator of ROS that, in the presence of ROS, undergoes oxidation, resulting in a green fluorescent signal. To assess intracellular ROS generation, SH-SY5Y cells were seeded at a density of 2 × 10
4 cells/well in 96-well black/clear bottom plates and left to grow for 24 h under controlled conditions (37 °C; 5% CO
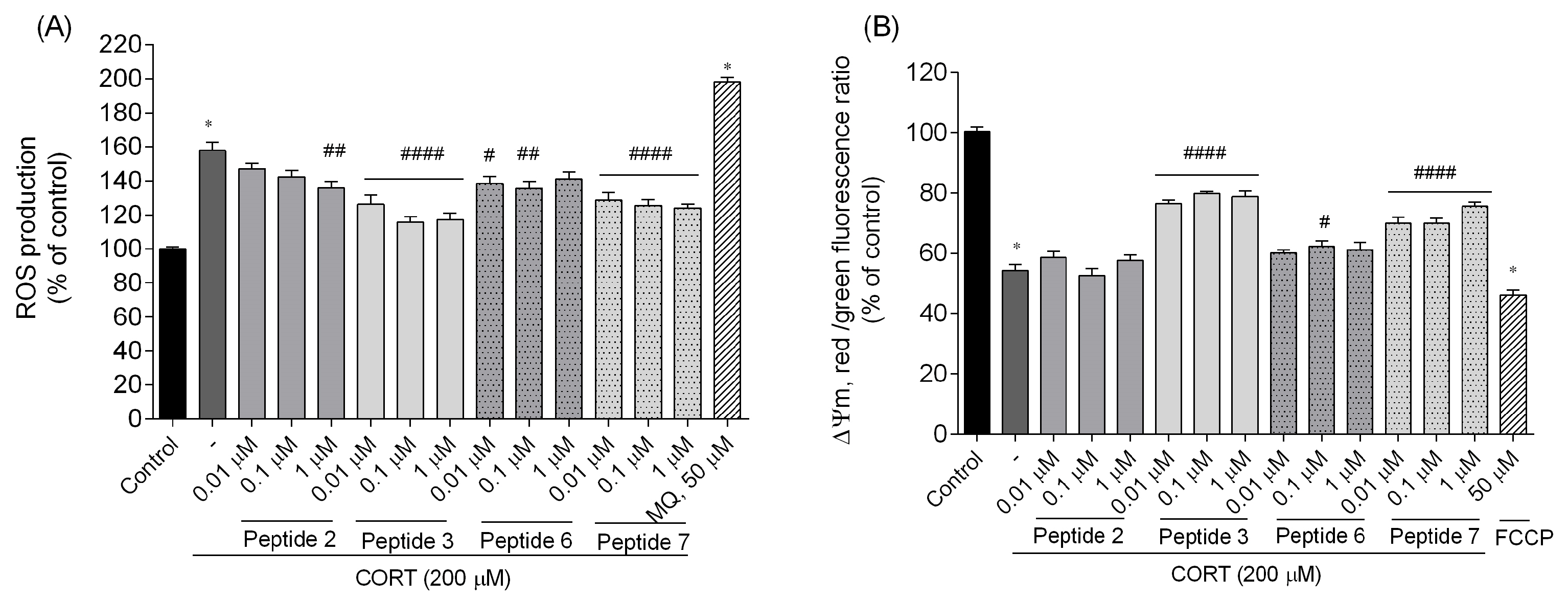
2). Afterwards, they were rinsed with HEPES-buffered salt solution (HBSS, ThermoFisher Scientific, Waltham, MA, USA) to remove traces of the medium, and carboxy-H2DCFDA at a final concentration of 10 μM in culture medium without FBS and phenol red was added. After 30 min of incubation at 37 °C, the carboxy-H2DCFDA-containing medium was removed, cells were washed with HBSS, and fresh medium containing peptides and CORT was added. After incubation, fluorescence intensity was monitored at wavelengths of 485 nm (excitation) and 535 nm (emission) using a FlexStation 3 Multi-Mode Microplate Reader (Molecular Devices, LLC, San Jose, CA, USA). The experimental design included control wells and menadione (MQ, 50 µM) as a positive control. The data was analyzed and expressed as a percentage of the control (non-treated cells).
2.7. Determination of Mitochondrial Membrane Potential Changes (∆Ψm)
Changes in the mitochondrial membrane potential (∆Ψm) were measured using the commercially available JC-10 assay kit (Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer’s instructions. The 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolocarbocyanine iodide (JC-10) is a cationic and lipophilic dye that can selectively penetrate mitochondria. It forms reversible red fluorescent JC-10 aggregates (λex = 540/λem = 590 nm) in the mitochondria of healthy cells with a polarized mitochondrial membrane. When ΔΨm is depolarized, the mitochondria fail to retain JC-10, leading to the dye reverting to its monomeric green fluorescent form (λex = 490/λem = 525 nm), indicating cells with inherently low ΔΨm, suggestive of apoptosis. Briefly, SH-SY5Y cells were seeded at a density of 2 × 104 cells/well in 96-well black/clear bottom plates and left to grow for 24 h under controlled conditions (37 °C; 5% CO2). Following treatment with CORT and peptides for 24 h, SH-SY5Y cells were loaded with 50 μL/well of JC-10 for 30 min. After incubation, 50 µL/well of Assay Buffer was added. Then fluorescence intensity was monitored at wavelengths of λex = 490/λem = 525 nm (green fluorescence monomer) and λex = 540/λem = 590 nm (red fluorescence aggregates), respectively, using FlexStation 3 Multi-Mode Microplate Reader (Molecular Devices, LLC, San Jose, CA, USA). The data was analyzed and expressed as a ratio of red (λex = 540/λem = 590 nm) to green (λex = 490/λem = 525 nm) fluorescence. The measured ratio values were normalized to the control (non-treated cells) and expressed as a percentage of control. A mitochondrial uncoupler, carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone (FCCP, Sigma-Aldrich, St. Louis, MO, USA) was used as a positive control (50 µM, 30 min treatment at 37 °C).
2.8. Measurement of Adenosine Triphosphate (ATP) Levels
An adenosine triphosphate (ATP) is the primary energy carrier in all metabolically active cells; thus, it is widely used as a marker of cell viability. When cells undergo necrosis or apoptosis, the existing ATP is quickly degraded, and its concentration rapidly declines. Therefore, measuring ATP levels can provide a reliable indication of the number of viable cells in a sample. To investigate the changes in cellular ATP levels, a luminescence ATP Detection Assay System ATPLite assay kit (PerkinElmer, Waltham, MA, USA) was used according to the manufacturer’s instructions. Briefly, SH-SY5Y cells were seeded at a density of 2 × 104 cells/well in 96-well white/clear bottom plates and left to grow for 24 h under controlled conditions (37 °C; 5% CO2). Following treatment with peptides for 24 h, SH-SY5Y cells were loaded and shaken with 50 μL/well of cell lysis solution for 5 min. After that, cells were treated with 50 μL/well of substrate solution and shaken for another 5 min. Then, after 10 min of incubation in the dark, the luminescence intensity was measured using a microplate reader FlexStation 3 Multi-Mode Microplate Reader (Molecular Devices, LLC., San Jose, CA, USA). The data was analyzed and expressed as a percentage of the control (non-treated cells).
2.9. Determination of Intracellular Calcium Ion [Ca2+]i Level
The intracellular calcium [Ca
2+]
i level, as a secondary cytotoxic hallmark, was determined using the fluorescent probe Fura-2-acetoxymethyl ester, Fura 2-AM (Fura-2 pentakis(acetoxymethyl) ester, Sigma-Aldrich, St. Louis, MO, USA) according to the described method [
25,
26], with minor changes. This membrane-permeable calcium indicator is used to measure cellular calcium concentrations by fluorescence. After entering the cell, cytosolic esterases convert Fura 2-AM to its active form, enabling it to bind calcium and emit fluorescence (380 nm). Briefly, SH-SY5Y cells were seeded at a density of 2 × 10
4 cells/well in 96-well plates and left to grow for 24 h under controlled conditions (37 °C; 5% CO
2). Following treatment with peptides and CORT for 24 h, SH-SY5Y cells were washed with HEPES buffer solution (HBSS, pH 7.4), and loaded with a loading buffer (HEPES buffer solution containing 135 mM NaCl, 5 mM KCl, 1 mM CaCl
2, 1 mM MgCl
2, 25 mM glucose, 10 mM HEPES, 0.1% bovine serum albumin, pH 7.4) and 0.5 µM of Fura 2-AM for 60 min in total darkness at 37 °C. Afterwards, they were rinsed with HBSS to remove traces of the medium with free Fura 2-AM. The fluorescence intensity was quantified at an excitation of 340/380 nm and an emission of 510 nm and monitored using FlexStation 3 Multi-Mode Microplate Reader (Molecular Devices, LLC, San Jose, CA, USA). The relative [Ca
2+]
i level was calculated from the ratio of the emission intensities with excitations of 340 nm and 380 nm (ratio 340/380).
2.10. Measurement of Caspase-3 Activity
Caspase-3 activity was measured using a colorimetric assay kit (Millipore, APT165, Billerica, MA, USA) following the manufacturer’s instructions. Briefly, SH-SY5Y cells were seeded at a density of 4 × 105 cells/well in 6-well plates and left to grow for 24 h under controlled conditions (37 °C; 5% CO2). Following treatment with peptides and CORT for 24 h, SH-SY5Y cells were harvested and lysed in an ice-cold lysis buffer, then centrifuged. Supernatants were collected, and protein concentrations were measured using the Bradford protein assay. The isolated supernatant was incubated with a reaction buffer containing the caspase-3 substrate acetyl-Asp-Glu-Val-Asp-p-nitroaniline (Ac-DEVD-pNa) at 37 °C for 2 h. Absorbance was measured at 405 nm at 37 °C using FlexStation 3 Multi-Mode Microplate Reader (Molecular Devices, LLC., San Jose, CA, USA). The experimental design included a p-nitroaniline (pNA) standard curve, control (non-treated cells), and blanks (wells without cells). The caspase activity was determined using a standard curve and expressed as the relative fold of caspase-3 activity of each sample compared with the control. The data were expressed as mean ± SEM of three independent experiments.
2.11. Real-Time Assessment of Cell Death Type
Cell death type was found using the RealTime-GloTM Annexin V Apoptosis and Necrosis Assay (Promega, Mannheim, Germany), according to the manufacturer’s guidelines and a previous paper [
23]. The test kit included Annexin V-LgBiT (1000×), Annexin V-SmBiT (1000×), CaCl
2 (1000×), Annexin V NanoBiT
® Substrate (1000×), and Necrosis Detection Reagent (1000×). Briefly, SH-SY5Y cells were seeded at a density of 2 × 10
4 cells/well in 96-well white plates and left to grow for 24 h under controlled conditions (37 °C; 5% CO
2). Following treatment with peptides and CORT for 24 h, 100 μL of 2× detection reagent was added to each well. Then, the plate was shaken for 30 s (at 500–700 rpm) and incubated in a humidified incubator. The luminescence signal and fluorescence (excitation of 485 nm, emission of 530 nm) intensity were analyzed using FlexStation 3 Multi-Mode Microplate Reader (Molecular Devices, LLC, San Jose, CA, USA). The measured values were compared to the control.
2.12. Quantitative Real-Time PCR Assay (qPCR)
The mRNA levels of Bax, Bcl-2, caspase-3, and BDNF genes were analyzed by qPCR. Briefly, SH-SY5Y cells were seeded on the 6-well plates (4.0 × 10
5 cells/well) and then incubated with peptides and CORT. Total RNA was extracted using the Total RNA Mini Kit (A&A Biotechnology, Gdynia, Poland) according to the manufacturer’s protocol. The concentration of RNA was measured using a sensitive single-tube fluorimeter for the fluorescence-based quantitation of nucleic acids and proteins (QuantiFluor
® RNA System, Promega, Mannheim, Germany). TranScriba Kit (A&A Biotechnology, Gdynia, Poland) was used for cDNA synthesis. Amplification of cDNA was performed using Real-Time 2×-PCR SYBR Master Mix (A&A Biotechnology, Gdynia, Poland) in Stratagene MX3005P QPCR System (Agilent Technologies, Inc., Santa Clara, CA, USA) according to the manufacturer’s instructions. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal reference gene to normalize the expression of investigated genes. The expression levels of the tested genes were determined by the 2
−∆∆CT method [
27]. The primer sequences used for qPCR are listed in
Table 2.
2.13. Estimation of Brain-Derived Neurotrophic Factor (BDNF) Using ELISA
Various treatments can influence BDNF levels in SH-SY5Y cells. Thus, we checked the impact of peptides on CORT-injured cells’ condition. Cells were seeded at a density of 4 × 10
5 cells/well in 6-well plates and left to grow for 24 h under controlled conditions (37 °C; 5% CO
2). Following treatment with peptides and CORT for 24 h, the supernatants were collected and centrifuged at 150×
g at 4 °C to remove floating cells. Then, the prepared supernatants were immediately added to a 96-well plate coated with a primary antibody specific to human BDNF according to the manufacturer’s instructions (Human BDNF SimpleStep ELISA
® Kit, Abcam, Cambridge, Great Britain) and our previous study [
17]. Briefly, 50 μL of all samples or standard was added to the appropriate wells, with 50 μL of the Antibody Cocktail. The plate was sealed and shaken (400 rpm) for 1 h at room temperature. Then, the plate was washed with 1X Wash Buffer PT (250 μL × 3 times), and TMB substrate solution was added and shaken (400 rpm) for 10 min in the dark. The color developed in proportion to the amount of bound BDNF. Finally, the stop solution changed the color from blue to yellow, and the intensity was measured at 450 nm. A standard curve was run for each assay, and all standards or samples were run in triplicate.
2.14. Western Blot Analysis
Western blot analysis was used for assaying BDNF protein expression. Briefly, SH-SY5Y cells were seeded at a density of 4 × 105 cells/well in 6-well plates and left to grow for 24 h under controlled conditions (37 °C; 5% CO2). Following treatment with peptides and CORT for 24 h, cells were washed 2 times with ice-cold PBS, and then 160 µL of ice-cold RIPA lysis buffer [with a cocktail of protease inhibitors (Hoffman-La Roche Ltd., Basel, Switzerland), pH 7.4] was added. Cells were incubated on ice for 15 min. Lysates were then centrifuged at 12,000× g for 10 min at 4 °C, and the supernatants were used as the total cell lysates. The protein content in the supernatants was quantified using the Bio-Rad protein assay kit. Equal amounts of protein were separated on 10% sodium dodecyl sulfate (SDS)–polyacrylamide gels and transferred onto a membrane using the Trans-Blot Turbo system (Bio-Rad, California, CA, USA), which allows for rapid and efficient protein transfer. Non-specific binding was blocked with tris-buffered saline Tween-20 (TBS-T: 150 mM NaCl, 20 mM Tris-HCl, 0.1% Tween 20, pH 7.5) containing 10% (w/v) nonfat milk for 1 h at room temperature. Then, the membrane was incubated overnight at 4 °C with one of the following specific primary antibodies: Vinculin (1:1000 dilution; Cell Signaling Technology, Danvers, MA, USA) or BDNF (1:1000 dilution; Cell Signaling Technology, Danvers, MA, USA), with 1% (w/v) bovine serum albumin. After incubation, the membranes were washed six times per 5 min with TBS-T. In the next step, membranes were incubated with HRP-conjugated secondary antibodies (1:1000 dilution; Cell Signaling Technology, Danvers, MA, USA) in TBS-T with 5% nonfat milk powder for 1 h at room temperature. Following six washes with TBS-T, the protein bands were detected with the Westar Supernova reagent from Cyanagen and visualized on a CCD camera in G:Box Chemi XR5, Syngene System. The band intensity was quantified by NIH ImageJ (version 3.0) densitometric analysis. Values of unstimulated samples were set to 1.0.
2.15. Secretion of Pro-Inflammatory Mediators
The level of nitric oxide (NO) production was determined in RAW 264.7 cells. Briefly, after cells’ attachment, they were treated with LPS (O55: B5 from Escherichia coli, Millipore Sigma, Darmstadt, Germany) at 1 µg/mL and 100 U/mL IFN-γ in medium with 3% of FBS for 24 h, and then the peptides were added for additional 24 h. After cells’ treatment, the medium was collected and the accumulation of NO metabolite in the cell culture supernatant was measured using Griess reagent (1% sulfanilamide and 0.1% naphthylethylenediamine dihydrochloride; Sigma-Aldrich), where 50 µL of the supernatant was mixed with 50 µL of Griess reagent (40 mg/mL) in a 96-well plate. After incubation at room temperature and darkness for 10 min, the absorbance was measured at 540 nm. Cells treated with LPS/IFN-γ without peptides were used as the positive control of the inflammatory response. Simultaneously, in the supernatants after RAW 264.7 cell treatment, the protein concentrations of interleukin 6 (IL-6) (Mouse IL6 ELISA kit, Biorbyt Ltd., Cambridge, UK) and tumor necrosis factor α (TNF-α) (Mouse TNFalpha ELISA kit, Biorbyt Ltd., Cambridge, UK) were determined by absorbance detection using ELISA kits, following the manufacturer’s instructions.
2.16. Statistical and Data Analyses
All data in this study were expressed as mean ± SEM of at least three to four independent experiments performed in triplicate. Statistical analyses were performed using one-way analysis of variance (ANOVA), followed by Dunnett’s multiple range test. Differences were considered statistically significant at p < 0.05. All the statistical analyses and constructions of graphs were performed using statistical software GraphPad Prism 6 (San Diego, CA, USA).
4. Discussion
The mechanisms underlying stress-induced damage to neurons were not fully understood, and ongoing research aimed to elucidate these mechanisms. The precise molecular and cellular pathways involved in stress-induced impairment can be complex and multifaceted. Some of the proposed mechanisms include glucocorticoid imbalance, oxidative stress, inflammation, altered neurotransmitter levels, or changes in synaptic plasticity. Here, we would like to focus on cellular changes, resulting in increased production of CORT, which have detrimental effects on neurons, including impaired stress response, mood regulation, neuroinflammation, and changes in synaptic plasticity, crucial for learning and memory. At the cellular level, stress can generate ROS and oxidative stress in neurons, leading to cellular damage involving DNA and protein damage, which may contribute to neurodegenerative conditions. As a result of stress, an inflammatory response is also generated in the brain, characterized by the activation of microglia and the release of pro-inflammatory cytokines. It is important to note that ongoing research continues to uncover new insights into the mechanisms of stress-induced neuronal damage. Using various models and techniques, including cell cultures, researchers investigate the effects of stress on neurons and their underlying mechanisms. Ultimately, a better understanding of these mechanisms helps to develop the potential treatments for stress-related neurological and psychiatric disorders. The human neuroblastoma SH-SY5Y cell line is widely used as a model cell system for studying neuronal cell death induced by various cellular stressors, including oxidative damage and mitochondrial dysfunction [
16,
46].
In this paper, we explored a CORT-induced in vitro injury model of stress using SH-SY5Y cells to mimic the cellular damage typically observed in depression. Although the model itself is well characterized [
31,
47], our study introduces a novel approach by examining peptides as possible neuroprotectants. The peptides may serve as promising candidates for mitigating neuronal alterations [
16,
48,
49]; that is why we focused on previously obtained peptides with opioid activity, which are analogs of EMs and exhibit enhanced biological activity compared to their parent compounds [
18,
19,
20]. The modifications introduced into the original sequence involved substituting Pro at position 2 with unnatural amino acids with six-membered heterocyclic rings, such as (
S)-Pip, (
R)-Nip, and Inp (
Figure 1) [
18,
19,
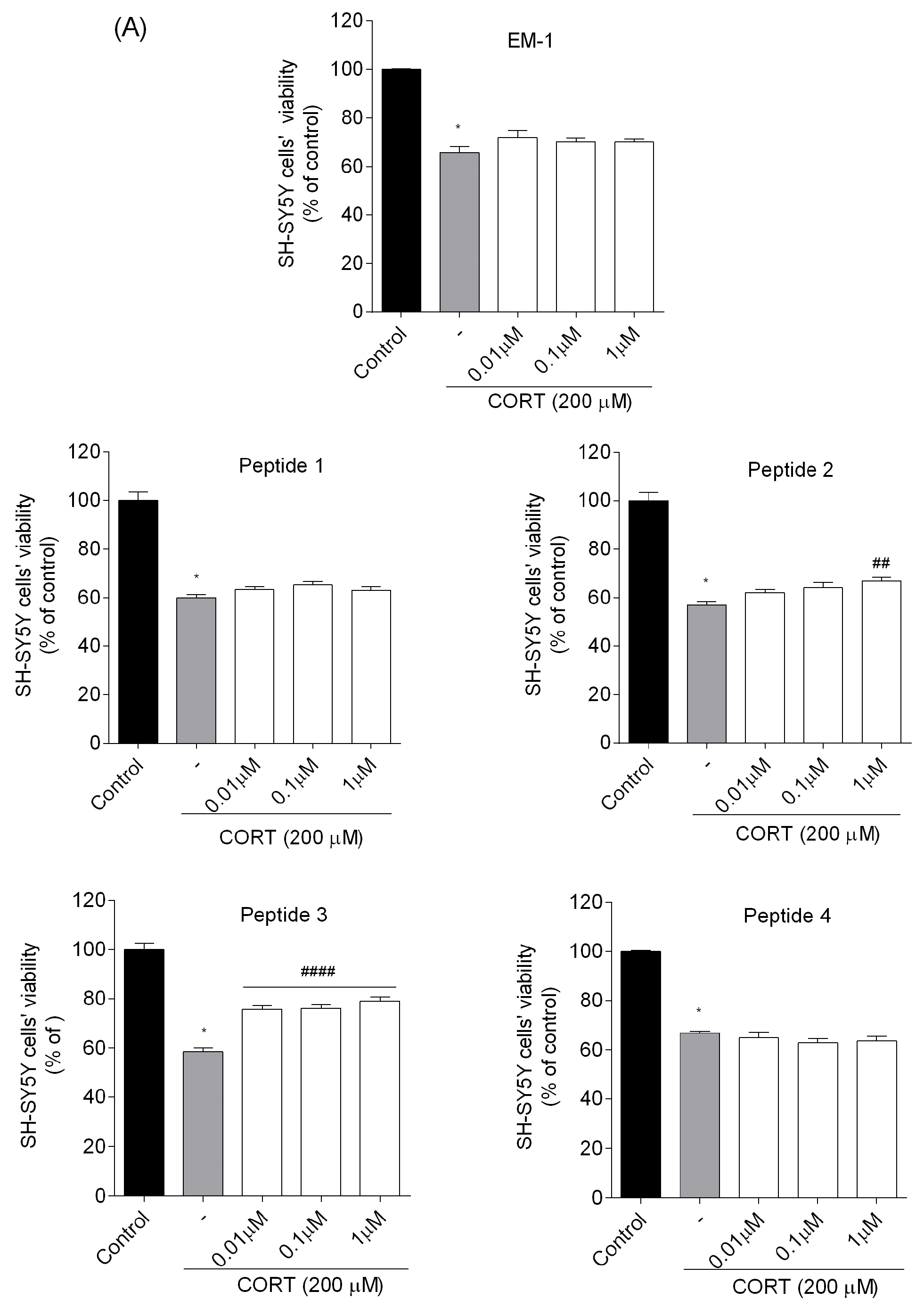
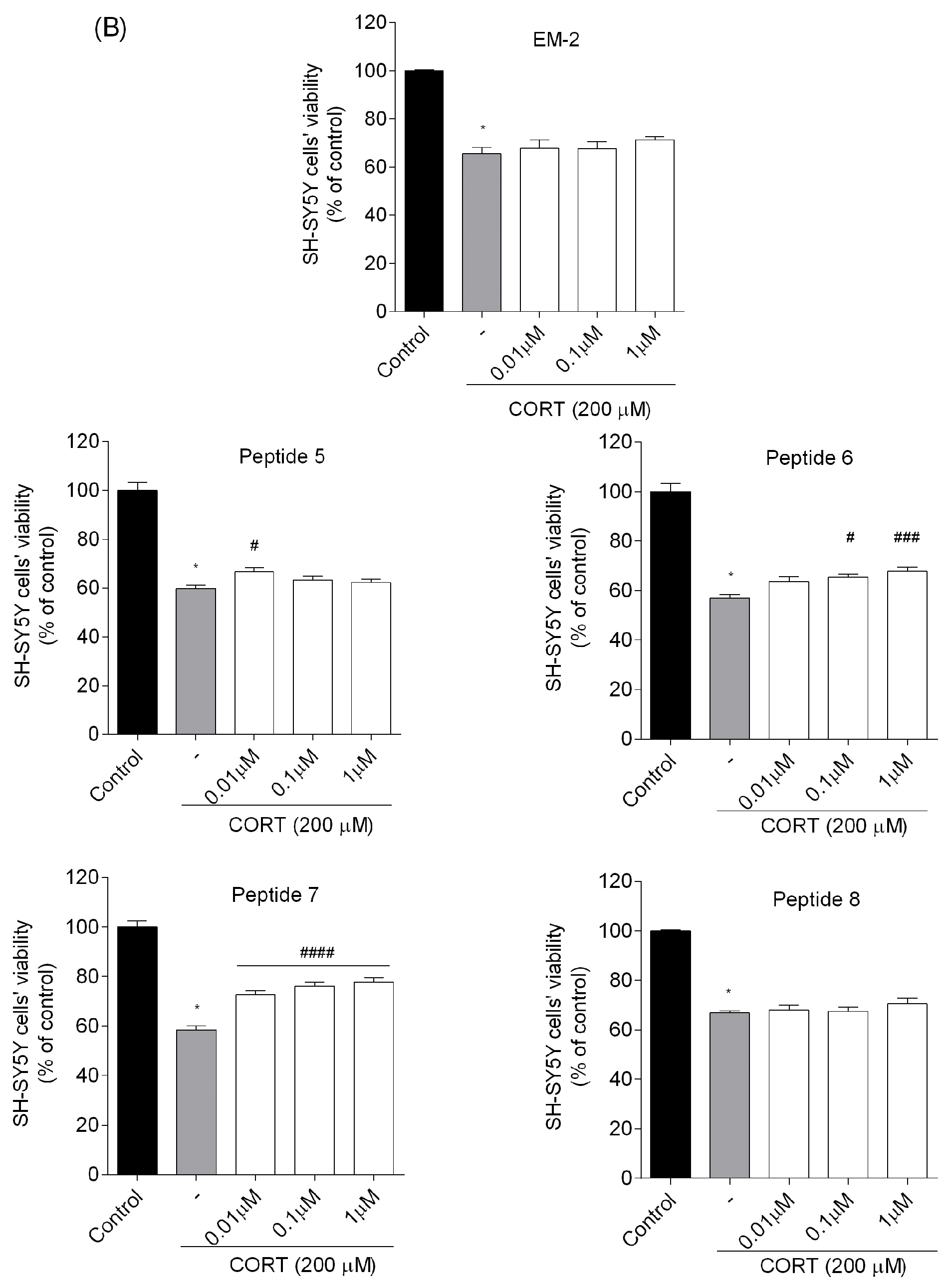
20]. Four peptides (analogs
2,
3,
6, and
7) were found to reduce SH-SY5Y cell stress caused by CORT in the tested concentration range, resulting in a markedly increased cell viability. Selected peptide analogs demonstrated superior neuroprotective activity compared to the parent endomorphins, which did not exhibit any protective effects in the selected neuronal damage model. This suggests that structural modifications introduced in the analogs were critical for enhancing biological efficacy, particularly in mitigating CORT-induced oxidative stress and cellular injury. Based on further research defining the mechanism of neuroprotection, we selected two peptides
3 and
7, with the following sequence Tyr-Inp-Trp-Phe-NH
2 and Tyr-Inp-Phe-Phe-NH
2, respectively. The remaining peptides, especially
2 and
6, also showed reparative effects, but the changes were less pronounced and not statistically significant; therefore, we focused primarily on peptides
3 and
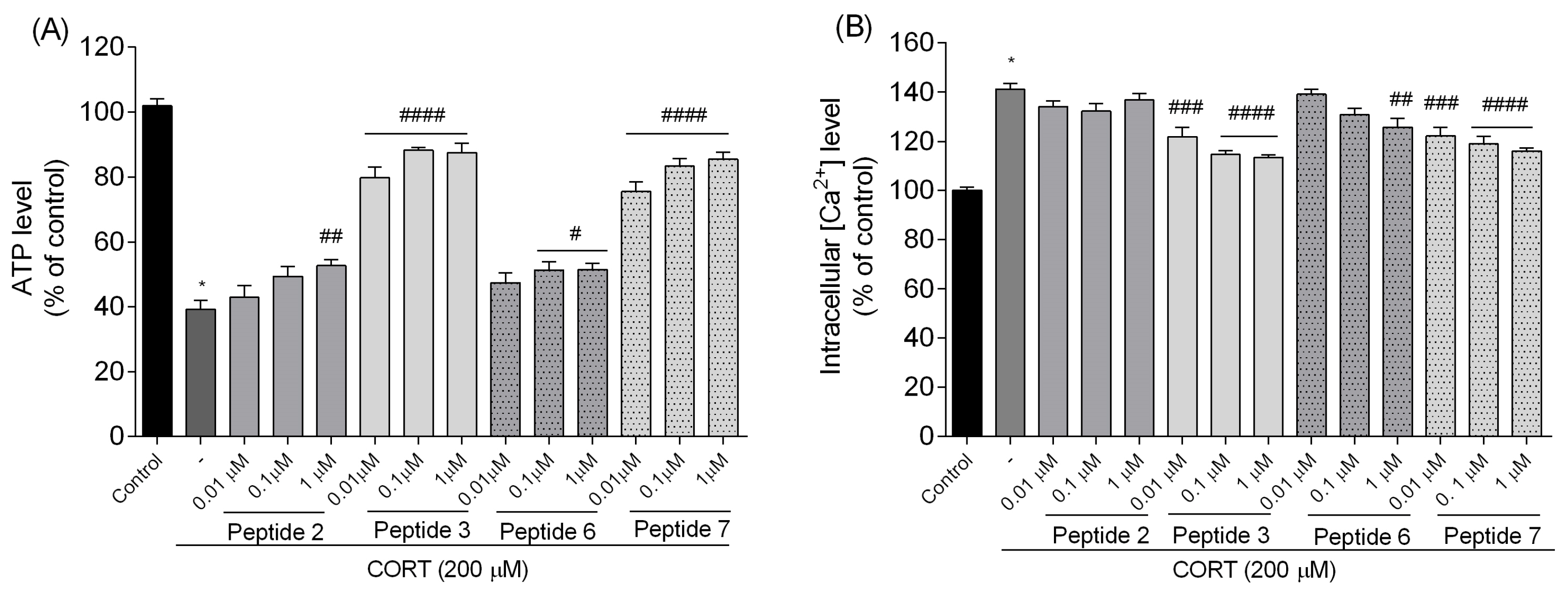
7. Those peptides reduced unfavorable morphological changes signaling apoptosis, such as cellular shrinkage, nuclear fragmentation, membrane blebbing, and separation from the surface. Peptide addition ensured healthy morphology and an unreduced number of viable cells. The CORT-induced changes, which led to the generation of ROS, mitochondrial dysfunction, ATP reduction, and disruption of calcium homeostasis, were alleviated by the administration of selected peptides. Peptides
3 and
7 showed the strongest neuroprotective effects, significantly reducing ROS levels and improving ΔΨm. Peptide
3, in particular, effectively prevented ATP depletion. Both peptides also reduced CORT-induced intracellular Ca
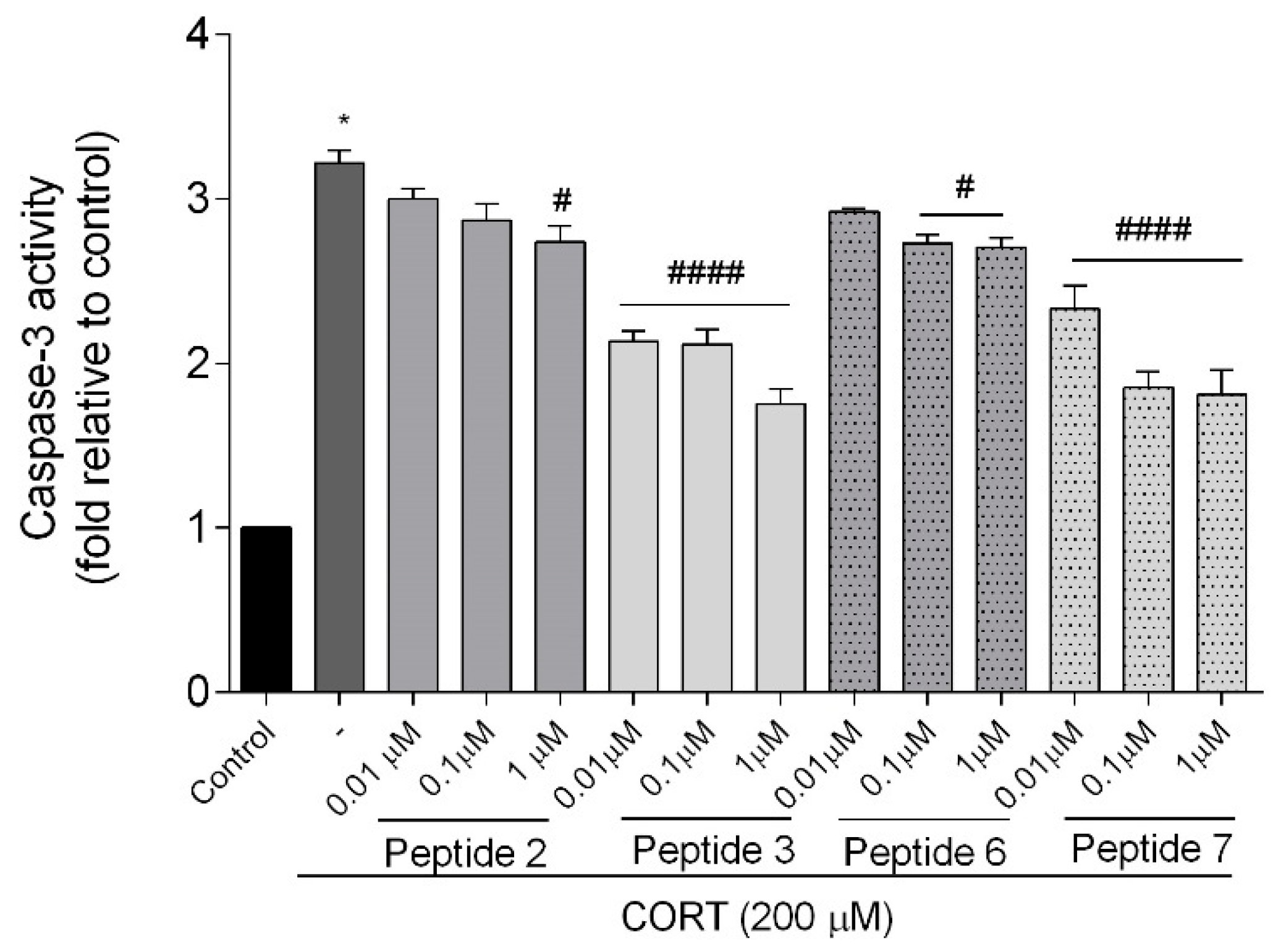
2+ overload by about 20% across all tested concentrations. Additionally, these peptides showed the highest effects on apoptotic markers induced by CORT. Co-administration of peptides with CORT significantly reduced caspase-3 activity, especially with peptides
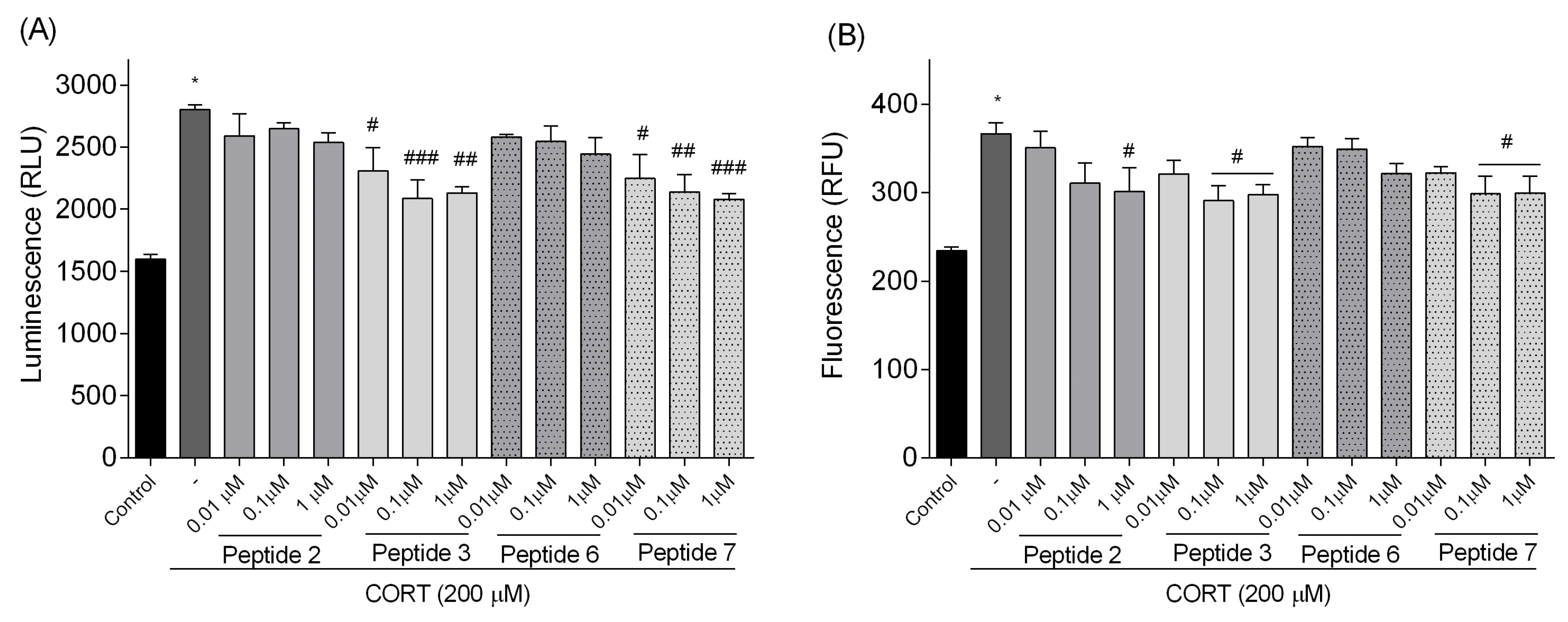
3 and
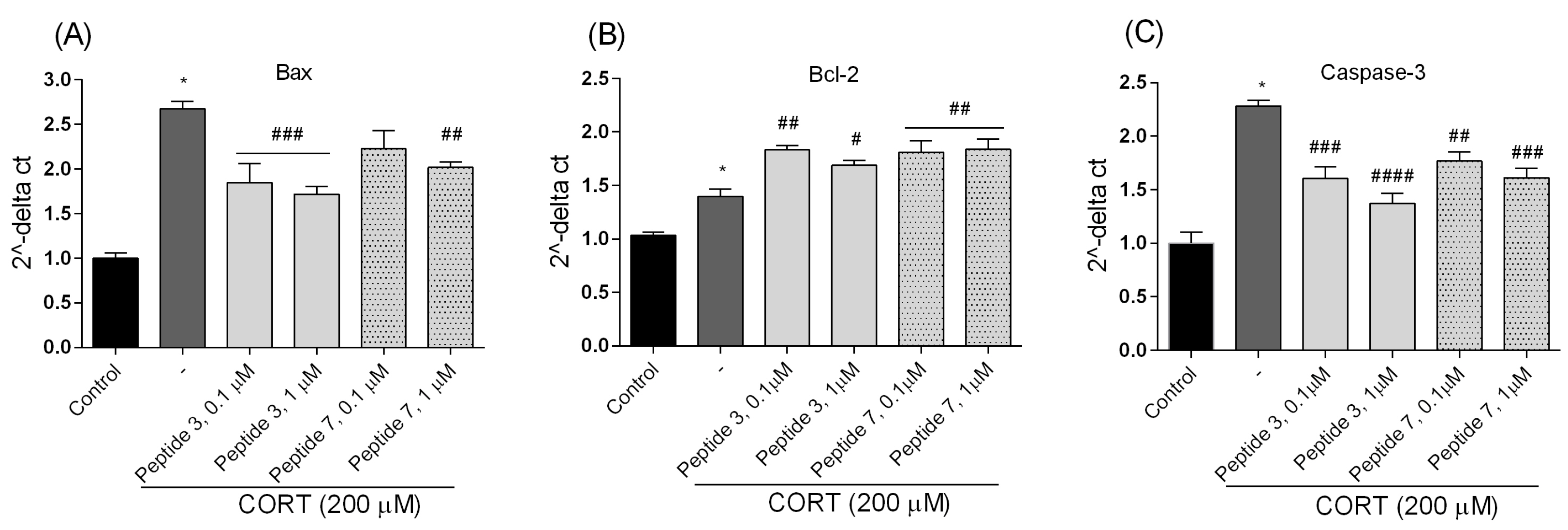
7, confirming their anti-apoptotic effects. These peptides also lowered luminescence and fluorescence signals, indicating reduced apoptosis and necrosis. We checked the mRNA expression levels of the pro-apoptotic Bax, anti-apoptotic Bcl-2, and caspase-3. Peptide treatment suppressed CORT-induced upregulation of Bax and caspase-3. This downregulation, consistent with reduced caspase-3 activity and cell death, confirms the anti-apoptotic effects of the selected peptides,
3 and
7.
From our point of view, the effect of peptides on CORT-induced BDNF level suppression was also important. Brain-derived neurotrophic factor (BDNF) plays an essential role in growth, differentiation, maintenance, and cell survival. In the context of depression, previous pharmacotherapies have focused on the theory of monoaminergic dysregulation [
50]. Recently, another hypothesis involving a deficiency in the neurotrophic pathway has attracted attention. This theory suggests that low levels of various neurotrophins, particularly BDNF, are linked to depressive disorders [
51]. Interestingly, several factors, such as environmental, physical activity, and many natural and synthetic compounds, may modulate BDNF expression or function [
51]. To date, various natural and synthetic compounds have demonstrated neuroprotective effects by boosting the expression of BDNF [
52]. Rodent and human studies showed that different drugs (e.g., the selective serotonin reuptake inhibitor fluoxetine (FLX), the selective norepinephrine reuptake inhibitor reboxetine (RBX), and the tricyclic antidepressant desipramine (DMI) predominantly inhibiting norepinephrine reuptake) may selectively influence the changes in BDNF gene expression [
53]. Moreover, BDNF has neuroprotective properties [
34,
35], which may be of great importance in the context of neurodegenerative diseases or neural injuries, because many factors may stimulate BDNF expression [
47,
54]. Our previous research has shown that 6-hydroxydopamine (6-OHDA)-reduced BDNF levels can be reversed by naturally occurring peptide, rubiscolin-6 (R-6) administration [
21].
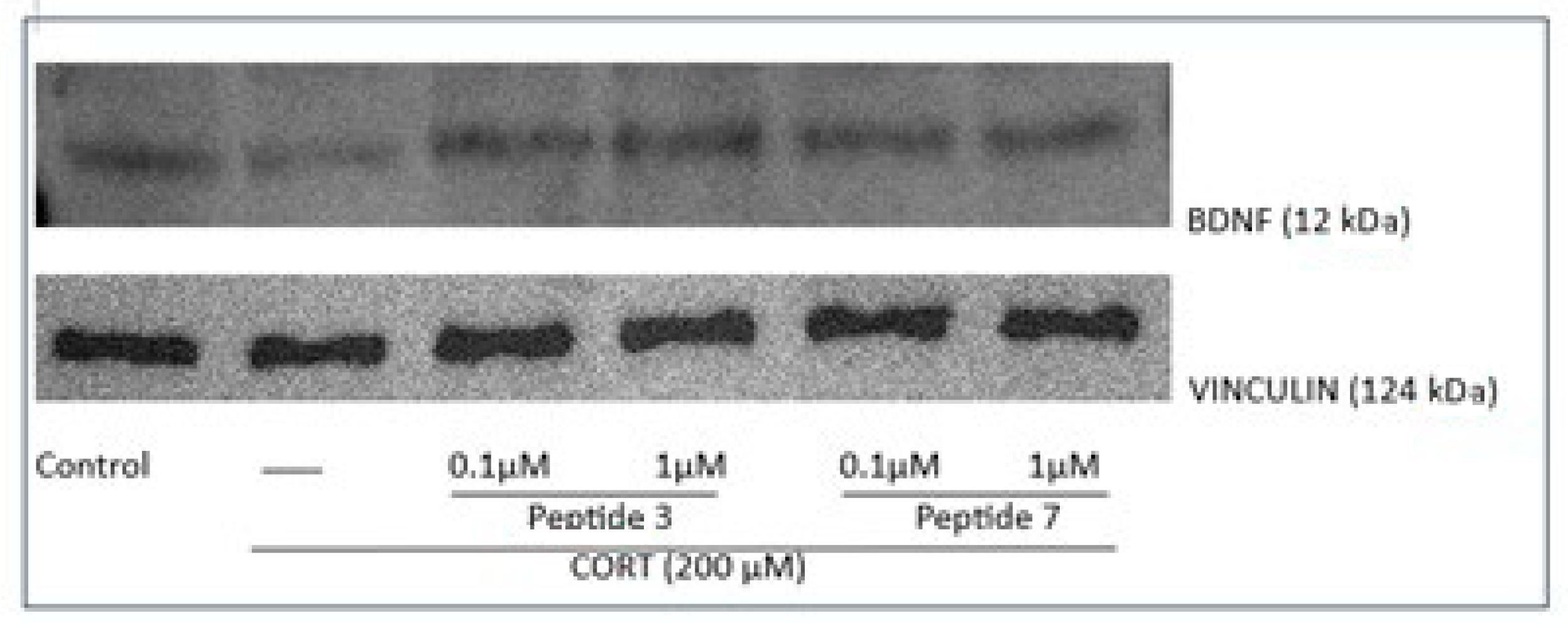
This time, our research showed that exposure of cells to CORT decreased the mRNA levels of BDNF, while treatment with the peptides 3 and 7 (at the dose of 0.1 and 1 µM) significantly increased BDNF mRNA levels. ELISA assay revealed that peptides increased the level of BDNF protein, and a more pronounced increase in BDNF protein levels was noted for peptide 3. Similarly, Western blot analysis showed that peptide 3 significantly increased BDNF levels, while peptide 7 caused a slight, non-significant increase.
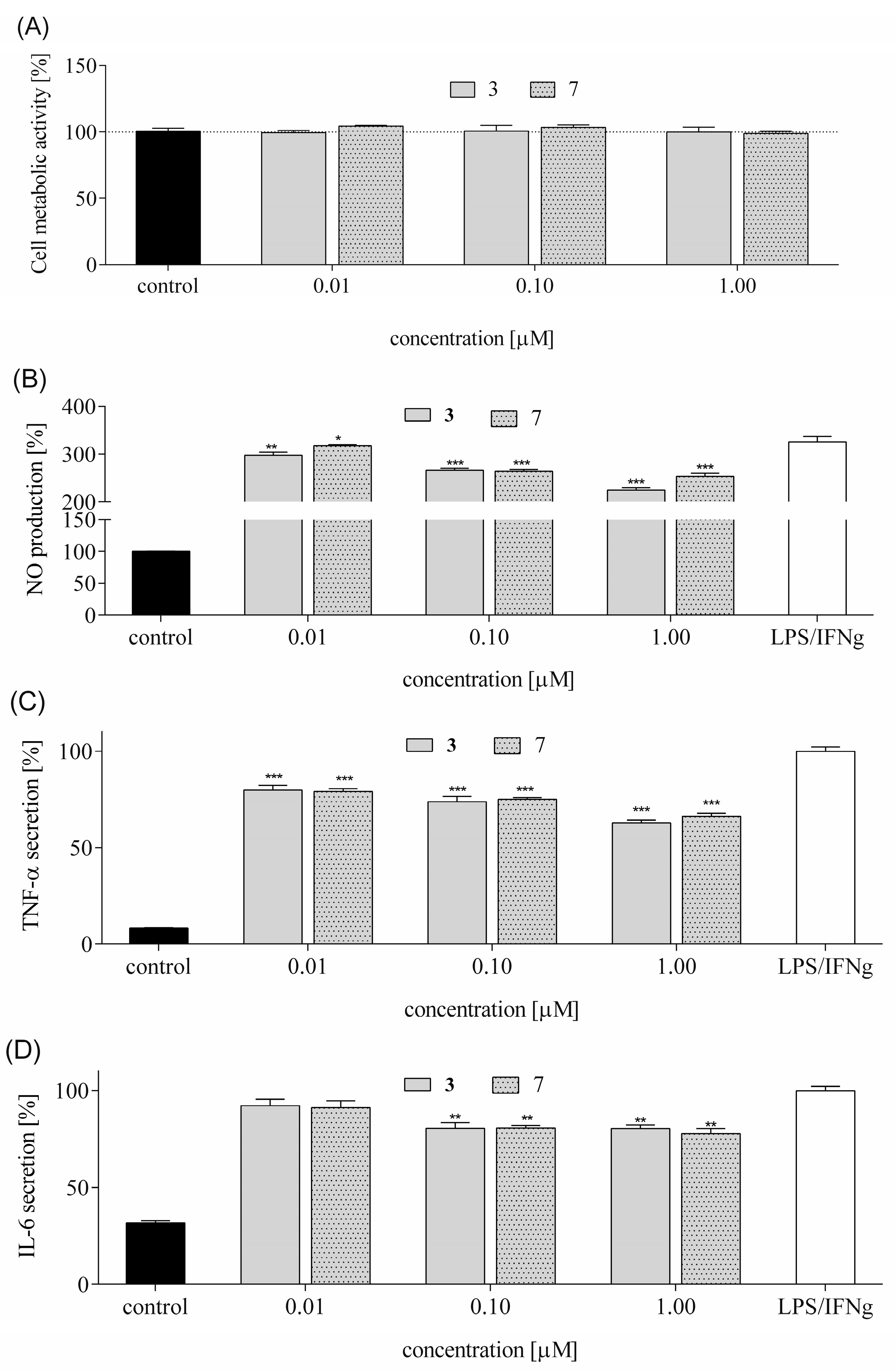
Chronic inflammation in the central nervous system contributes to neurotoxicity by promoting the release of pro-inflammatory cytokines, which can damage neurons and disrupt neural function [
55]. Therefore, we measure the levels of inflammatory cytokines such as NO, TNF-α, and IL-6 in LPS-stimulated RAW 264.7 cells. These cytokines serve as key biomarkers of neuroinflammatory activity. Elevated levels of these cytokines are associated with the breakdown of the blood–brain barrier (BBB), activation of microglia and astrocytes, and progression of neurodegenerative diseases. We observed that both peptides reduced NO levels, with peptide
3 showing greater efficacy. They reduced TNF-α secretion in a concentration-dependent manner. IL-6 secretion was also attenuated, albeit less effectively, with only the highest peptide concentration reducing levels by ~20%. These results suggest that tested peptides
3 and
7 possess anti-inflammatory properties, potentially through modulation of NO synthase expression and NF-κB activity. While NF-κB can promote neuroinflammation via cytokine release in glial cells, it may also exert neuroprotective effects in neurons by inducing anti-apoptotic and antioxidant pathways.
These findings suggest that the tested peptides exhibit promising neuroprotective properties, supporting the rationale for continued exploration of peptide-based compounds as potential therapeutic candidates.
A growing body of evidence supports the neuroprotective potential of peptides, as summarized in the reviews by Perlikowska [
16] or Patel K. and Mani A. [
48], which highlights various opioid peptides that modulate key pathological pathways in neurodegenerative conditions. Among opioid peptides evaluated for neuroprotection are both endogenous ligands, such as endomorphins (EM-1 and EM-2), and synthetic analogs including [D-Ala
2, N-MePhe
4, Gly-ol]-enkephalin (DAMGO), [D-Pen
2, D-Pen
5]-enkephalin (DPDPE), [D-Ala
2, D-Leu
5]-enkephalin (DADLE), [D-Ser
2, Leu
5, Thr
6]-enkephalin (DSLET), and biphalin ((Tyr-D-Ala-Gly-Phe-NH)
2) (see more in [
16]). Notably, EM-1 and EM-2 have demonstrated antioxidant and anti-apoptotic properties by scavenging free radicals, inhibiting lipid peroxidation, and protecting mitochondrial function. They also reduced amyloid β-induced neurotoxicity in vitro and in vivo, highlighting their potential as neuroprotective agents.
Our findings underscore the critical role of position 2 modifications in enhancing the biological activity of peptide analogs. Specifically, peptides 3 and 7, both modified with heterocyclic amino acids at this position, exhibited the most significant protective effects and demonstrated notable anti-inflammatory properties. Similar modifications in R-6 analogs also yielded promising results, suggesting a conserved mechanism of action. The incorporation of 4-carboxylic acid-containing amino acids (Inp) at position 2 appears to be a key determinant of bioactivity. These residues may enhance receptor binding affinity or selectivity, potentially through interactions with opioid receptors or other signaling pathways. The presence of a carboxylic acid moiety could contribute to improved peptide–receptor interactions via hydrogen bonding or electrostatic interactions, thereby modulating downstream biological responses. This highlights position 2 as a strategic site for rational design in the development of neuroprotective and anti-inflammatory peptide therapeutics.
The outcome of our study is the identification of peptide structures exhibiting both neuroprotective and anti-inflammatory properties. However, the most significant limitation of our findings is that the tested peptides are not able to pass the BBB, which poses a significant challenge for their direct application in treating central nervous system (CNS) disorders. Despite this limitation, we believe that further investigations are necessary to determine whether these peptides can exert neuroprotective effects in vivo, either through peripheral mechanisms or via modified delivery strategies. These efforts aim to establish a more comprehensive understanding of how opioid peptides may contribute to neuroprotection and stress resilience.
5. Conclusions
Our study focused on peptides structurally related to EMs, modified at position 2 with heterocyclic amino acids. Among the tested analogs, peptides 3 (Tyr-Inp-Trp-Phe-NH2) and 7 (Tyr-Inp-Phe-Phe-NH2) showed the most pronounced protective effects against CORT-induced cytotoxicity. These peptides improved cell viability, preserved healthy morphology, and significantly reduced ROS levels, mitochondrial dysfunction, intracellular Ca2+ overload, and ATP depletion. Both peptides also demonstrated anti-apoptotic activity by reducing caspase-3 activity and modulating the expression of Bax, Bcl-2, and caspase-3 genes. Additionally, they restored BDNF mRNA and protein levels, with peptide 3 showing a stronger effect, suggesting a role in neurotrophic support. Their anti-inflammatory potential was confirmed by reduced NO, TNF-α, and IL-6 levels, likely mediated through modulation of NO synthase and NF-κB signaling pathways.
Our findings highlight the significance of position 2 modifications in peptide analogs, particularly those incorporating heterocyclic amino acids. Interestingly, similar modifications in R-6 analogs also showed promising activity [
21], suggesting that the amino acid at position 2 may play a critical role in modulating biological function. This position could influence receptor binding affinity, potentially involving opioid receptors, and warrants further investigation to elucidate its mechanistic contribution to the observed bioactivity.
Overall, our findings support the therapeutic potential of peptides 3 and 7 as neuroprotective agents. These results align with recent reviews highlighting the promise of peptide-based strategies in neurodegenerative and stress-related disorders.