Integrated Multi-Omics Profiling Reveals That Highly Pyroptotic MDMs Contribute to Psoriasis Progression Through CXCL16
Abstract
1. Introduction
2. Materials and Methods
2.1. Transcriptomic Data Preparation
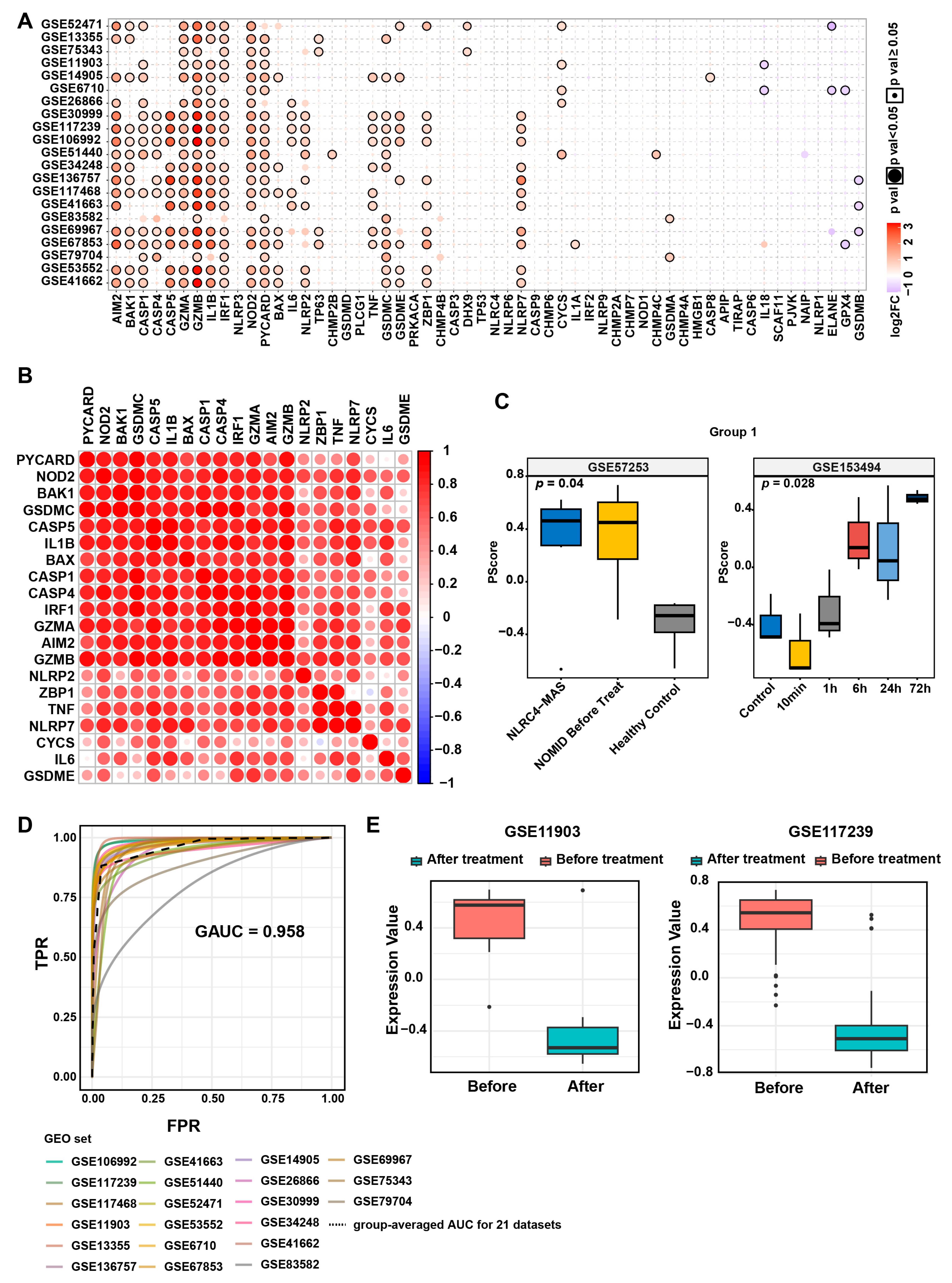
2.2. Construction of a Psoriasis Pyroptosis Scoring Tool
2.3. Analysis of Pyroptosis Scores and Psoriasis Characteristics
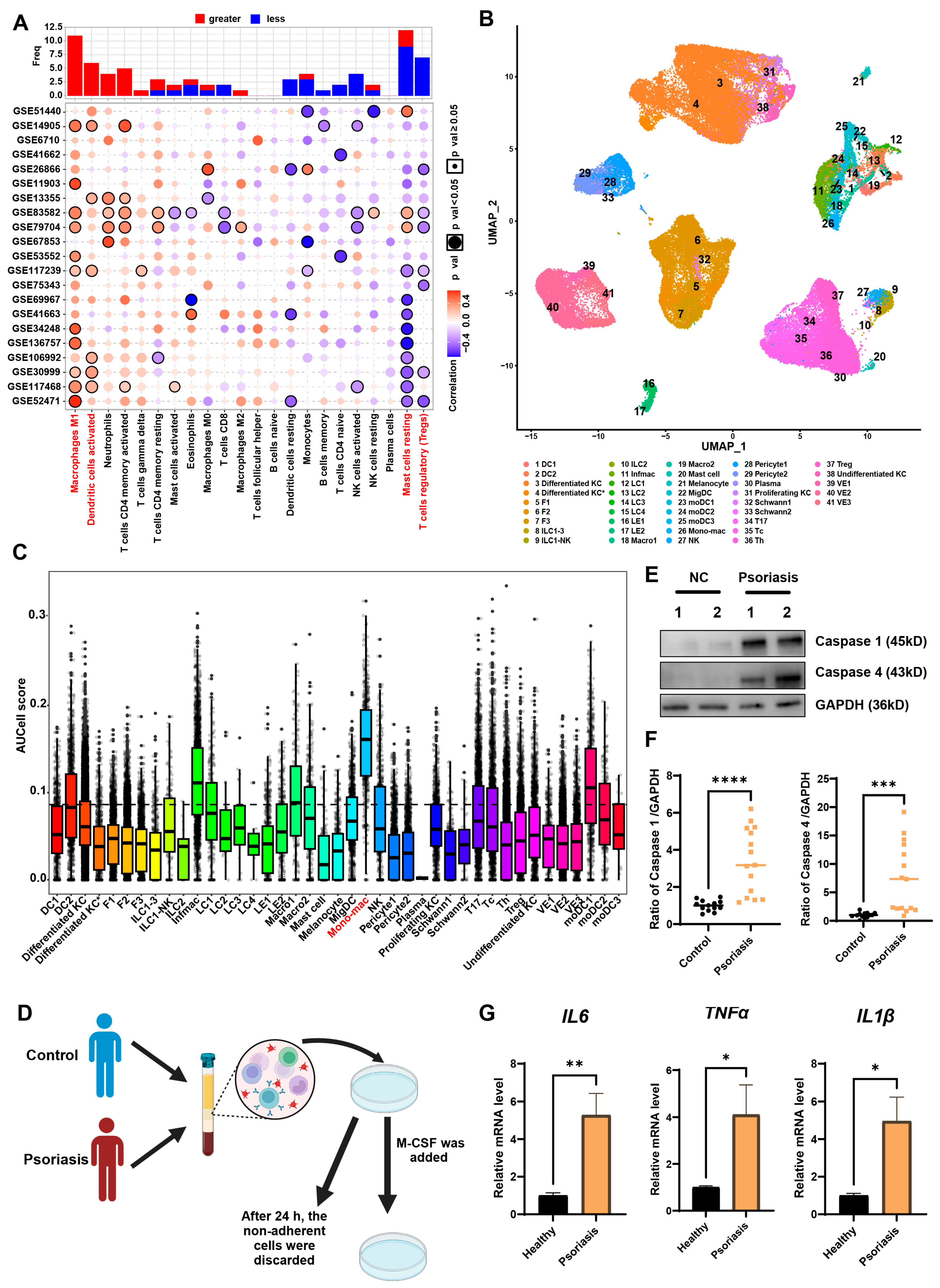
2.4. Immune Cell Infiltration Assessment
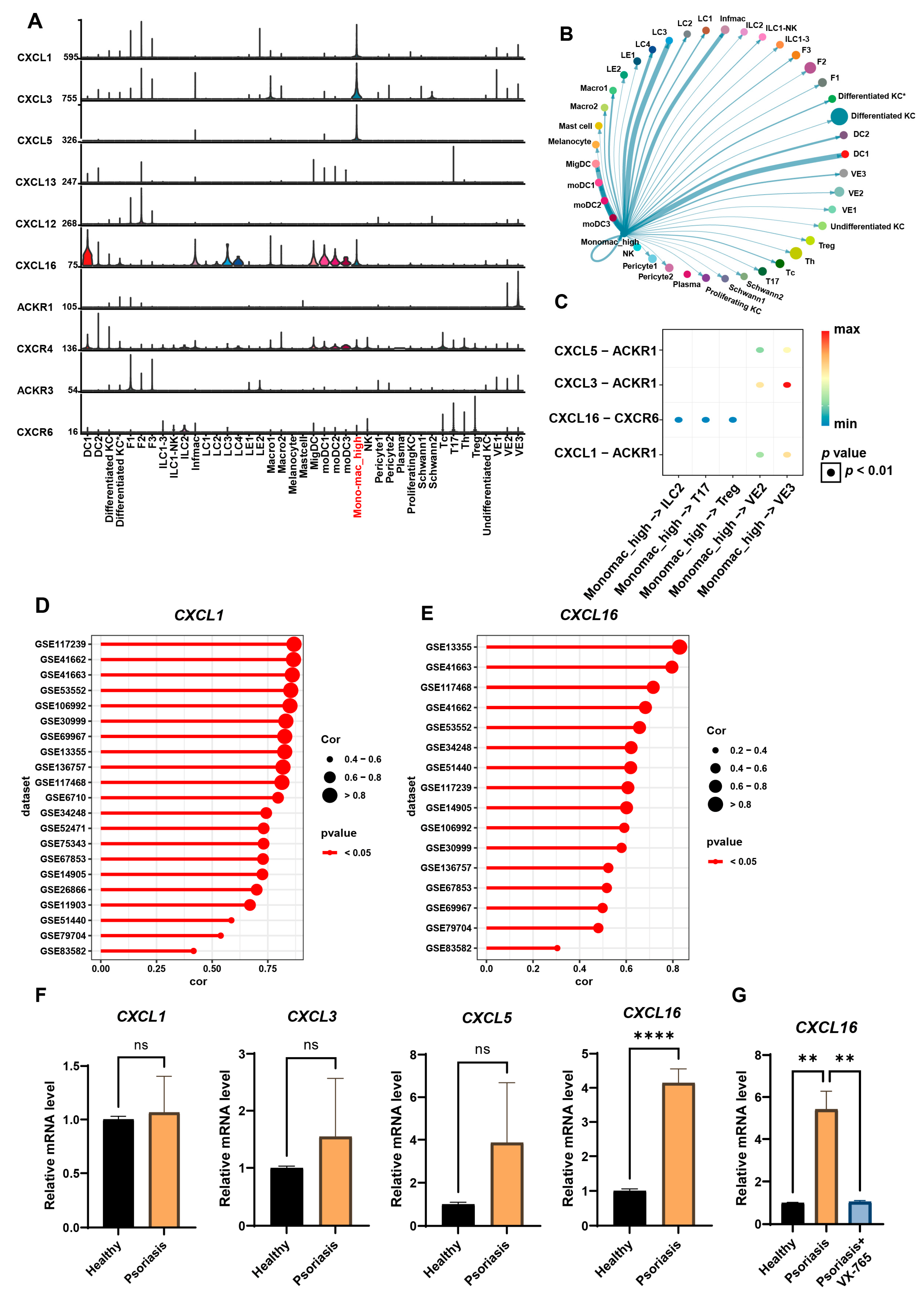
2.5. Single-Cell Analysis of Psoriasis Datasets
2.6. Human Monocyte Culture and Differentiation
2.7. RNA Isolation and Quantitative RT-PCR (RT-qPCR)
2.8. Western Blotting
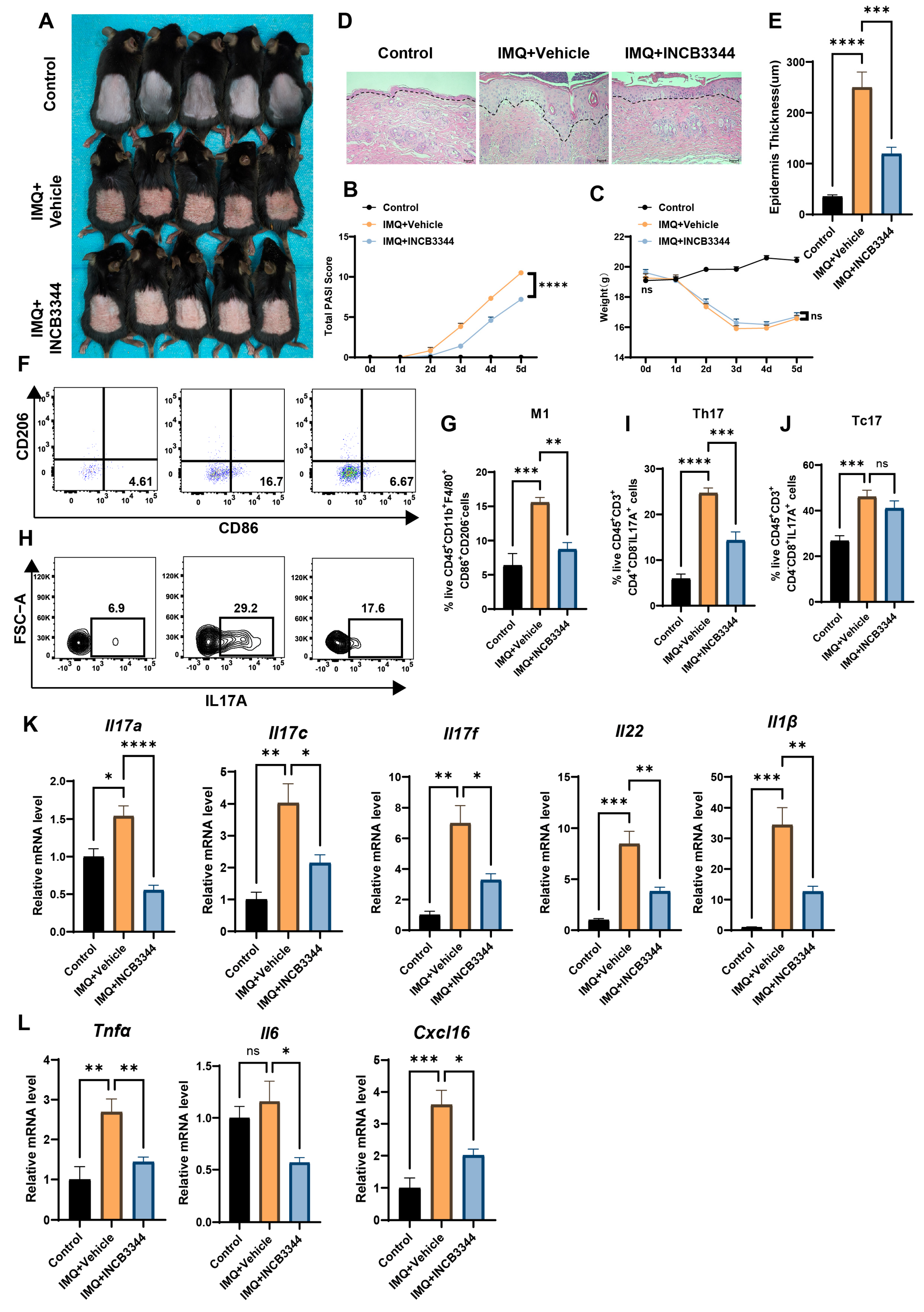
2.9. IMQ Model of Psoriasis
2.10. Animal Study
2.11. Skin Cell Isolation and Flow Cytometry
2.12. Statistical Analysis
3. Results
3.1. Elevated Pyroptosis Levels in Psoriatic Lesions
3.2. MDMs Exhibit the Highest Pyroptosis Levels in Psoriatic Lesions
3.3. Highly Pyroptotic MDMs Drive Psoriasis Progression Through CXCL16
3.4. Inhibition of MDMs Can Alleviate IMQ-Induced Psoriatic Dermatitis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Griffiths, C.E.M.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef] [PubMed]
- Shou, Y.; Yang, L.; Yang, Y.; Xu, J. Inhibition of keratinocyte ferroptosis suppresses psoriatic inflammation. Cell Death Dis. 2021, 12, 1009. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.W.; Zhang, W.J.; Wang, Y.; Tan, L.P.; Bao, Y.L.; Song, Z.B.; Yu, C.L.; Wang, S.Y.; Liu, L.; Li, Y.X. Convallatoxin induces HaCaT cell necroptosis and ameliorates skin lesions in psoriasis-like mouse models. Biomed. Pharmacother. 2020, 121, 109615. [Google Scholar] [CrossRef] [PubMed]
- Kenealy, S.; Manils, J.; Raverdeau, M.; Munoz-Wolf, N.; Barber, G.; Liddicoat, A.; Lavelle, E.C.; Creagh, E.M. Caspase-11-Mediated Cell Death Contributes to the Pathogenesis of Imiquimod-Induced Psoriasis. J. Investig. Dermatol. 2019, 139, 2389–2393.e3. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, X.; Cheng, H.; Zhou, F. AIM2 and Psoriasis. Front. Immunol. 2023, 14, 1085448. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target. Ther. 2021, 6, 128. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, S.B.; Miao, E.A. Gasdermins: Effectors of Pyroptosis. Trends Cell Biol. 2017, 27, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, Y.; Yang, F.; Liang, R.; Xu, W.; Li, Y.; Cheng, J.; Liang, B.; Tang, M.; Shi, X.; et al. Gasdermin E-mediated keratinocyte pyroptosis participates in the pathogenesis of psoriasis by promoting skin inflammation. Br. J. Dermatol. 2024, 191, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, Y.; Diao, Z.; Chen, D.; Xia, R.; Wang, B.; Yang, S.; Yin, Z. Gasdermin D-mediated neutrophil pyroptosis drives inflammation in psoriasis. eLife 2024, 13, RP101248. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.; Chen, H.; Ji, X.; Zhu, W.; Wu, Z.; Huang, S.; Lin, C.; Yang, T.; Zeng, Z.; Li, L. Knockdown of GSDMD inhibits pyroptosis in psoriasis by blocking the NOD-like receptor signaling pathway. Int. Immunopharmacol. 2025, 147, 114036. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Tang, B.; Zheng, X.; Luo, Q.; Bi, Y.; Deng, H.; Yu, J.; Lu, Y.; Han, L.; Chen, H.; et al. Analysis of the potential pyroptosis mechanism in psoriasis and experimental validation of NLRP3 in vitro and in vivo. Int. Immunopharmacol. 2023, 124, 110811. [Google Scholar] [CrossRef] [PubMed]
- Long, F.; Wei, X.; Chen, Y.; Li, M.; Lian, N.; Yu, S.; Chen, S.; Yang, Y.; Li, M.; Gu, H.; et al. Gasdermin E promotes translocation of p65 and c-jun into nucleus in keratinocytes for progression of psoriatic skin inflammation. Cell Death Dis. 2024, 15, 180. [Google Scholar] [CrossRef] [PubMed]
- Lian, N.; Chen, Y.; Chen, S.; Zhang, Y.; Chen, H.; Yang, Y.; Gu, H.; Chen, Q.; Li, M.; Chen, X. Gasdermin D-mediated keratinocyte pyroptosis as a key step in psoriasis pathogenesis. Cell Death Dis. 2023, 14, 595. [Google Scholar] [CrossRef] [PubMed]
- Gaujoux, R.; Seoighe, C. A flexible R package for nonnegative matrix factorization. BMC Bioinform. 2010, 11, 367. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.M.; Liu, C.L.; Green, M.R.; Gentles, A.J.; Feng, W.G.; Xu, Y.; Hoang, C.D.; Diehn, M.; Alizadeh, A.A. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods 2015, 12, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Williams, S. Pearson’s correlation coefficient. N. Z. Med. J. 1996, 109, 38. [Google Scholar] [PubMed]
- Reynolds, G.; Vegh, P.; Fletcher, J.; Poyner, E.F.M.; Stephenson, E.; Goh, I.; Botting, R.A.; Huang, N.; Olabi, B.; Dubois, A.; et al. Developmental cell programs are co-opted in inflammatory skin disease. Science 2021, 371, eaba6500. [Google Scholar] [CrossRef] [PubMed]
- Chaintreuil, P.; Laplane, L.; Esnault, F.; Ghesquier, V.; Savy, C.; Furstoss, N.; Arcangeli, M.L.; Cluzeau, T.; Robert, G.; Droin, N.; et al. Reprogramming monocyte-derived macrophages through caspase inhibition. Oncoimmunology 2022, 11, 2015859. [Google Scholar] [CrossRef] [PubMed]
- Daigneault, M.; Preston, J.A.; Marriott, H.M.; Whyte, M.K.; Dockrell, D.H. The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS ONE 2010, 5, e8668. [Google Scholar] [CrossRef] [PubMed]
- van der Fits, L.; Mourits, S.; Voerman, J.S.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.M.; Florencia, E.; Prens, E.P.; et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 2009, 182, 5836–5845. [Google Scholar] [CrossRef] [PubMed]
- Yong, L.; Yu, Y.; Li, B.; Ge, H.; Zhen, Q.; Mao, Y.; Yu, Y.; Cao, L.; Zhang, R.; Li, Z.; et al. Calcium/calmodulin-dependent protein kinase IV promotes imiquimod-induced psoriatic inflammation via macrophages and keratinocytes in mice. Nat. Commun. 2022, 13, 4255. [Google Scholar] [CrossRef] [PubMed]
- Hseu, J.H.; Chan, C.I.; Vadivalagan, C.; Chen, S.J.; Yen, H.R.; Hseu, Y.C.; Yang, H.L.; Wu, P.Y. Tranexamic acid improves psoriasis-like skin inflammation: Evidence from in vivo and in vitro studies. Biomed. Pharmacother. 2023, 166, 115307. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Shi, H.Y.; Lu, Y.Y.; Lin, J. Centella asiatica alleviates psoriasis through JAK/STAT3-mediated inflammation: An in vitro and in vivo study. J. Ethnopharmacol. 2023, 317, 116746. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Jiang, Q.; Huang, H.; Zhou, X. Topical histone deacetylase inhibitor remetinostat improves IMQ-induced psoriatic dermatitis via suppressing dendritic cell maturation and keratinocyte differentiation and inflammation. Eur. J. Pharmacol. 2024, 983, 177011. [Google Scholar] [CrossRef] [PubMed]
- Canna, S.W.; de Jesus, A.A.; Gounil, S.; Brooks, S.R.; Marrero, B.; Liu, Y.; DiMattia, M.A.; Zaal, K.J.M.; Sanchez, G.A.M.; Kim, H.; et al. An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat. Genet. 2014, 46, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.J.; Shen, J.W.; Li, Y.F.; Wu, J.W.; Luo, X.L.; Yu, Y.Y.; Zhang, Y.H.; Gu, L.; Zhang, X.B.; Jiang, C.Z.; et al. Pyroptosis inhibition improves the symptom of acute myocardial infarction. Cell Death Dis. 2021, 12, 852. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Xia, Y.; Huang, M.; Zhang, L.; Chen, L. Expression of NLPR3 in Psoriasis Is Associated with Enhancement of Interleukin-1beta and Caspase-1. Med. Sci. Monit. 2018, 24, 7909–7913. [Google Scholar] [CrossRef] [PubMed]
- Skendros, P.; Papagoras, C.; Lefaki, I.; Giatromanolaki, A.; Kotsianidis, I.; Speletas, M.; Bocly, V.; Theodorou, I.; Dalla, V.; Ritis, K. Successful response in a case of severe pustular psoriasis after interleukin-1β inhibition. Br. J. Dermatol. 2017, 176, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, Y.; Du, C.; Sang, L.; Liu, L.; Li, Y.; Wang, F.; Fan, W.; Tang, P.; Zhang, S.; et al. Evidence of pyroptosis and ferroptosis extensively involved in autoimmune diseases at the single-cell transcriptome level. J. Transl. Med. 2022, 20, 363. [Google Scholar] [CrossRef] [PubMed]
- Gaire, B.P.; Lee, C.H.; Kim, W.; Sapkota, A.; Lee, D.Y.; Choi, J.W. Lysophosphatidic Acid Receptor 5 Contributes to Imiquimod-Induced Psoriasis-Like Lesions through NLRP3 Inflammasome Activation in Macrophages. Cells 2020, 9, 1753. [Google Scholar] [CrossRef] [PubMed]
- Syed, S.N.; Weigert, A.; Brüne, B. Sphingosine Kinases are Involved in Macrophage NLRP3 Inflammasome Transcriptional Induction. Int. J. Mol. Sci. 2020, 21, 4733. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, R.; Li, L.; Zeng, Y.; Chen, J.; Wei, M.; Feng, Y.; Chen, G.; Wang, Y.; Lin, L.; et al. Pregnancy-induced changes to the gut microbiota drive macrophage pyroptosis and exacerbate septic inflammation. Immunity 2023, 56, 336–352.e9. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Yue, Z.; Nie, L.; Zhao, Z.; Wang, Q.; Chen, J.; Wang, Q. Hyperglycaemia-associated macrophage pyroptosis accelerates periodontal inflamm-aging. J. Clin. Periodontol. 2021, 48, 1379–1392. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; You, Y.; Zhang, J.; Ban, J.; Min, H.; Li, C.; Chen, J. Crystalline silica-induced macrophage pyroptosis interacting with mitophagy contributes to pulmonary fibrosis via modulating mitochondria homeostasis. J. Hazard. Mater. 2023, 454, 131562. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.S.; Gross, C.J.; Dreier, R.F.; Saller, B.S.; Mishra, R.; Gorka, O.; Heilig, R.; Meunier, E.; Dick, M.S.; Cikovic, T.; et al. The Inflammasome Drives GSDMD-Independent Secondary Pyroptosis and IL-1 Release in the Absence of Caspase-1 Protease Activity. Cell Rep. 2017, 21, 3846–3859. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, M.C.; Martin-Cleary, C.; Fernandez-Fernandez, B.; Elewa, U.; Sanchez-Niño, M.D.; Carrero, J.J.; Ortiz, A. CXCL16 in kidney and cardiovascular injury. Cytokine Growth Factor Rev. 2014, 25, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Bao, N.; Fu, B.; Zhong, X.; Jia, S.; Ren, Z.; Wang, H.; Wang, W.; Shi, H.; Li, J.; Ge, F.; et al. Role of the CXCR6/CXCL16 axis in autoimmune diseases. Int. Immunopharmacol. 2023, 121, 110530. [Google Scholar] [CrossRef] [PubMed]
- Hornburg, M.; Desbois, M.; Lu, S.; Guan, Y.; Lo, A.A.; Kaufman, S.; Elrod, A.; Lotstein, A.; DesRochers, T.M.; Munoz-Rodriguez, J.L.; et al. Single-cell dissection of cellular components and interactions shaping the tumor immune phenotypes in ovarian cancer. Cancer Cell 2021, 39, 928–944.e6. [Google Scholar] [CrossRef] [PubMed]
- Piehl, N.; van Olst, L.; Ramakrishnan, A.; Teregulova, V.; Simonton, B.; Zhang, Z.; Tapp, E.; Channappa, D.; Oh, H.; Losada, P.M.; et al. Cerebrospinal fluid immune dysregulation during healthy brain aging and cognitive impairment. Cell 2022, 185, 5028–5039.e13. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Jiao, W.; Shen, B. The role of proinflammatory cytokines and CXC chemokines (CXCL1-CXCL16) in the progression of prostate cancer: Insights on their therapeutic management. Cell. Mol. Biol. Lett. 2024, 29, 73. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, N.; Tran, T.; Sam, I.; Pourmir, I.; Gruel, N.; Granier, C.; Pineau, J.; Gey, A.; Kobold, S.; Fabre, E.; et al. CXCR6 expressing T cells: Functions and role in the control of tumors. Front. Immunol. 2022, 13, 1022136. [Google Scholar] [CrossRef] [PubMed]
- Korbecki, J.; Bajdak-Rusinek, K.; Kupnicka, P.; Kapczuk, P.; Simińska, D.; Chlubek, D.; Baranowska-Bosiacka, I. The Role of CXCL16 in the Pathogenesis of Cancer and Other Diseases. Int. J. Mol. Sci. 2021, 22, 3490. [Google Scholar] [CrossRef] [PubMed]
| Signaling Pathways | Signaling Pattern | High-Pyroptosis MDMs | Low-Pyroptosis MDMs |
|---|---|---|---|
| CXCL | signaling pattern 1 | √ | × |
| VISFATIN | signaling pattern 1 | √ | √ |
| MHC-II | signaling pattern 1 | √ | √ |
| IGTB2 | signaling pattern 1 | √ | √ |
| IL1 | signaling pattern 1 | √ | √ |
| EGF | signaling pattern 1 | √ | √ |
| NECTIN | signaling pattern 1 | √ | √ |
| TNF | signaling pattern 1 | √ | √ |
| CD86 | signaling pattern 1 | √ | √ |
| CD39 | signaling pattern 1 | √ | √ |
| CD80 | signaling pattern 1 | √ | √ |
| IL10 | signaling pattern 1 | √ | √ |
| ALCAM | signaling pattern 1 | √ | √ |
| SEMA7 | signaling pattern 1 | √ | √ |
| SEMA4 | signaling pattern 1 | √ | √ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, L.; Xie, X.; Zhang, M.; Zhu, W.; Zhang, G.; Chen, W. Integrated Multi-Omics Profiling Reveals That Highly Pyroptotic MDMs Contribute to Psoriasis Progression Through CXCL16. Biomedicines 2025, 13, 1763. https://doi.org/10.3390/biomedicines13071763
Jin L, Xie X, Zhang M, Zhu W, Zhang G, Chen W. Integrated Multi-Omics Profiling Reveals That Highly Pyroptotic MDMs Contribute to Psoriasis Progression Through CXCL16. Biomedicines. 2025; 13(7):1763. https://doi.org/10.3390/biomedicines13071763
Chicago/Turabian StyleJin, Liping, Xiaowen Xie, Mi Zhang, Wu Zhu, Guanxiong Zhang, and Wangqing Chen. 2025. "Integrated Multi-Omics Profiling Reveals That Highly Pyroptotic MDMs Contribute to Psoriasis Progression Through CXCL16" Biomedicines 13, no. 7: 1763. https://doi.org/10.3390/biomedicines13071763
APA StyleJin, L., Xie, X., Zhang, M., Zhu, W., Zhang, G., & Chen, W. (2025). Integrated Multi-Omics Profiling Reveals That Highly Pyroptotic MDMs Contribute to Psoriasis Progression Through CXCL16. Biomedicines, 13(7), 1763. https://doi.org/10.3390/biomedicines13071763






