Cardioneuroablation for Vasovagal Syncope: An Updated Systematic Review and Single-Arm Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
3. Results
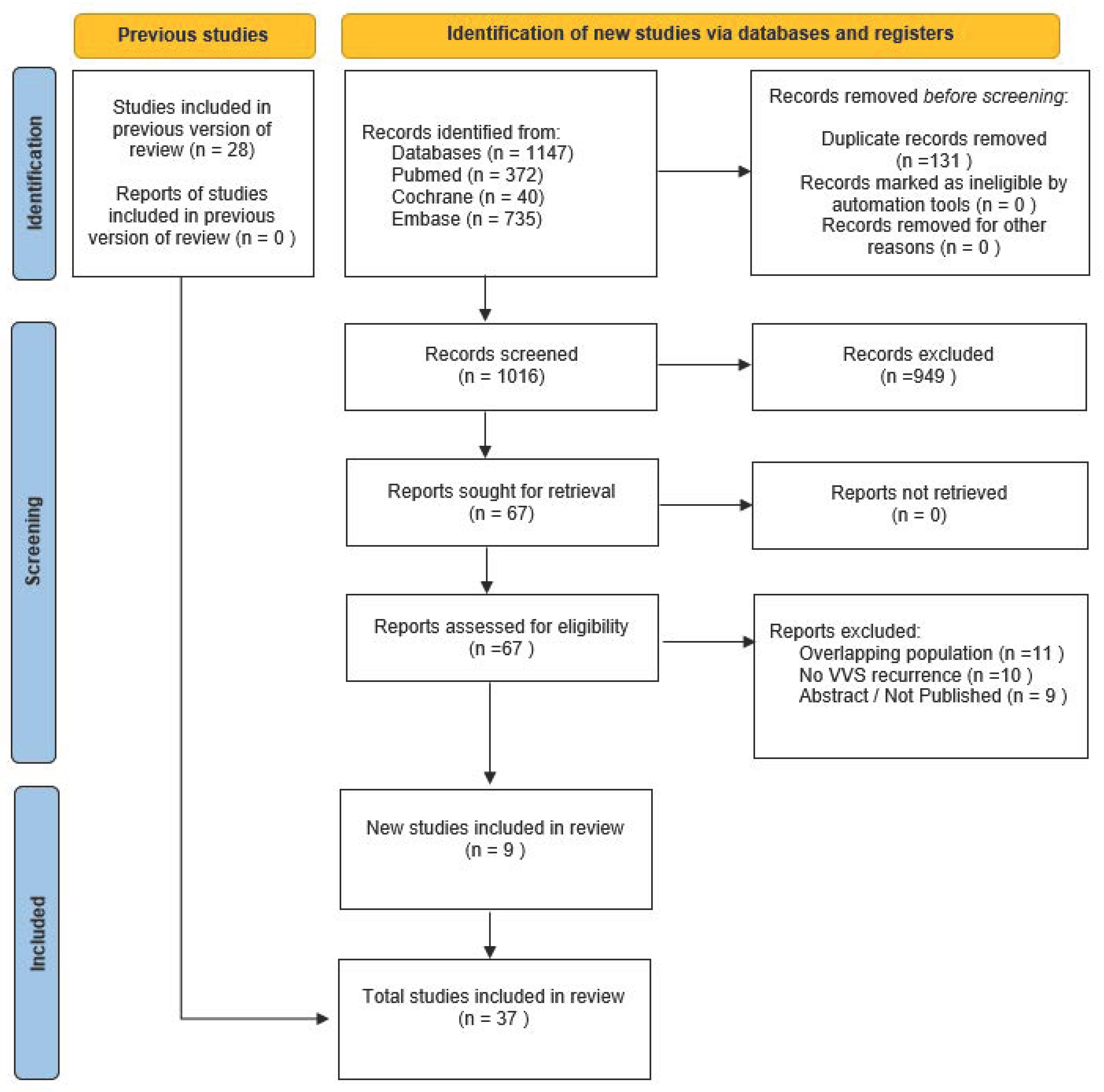
3.1. Study Selection and Baseline Characteristics
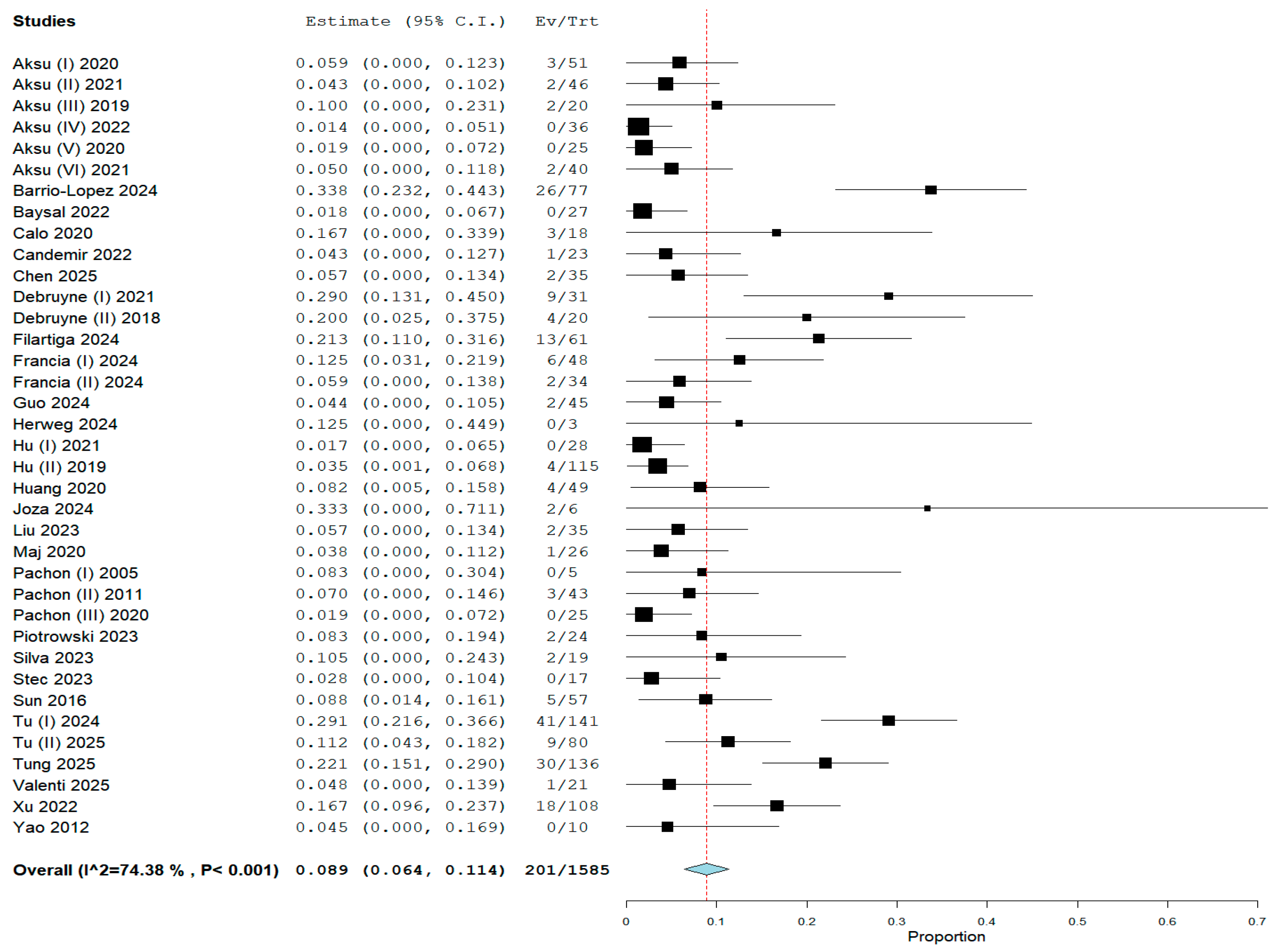
3.2. Pooled Analysis of the Primary Endpoint
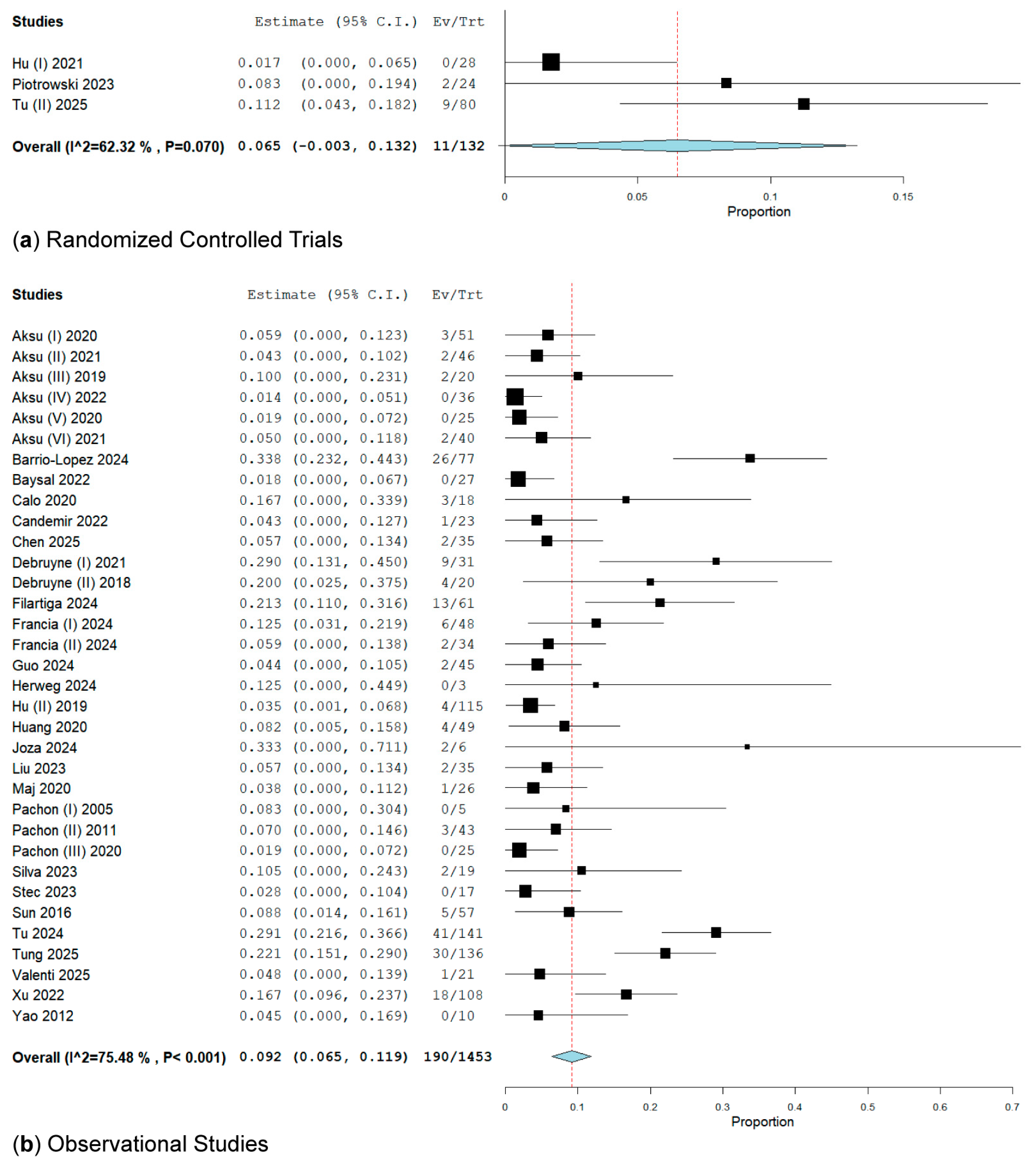
3.2.1. Subgroup Analysis of RCTs and Observational Studies on VVS Recurrence
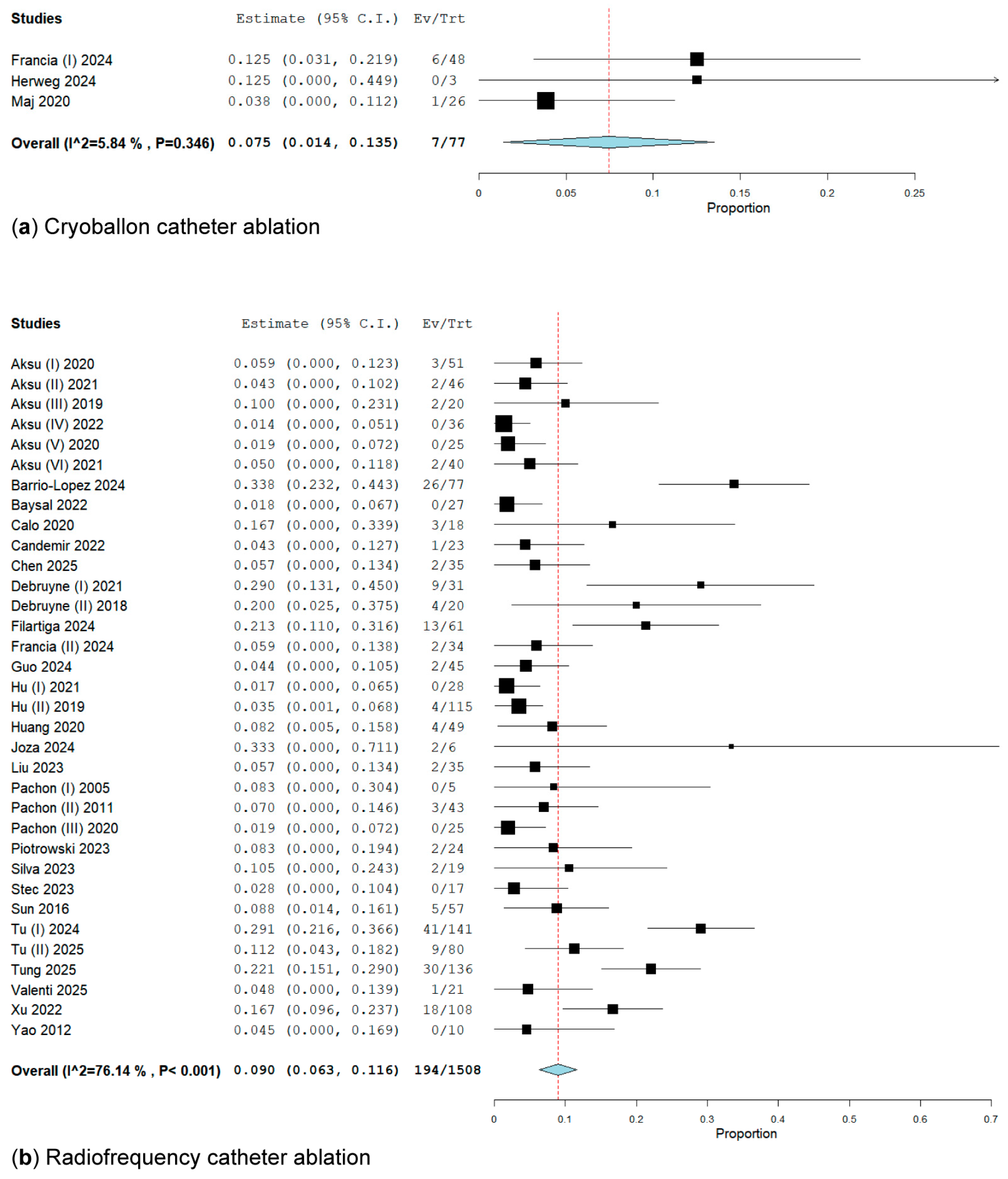
3.2.2. Subgroup Analysis of Energy Ablation Concerning the Primary Endpoint
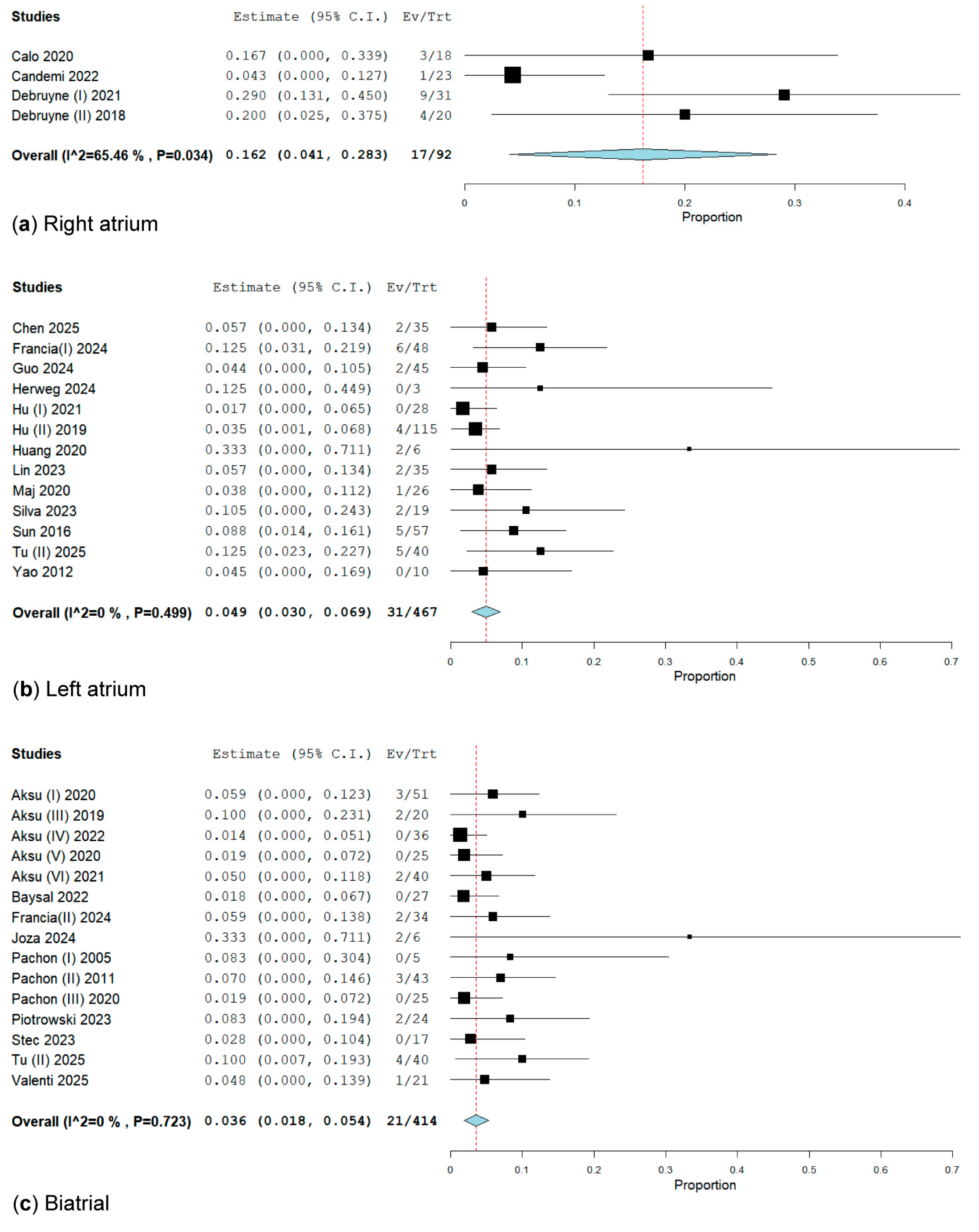
3.2.3. Subgroup Analysis of CNA Location for the Primary Endpoint
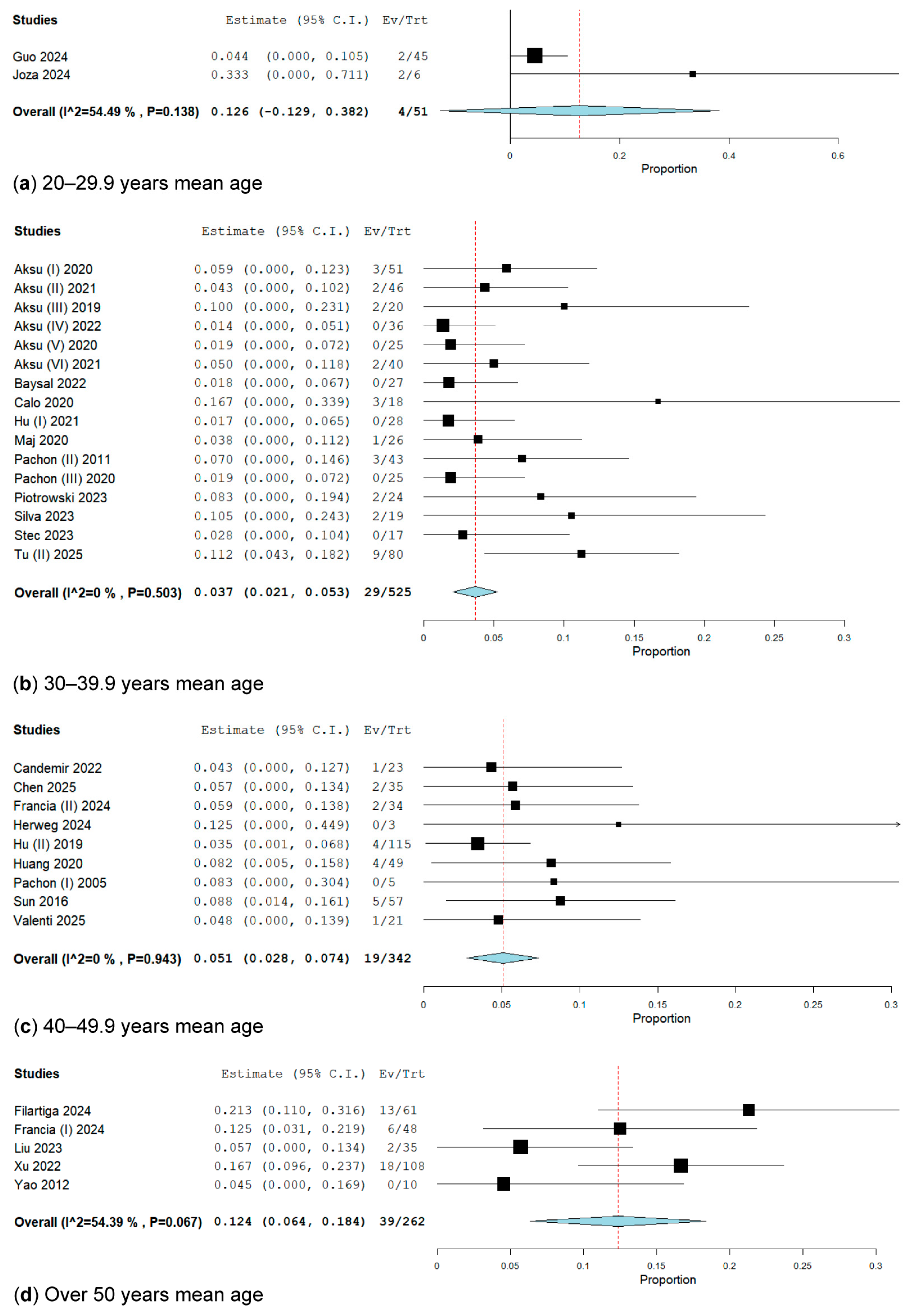
3.2.4. Subgroup Analysis by Age
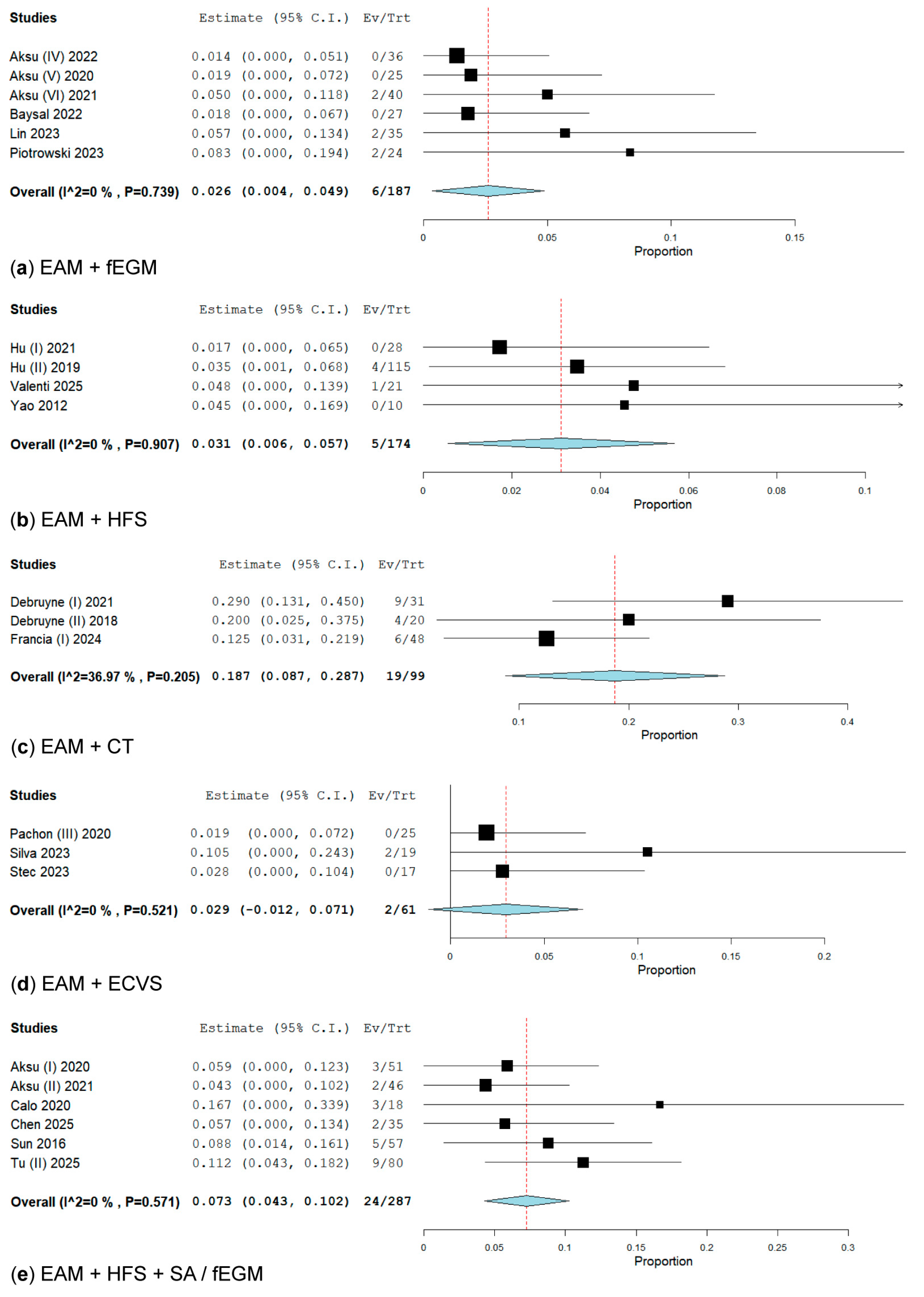
3.2.5. Subanalysis on an Additional Technique for Electrical Anatomical Mapping (EAM) When Performing GP Ablation
3.3. Secondary Outcomes Analysis
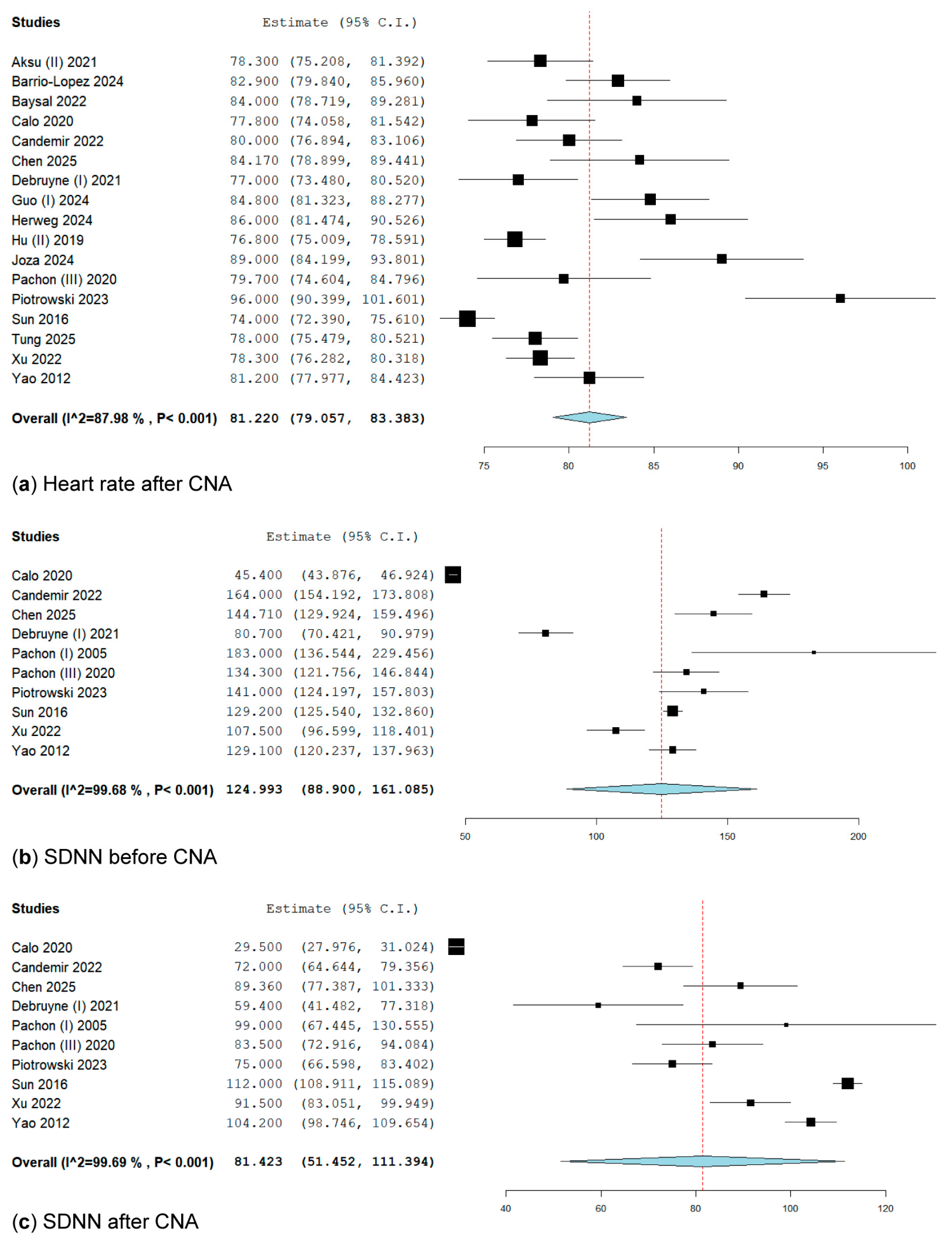
3.3.1. Heart Rate After Cardioneuroablation
3.3.2. SDNN Before Cardioneuroablation
3.3.3. SDNN After Cardioneuroablation
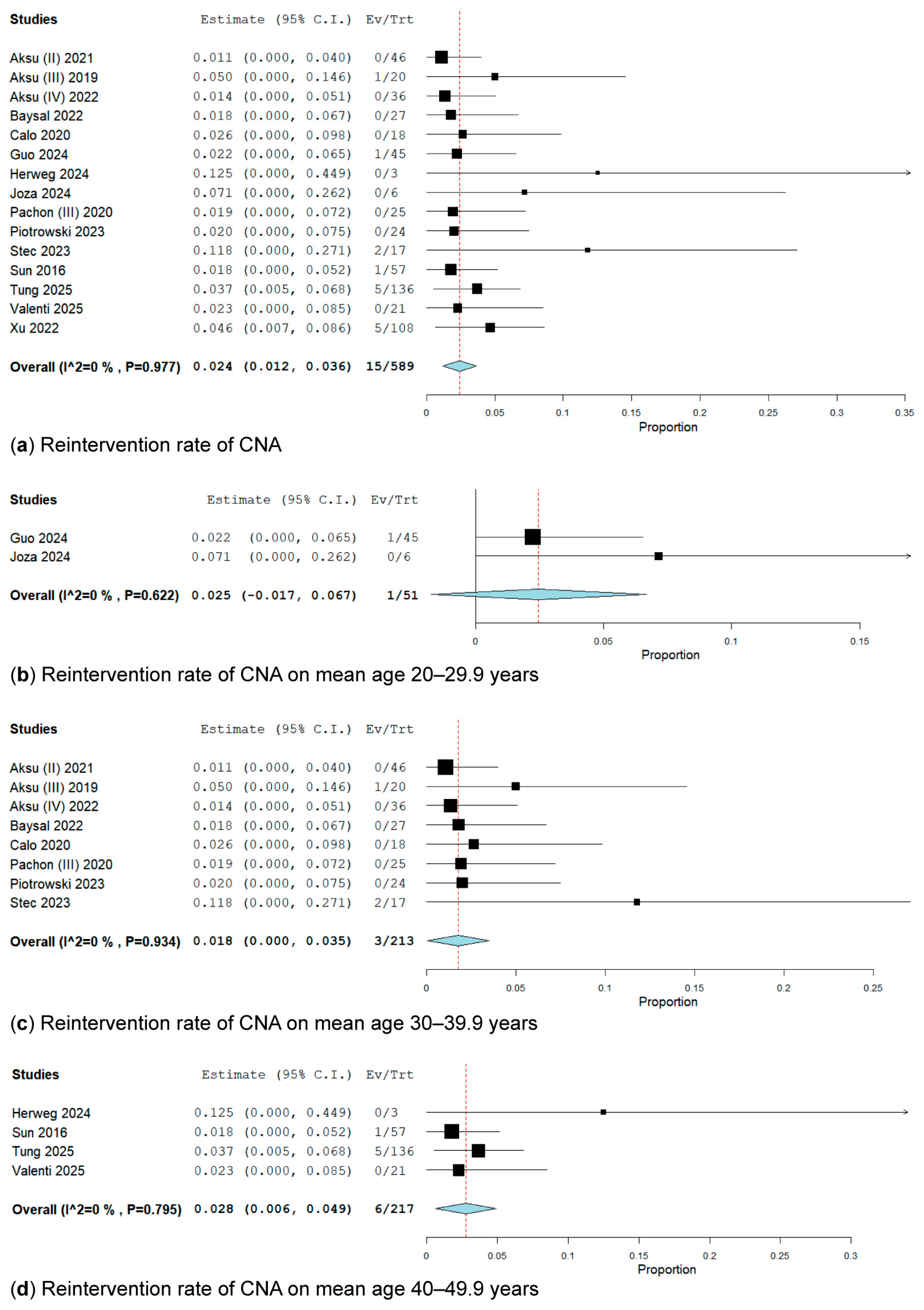
3.3.4. Reintervention Rate
3.4. Additional Heterogeneity Analysis: Meta-Regression and Baujat Plot
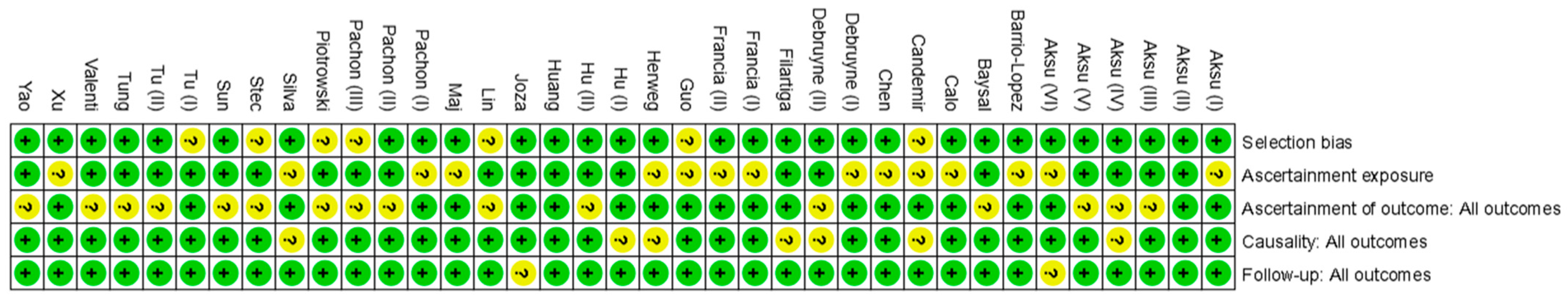
3.5. Quality Assessment
4. Discussion
5. Limitations, Strengths, and Future Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AV | Atrioventricular |
| BA | Biatrial |
| BPM | Beats per minute |
| CBA | Cryoballoon ablation |
| CI | Cardioinhibitory |
| CNA | Cardioneuroablation |
| CT | Computerised tomography |
| ECVS | Extracardiac vagal stimulation |
| EAM | Electroanatomic mapping |
| fEGM | Fractionated electrograms |
| GP | Ganglionated plexus |
| HDFM | High-density fractionation mapping |
| HFS | High-frequency stimulation |
| HR | Heart rate |
| I2 | I-squared statistic (heterogeneity) |
| LA | Left atrium |
| M | Mixed (type of syncope) |
| POTS | Postural orthostatic tachycardia syndrome |
| PC | Prospective cohort |
| PRISMA | Preferred reporting items for systematic reviews and meta-analyses |
| QM | Q-test for moderators |
| RCT | Randomised controlled trial |
| RA | Right atrium |
| RFA | Radiofrequency ablation |
| R | Recurrent (type of syncope) |
| REML | Restricted maximum likelihood |
| SA | Spectral analysis |
| SDNN | Standard deviation of normal-to-normal intervals |
| V | Vasodepressor (type of syncope) |
| VVS | Vasovagal syncope |
References
- Adkisson, W.O.; Benditt, D.G. Pathophysiology of reflex syncope: A review. J. Cardiovasc. Electrophysiol. 2017, 28, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Brignole, M.; Moya, A.; de Lange, F.J.; Deharo, J.-C.; Elliott, P.M.; Fanciulli, A.; Fedorowski, A.; Furlan, R.; Kenny, R.A.; Martín, A.; et al. 2018 ESC Guidelines for the diagnosis and management of syncope. Eur. Hear. J. 2018, 39, 1883–1948. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Karimi, Z.; Hemmati, M.; Mohammadi, A.; Shohaimi, S.; Mohammadi, M. Global prevalence of vasovagal syncope: A systematic review and meta-analysis. Glob. Epidemiol. 2024, 7, 100136. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.S.; Sheldon, A.G.; Connolly, S.J.; Morillo, C.A.; Klingenheben, T.; Krahn, A.D.; Koshman, M.; Ritchie, D.; for the Investigators of the Syncope Symptom Study and the Prevention of Syncope Trial. Age of First Faint in Patients with Vasovagal Syncope. J. Cardiovasc. Electrophysiol. 2006, 17, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Koene, R.J.; Adkisson, W.O.; Benditt, D.G. Syncope and the risk of sudden cardiac death: Evaluation, management, and prevention. J. Arrhythmia 2017, 33, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Barón-Esquivias, G.; Quintanilla, M.; Díaz-Martín, A.J.; Barón-Solís, C.; Almeida-González, C.V.; García-Romero, C.; Paneque, I.; Rubio-Guerrero, C.; Rodríguez-Corredor, R.; Valle-Racero, J.I.; et al. Long-term recurrences and mortality in patients with noncardiac syncope. Rev. Esp. Cardiol. 2021, 75, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Tajdini, M.; Tavolinejad, H.; Aminorroaya, A.; Aryan, Z.; Jalali, A.; Alaeddini, F.; Sadeghian, S.; Yadangi, S.; Vasheghani-Farahani, A.; Kalhor, P.; et al. Clinical Associations of Injuries Caused by Vasovagal Syncope: A Cohort Study From a Tertiary Syncope Unit. J. Am. Hear. Assoc. 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.S.; Seifer, C.; Parkash, R.; Sandhu, R.K.; Hamzeh, R.; Raj, S.R. Atomoxetine for suppression of vasovagal syncope. Clin. Auton. Res. 2022, 33, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Raviele, A.; Brignole, M.; Sutton, R.; Alboni, P.; Giani, P.; Menozzi, C.; Moya, A. Effect of Etilefrine in Preventing Syncopal Recurrence in Patients With Vasovagal Syncope. Circulation 1999, 99, 1452–1457. [Google Scholar] [CrossRef] [PubMed]
- Izcovich, A.; Malla, C.G.; Manzotti, M.; Catalano, H.N.; Guyatt, G. Midodrine for orthostatic hypotension and recurrent reflex syncope. Neurology 2014, 83, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Flevari, P.; Leftheriotis, D.; Repasos, E.; Katsaras, D.; Katsimardos, A.; Lekakis, J. Fluoxetine vs. placebo for the treatment of recurrent vasovagal syncope with anxiety sensitivity. EP Eur. 2016, 19, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Tajdini, M.; Behnoush, A.H.; Khalaji, A.; Raj, S.R. Vasovagal Syncope: A Review of Current and Emerging Therapies for a Common Cardiology Condition. J. Tehran Univ. Hear. Cent. 2024, 19, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Aksu, T.; Guler, T.E.; Bozyel, S.; Ozcan, K.S.; Yalin, K.; Mutluer, F.O. Cardioneuroablation in the treatment of neurally mediated reflex syncope: A review of the current literature. Turk Kardiyol. Dern. Ars.-Arch. Turk. Soc. Cardiol. 2016, 45, 4–41. [Google Scholar] [CrossRef] [PubMed]
- Aksu, T.; Brignole, M.; Calo, L.; Debruyne, P.; Di Biase, L.; Deharo, J.C.; Fanciulli, A.; Fedorowski, A.; Kulakowski, P.; Morillo, C.; et al. Cardioneuroablation for the treatment of reflex syncope and functional bradyarrhythmias: A Scientific Statement of the European Heart Rhythm Association (EHRA) of the ESC, the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS) and the Latin American Heart Rhythm Society (LAHRS). EP Eur. 2024, 26. [Google Scholar] [CrossRef]
- Pachon, J.C.; Pachon, E.I.; Pachon, M.Z.C.; Lobo, T.J.; Pachon, J.C.; Santillana, T.G. Catheter ablation of severe neurally meditated reflex (neurocardiogenic or vasovagal) syncope: Cardioneuroablation long-term results. EP Eur. 2011, 13, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Brignole, M.; Arabia, F.; Ammirati, F.; Tomaino, M.; Quartieri, F.; Rafanelli, M.; Del Rosso, A.; Vecchi, M.R.; Russo, V.; Gaggioli, G. Standardized algorithm for cardiac pacing in older patients affected by severe unpredictable reflex syncope: 3-year insights from the Syncope Unit Project 2 (SUP 2) study. Europace 2015, 18, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Aksu, T.; Guler, T.E.; Bozyel, S.; Yalin, K.; Gopinathannair, R. Usefulness of post-procedural heart rate response to predict syncope recurrence or positive head up tilt table testing after cardioneuroablation. EP Eur. 2020, 22, 1320–1327. [Google Scholar] [CrossRef] [PubMed]
- Aksu, T.; Guler, T.E.; Bozyel, S.; Golcuk, S.E.; Yalin, K.; Lakkireddy, D.; Gopinathannair, R. Medium-term results of cardioneuroablation for clinical bradyarrhythmias and vasovagal syncope: Effects on QT interval and heart rate. J. Interv. Card. Electrophysiol. 2020, 60, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Aksu, T.; Guler, T.E.; Mutluer, F.O.; Bozyel, S.; Golcuk, S.E.; Yalin, K. Electroanatomic-mapping-guided cardioneuroablation versus combined approach for vasovagal syncope: A cross-sectional observational study. J. Interv. Card. Electrophysiol. 2018, 54, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Aksu, T.; De Potter, T.; John, L.; Osorio, J.; Singh, D.; Alyesh, D.; Baysal, E.; Kumar, K.; Mikaeili, J.; Forno, A.D.; et al. Procedural and short-term results of electroanatomic-mapping-guided ganglionated plexus ablation by first-time operators: A multicenter study. J. Cardiovasc. Electrophysiol. 2021, 33, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Aksu, T.; Guler, T.E.; Bozyel, S.; Yalin, K. Vagal responses during cardioneuroablation on different ganglionated plexi: Is there any role of ablation strategy? Int. J. Cardiol. 2020, 304, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Aksu, T.; Yalin, K.; John, L.; Osorio, J.; Winterfield, J.; Aras, D.; Gopinathannair, R. Effect of conscious sedation and deep sedation on the vagal response characteristics during ganglionated plexus ablation. J. Cardiovasc. Electrophysiol. 2021, 32, 2333–2336. [Google Scholar] [CrossRef] [PubMed]
- Barrio-Lopez, M.T.; Álvarez-Ortega, C.; Minguito-Carazo, C.; Franco, E.; García-Granja, P.E.; Alcalde-Rodríguez, Ó.; Salvador-Montañés, Ó.; Francisco-Pascual, J.; Macías-Ruíz, R.; Del Castillo, Á.M.; et al. Predictors of Clinical Success of Cardioneuroablation in Patients With Syncope. JACC Clin. Electrophysiol. 2024, 10, 2711–2724. [Google Scholar] [CrossRef] [PubMed]
- Baysal, E.; Mutluer, F.O.; Dagsali, A.E.; Kumrulu, U.C.; Huang, H.D.; Aksu, T. Improved health-related quality of life after cardioneuroablation in patients with vasovagal syncope. J. Interv. Card. Electrophysiol. 2022, 68, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Calo, L.; Rebecchi, M.; Sette, A.; Sciarra, L.; Borrelli, A.; Scara, A.; Grieco, D.; Politano, A.; Sgueglia, M.; De Luca, L.; et al. Catheter ablation of right atrial ganglionated plexi to treat cardioinhibitory neurocardiogenic syncope: A long-term follow-up prospective study. J. Interv. Card. Electrophysiol. 2020, 61, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Candemir, B.; Baskovski, E.; Beton, O.; Cardiology, D.B.N.P.H.C.O.; Shanableh, N.; Akbulut, I.M.; Kozluca, V.; Esenboga, K.; Tan, T.S.; Altin, T.; et al. Procedural Characteristics, Safety, and Follow-up of Modified Right-Sided Approach for Cardioneuroablation. Anatol. J. Cardiol. 2022, 26, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Li, Y.; Liu, Y.; Shen, G.; Xiong, G.; Wu, B.; Liu, Y.; Huang, X.; Li, H.; Zhou, H.; et al. Efficacy of cardioneuroablation for vasodepressor vasovagal syncope. Front. Neurosci. 2025, 19, 1514513. [Google Scholar] [CrossRef] [PubMed]
- Debruyne, P.; Rossenbacker, T.; Janssens, L.; Collienne, C.; Ector, J.; Haemers, P.; Waroux, J.-B.l.P.d.; Bazelmans, C.; Boussy, T.; Wijns, W. Durable Physiological Changes and Decreased Syncope Burden 12 Months After Unifocal Right-Sided Ablation Under Computed Tomographic Guidance in Patients With Neurally Mediated Syncope or Functional Sinus Node Dysfunction. Circ. Arrhythmia Electrophysiol. 2021, 14, e009747. [Google Scholar] [CrossRef] [PubMed]
- Debruyne, P.; Rossenbacker, T.; Collienne, C.; Roosen, J.; Ector, B.; Janssens, L.; Charlier, F.; Vankelecom, B.; Dewilde, W.; Wijns, W. Unifocal Right-Sided Ablation Treatment for Neurally Mediated Syncope and Functional Sinus Node Dysfunction Under Computed Tomographic Guidance. Circ. Arrhythmia Electrophysiol. 2018, 11, e006604. [Google Scholar] [CrossRef] [PubMed]
- Filartiga, D.V.; Francia, P.; Penela, D.; Falasconi, G.; Soto-Iglesias, D.; Ocana, P.F.; Dharandas, R.S.A.; Armenta, J.F.; Alderete, J.; Bellido, A.; et al. Differential and synergistic effects of left and right atrial ablation of paraseptal ganglionated plexuses in a cohort of consecutive patients undergoing cardioneuroablation. EP Eur. 2024, 26. [Google Scholar] [CrossRef]
- Francia, P.; Viveros, D.; Gigante, C.; Falasconi, G.; Penela, D.; Soto-Iglesias, D.; Landra, F.; Teresi, L.; Marti-Almor, J.; Alderete, J.; et al. Differential and synergistic effects of right and left atrial ganglionated plexi ablation in patients undergoing cardioneuroablation: Results from the ELEGANCE multicenter study. J. Interv. Card. Electrophysiol. 2024, 68, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Francia, P.; Viveros, D.; Falasconi, G.; Penela, D.; Soto-Iglesias, D.; Martí-Almor, J.; Alderete, J.; Saglietto, A.; Bellido, A.F.; Franco-Ocaña, P.; et al. Clinical impact of aging on outcomes of cardioneuroablation for reflex syncope or functional bradycardia: Results from the cardionEuroabLation: PatiEnt selection, imaGe integrAtioN and outComEs—The ELEGANCE multicenter study. Hear. Rhythm. 2023, 20, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, Y.; Li, S.; Ma, J.; Liu, J.; Ruan, Y.; Zhang, J. Comparative study of the therapeutic effects of radiofrequency ablation of ganglionated plexi guided by high-frequency stimulation and anatomical localization methods in the treatment of vagal syncope in young people. Cardiology 2024, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Herweg, B.; Patel, R.S.; Noujaim, S.; Spano, J.; Mencer, N.; Vijayaraman, P. Cryoballoon cardioneuroablation: New electrophysiological insights. Hear. Rhythm. O2 2024, 5, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Zheng, L.; Liang, E.; Ding, L.; Wu, L.; Chen, G.; Fan, X.; Yao, Y. Right anterior ganglionated plexus: The primary target of cardioneuroablation? Hear. Rhythm. 2019, 16, 1545–1551. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Zheng, L.; Liu, S.; Shen, L.; Liang, E.; Liu, L.; Wu, L.; Ding, L.; Yao, Y. The impacts of the ganglionated plexus ablation sequence on the vagal response, heart rate, and blood pressure during cardioneuroablation. Auton. Neurosci. 2021, 233, 102812. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, Y.; Huang, Y.; Zhao, H.; He, L.; Tan, Z.; Xu, D.; Peng, J. Comparative effects of intensive ganglionated plexus ablation in treating paroxysmal atrial fibrillation and vasovagal syncope. Clin. Cardiol. 2020, 43, 1326–1333. [Google Scholar] [CrossRef] [PubMed]
- Joza, J.; da Rosa, L.G.B.; Alturki, A.; Anglesio, V.; Sanchez-Somonte, P.; Poletaev, V.; Bernier, M.; Verma, A.; Essebag, V. Cardioneuroablation as a strategy to prevent pacemaker implantation in young patients with vasovagal syncope. IJC Hear. Vasc. 2024, 51, 101360. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Liu, Q.; Jiang, R.; Chen, S.; Yu, L.; Jiang, C. Selective anatomical catheter ablation of left atrial ganglionated plexus for vasovagal syncope: Left superior and right anterior ganglionated plexus ablation. Pacing Clin. Electrophysiol. 2023, 46, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Maj, R.; Osório, T.G.; Borio, G.; Iacopino, S.; Ströker, E.; Sieira, J.; Terasawa, M.; Kazawa, S.; Rizzo, A.; Galli, A.; et al. A novel strategy to treat vaso-vagal syncope: Cardiac neuromodulation by cryoballoon pulmonary vein isolation. Indian Pacing Electrophysiol. J. 2020, 20, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Pachon, J.C.; Pachon, E.I.; Lobo, T.J.; Pachon, M.Z.; Vargas, R.N.; Jatene, A.D. “Cardioneuroablation”—New treatment for neurocardiogenic syncope, functional AV block and sinus dysfunction using catheter RF-ablation. EP Eur. 2005, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Pachon-M, E.I.; Pachon, C.T.C.; Santillana-P, T.G.; Lobo, T.J.; Pachon-M, J.C.; Zerpa-A, J.C.; Cunha-P, M.Z.; Higuti, C.; Ortencio, F.A.; Amarante, R.C.; et al. Long-Term Evaluation of the Vagal Denervation by Cardioneuroablation Using Holter and Heart Rate Variability. Circ. Arrhythmia Electrophysiol. 2020, 13. [Google Scholar] [CrossRef] [PubMed]
- Piotrowski, R.; Baran, J.; Sikorska, A.; Krynski, T.; Kulakowski, P. Cardioneuroablation for Reflex Syncope. JACC Clin. Electrophysiol. 2022, 9, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zheng, L.; Qiao, Y.; Shi, R.; Hou, B.; Wu, L.; Guo, J.; Zhang, S.; Yao, Y. Catheter Ablation as a Treatment for Vasovagal Syncope: Long-Term Outcome of Endocardial Autonomic Modification of the Left Atrium. J. Am. Hear. Assoc. 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.S.; Fonseca, P.; Cardoso, F.; Almeida, J.; Ribeiro, S.; Oliveira, M.; Sanfins, V.; Gonçalves, H.; Pachon, M.J.C.; Barra, S.; et al. Cardioneuroablation for severe neurocardiogenic syncope. Rev. Port. Cardiol. 2023, 42, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Stec, S.; Wileczek, A.; Reichert, A.; Śledź, J.; Kosior, J.; Jagielski, D.; Polewczyk, A.; Zając, M.; Kutarski, A.; Karbarz, D.; et al. Shared Decision Making and Cardioneuroablation Allow Discontinuation of Permanent Pacing in Patients with Vagally Mediated Bradycardia. J. Cardiovasc. Dev. Dis. 2023, 10, 392. [Google Scholar] [CrossRef] [PubMed]
- Tu, B.; Lai, Z.-H.; Chen, A.-Y.; Weng, Z.-Y.; Cai, S.-M.; Zhang, Z.-X.; Zhou, L.-K.; Zheng, L.-H.; Yao, Y. Effectiveness of cardioneuroablation in different subtypes of vasovagal syncope. J. Geriatr. Cardiol. 2024, 21, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Tu, B.; Chen, A.; Cai, S.; Zhang, Z.; Zhou, L.; Lai, Z.; Maimaitijiang, P.; Hu, Z.; Wu, L.; Ding, L.; et al. The Efficacy of Left Atrial vs Biatrial Cardioneuroablation in Patients With Vasovagal Syncope. JACC: Clin. Electrophysiol. 2025, 11, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- Tung, R.; Pujol-Lopez, M.; Locke, A.H.; Alyesh, D.M.; Sundaram, S.; Shah, A.D.; Kumar, V.; Kowlgi, G.; Kumar, K.; Shvilkin, A.; et al. Cardioneuroablation for functional bradycardia and vasovagal syncope outcomes from the US multicenter cna registry. JACC Clin. Electrophysiol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Valenti, N.; Di Monaco, A.; Romanazzi, I.; Vitulano, N.; Troisi, F.; Quadrini, F.; Vitullo, A.; Sgarra, L.; Caruso, R.; Anzelmo, V.; et al. Cardioneuroablation for reflex syncope or functional bradyarrhytmias: New insight from a single center experience. Front. Cardiovasc. Med. 2025, 11, 1526825. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhao, Y.; Duan, Y.; Wang, R.; Hou, J.; Wang, J.; Chen, B.; Yang, Y.; Xue, X.; Zhao, Y.; et al. Clinical Efficacy of Catheter Ablation in the Treatment of Vasovagal Syncope. J. Clin. Med. 2022, 11, 5371. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Shi, R.; Wong, T.; Zheng, L.; Chen, W.; Yang, L.; Huang, W.; Bao, J.; Zhang, S. Endocardial Autonomic Denervation of the Left Atrium to Treat Vasovagal Syncope. Circ. Arrhythmia Electrophysiol. 2012, 5, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Vandenberk, B.; Lei, L.Y.; Ballantyne, B.; Vickers, D.; Liang, Z.; Sheldon, R.S.; Chew, D.S.; Aksu, T.; Raj, S.R.; Morillo, C.A. Cardioneuroablation for vasovagal syncope: A systematic review and meta-analysis. Hear. Rhythm. 2022, 19, 1804–1812. [Google Scholar] [CrossRef] [PubMed]
- Prata, A.A.; Katsuyama, E.; Scardini, P.; Antunes, V.; Granja, J.; Coan, A.C.; Fukunaga, C.; Mateos, J.C.P. Cardioneuroablation in patients with vasovagal syncope: An updated systematic review and meta-analysis. Hear. Rhythm. 2024, 22, 526–535. [Google Scholar] [CrossRef] [PubMed]
| Study and Year | Design | Sample Size | Location of Ablation | Type of Ablation | Method of Ablation | Age | Female, n (%) | Type of Syncope | Syncope’s Burden | Follow-up (Months) |
|---|---|---|---|---|---|---|---|---|---|---|
| Aksu (I) (2020) [18] | RCT | 51 | BA | RFA | EAM/HFS + SA | 35.5 ± 12.2 | 19 (37.25) | R | 5.5 ± 2.9 | 22.2 |
| Aksu (II) (2021) [19] | PC | 46 | BA | RFA | EAM + HFS + SA fEGM | 39.4 ± 14 | 27 (41.5) | R | 4.7 ± 3 | 14.9 |
| RA | RFA | HFS + SA/EAM | ||||||||
| Aksu (III) (2019) [20] | PC | 20 | BA | RFA | EAM + fEGM | 36 ± 12.8 | 10 (50) | R | NA | 24 |
| RFA | EAM + HFS | |||||||||
| Aksu (IV). (2022) [21] | RC | 36 | BA | RFA | EAM + fEGM | 35.1 ± 14 | 19 (52.77) | R | 6.7 ± 4 | 8 |
| Aksu (V) (2020) [22] | RC | 25 | BA | RFA | EAM + fEGM | 39.6. ± 14 | 16 (32.7) | R | 2.8 ± 1.9 | 9.5 |
| Aksu (VI) (2021) [23] | RC | 40 | BA | RFA | EAM + fEGM | 36 ± 13 | 17 (42.5) | R, CI | 6.2 ± 3.4 | 12.1 |
| Barrio-Lopez (2024) [24] | RC | 77 | BA, RA, LA | RFA | EAM + fEGM + HFS + ECVS | 49.3 ± 13.4 | 42 (54.5) | R, CI, M | 6.9 ± 3.11 | 6 |
| Baysal (2022) [25] | PC | 27 | BA | RFA | EAM + fEGM | 34 ± 14 | 13 (48.14) | R | 5.5 ± 5.56 | 12 |
| Calo (2020) [26] | PC | 18 | RA | RFA | EAM + fEGM + HFS + SA | 36.9 ± 11.2 | 10 (55.55) | CI | 5.2 ± 1.4 | 34.1 |
| Candemir (2022) [27] | RC | 23 | RA | RFA | EAM | 40.7 ± 13.2 | 10 (43.47) | R, CI, M | 3.3 ± 1.3 | 10 |
| Chen (2025) [28] | RC | 35 | LA | RFA | HFS + EAM | 47.48 ± 16.49 | 17 (48.57) | R, CI, M, V | 4.14 ± 4.45 | 11 |
| Debruyne (I) (2021) [29] | PC | 31 | RA | RFA | EAM + CT | 39.6 ± 9 | 16 (51.61) | R | 13.3 ± 22.3 | 12 |
| Debruyne (II) (2018) [30] | PC | 20 | RA | RFA | EAM + CT | NA | 6 (50) | M, CI | NA | 8.7 |
| Filartiga (2024) [31] | PC | 61 | BA | RFA | EAM | 50 ± 15 | 24 (39) | NA | NA | 16 |
| BA | 19 | |||||||||
| Francia (I) (2024) [32] | RC | 48 | LA | CBA | EAM + CT | 51 ± 16 | 23 (47.91) | R | NA | 10.2 |
| Francia (II) (2024) [33] | RC | 34 | BA | RFA | EAM + CT + fEGM | 47.9 ± 17.2 | 11 (32.35) | R | 4.1 ± 4.03 | 24.5 |
| Guo (2024) [34] | RC | 45 | LA | RFA | EAM | 22.5 ± 4.4 | 2 (4.44) | R | 3.44 ± 1 | 15 |
| RFA | HFS | |||||||||
| Herweg (2024) [35] | PC | 3 | LA | CBA | EAM | 48 ± 14.7 | 1 (33.33) | R | 2.6 ± 0.6 | 5.94 |
| Hu (I) (2021) [36] | RCT | 28 | LA | RFA | EAM + HFS | 39.5 ± 11.06 | 20 (71.42) | R | 5 ± 3.51 | 16.5 |
| Hu (II) (2019) [37] | RC | 115 | LA | RFA | EAM + HFS | 42.9 ± 17.9 | 69 (60) | R | 3.5 ± 4.4 | 21.4 |
| Huang (2020) [38] | PC | 49 | LA | RFA | EAM | 42.4 ± 16.1 | 27 (55.1) | R | NA | 16.4 |
| Joza (2024) [39] | PC | 6 | BA | RFA | EAM + fEGM + HFS | 29 ± 4 | 4 (67) | CI | 14 ± 7.2 | 13.4 |
| Lin (2023) [40] | RC | 35 | LA | RFA | EAM + fEGM | 53.9 ± 13.6 | 42 (60) | R, CI, M, V | 9.6 ± 15.4 | 12 |
| Maj (2020) [41] | RC | 26 | LA | CBA | NA | 37.5 ± 9.0 | 6 (23.1) | CI | 2.6 ± 0.8 | 20.1 |
| Pachon (I) (2005) [42] | RC | 5 | BA | RFA | EAM + SA | 47.5 ± 16 | NA | CI | NA | 9.2 |
| Pachon (II) (2011) [15] | PC | 43 | BA | RFA | EAM + SA | 32.9 ± 15 | 18 (41.9) | M, CI | 4.7 ± 2 | 45.1 |
| Pachon (III) (2020) [43] | PC | 25 | BA | RFA | EAM + ECVS | 36.3 ± 19 | 40 (48) | R | 3.8 ± 2 | 40 |
| Piotrowski (2022) [44] | RCT | 24 | BA | RFA | EAM + fEGM | 38 ± 10 | 26 (54.16) | R | 3 ± 3 | 24 |
| Sun (2016) [45] | PC | 57 | LA | RFA | EAM + HFS | 43.2 ± 13.4 | 35 (61.4) | R | 3 ± 0.87 | 36.4 |
| Silva (2023) [46] | PC | 19 | LA | RFA | EAM + ECVS | 37.8 ± 12.9 | 6 (32) | R | NA | 21.0 |
| Stec (2023) [47] | PC | 17 | BA | RFA | EAM + ECVS | 37.7 ± 1.47 | 8 (40) | CI | NA | 20.8 |
| Tu (I) (2024) [48] | RC | 141 | LA | RFA | EAM + HFS | 40 ± 18 | 90 (63.82) | R, CI, M, V | 2.6 ± 2.22 | 51.6 |
| Tu (II) (2025) [49] | RCT | 80 | BA | RFA | EAM + HFS + SA/fEGM | 38 ± 16 | 43 (53.8) | M, CI | 3.06 ± 0.62 | 12 |
| LA | RFA | HFS + SA/EAM | ||||||||
| Tung (2025) [50] | RC | 136 | BA, RA, LA | RFA | HFS + HDFM | 47.4 ± 17 | 18 (41.9) | R, M, V | 7.16 ± 2 | 14 |
| Valenti (2025) [51] | RC | 21 | BA | RFA | EAM + HFS | 42 ± 21 | 8 (38) | R | NA | 12 |
| Xu (2022) [52] | RC | 108 | LA | RFA | EAM | 51.2 ± 15.3 | 48 (44.44) | M, V | 2.58 ± 1.77 | 10 |
| RFA | HFS | |||||||||
| Yao (2012) [53] | PC | 10 | LA | RFA | EAM + HFS | 50.4 ± 6.4 | 7 (70) | R | 7.1 ± 13.33 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ababei, A.; Ursu, C.G.; Bistriceanu, M.I.A.; Andreescu, D.I.; Iurea, I.-M.; Budeanu, B.; Dumitrache, A.E.; Hostiuc, A.; Sturz-Lazar, M.-C.; Toma, C.-V.; et al. Cardioneuroablation for Vasovagal Syncope: An Updated Systematic Review and Single-Arm Meta-Analysis. Biomedicines 2025, 13, 1758. https://doi.org/10.3390/biomedicines13071758
Ababei A, Ursu CG, Bistriceanu MIA, Andreescu DI, Iurea I-M, Budeanu B, Dumitrache AE, Hostiuc A, Sturz-Lazar M-C, Toma C-V, et al. Cardioneuroablation for Vasovagal Syncope: An Updated Systematic Review and Single-Arm Meta-Analysis. Biomedicines. 2025; 13(7):1758. https://doi.org/10.3390/biomedicines13071758
Chicago/Turabian StyleAbabei, Alexandru, Cosmin Gabriel Ursu, Mircea Ioan Alexandru Bistriceanu, Darie Ioan Andreescu, Iasmina-Maria Iurea, Beatrice Budeanu, Adriana Elena Dumitrache, Alexandra Hostiuc, Maria-Celina Sturz-Lazar, Cristian-Valentin Toma, and et al. 2025. "Cardioneuroablation for Vasovagal Syncope: An Updated Systematic Review and Single-Arm Meta-Analysis" Biomedicines 13, no. 7: 1758. https://doi.org/10.3390/biomedicines13071758
APA StyleAbabei, A., Ursu, C. G., Bistriceanu, M. I. A., Andreescu, D. I., Iurea, I.-M., Budeanu, B., Dumitrache, A. E., Hostiuc, A., Sturz-Lazar, M.-C., Toma, C.-V., Busnatu, S. S., Deaconu, A., & Bogdan, S. (2025). Cardioneuroablation for Vasovagal Syncope: An Updated Systematic Review and Single-Arm Meta-Analysis. Biomedicines, 13(7), 1758. https://doi.org/10.3390/biomedicines13071758











