State of the Art in Pulmonary Arterial Hypertension: Molecular Basis, Imaging Modalities, and Right Heart Failure Treatment
Abstract
1. Introduction
1.1. Overview of Pulmonary Arterial Hypertension
1.2. Importance of Comprehending the Molecular and Hemodynamic Bases
1.3. Significance of Right-Sided Heart Failure in PAH
2. Molecular Basis in PAH
2.1. Genetic Factors and Mutation
2.2. Pathophysiology at the Molecular Level
2.3. Key Molecular Pathways
3. Hemodynamic Basis of PAH
3.1. Hemodynamic Factors in PAH
3.2. Pulmonary Vascular Alterations
3.3. Influence on Right-Sided Heart Function
4. Relation Between Molecular and Hemodynamic Factors
4.1. Interaction Between Molecular and Hemodynamic Mechanisms
4.2. Implications for Disease Progression
5. State-of-the-Art Imaging Approaches
5.1. Advanced Imaging Techniques
5.2. Echocardiography
5.3. Computed Tomography
5.4. Nuclear Medicine
5.5. Cardiac Magnetic Resonance
6. Treatment Strategies for PAH and Right-Sided Heart Failure
6.1. Current Treatment Options
6.2. Novel/Investigational Therapeutic Approaches
6.3. Integrated Imaging and Therapeutic Strategies
6.4. Precision Medicine in PAH Management
7. Future Directions
8. Discussion and Conclusions
8.1. Summary of Key Findings
8.2. Importance of an Integrative Approach in PAH Management
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Badesch, D.B.; Raskob, G.E.; Elliott, C.G.; Krichman, A.M.; Farber, H.W.; Frost, A.E.; Barst, R.J.; Benza, R.L.; Liou, T.G.; Turner, M. Pulmonary arterial hypertension: Baseline characteristics from the REVEAL Registry. Chest 2010, 137, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.; Brida, M.; Carlsen, J.; Coats, A.J.; Escribano-Subias, P.; Ferrari, P. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: Developed by the task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Endorsed by the International Society for Heart and Lung Transplantation (ISHLT) and the European Reference Network on rare respiratory diseases (ERN-LUNG). Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [PubMed]
- Lau, E.M.; Giannoulatou, E.; Celermajer, D.S.; Humbert, M. Epidemiology and treatment of pulmonary arterial hypertension. Nat. Rev. Cardiol. 2017, 14, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Leary, P.J.; Lindstrom, M.; Johnson, C.O.; Emmons-Bell, S.; Rich, S.; Corris, P.A.; DuBrock, H.M.; Ventetuolo, C.E.; Abate, Y.H.; Abdelmasseh, M. Global, regional, and national burden of pulmonary arterial hypertension, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Respir. Med. 2025, 13, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Stacher, E.; Graham, B.B.; Hunt, J.M.; Gandjeva, A.; Groshong, S.D.; McLaughlin, V.V.; Jessup, M.; Grizzle, W.E.; Aldred, M.A.; Cool, C.D. Modern age pathology of pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2012, 186, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Tuder, R.M. Pulmonary vascular remodeling in pulmonary hypertension. Cell Tissue Res. 2017, 367, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Xanthouli, P.; Jordan, S.; Milde, N.; Marra, A.; Blank, N.; Egenlauf, B.; Gorenflo, M.; Harutyunova, S.; Lorenz, H.-M.; Nagel, C. Haemodynamic phenotypes and survival in patients with systemic sclerosis: The impact of the new definition of pulmonary arterial hypertension. Ann. Rheum. Dis. 2020, 79, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Pieske, B.; Tschöpe, C.; De Boer, R.A.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S. How to diagnose heart failure with preserved ejection fraction: The HFA–PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. Heart J. 2019, 40, 3297–3317. [Google Scholar] [CrossRef] [PubMed]
- Vonk-Noordegraaf, A.; Haddad, F.; Chin, K.M.; Forfia, P.R.; Kawut, S.M.; Lumens, J.; Naeije, R.; Newman, J.; Oudiz, R.J.; Provencher, S. Right heart adaptation to pulmonary arterial hypertension: Physiology and pathobiology. J. Am. Coll. Cardiol. 2013, 62, D22–D33. [Google Scholar] [CrossRef] [PubMed]
- Ullah, W.; Minalyan, A.; Saleem, S.; Nadeem, N.; Abdullah, H.M.; Abdalla, A.; Chan, V.; Saeed, R.; Khan, M.; Collins, S. Comparative accuracy of non-invasive imaging versus right heart catheterization for the diagnosis of pulmonary hypertension: A systematic review and meta-analysis. IJC Heart Vasc. 2020, 29, 100568. [Google Scholar] [CrossRef] [PubMed]
- Lane, K.B.; Machado, R.D.; Pauciulo, M.W.; Thomson, J.R.; Phillips, J.A.; Loyd, J.E.; Nichols, W.C.; Trembath, R.C. Heterozygous germline mutations in BMPR2, encoding a TGF-β receptor, cause familial primary pulmonary hypertension. Nat. Genet. 2000, 26, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Morse, J.H.; Slager, S.L.; Cuervo, N.; Moore, K.J.; Venetos, G.; Kalachikov, S.; Cayanis, E.; Fischer, S.G.; Barst, R.J. Familial primary pulmonary hypertension (Gene PPH1) is caused by mutations in the bone morphogenetic protein receptor–II gene. Am. J. Hum. Genet. 2000, 67, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Morrell, N.W.; Aldred, M.A.; Chung, W.K.; Elliott, C.G.; Nichols, W.C.; Soubrier, F.; Trembath, R.C.; Loyd, J.E. Genetics and genomics of pulmonary arterial hypertension. Eur. Respir. J. 2019, 53, 1801899. [Google Scholar] [CrossRef] [PubMed]
- Austin, E.D.; Aldred, M.A.; Alotaibi, M.; Gräf, S.; Nichols, W.C.; Trembath, R.C.; Chung, W.K. Genetics and precision genomics approaches to pulmonary hypertension. Eur. Respir. J. 2024, 64, 2401370. [Google Scholar] [CrossRef] [PubMed]
- Naeije, R.; Richter, M.J.; Rubin, L.J. The physiological basis of pulmonary arterial hypertension. Eur. Respir. J. 2022, 59, 2102334. [Google Scholar] [CrossRef] [PubMed]
- Wagenvoort, C.; Wagenvoort, N. Primary pulmonary hypertension: A pathologic study of the lung vessels in 156 clinically diagnosed cases. Circulation 1970, 42, 1163–1184. [Google Scholar] [CrossRef]
- St. Croix, C.M.; Steinhorn, R.H. New thoughts about the origin of plexiform lesions. Am. J. Respir. Crit. Care Med. 2016, 193, 484–485. [Google Scholar] [CrossRef] [PubMed]
- Cracowski, J.-L.; Chabot, F.; Labarère, J.; Faure, P.; Degano, B.; Schwebel, C.; Chaouat, A.; Reynaud-Gaubert, M.; Cracowski, C.; Sitbon, O. Proinflammatory cytokine levels are linked to death in pulmonary arterial hypertension. Eur. Respir. J. 2014, 43, 915–917. [Google Scholar] [CrossRef] [PubMed]
- Ricard, N.; Tu, L.; Le Hiress, M.; Huertas, A.; Phan, C.; Thuillet, R.; Sattler, C.; Fadel, E.; Seferian, A.; Montani, D. Increased pericyte coverage mediated by endothelial-derived fibroblast growth factor-2 and interleukin-6 is a source of smooth muscle–like cells in pulmonary hypertension. Circulation 2014, 129, 1586–1597. [Google Scholar] [CrossRef] [PubMed]
- Rol, N.; Kurakula, K.B.; Happé, C.; Bogaard, H.J.; Goumans, M.-J. TGF-β and BMPR2 signaling in PAH: Two black sheep in one family. Int. J. Mol. Sci. 2018, 19, 2585. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.; Sommer, N.; Cox-Flaherty, K.; Weissmann, N.; Ventetuolo, C.E.; Maron, B.A. Pulmonary hypertension: A contemporary review. Am. J. Respir. Crit. Care Med. 2023, 208, 528–548. [Google Scholar] [CrossRef] [PubMed]
- Martin de Miguel, I.; Cruz-Utrilla, A.; Oliver, E.; Escribano-Subias, P. Novel molecular mechanisms involved in the medical treatment of pulmonary arterial hypertension. Int. J. Mol. Sci. 2023, 24, 4147. [Google Scholar] [CrossRef] [PubMed]
- Christou, H.; Khalil, R.A. Mechanisms of pulmonary vascular dysfunction in pulmonary hypertension and implications for novel therapies. Am. J. Physiol.-Heart Circ. Physiol. 2022, 322, H702–H724. [Google Scholar] [CrossRef] [PubMed]
- Panchal, J.; Jaiswal, S.; Jain, S.; Kumawat, J.; Sharma, A.; Jain, P.; Jain, S.; Verma, K.; Dwivedi, J.; Sharma, S. Development of novel bosentan analogues as endothelin receptor antagonists for pulmonary arterial hypertension. Eur. J. Med. Chem. 2023, 259, 115681. [Google Scholar] [CrossRef] [PubMed]
- Tabeling, C.; González Calera, C.R.; Lienau, J.; Höppner, J.; Tschernig, T.; Kershaw, O.; Gutbier, B.; Naujoks, J.; Herbert, J.; Opitz, B.; et al. Endothelin B Receptor Immunodynamics in Pulmonary Arterial Hypertension. Front. Immunol. 2022, 13, 895501. [Google Scholar] [CrossRef] [PubMed]
- Pullamsetti, S.S.; Mamazhakypov, A.; Weissmann, N.; Seeger, W.; Savai, R. Hypoxia-inducible factor signaling in pulmonary hypertension. J. Clin. Investig. 2020, 130, 5638–5651. [Google Scholar] [CrossRef] [PubMed]
- Ball, M.K.; Waypa, G.B.; Mungai, P.T.; Nielsen, J.M.; Czech, L.; Dudley, V.J.; Beussink, L.; Dettman, R.W.; Berkelhamer, S.K.; Steinhorn, R.H.; et al. Regulation of hypoxia-induced pulmonary hypertension by vascular smooth muscle hypoxia-inducible factor-1α. Am. J. Respir. Crit. Care Med. 2014, 189, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Li, M.; Wharton, J.; Zhu, M.M.; Zhao, Y.Y. Prolyl-4 Hydroxylase 2 (PHD2) Deficiency in Endothelial Cells and Hematopoietic Cells Induces Obliterative Vascular Remodeling and Severe Pulmonary Arterial Hypertension in Mice and Humans Through Hypoxia-Inducible Factor-2α. Circulation 2016, 133, 2447–2458. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; He, Y.-Y.; Jiang, X.; Wang, Y.; Chen, J.-W.; Zhao, J.-H.; Ye, J.; Lian, T.-Y.; Zhang, X.; Zhang, R.-J.; et al. DNA methyltransferase 3B deficiency unveils a new pathological mechanism of pulmonary hypertension. Sci. Adv. 2020, 6, eaba2470. [Google Scholar] [CrossRef] [PubMed]
- Potus, F.; Pauciulo, M.W.; Cook, E.K.; Zhu, N.; Hsieh, A.; Welch, C.L.; Shen, Y.; Tian, L.; Lima, P.; Mewburn, J. Novel mutations and decreased expression of the epigenetic regulator TET2 in pulmonary arterial hypertension. Circulation 2020, 141, 1986–2000. [Google Scholar] [CrossRef] [PubMed]
- Omura, J.; Habbout, K.; Shimauchi, T.; Wu, W.H.; Breuils-Bonnet, S.; Tremblay, E.; Martineau, S.; Nadeau, V.; Gagnon, K.; Mazoyer, F.; et al. Identification of Long Noncoding RNA H19 as a New Biomarker and Therapeutic Target in Right Ventricular Failure in Pulmonary Arterial Hypertension. Circulation 2020, 142, 1464–1484. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wang, J.; Huang, H.; Yu, Y.; Ding, J.; Yu, Y.; Li, K.; Wei, D.; Ye, Q.; Wang, F. YTHDF1 regulates pulmonary hypertension through translational control of MAGED1. Am. J. Respir. Crit. Care Med. 2021, 203, 1158–1172. [Google Scholar] [CrossRef] [PubMed]
- Kazmirczak, F.; Vogel, N.T.; Prisco, S.Z.; Patterson, M.T.; Annis, J.; Moon, R.T.; Hartweck, L.M.; Mendelson, J.B.; Kim, M.; Calixto Mancipe, N.; et al. Ferroptosis-Mediated Inflammation Promotes Pulmonary Hypertension. Circ. Res. 2024, 135, 1067–1083. [Google Scholar] [CrossRef] [PubMed]
- Galiè, N.; McLaughlin, V.V.; Rubin, L.J.; Simonneau, G. An overview of the 6th World Symposium on Pulmonary Hypertension. Eur. Respir. J. 2019, 53, 1802148. [Google Scholar] [CrossRef] [PubMed]
- Gan, C.T.-J.; Lankhaar, J.-W.; Westerhof, N.; Marcus, J.T.; Becker, A.; Twisk, J.W.; Boonstra, A.; Postmus, P.E.; Vonk-Noordegraaf, A. Noninvasively assessed pulmonary artery stiffness predicts mortality in pulmonary arterial hypertension. Chest 2007, 132, 1906–1912. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-F.; Nambiar Veetil, N.; Li, Q.; Kucherenko, M.M.; Knosalla, C.; Kuebler, W.M. Pulmonary hypertension: Linking inflammation and pulmonary arterial stiffening. Front. Immunol. 2022, 13, 959209. [Google Scholar] [CrossRef] [PubMed]
- Reiter, G.; Reiter, U.; Kovacs, G.; Olschewski, H.; Fuchsjäger, M. Blood flow vortices along the main pulmonary artery measured with MR imaging for diagnosis of pulmonary hypertension. Radiology 2015, 275, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chesler, N.C. Pulmonary vascular wall stiffness: An important contributor to the increased right ventricular afterload with pulmonary hypertension. Pulm. Circ. 2011, 1, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Westerhof, B.E.; Saouti, N.; Van Der Laarse, W.J.; Westerhof, N.; Vonk Noordegraaf, A. Treatment strategies for the right heart in pulmonary hypertension. Cardiovasc. Res. 2017, 113, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Vanderpool, R.R.; Avazmohammadi, R.; Lapshin, E.; Bachman, T.N.; Sacks, M.; Simon, M.A. Biomechanical and hemodynamic measures of right ventricular diastolic function: Translating tissue biomechanics to clinical relevance. J. Am. Heart Assoc. 2017, 6, e006084. [Google Scholar] [CrossRef] [PubMed]
- Vonk Noordegraaf, A.; Westerhof, B.E.; Westerhof, N. The relationship between the right ventricle and its load in pulmonary hypertension. J. Am. Coll. Cardiol. 2017, 69, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Boulate, D.; Mercier, O.; Guihaire, J.; Fadel, E.; Naeije, R.; Haddad, F.; Rischard, F. Pulmonary circulatory–right ventricular uncoupling: New insights into pulmonary hypertension pathophysiology. In Pulmonary Hypertension: Basic Science to Clinical Medicine; Springer International Publishing: Cham, Switzerland, 2016; pp. 241–253. [Google Scholar]
- Driss, A.B.; Devaux, C.; Henrion, D.; Duriez, M.; Thuillez, C.; Levy, B.I.; Michel, J.-B. Hemodynamic stresses induce endothelial dysfunction and remodeling of pulmonary artery in experimental compensated heart failure. Circulation 2000, 101, 2764–2770. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, M.; Kheyfets, V.; Schroeder, J.; Dunning, J.; Shandas, R.; Buckner, J.; Browning, J.; Hertzberg, J.; Hunter, K.; Fenster, B. Main pulmonary arterial wall shear stress correlates with invasive hemodynamics and stiffness in pulmonary hypertension. Pulm. Circ. 2016, 6, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.; Bernstein, D. Molecular mechanisms of right ventricular failure. Circulation 2015, 132, 1734–1742. [Google Scholar] [CrossRef] [PubMed]
- Hemnes, A.R.; Brittain, E.L.; Trammell, A.W.; Fessel, J.P.; Austin, E.D.; Penner, N.; Maynard, K.B.; Gleaves, L.; Talati, M.; Absi, T. Evidence for right ventricular lipotoxicity in heritable pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2014, 189, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Al-Qazazi, R.; Lima, P.D.; Prisco, S.Z.; Potus, F.; Dasgupta, A.; Chen, K.-H.; Tian, L.; Bentley, R.E.; Mewburn, J.; Martin, A.Y. Macrophage–NLRP3 activation promotes right ventricle failure in pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2022, 206, 608–624. [Google Scholar] [CrossRef] [PubMed]
- Galderisi, M.; Cosyns, B.; Edvardsen, T.; Cardim, N.; Delgado, V.; Di Salvo, G.; Donal, E.; Sade, L.E.; Ernande, L.; Garbi, M. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: An expert consensus document of the European Association of Cardiovascular Imaging. Eur. Heart J.-Cardiovasc. Imaging 2017, 18, 1301–1310. [Google Scholar] [PubMed]
- Mysore, M.M.; Bilchick, K.C.; Ababio, P.; Ruth, B.K.; Harding, W.C.; Breathett, K.; Chadwell, K.; Patterson, B.; Mwansa, H.; Jeukeng, C.M. Right atrial to left atrial volume index ratio is associated with increased mortality in patients with pulmonary hypertension. Echocardiography 2018, 35, 1729–1735. [Google Scholar] [CrossRef] [PubMed]
- Augustine, D.X.; Coates-Bradshaw, L.D.; Willis, J.; Harkness, A.; Ring, L.; Grapsa, J.; Coghlan, G.; Kaye, N.; Oxborough, D.; Robinson, S.; et al. Echocardiographic assessment of pulmonary hypertension: A guideline protocol from the British Society of Echocardiography. Echo Res. Pract. 2018, 5, G11–G24. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, Y.; Kumasawa, J.; Shimizu, S.; Nakano, Y.; Kataoka, Y.; Tsujimoto, H.; Kono, M.; Okabayashi, S.; Imura, H.; Mizuta, T. Doppler trans-thoracic echocardiography for detection of pulmonary hypertension in adults. Cochrane Database Syst. Rev. 2022, 5, Cd012809. [Google Scholar] [CrossRef] [PubMed]
- Evaldsson, A.W.; Lindholm, A.; Jumatate, R.; Ingvarsson, A.; Smith, G.J.; Waktare, J.; Rådegran, G.; Roijer, A.; Meurling, C.; Ostenfeld, E. Right ventricular function parameters in pulmonary hypertension: Echocardiography vs. cardiac magnetic resonance. BMC Cardiovasc. Disord. 2020, 20, 259. [Google Scholar] [CrossRef] [PubMed]
- Tello, K.; Wan, J.; Dalmer, A.; Vanderpool, R.; Ghofrani, H.A.; Naeije, R.; Roller, F.; Mohajerani, E.; Seeger, W.; Herberg, U. Validation of the tricuspid annular plane systolic excursion/systolic pulmonary artery pressure ratio for the assessment of right ventricular-arterial coupling in severe pulmonary hypertension. Circ. Cardiovasc. Imaging 2019, 12, e009047. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Dixon, D.; Labate, V.; Beussink-Nelson, L.; Bandera, F.; Cuttica, M.J.; Shah, S.J. RV contractile function and its coupling to pulmonary circulation in heart failure with preserved ejection fraction: Stratification of clinical phenotypes and outcomes. JACC Cardiovasc. Imaging 2017, 10, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Takahama, H.; McCully, R.B.; Frantz, R.P.; Kane, G.C. Unraveling the RV ejection Doppler envelope: Insight into pulmonary artery hemodynamics and disease severity. JACC Cardiovasc. Imaging 2017, 10, 1268–1277. [Google Scholar] [CrossRef] [PubMed]
- Arkles, J.S.; Opotowsky, A.R.; Ojeda, J.; Rogers, F.; Liu, T.; Prassana, V.; Marzec, L.; Palevsky, H.I.; Ferrari, V.A.; Forfia, P.R. Shape of the right ventricular Doppler envelope predicts hemodynamics and right heart function in pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2011, 183, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.X.; Zhu, Q.; Wang, S.T.; Wang, Y.H.; Li, G.Y.; Kong, F.X.; Ma, C.Y. Diagnostic and prognostic value of echocardiography in pulmonary hypertension: An umbrella review of systematic reviews and meta-analyses. BMC Pulm. Med. 2023, 23, 253. [Google Scholar] [CrossRef] [PubMed]
- Bossone, E.; D’Andrea, A.; D’Alto, M.; Citro, R.; Argiento, P.; Ferrara, F.; Cittadini, A.; Rubenfire, M.; Naeije, R. Echocardiography in pulmonary arterial hypertension: From diagnosis to prognosis. J. Am. Soc. Echocardiogr. 2013, 26, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, M.; Rudski, L.G.; Addetia, K.; Afilalo, J.; D’Alto, M.; Freed, B.H.; Friend, L.B.; Gargani, L.; Grapsa, J.; Hassoun, P.M. Guidelines for the echocardiographic assessment of the right heart in adults and special considerations in pulmonary hypertension: Recommendations from the American society of echocardiography. J. Am. Soc. Echocardiogr. 2025, 38, 141–186. [Google Scholar] [CrossRef] [PubMed]
- Kadoglou, N.P.E.; Khattab, E.; Velidakis, N.; Gkougkoudi, E.; Myrianthefs, M.M. The Role of Echocardiography in the Diagnosis and Prognosis of Pulmonary Hypertension. J. Pers. Med. 2024, 14, 474. [Google Scholar] [CrossRef] [PubMed]
- Broncano, J.; Bhalla, S.; Gutierrez, F.R.; Vargas, D.; Williamson, E.E.; Makan, M.; Luna, A. Cardiac MRI in Pulmonary Hypertension: From Magnet to Bedside. Radiographics 2020, 40, 982–1002. [Google Scholar] [CrossRef] [PubMed]
- Lambert, L.; Michalek, P.; Burgetova, A. The diagnostic performance of CT pulmonary angiography in the detection of chronic thromboembolic pulmonary hypertension-systematic review and meta-analysis. Eur. Radiol. 2022, 32, 7927–7935. [Google Scholar] [CrossRef] [PubMed]
- Duijnhouwer, A.L.; Lemmers, J.; Smit, J.; van Haren-Willems, J.; Knaapen-Hans, H.; ten Cate, T.; Hagmolen Of ten Have, W.; de Boer, M.-J.; Roos-Hesselink, J.; Vonk, M. The outcome of pulmonary hypertension and its association with pulmonary artery dilatation. Neth. Heart J. 2020, 28, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Żyłkowska, J.; Kurzyna, M.; Florczyk, M.; Burakowska, B.; Grzegorczyk, F.; Burakowski, J.; Wieteska, M.; Oniszh, K.; Biederman, A.; Wawrzyńska, L. Pulmonary artery dilatation correlates with the risk of unexpected death in chronic arterial or thromboembolic pulmonary hypertension. Chest 2012, 142, 1406–1416. [Google Scholar] [CrossRef] [PubMed]
- Xi, Q.; Liu, Z.; Xiong, C.; Luo, Q.; Zhao, Z.; Zhao, Q.; Yang, T.; Zeng, Q.; Li, P.; Qiu, L. Pulmonary artery dilatation in different causes of pulmonary hypertension. Pulm. Circ. 2023, 13, e12313. [Google Scholar] [CrossRef] [PubMed]
- Truong, Q.A.; Massaro, J.M.; Rogers, I.S.; Mahabadi, A.A.; Kriegel, M.F.; Fox, C.S.; O’Donnell, C.J.; Hoffmann, U. Reference values for normal pulmonary artery dimensions by noncontrast cardiac computed tomography: The Framingham Heart Study. Circ. Cardiovasc. Imaging 2012, 5, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhuang, B.; Yin, G.; Yang, X.; Zhao, S.; Lu, M. Reference values of thoracic aorta and pulmonary artery diameters by age and gender in healthy Chinese adults assessed by cardiac magnetic resonance imaging: Data from National Center for Cardiovascular Diseases of China. Int. J. Cardiovasc. Imaging 2021, 37, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Kligerman, S.J.; Henry, T.; Lin, C.T.; Franks, T.J.; Galvin, J.R. Mosaic Attenuation: Etiology, Methods of Differentiation, and Pitfalls. Radiographics 2015, 35, 1360–1380. [Google Scholar] [CrossRef] [PubMed]
- Worthy, S.A.; Müller, N.L.; Hartman, T.E.; Swensen, S.J.; Padley, S.; Hansell, D.M. Mosaic attenuation pattern on thin-section CT scans of the lung: Differentiation among infiltrative lung, airway, and vascular diseases as a cause. Radiology 1997, 205, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Resten, A.; Maître, S.; Humbert, M.; Sitbon, O.; Capron, F.; Simoneau, G.; Musset, D. Pulmonary arterial hypertension: Thin-section CT predictors of epoprostenol therapy failure. Radiology 2002, 222, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Montani, D.; Lau, E.M.; Dorfmüller, P.; Girerd, B.; Jaïs, X.; Savale, L.; Perros, F.; Nossent, E.; Garcia, G.; Parent, F.; et al. Pulmonary veno-occlusive disease. Eur. Respir. J. 2016, 47, 1518–1534. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, S.; Swift, A.J.; Condliffe, R.; Johns, C.; Elliot, C.A.; Hill, C.; Davies, C.; Hurdman, J.; Sabroe, I.; Wild, J.M.; et al. CT features of pulmonary arterial hypertension and its major subtypes: A systematic CT evaluation of 292 patients from the ASPIRE Registry. Thorax 2015, 70, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Resten, A.; Maitre, S.; Humbert, M.; Rabiller, A.; Sitbon, O.; Capron, F.; Simonneau, G.; Musset, D. Pulmonary hypertension: CT of the chest in pulmonary venoocclusive disease. Am. J. Roentgenol. 2004, 183, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Kacprzak, A.; Burakowska, B.; Kurzyna, M.; Fijałkowska, A.; Florczyk, M.; Wieteska-Miłek, M.; Darocha, S.; Torbicki, A.; Szturmowicz, M. Predictive value of chest HRCT for survival in idiopathic pulmonary arterial hypertension. Respir. Res. 2021, 22, 293. [Google Scholar] [CrossRef] [PubMed]
- Ascha, M.; Renapurkar, R.D.; Tonelli, A.R. A review of imaging modalities in pulmonary hypertension. Ann. Thorac. Med. 2017, 12, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Fuld, M.K.; Halaweish, A.F.; Haynes, S.E.; Divekar, A.A.; Guo, J.; Hoffman, E.A. Pulmonary perfused blood volume with dual-energy CT as surrogate for pulmonary perfusion assessed with dynamic multidetector CT. Radiology 2013, 267, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Giordano, J.; Khung, S.; Duhamel, A.; Hossein-Foucher, C.; Bellèvre, D.; Lamblin, N.; Remy, J.; Remy-Jardin, M. Lung perfusion characteristics in pulmonary arterial hypertension (PAH) and peripheral forms of chronic thromboembolic pulmonary hypertension (pCTEPH): Dual-energy CT experience in 31 patients. Eur. Radiol. 2017, 27, 1631–1639. [Google Scholar] [CrossRef] [PubMed]
- Dournes, G.; Verdier, D.; Montaudon, M.; Bullier, E.; Rivière, A.; Dromer, C.; Picard, F.; Billes, M.-A.; Corneloup, O.; Laurent, F. Dual-energy CT perfusion and angiography in chronic thromboembolic pulmonary hypertension: Diagnostic accuracy and concordance with radionuclide scintigraphy. Eur. Radiol. 2014, 24, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, B.; Kyheng, M.; Giordano, J.; Lamblin, N.; De Groote, P.; Fertin, M.; Delobelle, M.; Perez, T.; Faivre, J.-B.; Remy, J. Dual-energy CT lung perfusion characteristics in pulmonary arterial hypertension (PAH) and pulmonary veno-occlusive disease and/or pulmonary capillary hemangiomatosis (PVOD/PCH): Preliminary experience in 63 patients. Eur. Radiol. 2022, 32, 4574–4586. [Google Scholar] [CrossRef] [PubMed]
- Bajc, M.; Schümichen, C.; Grüning, T.; Lindqvist, A.; Le Roux, P.-Y.; Alatri, A.; Bauer, R.W.; Dilic, M.; Neilly, B.; Verberne, H.J. EANM guideline for ventilation/perfusion single-photon emission computed tomography (SPECT) for diagnosis of pulmonary embolism and beyond. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2429–2451. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Delcroix, M.; Jais, X.; Madani, M.M.; Matsubara, H.; Mayer, E.; Ogo, T.; Tapson, V.F.; Ghofrani, H.A.; Jenkins, D.P. Chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801915. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Fang, W.; Lv, B.; He, J.G.; Xiong, C.M.; Liu, Z.H.; He, Z.X. Diagnosis of chronic thromboembolic pulmonary hypertension: Comparison of ventilation/perfusion scanning and multidetector computed tomography pulmonary angiography with pulmonary angiography. Nucl. Med. Commun. 2012, 33, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Carrabba, N.; Amico, M.A.; Guaricci, A.I.; Carella, M.C.; Maestrini, V.; Monosilio, S.; Pedrotti, P.; Ricci, F.; Monti, L.; Figliozzi, S. CMR Mapping: The 4th-Era Revolution in Cardiac Imaging. J. Clin. Med. 2024, 13, 337. [Google Scholar] [CrossRef] [PubMed]
- Grothues, F.; Moon, J.C.; Bellenger, N.G.; Smith, G.S.; Klein, H.U.; Pennell, D.J. Interstudy reproducibility of right ventricular volumes, function, and mass with cardiovascular magnetic resonance. Am. Heart J. 2004, 147, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Alabed, S.; Shahin, Y.; Garg, P.; Alandejani, F.; Johns, C.S.; Lewis, R.A.; Condliffe, R.; Wild, J.M.; Kiely, D.G.; Swift, A.J. Cardiac-MRI predicts clinical worsening and mortality in pulmonary arterial hypertension: A systematic review and meta-analysis. Cardiovasc. Imaging 2021, 14, 931–942. [Google Scholar]
- Messroghli, D.R.; Plein, S.; Higgins, D.M.; Walters, K.; Jones, T.R.; Ridgway, J.P.; Sivananthan, M.U. Human myocardium: Single-breath-hold MR T1 mapping with high spatial resolution—Reproducibility study. Radiology 2006, 238, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.; Nielsen-Kudsk, J.E.; Vonk Noordegraaf, A.; de Man, F.S. Right ventricular fibrosis: A pathophysiological factor in pulmonary hypertension? Circulation 2019, 139, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Alabed, S.; Saunders, L.; Garg, P.; Shahin, Y.; Alandejani, F.; Rolf, A.; Puntmann, V.O.; Nagel, E.; Wild, J.M.; Kiely, D.G. Myocardial T1-mapping and extracellular volume in pulmonary arterial hypertension: A systematic review and meta-analysis. Magn. Reson. Imaging 2021, 79, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Blyth, K.G.; Groenning, B.A.; Martin, T.N.; Foster, J.E.; Mark, P.B.; Dargie, H.J.; Peacock, A.J. Contrast enhanced-cardiovascular magnetic resonance imaging in patients with pulmonary hypertension. Eur. Heart J. 2005, 26, 1993–1999. [Google Scholar] [CrossRef] [PubMed]
- Sanz, J.; Dellegrottaglie, S.; Kariisa, M.; Sulica, R.; Poon, M.; O’Donnell, T.P.; Mehta, D.; Fuster, V.; Rajagopalan, S. Prevalence and correlates of septal delayed contrast enhancement in patients with pulmonary hypertension. Am. J. Cardiol. 2007, 100, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Freed, B.H.; Gomberg-Maitland, M.; Chandra, S.; Mor-Avi, V.; Rich, S.; Archer, S.L.; Jamison, E.B., Jr.; Lang, R.M.; Patel, A.R. Late gadolinium enhancement cardiovascular magnetic resonance predicts clinical worsening in patients with pulmonary hypertension. J. Cardiovasc. Magn. Reson. 2012, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- De Siqueira, M.E.M.; Pozo, E.; Fernandes, V.R.; Sengupta, P.P.; Modesto, K.; Gupta, S.S.; Barbeito-Caamaño, C.; Narula, J.; Fuster, V.; Caixeta, A. Characterization and clinical significance of right ventricular mechanics in pulmonary hypertension evaluated with cardiovascular magnetic resonance feature tracking. J. Cardiovasc. Magn. Reson. 2016, 18, 39. [Google Scholar] [CrossRef] [PubMed]
- Stankovic, Z.; Allen, B.D.; Garcia, J.; Jarvis, K.B.; Markl, M. 4D flow imaging with MRI. Cardiovasc. Diagn. Ther. 2014, 4, 173. [Google Scholar] [PubMed]
- Cerne, J.W.; Pathrose, A.; Gordon, D.Z.; Sarnari, R.; Veer, M.; Blaisdell, J.; Allen, B.D.; Avery, R.; Markl, M.; Ragin, A. Evaluation of pulmonary hypertension using 4D flow MRI. J. Magn. Reson. Imaging 2022, 56, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Reiter, U.; Kovacs, G.; Reiter, C.; Kräuter, C.; Nizhnikava, V.; Fuchsjäger, M.; Olschewski, H.; Reiter, G. MR 4D flow-based mean pulmonary arterial pressure tracking in pulmonary hypertension. Eur. Radiol. 2021, 31, 1883–1893. [Google Scholar] [CrossRef] [PubMed]
- Elbaz, M.S.; Shafeghat, M.; Freed, B.H.; Sarnari, R.; Zilber, Z.; Avery, R.; Markl, M.; Allen, B.D.; Carr, J. 3D vortex-energetics in the left pulmonary artery for differentiating pulmonary arterial hypertension and pulmonary venous hypertension groups using 4D flow MRI. J. Magn. Reson. Imaging 2025, 61, 2130–2143. [Google Scholar] [CrossRef] [PubMed]
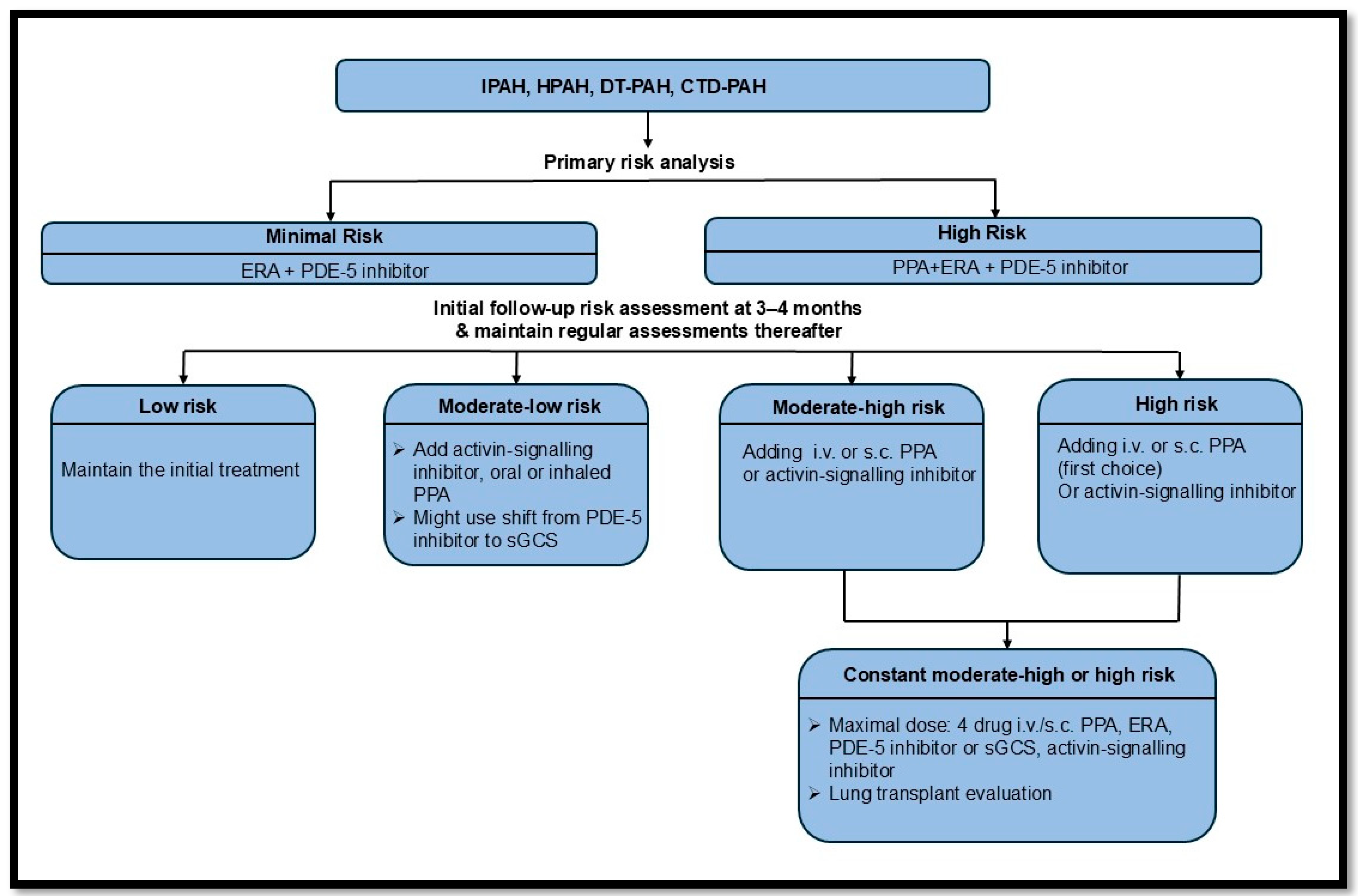
- Chin, K.M.; Gaine, S.P.; Gerges, C.; Jing, Z.-C.; Mathai, S.C.; Tamura, Y.; McLaughlin, V.V.; Sitbon, O. Treatment algorithm for pulmonary arterial hypertension. Eur. Respir. J. 2024, 64, 2401325. [Google Scholar] [CrossRef] [PubMed]
- Kolaitis, N.A. Lung transplantation for pulmonary arterial hypertension. Chest 2023, 164, 992–1006. [Google Scholar] [CrossRef] [PubMed]
- Galiè, N.; Channick, R.N.; Frantz, R.P.; Grünig, E.; Jing, Z.C.; Moiseeva, O.; Preston, I.R.; Pulido, T.; Safdar, Z.; Tamura, Y. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur. Respir. J. 2019, 53, 1801889. [Google Scholar] [CrossRef] [PubMed]
- Dhoble, S.; Patravale, V.; Weaver, E.; Lamprou, D.A.; Patravale, T. Comprehensive review on novel targets and emerging therapeutic modalities for pulmonary arterial Hypertension. Int. J. Pharm. 2022, 621, 121792. [Google Scholar] [CrossRef] [PubMed]
- Galiè, N.; Brundage, B.H.; Ghofrani, H.A.; Oudiz, R.J.; Simonneau, G.; Safdar, Z.; Shapiro, S.; White, R.J.; Chan, M.; Beardsworth, A. Tadalafil therapy for pulmonary arterial hypertension. Circulation 2009, 119, 2894–2903. [Google Scholar] [CrossRef] [PubMed]
- Galiè, N.; Ghofrani, H.A.; Torbicki, A.; Barst, R.J.; Rubin, L.J.; Badesch, D.; Fleming, T.; Parpia, T.; Burgess, G.; Branzi, A. Sildenafil citrate therapy for pulmonary arterial hypertension. N. Engl. J. Med. 2005, 353, 2148–2157. [Google Scholar] [CrossRef] [PubMed]
- Hoeper, M.M.; Al-Hiti, H.; Benza, R.L.; Chang, S.A.; Corris, P.A.; Gibbs, J.S.R.; Grünig, E.; Jansa, P.; Klinger, J.R.; Langleben, D.; et al. Switching to riociguat versus maintenance therapy with phosphodiesterase-5 inhibitors in patients with pulmonary arterial hypertension (REPLACE): A multicentre, open-label, randomised controlled trial. Lancet. Respir. Med. 2021, 9, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Grünig, E.; Jansa, P.; Fan, F.; Hauser, J.A.; Pannaux, M.; Morganti, A.; Rofael, H.; Chin, K.M. Randomized trial of macitentan/tadalafil single-tablet combination therapy for pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2024, 83, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Channick, R.N.; Simonneau, G.; Sitbon, O.; Robbins, I.M.; Frost, A.; Tapson, V.F.; Badesch, D.B.; Roux, S.; Rainisio, M.; Bodin, F. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: A randomised placebocontrolled study. Lancet 2001, 358, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.J.; Badesch, D.B.; Barst, R.J.; Galie, N.; Black, C.M.; Keogh, A.; Pulido, T.; Frost, A.; Roux, S.; Leconte, I. Bosentan therapy for pulmonary arterial hypertension. N. Engl. J. Med. 2002, 346, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Galiè, N.; Barberà, J.A.; Frost, A.E.; Ghofrani, H.-A.; Hoeper, M.M.; McLaughlin, V.V.; Peacock, A.J.; Simonneau, G.; Vachiery, J.-L.; Grünig, E. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N. Engl. J. Med. 2015, 373, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Sitbon, O.; Bosch, J.; Cottreel, E.; Csonka, D.; de Groote, P.; Hoeper, M.M.; Kim, N.H.; Martin, N.; Savale, L.; Krowka, M. Macitentan for the treatment of portopulmonary hypertension (PORTICO): A multicentre, randomised, double-blind, placebo-controlled, phase 4 trial. Lancet. Respir. Med. 2019, 7, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.; Gu, Z.; Li, J.; Liu, X.; Wu, X.; Han, Y.; Pu, J. Clinical adverse effects of endothelin receptor antagonists: Insights from the meta-analysis of 4894 patients from 24 randomized double-blind placebo-controlled clinical trials. J. Am. Heart Assoc. 2016, 5, e003896. [Google Scholar] [CrossRef] [PubMed]
- Sitbon, O.; Noordegraaf, A.V. Epoprostenol and pulmonary arterial hypertension: 20 years of clinical experience. Eur. Respir. Rev. 2017, 26, 160055. [Google Scholar] [CrossRef] [PubMed]
- Barst, R.J.; Rubin, L.J.; Long, W.A.; McGoon, M.D.; Rich, S.; Badesch, D.B.; Groves, B.M.; Tapson, V.F.; Bourge, R.C.; Brundage, B.H. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N. Engl. J. Med. 1996, 334, 296–301. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, V.V.; Benza, R.L.; Rubin, L.J.; Channick, R.N.; Voswinckel, R.; Tapson, V.F.; Robbins, I.M.; Olschewski, H.; Rubenfire, M.; Seeger, W. Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: A randomized controlled clinical trial. J. Am. Coll. Cardiol. 2010, 55, 1915–1922. [Google Scholar] [CrossRef] [PubMed]
- Frost, A.; Janmohamed, M.; Fritz, J.S.; McConnell, J.W.; Poch, D.; Fortin, T.A.; Miller, C.E.; Chin, K.M.; Fisher, M.; Eggert, M. Safety and tolerability of transition from inhaled treprostinil to oral selexipag in pulmonary arterial hypertension: Results from the TRANSIT-1 study. J. Heart Lung Transplant. 2019, 38, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Sitbon, O.; Channick, R.; Chin, K.M.; Frey, A.; Gaine, S.; Galiè, N.; Ghofrani, H.-A.; Hoeper, M.M.; Lang, I.M.; Preiss, R. Selexipag for the treatment of pulmonary arterial hypertension. N. Engl. J. Med. 2015, 373, 2522–2533. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; McLaughlin, V.; Gibbs, J.S.R.; Gomberg-Maitland, M.; Hoeper, M.M.; Preston, I.R.; Souza, R.; Waxman, A.; Escribano Subias, P.; Feldman, J. Sotatercept for the treatment of pulmonary arterial hypertension. N. Engl. J. Med. 2021, 384, 1204–1215. [Google Scholar] [CrossRef] [PubMed]
- Hoeper, M.M.; Badesch, D.B.; Ghofrani, H.A.; Gibbs, J.S.R.; Gomberg-Maitland, M.; McLaughlin, V.V.; Preston, I.R.; Souza, R.; Waxman, A.B.; Grünig, E. Phase 3 trial of sotatercept for treatment of pulmonary arterial hypertension. N. Engl. J. Med. 2023, 388, 1478–1490. [Google Scholar] [CrossRef] [PubMed]
- Sitbon, O.; Sattler, C.; Bertoletti, L.; Savale, L.; Cottin, V.; Jaïs, X.; De Groote, P.; Chaouat, A.; Chabannes, C.; Bergot, E. Initial dual oral combination therapy in pulmonary arterial hypertension. Eur. Respir. J. 2016, 47, 1727–1736. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.M.; Sitbon, O.; Doelberg, M.; Feldman, J.; Gibbs, J.S.R.; Grünig, E.; Hoeper, M.M.; Martin, N.; Mathai, S.C.; McLaughlin, V.V. Three-versus two-drug therapy for patients with newly diagnosed pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2021, 78, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Ghofrani, H.-A.; Gomberg-Maitland, M.; Zhao, L.; Grimminger, F. Mechanisms and treatment of pulmonary arterial hypertension. Nat. Rev. Cardiol. 2025, 22, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Capdeville, R.; Buchdunger, E.; Zimmermann, J.; Matter, A. Glivec (STI571, imatinib), a rationally developed, targeted anticancer drug. Nat. Rev. Drug Discov. 2002, 1, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Ghofrani, H.A.; Seeger, W.; Grimminger, F. Imatinib for the treatment of pulmonary arterial hypertension. N. Engl. J. Med. 2005, 353, 1412–1413. [Google Scholar] [CrossRef] [PubMed]
- Hoeper, M.M.; Barst, R.J.; Bourge, R.C.; Feldman, J.; Frost, A.E.; Galié, N.; Gómez-Sánchez, M.A.; Grimminger, F.; Grünig, E.; Hassoun, P.M. Imatinib mesylate as add-on therapy for pulmonary arterial hypertension: Results of the randomized IMPRES study. Circulation 2013, 127, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Frost, A.E.; Barst, R.J.; Hoeper, M.M.; Chang, H.-J.; Frantz, R.P.; Fukumoto, Y.; Galié, N.; Hassoun, P.M.; Klose, H.; Matsubara, H. Long-term safety and efficacy of imatinib in pulmonary arterial hypertension. J. Heart Lung Transplant. 2015, 34, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Gillies, H.; Niven, R.; Dake, B.T.; Chakinala, M.M.; Feldman, J.P.; Hill, N.S.; Hoeper, M.M.; Humbert, M.; McLaughlin, V.V.; Kankam, M. AV-101, a novel inhaled dry-powder formulation of imatinib, in healthy adult participants: A phase 1 single and multiple ascending dose study. ERJ Open Res. 2023, 9, 00433–02022. [Google Scholar] [CrossRef] [PubMed]
- Frantz, R.P.; McLaughlin, V.V.; Sahay, S.; Subías, P.E.; Zolty, R.L.; Benza, R.L.; Channick, R.N.; Chin, K.M.; Hemnes, A.R.; Howard, L.S. Seralutinib in adults with pulmonary arterial hypertension (TORREY): A randomised, double-blind, placebo-controlled phase 2 trial. Lancet Respir. Med. 2024, 12, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Upton, P.D.; Dunmore, B.J.; Li, W.; Morrell, N.W. An emerging class of new therapeutics targeting TGF, Activin, and BMP ligands in pulmonary arterial hypertension. Dev. Dyn. 2023, 252, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Peacock, A.J.; Crawley, S.; McLure, L.; Blyth, K.G.; Vizza, C.D.; Poscia, R.; Francone, M.; Iacucci, I.; Olschewski, H.; Kovacs, G. Changes in right ventricular function measured by cardiac magnetic resonance imaging in patients receiving pulmonary arterial hypertension–targeted therapy: The EURO-MR Study. Circ. Cardiovasc. Imaging 2014, 7, 107–114. [Google Scholar] [CrossRef] [PubMed]
- van de Veerdonk, M.C.; Marcus, J.T.; Westerhof, N.; Heymans, M.W.; Bogaard, H.-J.; Vonk-Noordegraaf, A. Upfront combination therapy reduces right ventricular volumes in pulmonary arterial hypertension. Eur. Respir. J. 2017, 49, 1700007. [Google Scholar] [CrossRef] [PubMed]
- Galiè, N.; Hinderliter, A.L.; Torbicki, A.; Fourme, T.; Simonneau, G.; Pulido, T.; Espinola-Zavaleta, N.; Rocchi, G.; Manes, A.; Frantz, R. Effects of the oral endothelin-receptorantagonist bosentan on echocardiographicand doppler measures in patients with pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2003, 41, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Tello, K.; Seeger, W.; Naeije, R.; Vanderpool, R.; Ghofrani, H.A.; Richter, M.; Tedford, R.J.; Bogaard, H.J. Right heart failure in pulmonary hypertension: Diagnosis and new perspectives on vascular and direct right ventricular treatment. Br. J. Pharmacol. 2021, 178, 90–107. [Google Scholar] [CrossRef] [PubMed]
- Konstam, M.A.; Kiernan, M.S.; Bernstein, D.; Bozkurt, B.; Jacob, M.; Kapur, N.K.; Kociol, R.D.; Lewis, E.F.; Mehra, M.R.; Pagani, F.D. Evaluation and management of right-sided heart failure: A scientific statement from the American Heart Association. Circulation 2018, 137, e578–e622. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Arroyo, J.; Sakagami, M.; Syed, A.A.; Farkas, L.; Van Tassell, B.; Kraskauskas, D.; Mizuno, S.; Abbate, A.; Bogaard, H.J.; Byron, P.R. Iloprost reverses established fibrosis in experimental right ventricular failure. Eur. Respir. J. 2015, 45, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Toshner, M.; Church, C.; Harbaum, L.; Rhodes, C.; Moreschi, S.S.V.; Liley, J.; Jones, R.; Arora, A.; Batai, K.; Desai, A.A. Mendelian randomisation and experimental medicine approaches to interleukin-6 as a drug target in pulmonary arterial hypertension. Eur. Respir. J. 2022, 59, 2002463. [Google Scholar] [CrossRef] [PubMed]
- Benza, R.L.; Gomberg-Maitland, M.; Demarco, T.; Frost, A.E.; Torbicki, A.; Langleben, D.; Pulido, T.; Correa-Jaque, P.; Passineau, M.J.; Wiener, H.W.; et al. Endothelin-1 Pathway Polymorphisms and Outcomes in Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2015, 192, 1345–1354. [Google Scholar] [CrossRef] [PubMed]
- Hoeper, M.M.; Pausch, C.; Olsson, K.M.; Huscher, D.; Pittrow, D.; Grünig, E.; Staehler, G.; Vizza, C.D.; Gall, H.; Distler, O. COMPERA 2.0: A refined four-stratum risk assessment model for pulmonary arterial hypertension. Eur. Respir. J. 2022, 60, 2102311. [Google Scholar] [CrossRef] [PubMed]
- Benza, R.L.; Kanwar, M.K.; Raina, A.; Scott, J.V.; Zhao, C.L.; Selej, M.; Elliott, C.G.; Farber, H.W. Development and validation of an abridged version of the REVEAL 2.0 risk score calculator, REVEAL Lite 2, for use in patients with pulmonary arterial hypertension. Chest 2021, 159, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Legchenko, E.; Chouvarine, P.; Borchert, P.; Fernandez-Gonzalez, A.; Snay, E.; Meier, M.; Maegel, L.; Mitsialis, S.A.; Rog-Zielinska, E.A.; Kourembanas, S. PPARγ agonist pioglitazone reverses pulmonary hypertension and prevents right heart failure via fatty acid oxidation. Sci. Transl. Med. 2018, 10, eaao0303. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.J.; Beckmann, T.; Vorla, M.; Kalra, D.K. New Drugs and Therapies in Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2023, 24, 5850. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, C.J.; Batai, K.; Bleda, M.; Haimel, M.; Southgate, L.; Germain, M.; Pauciulo, M.W.; Hadinnapola, C.; Aman, J.; Girerd, B. Genetic determinants of risk in pulmonary arterial hypertension: International genome-wide association studies and meta-analysis. Lancet Respir. Med. 2019, 7, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Harbaum, L.; Rhodes, C.J.; Wharton, J.; Lawrie, A.; Karnes, J.H.; Desai, A.A.; Nichols, W.C.; Humbert, M.; Montani, D.; Girerd, B. Mining the plasma proteome for insights into the molecular pathology of pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2022, 205, 1449–1460. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, C.J.; Ghataorhe, P.; Wharton, J.; Rue-Albrecht, K.C.; Hadinnapola, C.; Watson, G.; Bleda, M.; Haimel, M.; Coghlan, G.; Corris, P.A. Plasma metabolomics implicates modified transfer RNAs and altered bioenergetics in the outcomes of pulmonary arterial hypertension. Circulation 2017, 135, 460–475. [Google Scholar] [CrossRef] [PubMed]
- Kelly, N.J.; Chan, S.Y. Pulmonary arterial hypertension: Emerging principles of precision medicine across basic science to clinical practice. Rev. Cardiovasc. Med. 2022, 23, 378. [Google Scholar] [CrossRef] [PubMed]
- Willemink, M.J.; Persson, M.; Pourmorteza, A.; Pelc, N.J.; Fleischmann, D. Photon-counting CT: Technical principles and clinical prospects. Radiology 2018, 289, 293–312. [Google Scholar] [CrossRef] [PubMed]
- Hagar, M.T.; Soschynski, M.; Saffar, R.; Rau, A.; Taron, J.; Weiss, J.; Stein, T.; Faby, S.; von Zur Muehlen, C.; Ruile, P. Accuracy of ultrahigh-resolution photon-counting CT for detecting coronary artery disease in a high-risk population. Radiology 2023, 307, e223305. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Montoya, J.; Gutjahr, R.; Ferrero, A.; Halaweish, A.; Kappler, S.; McCollough, C.; Leng, S. Lung nodule volume quantification and shape differentiation with an ultra-high resolution technique on a photon-counting detector computed tomography system. J. Med. Imaging 2017, 4, 043502. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, S.; Bogaard, H.J.; Elbaz, M.S.; Freed, B.H.; Remy-Jardin, M.; van Beek, E.J.; Gopalan, D.; Kiely, D.G. Emerging multimodality imaging techniques for the pulmonary circulation. Eur. Respir. J. 2024, 64, 2401128. [Google Scholar] [CrossRef] [PubMed]

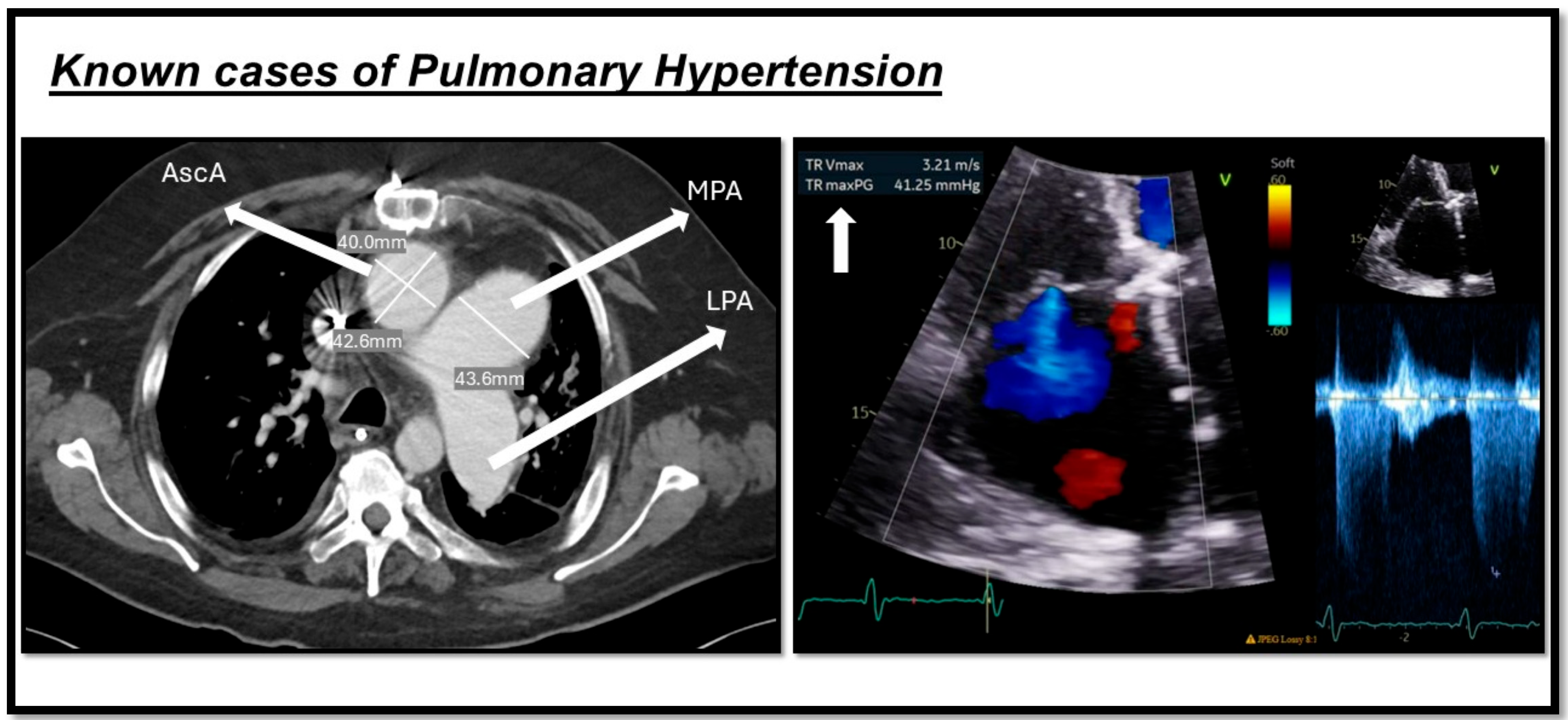
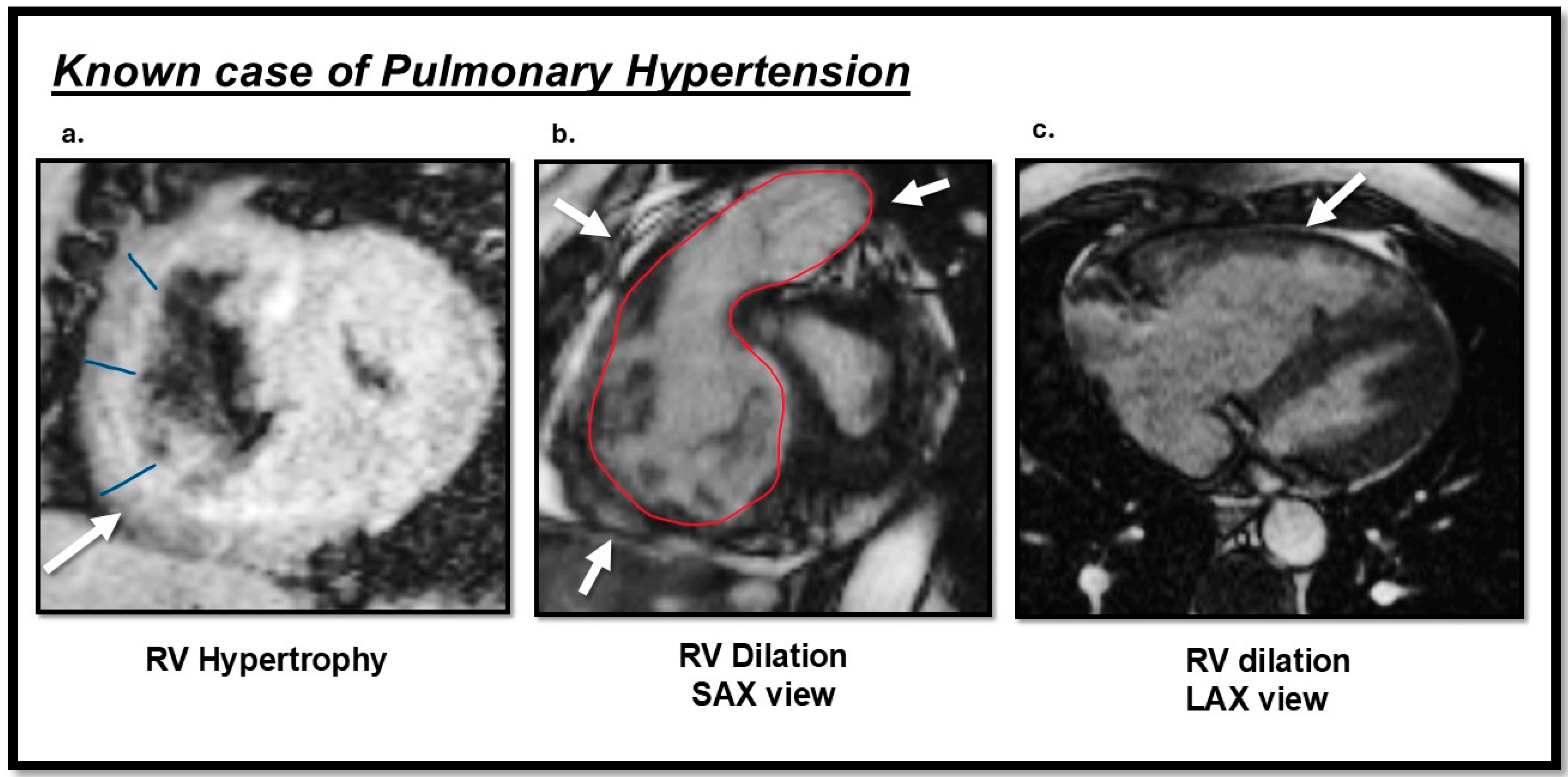
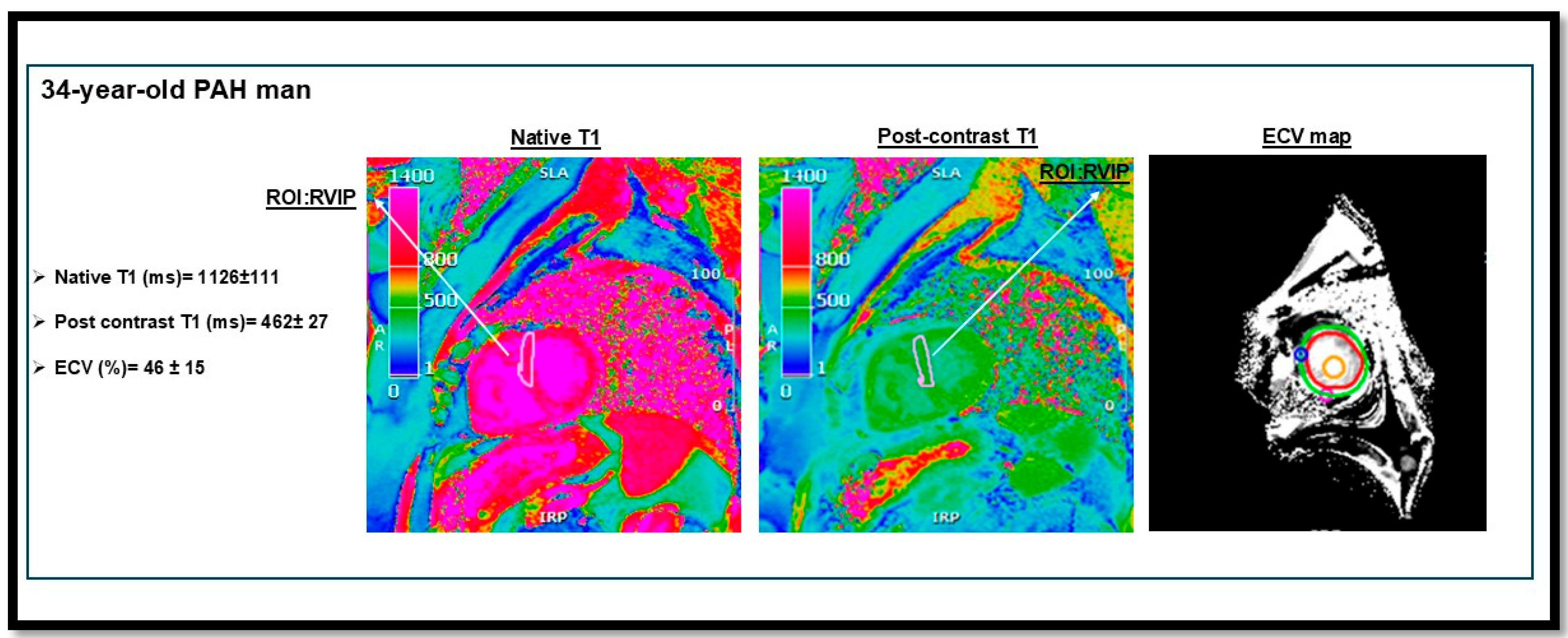
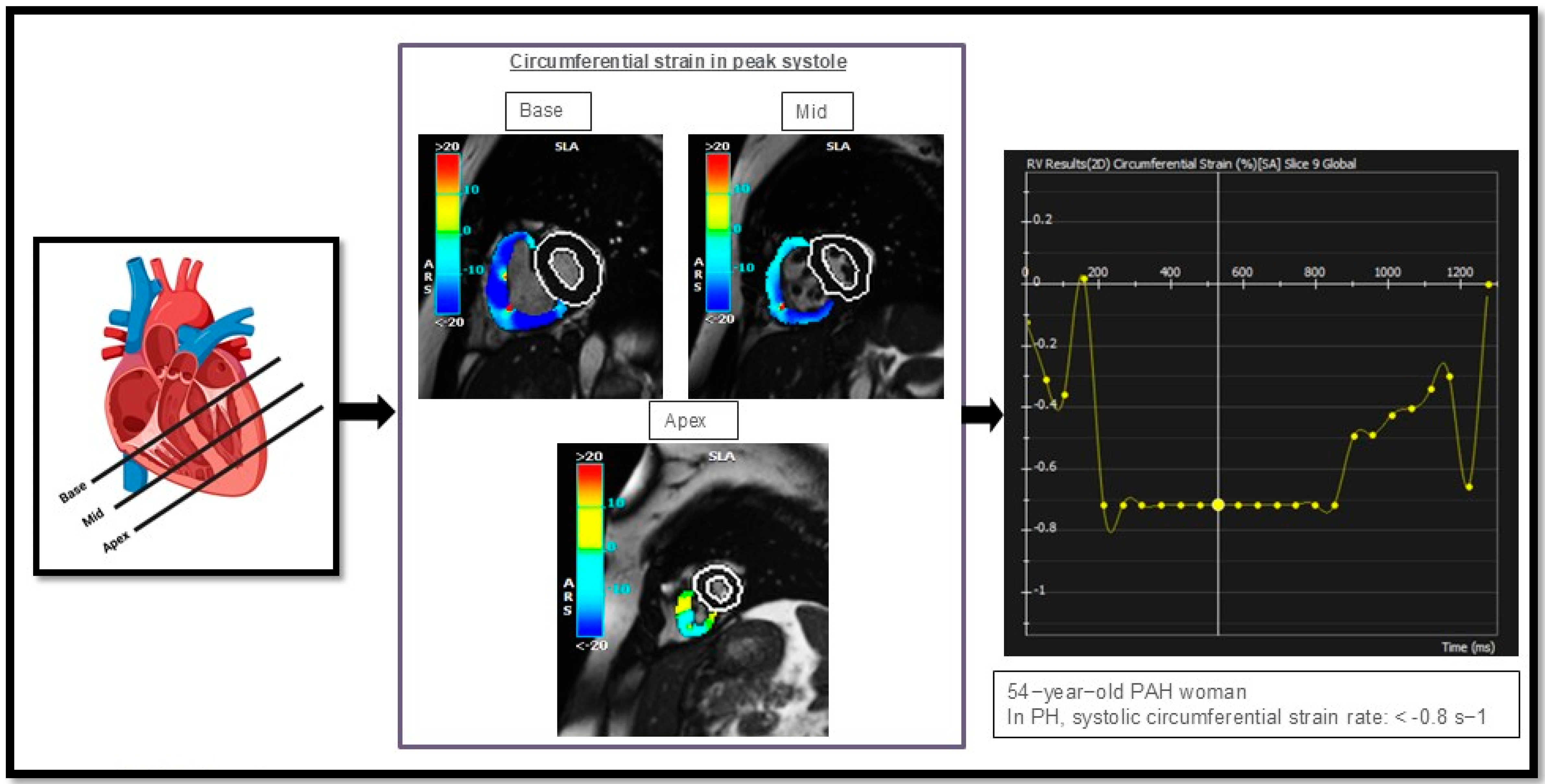
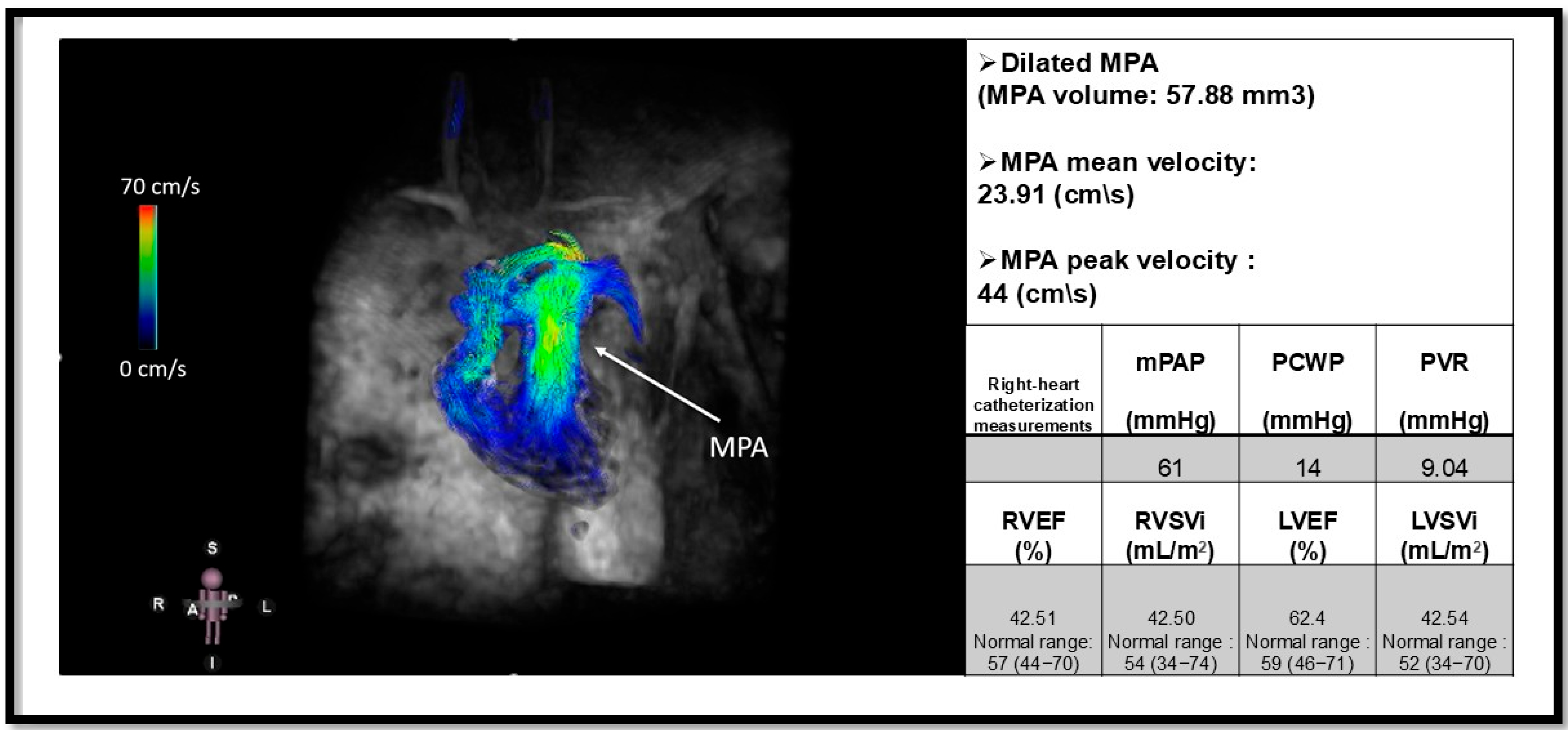
| Pulmonary arterial hypertension (PAH): | 1. Idiopathic (IPAH): Sparing pulmonary veins | |
| 2. Heritable: BMPR2 mutations/other mutations | ||
| 3. Drugs\toxins induced: Methamphetamine, Dasatinib (targeted cancer therapy tyrosine kinase inhibitor) | ||
| 4. Associated with other disease (APAH): | 4.1. Connective tissue disease Mixed connective tissue disease (MCTD) Systemic lupus erythematosus (SLE) | |
| 4.2. Infection with HIV | ||
| 4.3. Portal hypertension Liver disease | ||
| 4.4. Congenital heart disease Eisenmenger syndrome, PAH related to systemic-to-pulmonary shunts, PAH associated with small cardiac defects (ASD, VSD), and PAH after cardiac defect closure | ||
| 4.5. Schistosomiasis | ||
| 4.6. Chronic hemolytic anemia Beta thalassemia Sickle cell disease | ||
| 5. Long-term responders to calcium channel blockers (CCBs): Improvement with CCBs | ||
| 6. Features of venous-capillary involvement: Pulmonary veno-occlusive disease | ||
| 7. Persistent PH of the newborn | ||
| Modality | Main Diagnostic Features | Benefits | limitations | |
|---|---|---|---|---|
| Right heart catheterization (RHC) | Direct pressure measurements: mPAP, PVR, PCWP, and cardiac output. |
|
| |
| Echocardiography |
|
|
| |
| V/Q Scan |
|
|
| |
| Computed Tomography (CT) |
|
|
| |
| Cardiac Magnetic Resonance (CMR) | Cine |
|
|
|
| LGE | Fibrosis in RV insertion points or RV free wall. | |||
| T1 mapping | Elevated T1 relacation time and ECV in diffuse fibrosis | |||
| Strain/Strain Rate Analysis (Feature Tracking) | Myocardial Deformation, reduced strain rate in three directions, longitudinal, circumferential and radial | |||
| Flow and Velocity, 4D-flow MRI | Measuring peak and mean velocity in the MPA, LPA and RPA | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shafeghat, M.; Raza, Y.; Catania, R.; Rahsepar, A.A.; Tilkens, B.; Cuttica, M.J.; Freed, B.H.; Dai, J.; Zhao, Y.-Y.; Carr, J.C. State of the Art in Pulmonary Arterial Hypertension: Molecular Basis, Imaging Modalities, and Right Heart Failure Treatment. Biomedicines 2025, 13, 1773. https://doi.org/10.3390/biomedicines13071773
Shafeghat M, Raza Y, Catania R, Rahsepar AA, Tilkens B, Cuttica MJ, Freed BH, Dai J, Zhao Y-Y, Carr JC. State of the Art in Pulmonary Arterial Hypertension: Molecular Basis, Imaging Modalities, and Right Heart Failure Treatment. Biomedicines. 2025; 13(7):1773. https://doi.org/10.3390/biomedicines13071773
Chicago/Turabian StyleShafeghat, Melika, Yasmin Raza, Roberta Catania, Amir Ali Rahsepar, Blair Tilkens, Michael J. Cuttica, Benjamin H. Freed, Jingbo Dai, You-Yang Zhao, and James C. Carr. 2025. "State of the Art in Pulmonary Arterial Hypertension: Molecular Basis, Imaging Modalities, and Right Heart Failure Treatment" Biomedicines 13, no. 7: 1773. https://doi.org/10.3390/biomedicines13071773
APA StyleShafeghat, M., Raza, Y., Catania, R., Rahsepar, A. A., Tilkens, B., Cuttica, M. J., Freed, B. H., Dai, J., Zhao, Y.-Y., & Carr, J. C. (2025). State of the Art in Pulmonary Arterial Hypertension: Molecular Basis, Imaging Modalities, and Right Heart Failure Treatment. Biomedicines, 13(7), 1773. https://doi.org/10.3390/biomedicines13071773







